Abstract
Background
Early decrease in Ki67 after a short preoperative course of endocrine therapy (ET) has shown prognostic and predictive value in clinical research, but its applicability and reproducibility in routine clinical practice remain largely unknown. We therefore assessed on-treatment Ki67 changes following a short preoperative ET and its association with biological variables, such as intrinsic subtype and risk of recurrence (ROR), plus long-term outcomes, in a real-world cohort of patients with early estrogen receptor-positive, human epidermal growth factor receptor 2-negative (ER+/HER2-negative) breast cancer.
Methods
We conducted a retrospective, registry-based analysis of 230 consecutive patients with early ER+/HER2− breast cancer treated as per standard clinical care at the Breast Unit of the Clinic Barcelona Comprehensive Cancer Center between 2014 and 2023. All patients received preoperative ET, tamoxifen, or an aromatase inhibitor (AI), for 2-12 weeks before surgery. Clinical and pathological variables were collected and stratified by Ki67 response: “responders” (post-treatment Ki67 0% to 10%) and “complete cell cycle arrest (CCCA) responders” (Ki67 ≤2.7%). PAM50/Prosigna was used to determine intrinsic subtypes and ROR-score. Event-free survival was estimated using Kaplan–Meier curves, and associations were tested using Cox proportional hazards regression.
Results
The median duration of preoperative ET was 5 weeks (min-max range, 2-12 weeks). Overall, 196 patients (85.2%) met the Ki67 response criterion and 111 (48.3%) achieved CCCA. Response rates were significantly higher in postmenopausal compared with premenopausal women (P = 0.004). Notably, 95.6% of postmenopausal patients received an AI, whereas all premenopausal women were treated with tamoxifen. Additionally, response varied by intrinsic subtype, favoring Luminal A tumors (P = 0.047). In multivariable models, postmenopausal status and higher baseline ER expression were independently associated with both Ki67 response and CCCA, whereas a lower baseline ROR-score predicted CCCA. After a median follow-up of 47 months, CCCA was associated with significantly improved event-free survival [hazard ratio (HR) = 0.19; 95% CI (confidence interval) 0.05-0.72; P value = 0.012].
Conclusion
In routine practice, a short course of preoperative ET yields substantial reductions in tumor proliferation. Early assessment of Ki67 suppression offers a readily accessible indicator of endocrine sensitivity, and achieving CCCA identifies patients who have a more favorable prognosis and thus are potentially eligible to de-escalate in treatment strategies.
Key words: preoperative endocrine therapy, Ki67, complete cell cycle arrest, dynamic biomarkers
Highlights
-
•
Short-course preoperative ET suppresses Ki67 in early ER+/HER2-negative BC.
-
•
CCCA (Ki67 ≤2.7%) predicts better EFS.
-
•
Biological features (ER high, Luminal A, ROR-low) associate with a stronger ET response.
-
•
Short-course ET is a feasible and easy strategy to assess endocrine sensitivity.
Introduction
Breast cancer (BC) is the most common malignancy in women worldwide, with estrogen receptor-positive and human epidermal growth factor receptor 2-negative (ER+/HER2-negative) tumors representing the most frequent subtype.1 Endocrine therapy (ET) is the cornerstone of treatment across the disease spectrum, improving survival and reducing recurrence or progression in both early-stage and metastatic settings.2, 3, 4 Although neoadjuvant chemotherapy is widely adopted, preoperative ET remains underused,5, 6, 7 despite its favorable safety profile and its potential to inform in vivo endocrine sensitivity.
A brief course of ET before surgery is not intended to elicit dramatic radiological shrinkage, but rather to induce early biological changes, specifically, suppression of tumor proliferation, which may inform adjuvant treatment decisions. Previous studies demonstrate that a substantial on-treatment reduction in proliferation predicts excellent outcomes with ET alone and can identify patients who may safely omit chemotherapy.8,9 The Preoperative Endocrine Prognostic Index, which integrates post-treatment tumor size, nodal status, hormone–receptor expression, and proliferation levels, has been validated as a tool to guide escalation or de-escalation of adjuvant therapy.8
Moreover, combining dynamic proliferation assessment with genomic risk scores (e.g. Oncotype DX) refines individual risk stratification beyond baseline molecular staging.9,10 Even patients with N1 disease, if classified as intermediate risk by Oncotype DX and showing marked proliferation suppression, may be candidates for de-escalated regimens, whereas those with minimal change could be considered for treatment intensification.9,11
Moreover, recent neoadjuvant trials have highlighted the value of achieving complete cell cycle arrest (CCCA), a near-complete halt in proliferation (Ki67 ≤2.7%), as a robust surrogate marker of endocrine sensitivity and a predictor of long-term survival outcomes.12,13
The applicability and reproducibility of these strategies in routine clinical practice remain largely unexplored. In this article we therefore evaluate early proliferation dynamics induced by a short-term preoperative ET in a real-world cohort of patients with early ER+/HER2-negative BC managed at a single institution, and explore how these changes correlate with clinical, pathological, and genomic characteristics, as well as patient outcomes.
Methods
Study design and patient population
This registry-based retrospective cohort study included 230 consecutive patients treated according to standard clinical practice. Eligible patients had unicentric, unilateral stage I-III ER+/HER2-negative BC [per American Joint Committee on Cancer Cancer Staging Manual, 8th edition] and received short preoperative ET between 2014 and 2023 at the Hospital Clinic of Barcelona, Spain. As per physician discretion, patients received 2-12 weeks of ET before definitive surgery, mainly when uncertainty was present regarding the benefit of chemotherapy. This approach was used as a real-world strategy to better assess in vivo endocrine sensitivity and inform adjuvant treatment planning. The duration of preoperative ET was not standardized and in many cases was influenced by surgical scheduling and operating room availability rather than a predefined protocol.
Data collected
Electronic medical records were used to collect baseline clinical data, including epidemiological characteristics such as age, menopausal status, body mass index, and on-treatment information. Radiological data were obtained from mammography or magnetic resonance imaging carried out at baseline as part of standard care. Baseline core biopsies and surgical specimens were analyzed by immunohistochemistry (IHC). ER positivity was defined as ≥1% expression, and HER2 negativity was defined according to American Society of Clinical Oncology/College of American Pathologists guidelines as IHC 0-1+, or IHC 2+ with non-amplified in situ hybridization (ISH) results.14,15 Ki67 was assessed per local clinical guidelines by the same team of pathologists in baseline core biopsies and surgical samples. Tumor sections (3-4 μm) were stained with rabbit anti-Ki67 monoclonal antibody (clone 30.9, Roche, Basel, Switzerland) using an automated Ventana Benchmark Ultra stainer (Roche, Basel). IHC-based subtypes were defined as follows: luminal A-like (ER+/HER2-negative, progesterone receptor (PR) ≥20%, Ki67<14%) and luminal B-like (ER+/HER2−-negative/Ki67<14%/PR<20% or ER+/HER2-negative/Ki67 ≥14%).16 PAM50 intrinsic subtype and risk of recurrence (ROR) score were determined at the Hospital Clinic of Barcelona using the Prosigna assay in 138 baseline primary tumor core biopsies and 44 surgical samples. Cut-offs for ROR-score were defined according to PAM50 (Prosigna) assay certification17: low-ROR was defined as 40 or fewer points if node-negative and under 15 points if one to three positive nodes were present. Intermediate-ROR disease was defined as 41-60 points if node-negative and 16-40 points if one to three positive nodes were present. High-ROR disease was defined as 61-100 points if node- negative and 41-100 points if one to three positive nodes were present.
Endpoints
Endocrine therapy response was categorized according to the on-treatment proliferation index as follows: “responders” (post-therapy proliferation of 0% to 10%) and a subgroup defined as CCCA responders (Ki67 ≤2.7%). Prognostic performance was evaluated by event-free survival (EFS), defined as the interval from ET initiation to first recurrence, or death from any cause.
Statistical analysis
Continuous variables were summarized as medians (ranges) and categorical variables as counts (percentages). Logistic regression, both univariable and multivariable models adjusted for key clinical covariates, was used to identify factors associated with response and CCCA. EFS was estimated using Kaplan–Meier curves and compared using log-rank tests; hazard ratios (HRs), and 95% confidence intervals (CIs) were derived from univariable Cox proportional hazards models. All analyses were conducted using R (version 3.6.2).
Ethical considerations
The study was conducted in accordance with EMA/CHMP/ICH/135/1995 standards and was approved by the Ethics Committee of Hospital Clinic of Barcelona (HCB/2024/0328). All patients provided the written informed consent form of the “Biospecimens of patients with solid tumors at Hospital Clinic of Barcelona” collection (reference C.0004038).
Results
Clinicopathological variables are summarized in Table 1. Among the 230 patients included, the median age was 62 years (range, 35-95 years), and 181 (78.7%) were postmenopausal. Body mass index data were available for 227 patients, of whom more than half (54.6%) were classified as overweight or obese. Most had invasive carcinoma of no special type (NST) (87.0%), grade 2 histology (52.2%), clinical T1 tumors (74.8%), and no clinical nodal involvement (96.5%). The median baseline proliferation index (Ki67) was 12% (range, 1%-80%); 44.3% had initial Ki67 of ≤10%, and 12.2% had Ki67 of ≥30%. Preoperative ET was administered for a median of 5 weeks (range, 2-12 weeks) and selected based on menopausal status: tamoxifen (TAM) was used for premenopausal women and eight postmenopausal women (24.8% of cases), while ovarian suppression was not used in any patient.
Table 1.
Tumor, patient and treatment characteristics
| Characteristic | Overall population (N = 230) |
|---|---|
| Age (years) – median (range) | 62 (35-95) |
| BMI – median (range) | 25.3 (17.2-48.8) |
| Underweight/normal | 103 (45.4%) |
| Overweight | 76 (33.5%) |
| Obese | 48 (21.1%) |
| Menopausal status | |
| Premenopausal | 49 (21.3%) |
| Postmenopausal | 181 (78.7%) |
| Tumor histology | |
| NST | 200 (87.0%) |
| ILC | 21 (9.1%) |
| Other | 9 (3.9%) |
| Histological grade | |
| 1 | 97 (42.1%) |
| 2 | 120 (52.5%) |
| 3 | 7 (3.0%) |
| Unknown | 6 (2.6%) |
| Clinical T stage | |
| 1 | 172 (74.8%) |
| 2 | 50 (21.7%) |
| 3 | 6 (2.6%) |
| 4 | 2 (0.9%) |
| Clinical N stage | |
| N0 | 222 (96.5%) |
| N1 | 6 (2.6%) |
| Nx | 2 (0.9%) |
| Baseline Ki67 results – median (range) | 12 (1-80) |
| ≤10% | 102 (44.3%) |
| 11%-29% | 100 (43.5%) |
| ≥30% | 28 (12.2%) |
| Baseline ER status | |
| 1%-10% | 2 (0.9%) |
| ≥10% | 228 (99.1%) |
| Baseline PR status | |
| <10% | 32 (13.9%) |
| ≥10% | 198 (86.1%) |
| ET duration (weeks) – median (range) | 5 (2-12) |
| Endocrine treatment | |
| TAM | 57 (24.8%) |
| AI | 173 (75.2%) |
| Postsurgical Ki67 results – median (range) | 3 (1-75) |
| ≤10% | 196 (85.2%) |
| 11%-29% | 27 (11.7%) |
| ≥30% | 7 (3.0%) |
| Pathological T stage | |
| 1 | 180 (78.6%) |
| 2 | 43 (18.8%) |
| 3 | 5 (2.2%) |
| 4 | 1 (0.4%) |
| Unknown | 1 |
| Pathological N stage | |
| N0 | 160 (73.1%) |
| N1 | 52 (23.6%) |
| N2 | 6 (2.7%) |
| N3 | 1 (0.5%) |
| Unknown | 11 |
| Adjuvant chemotherapy | |
| Yes | 51 (22.2%) |
| No | 178 (77.4%) |
| Unknown | 1 (0.4%) |
AI, aromatase inhibitor; BMI, body mass index; ER, estrogen receptor; ET, endocrine therapy; ILC, invasive lobular carcinoma; NST, no special type; PR, progesterone receptor; TAM, tamoxifen.
Among the 138 patients with available baseline PAM50 data (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2025.105845), 67.4% were classified as Luminal A, 31.2% as Luminal B, and 1.4% as Basal-like. None were HER2-enriched. ROR scores were low in 45.0%, intermediate in 34.8%, and high in 20.1% of these patients.
Following short-course preoperative ET, the median Ki67 proliferation index declined from 12% to 3%, though post-treatment values ranged from 1% to 75%. Overall, 196 patients (85.2%) achieved a post-treatment Ki67 of ≤10%, and 111 (48.3%) met criteria for CCCA. Only 3.0% of cases exhibited a post-treatment Ki67 of ≥30%. Among patients with elevated baseline Ki67 (>10%; n = 128), 75.8% achieved a Ki67 response and 39.1% reached CCCA (Figure 1A). In contrast, tumors with low baseline Ki67 (≤10%) largely maintained low proliferation after treatment, with only 2.9% showing a post-treatment increase >10%. Median ET duration was similar between responders and non-responders (5 versus 4 weeks, respectively; P = 0.406), indicating that treatment length within this short window did not significantly influence Ki67 suppression (Table 2).
Figure 1.
(A) Response rate (Ki67 ≤10%) and complete cell cycle arrest (CCCA) for the entire cohort and for patients with baseline Ki67 > 10%. (B) Response rate (Ki67 ≤10%) and CCCA according to menopausal status. (C) Response rate (Ki67 ≤10%) and CCCA by intrinsic subtype.
Table 2.
Clinical and biological differences between responders and non-responders
| Characteristics | Non-responders (N = 34) | Responders (N = 196) | P value |
|---|---|---|---|
| Age years – median (range) | 61.5 (35-84) | 62.0 (36-95) | 0.080 |
| BMI – median (range) | 24.7 (20.89-27.4) | 25.5 (23.1-29.8) | |
| Underweight/normal | 18 (52.9%) | 86 (44.6%) | |
| Overweight | 14 (41.2%) | 61 (31.6%) | |
| Obese | 2 (5.9%) | 46 (23.8%) | 0.030 |
| Menopausal status | |||
| Premenopausal | 14 (41.2%) | 35 (17.9%) | 0.004 |
| Postmenopausal | 20 (58.8%) | 161 (82.1%) | |
| Tumor histology | |||
| NST | 32 (94.1%) | 168 (85.7%) | |
| ILC | 2 (5.9%) | 19 (9.7%) | 0.168 |
| Other | 0 (0.0%) | 9 (4.6%) | |
| Histological grade | |||
| 1 | 7 (20.6%) | 90 (45.9%) | |
| 2 | 23 (67.6%) | 97 (49.5%) | <0.001 |
| 3 | 4 (11.8%) | 3 (1.5%) | |
| Unknown | 0 (0.0%) | 6 (3.0%) | |
| Clinical T stage | 0.648 | ||
| 1 | 24 (70.6) | 148 (75.5%) | |
| 2 | 10 (29.4) | 40 (20.4%) | |
| 3 | 0 (0.0%) | 6 (3.1%) | |
| 4 | 0 (0.9%) | 2 (1.0%) | |
| Clinical N stage | |||
| N0 | 33 (97.1%) | 189 (96.4%) | 0.850 |
| N1 | 1 (2.9%) | 5 (2.5%) | |
| Ki67 baseline – median (range) | 21.0 (5.0-80.0) | 10.0 (1.0-55.0) | |
| <10% | 3 (8.8%) | 99 (50.5%) | |
| ≥10%-29% | 17 (50%) | 83 (42.3%) | <0.001 |
| ≥30% | 14 (41.2%) | 14 (7.1%) | |
| Baseline ER status | |||
| 1-10% | 1 (2.9%) | 1 (0.5%) | 0.683 |
| ≥10% | 33 (97.1%) | 195 (99.5%) | |
| Baseline PR status | |||
| <10% | 3 (8.8%) | 33 (16.8%) | 0.509 |
| ≥10% | 31 (91.2%) | 163 (83.2%) | |
| Clinical intrinsic subtype | |||
| Luminal A-like | 5 (14.7%) | 100 (51%) | |
| Luminal B-like | 29 (85.3%) | 96 (49%) | <0.001 |
| Baseline PAM50 intrinsic subtype | 0.047 | ||
| Luminal A | 13 (48.1%) | 80 (72.1%) | |
| Luminal B | 13 (48.1%) | 30 (27.0%) | |
| Basal-like | 1 (3.7%) | 1 (0.9%) | |
| Baseline ROR | |||
| Low-risk | 6 (22.2%) | 56 (50.5%) | |
| Intermediate-risk | 12 (44.4%) | 36 (32.4%) | 0.023 |
| High-risk | 9 (33.3%) | 19 (17.1%) | |
| ET duration (weeks) – median (range) | 4 (2-11) | 5 (2-12) | 0.406 |
| Endocrine treatment | |||
| TAM | 18 (52.9%) | 39 (19.9%) | <0.001 |
| AI | 16 (47.0%) | 157 (80.1%) | |
| Adjuvant chemotherapy | |||
| Yes | 20 (58.8%) | 31 (15.9%) | <0.001 |
| No | 14 (41.2%) | 164 (84.1%) |
Bold values: statistically significant (P < 0.05).
AI, aromatase inhibitor; BMI, body mass index; ER, estrogen receptor; ET, endocrine therapy; ILC, invasive lobular carcinoma; NST, no special type; PR, progesterone receptor; ROR, risk of recurrence; TAM, tamoxifen.
Postmenopausal women showed significantly higher response rates than premenopausal patients (88.9% versus 71.4%, P = 0.004) (Figure 1B), although all premenopausal patients received TAM, compared with only 8 (4.4%) from the postmenopausal group. Additionally, response rates declined with increasing histological grade (grade 1: 92.8%, grade 2: 80.8%, grade 3: 42.9%; P < 0.001) (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2025.105845). Adjuvant chemotherapy was administered to 22.3% of the cohort, with a higher proportion among non-responders than responders (58.8% versus 15.9%, P < 0.001) (Table 2).
PAM50 intrinsic subtypes were significantly associated with ET response. Luminal A tumors (n = 93) had higher response rates than Luminal B tumors (n = 43), (86.0% versus 69.8%; P = 0.047) and showed a trend toward higher CCCA rates (47.3% versus 27.9%; P = 0.051) (Figure 1C). Among patients with Luminal A tumors (n = 93), those who were postmenopausal at diagnosis (n = 68) had significantly higher response (94.1%) and CCCA (60.3%) rates than premenopausal women (n = 25) (response: 64.0%, CCCA: 12.0%; P < 0.001 for both) (Supplementary Figure S2A, available at https://doi.org/10.1016/j.esmoop.2025.105845). In contrast, for Luminal B tumors (n = 43), postmenopausal patients (n = 36), 69.4%, and 27.8%, achieved response and CCCA rates, respectively, compared with 71.4% and 28.6% among premenopausal women (n = 7), with no statistically significant differences (P = 0.899 for response, P = 0.945 for CCCA) (Supplementary Figure S2B, available at https://doi.org/10.1016/j.esmoop.2025.105845). These findings occurred in the context of different treatment strategies which were designated according to menopausal status.
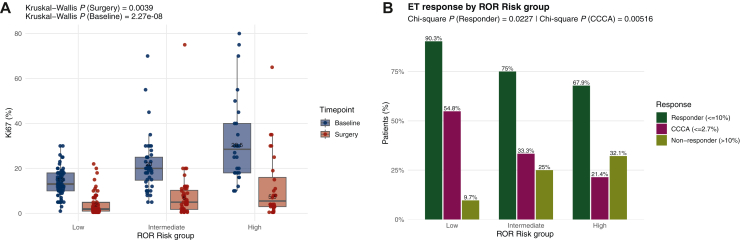
Both baseline and post-operative Ki67 varied by baseline ROR-score (Figure 2A), with significant differences regarding proliferation responses across risk groups (P = 0.023). Higher response rates were observed in low-risk tumors (n = 62, 90.3%), followed by intermediate (n = 48, 75.0%) and high-risk (n = 28, 67.9%) groups. CCCA was also associated with initial ROR category (P = 0.005), with rates of 54.8%, 33.3%, and 21.4% for low-, intermediate-, and high-risk tumors, respectively (Figure 2B).
Figure 2.
(A) Ki67 level at baseline and at surgery by ROR-score group: high-, intermediate-, and low-risk. (B) Endocrine therapy response by baseline ROR-score group.
Among patients with baseline proliferation >10% and available PAM50 data (n = 108, 46.9%), a trend toward a lower Ki67 response was observed across increasing ROR risk categories: 86.0% (low), n = 43, 69.2% (intermediate), n = 39, and 65.4% (high), n = 26, respectively (P = 0.092). In contrast, CCCA rates differed significantly by ROR category: 55.8% (low), 28.2% (intermediate), and 19.2% (high), respectively (P = 0.003) (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2025.105845).
Multivariable logistic regression identified postmenopausal status (95.6% receiving AI) as a strong predictor of response [odds ratio (OR) 33.39; 95% CI 2.78-401.74; P = 0.006], along with higher baseline ER expression (OR 1.12 per 1%; 95% CI 1.02-1.24; P = 0.020) and lower baseline Ki67 (OR 0.81 per 1%; 95% CI 0.69-0.95; P = 0.008) (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2025.105845). For CCCA, postmenopausal status (OR 9.82; 95% CI 2.59-37.22; P < 0.001), baseline ER expression (OR 1.06; 95% CI 1.00-1.12; P = 0.048), and higher ROR-score (OR 0.94; 95% CI 0.90-0.99; P = 0.016) remained independently associated (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2025.105845). Univariate analyses are reported in Supplementary Material (Supplementary Tables S5 and S6, available at https://doi.org/10.1016/j.esmoop.2025.105845).
Among 44 patients with post-treatment PAM50 data, 81.8% were Luminal A and 13.6% Luminal B, with median post-treatment proliferation values (Ki67) of 5% and 18%, respectively. Of 24 patients with both baseline and post-treatment subtype data, 50% (n = 12) were Luminal A at both time points. Notably, 88.9% (n = 8) of those initially classified as Luminal B shifted to Luminal A after ET. Basal-like tumors remained stable (n = 2), (Supplementary Table S7, available at https://doi.org/10.1016/j.esmoop.2025.105845). Additionally, patients who achieved CCCA had significantly lower post-treatment ROR scores (median 22 versus 32; P = 0.045) (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2025.105845).
At a median follow-up of 47 months, 16 patients (6.9%) experienced recurrence or death. The 5-year EFS was 92.1% (95% CI 88.0%-96.4%) for the entire cohort (Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2025.105845). There was no significant difference in EFS by post-treatment proliferation response (≤10% versus >10%; 92.4% versus 89.0%, P = 0.790) (Figure 3A), nor across endocrine response profiles based on baseline or post-treatment cut-offs (log-rank P = 0.893) (Supplementary Figure S5, available at https://doi.org/10.1016/j.esmoop.2025.105845).
Figure 3.
(A) Event-free survival according to post-treatment Ki67 response. (B) Event-free survival according to post-treatment complete cell cycle arrest (CCCA).
In contrast, patients achieving CCCA had significantly higher 5-year EFS (96.6% versus 87.4%; P = 0.012), with an unadjusted HR of 0.19 (95% CI 0.05-0.72; P = 0.012) (Figure 3B). Neither intrinsic subtype nor baseline ROR-score predicted EFS in this clinically low-risk cohort (Supplementary Table S8, available at https://doi.org/10.1016/j.esmoop.2025.105845).
In an exploratory multivariable Cox regression model including tumor size, nodal status, baseline proliferation, and adjuvant chemotherapy, CCCA remained an independent prognostic factor (adjusted HR 0.25; 95% CI 0.07-0.92; P = 0.037), as did tumor size (HR 1.04 per mm; 95% CI 1.00-1.09; P = 0.029) (Supplementary Table S9, available at https://doi.org/10.1016/j.esmoop.2025.105845).
Discussion
This study supports the clinical utility of short-term preoperative ET as a biologically informative strategy in early ER-positive/HER2-negative BC. A substantial proportion of tumors demonstrated a meaningful antiproliferative response, particularly among postmenopausal individuals, the majority of whom were treated with AI, compared with premenopausal patients who received TAM. Additionally, biological characteristics, such as high baseline ER expression, Luminal A subtype, and ROR-low category, were strong predictors of response, as they all reflect a predominantly endocrine-driven disease. Moreover, the value of this study lies in its real-world setting, reflecting routine clinical practice, primarily used when the benefit of chemotherapy was uncertain. This approach offered a simple, low-cost intervention, that allowed an in vivo assessment of endocrine sensitivity before definitive treatment. In addition, the duration of preoperative ET was often influenced by surgical rather than strict protocols; importantly, this heterogeneity did not impact Ki67 response, further supporting the feasibility of integrating short-course ET into daily practice. As such, preoperative ET can be readily implemented as a prognostic and predictive tool across health care systems, making it especially attractive for resource-constrained environments.
One of the key findings in this cohort was that achieving CCCA was independently associated with significantly better EFS, even after adjusting for conventional clinical-pathological features. These findings align with previous clinical trials showing that dynamic changes in tumor proliferation after brief ET are more predictive of long-term outcomes than baseline biomarkers alone.12 Notably, while a 10% proliferation threshold (as used in ADAPT) has been validated as a marker of endocrine sensitivity, it was not prognostic in our cohort. This may be attributed to the relatively short follow-up, limited number of events, and the predominance of early-stage, low-burden tumors.11
Our data also highlight the intertwined influence of menopausal status, treatment type, and treatment intensity on endocrine response. In our cohort, response rates were significantly lower among premenopausal women; however, all of these patients received TAM monotherapy. This treatment uniformity limits our ability to determine whether lower response rates were attributable to menopausal status per se or to differences in ET. This contrasts with findings from the ADAPT cycle study, where premenopausal patients, including those <40 years, achieved response rates comparable with postmenopausal women when treated with AI in combination with ovarian function suppression. These data underscore the importance of intensified endocrine blockade in younger patients and suggest that treatment regimen, rather than menopausal status alone, may be the key determinant of response.9 Ongoing studies, including EMPRESS (NCT05659563) and PREMIERE (NCT05982093), are evaluating whether oral selective estrogen receptor degraders (SERDs) can achieve meaningful responses in premenopausal patients without ovarian suppression.
Beyond static biomarkers, our findings reinforce the potential value of dynamic endpoints, such as CCCA and the potential role of molecular subtype shifts, for refining treatment strategies. In our cohort, a notable fraction of Luminal B tumors demonstrated significant proliferation suppression, and many converted to Luminal A after short-course ET, just as observed with longer neoadjuvant ET duration.18,19 This supports the concept of molecular downstaging as a surrogate of endocrine sensitivity, as previously reported in trials such as CORALLEEN,20 and currently being evaluated in the RIBOLARIS trial (NCT05296746), which aims to evaluate whether patients achieving a ROR-low status after neoadjuvant ribociclib and ET can safely forgo chemotherapy.21 Notably, in our cohort, patients who achieved a Ki67 response were significantly less likely to receive chemotherapy. These findings suggest that biological response may have already influenced clinical decision making by treating physicians.
The transient nature of these changes, however, raises important questions. Previous studies have reported that proliferation levels and intrinsic subtypes can revert after discontinuation of ET, suggesting that short-term responses may reflect reversible suppression rather than durable reprogramming.13,22,23 This has implications for interpreting dynamic biomarkers, especially those largely driven by proliferation such as ROR-score. Moreover, recent evidence points to genomic and epigenetic factors, such as TP53 mutations and chromatin remodeling, as potential determinants of sustained endocrine response.24 Immunological remodeling during ET is also emerging as a relevant layer of tumor evolution, further contributing to the heterogeneity in response and rebound risk.20
This study has several limitations. Firstly, its retrospective design and short follow-up, which is insufficient to fully capture the long-term survival impact in HR-positive–BC. Moreover, our cohort was predominantly low-risk, with a limited number of events, constraining the robustness of prognostic conclusions. Secondly, the decision to administer adjuvant chemotherapy was not standardized and may have confounded survival analyses. Thirdly, only a subset of patients had paired molecular data pre- and post-treatment, limiting our ability to fully assess transcriptional changes, plus correlations with commercial genomic assays such as Oncotype DX or MammaPrint were not feasible, as these data were not available in our registry. Additionally, we could not obtain Preoperative Endocrine Prognostic Index score results as a prognostic tool; patients received short-course preoperative ET with variable duration; thus reliable calculation was not feasible. Fourthly, compliance and tolerability data were not systematically collected; however, given the short treatment duration, compliance was not perceived as a relevant issue in routine clinical practice. Finally, because pre- and postmenopausal patients received different ET, we are unable to determine whether the observed differences in response are due to menopausal status itself or to the type of treatment administered.
Taken together, our findings add to the growing body of evidence supporting the integration of dynamic biomarkers into clinical decision making. Additionally, CCCA emerges as a robust surrogate of endocrine sensitivity that correlates with favorable biology and long-term outcomes.
Conclusion
Short-term preoperative ET offers a practical, real-time method to assess endocrine sensitivity in early ER-positive/HER2-negative–BC. While overall proliferation suppression was common, CCCA seems to be the most reliable marker of favorable prognosis. Molecular downstaging and intrinsic subtype shifts further suggest biologically meaningful responses. Finally, due to its simplicity, low cost, and minimal infrastructure requirements, this strategy can be widely implemented across diverse health care settings, including those with limited access to genomic testing.
Acknowledgements
MVL and BW have been awarded a research grant from Sociedad Española Oncología Médica (SEOM). ES is supported by a SEOM Research Fellowship (2024-2026). FS is supported by a Juan Rodés 2024 Clinical Research Contract from the Instituto de Salud Carlos III (ISCIII, JR24/00024). FB-M received funding from Fundación científica AECC Ayudas Investigador AECC 2021 (INVES21943BRAS). MBS is supported by a 2024-2027 BBVA Foundation/the Hospital Clinic of Barcelona Joan Rodés-Josep Baselga Advanced Research Contracts in Oncology.
Disclosure
Potential conflicts of interest are the following: RGB reports lecture fees from Daiichi Sankyo, Novartis and MSD. BW reports lectures fees from AstraZeneca and advisory/consulting fees from Novartis. MBS declares advisor or consulting fees from Pfizer, Lilly, Novartis and AstraZeneca and travel expenses from Novartis, Pfizer, Gilead and AstraZeneca. OMS reports advisory/consulting fees from Reveal Genomics, Roche, and AstraZeneca, lecture fees from Daiichi Sankyo, Novartis, Pfizer, and Eisai and travel expenses from Gilead and Novartis. ES reports advisory board or speaker honoraria from AstraZeneca and Sysmex; and consultant of Reveal Genomics. PG reports part-time employment from Reveal Genomics. AP reports advisory and consulting fees from AstraZeneca, Roche, Novartis, Daiichi Sankyo, and Ona Therapeutics, lecture fees from AstraZeneca, Roche, Novartis, and Daiichi Sankyo, institutional financial interests from AstraZeneca, Novartis, Roche, and Daiichi Sankyo; stockholder and employee of Reveal Genomics; patents filed PCT/EP2016/080056, PCT/EP2022/086493, PCT/EP2023/060810, EP23382703 and EP23383369. FB-M reports part-time employment from Reveal Genomics and has patents filed: PCT/EP2022/086493, PCT/EP2023/060810, EP23382703, and EP23383369B. FS reports honoraria from Novartis, Gilead, Veracyte and Daiichy Sankyo for educational events/materials, advisory fees from Pfizer, Daiichi Sankyo and Veracyte, and travel expenses from Novartis, Gilead, and Daiichy Sankyo. ES declares personal fees for educational events and/or material from Novartis, Pfizer, Eisai, and Daiichi Sankyo; advisory fees from Pfizer and Seagen; and travel and accommodation expenses from Gilead, Daiichi Sankyo, Novartis, and Lilly. TP reports advisory and consulting fees or speaker honoraria from Novartis, AstraZeneca, Lilly, Pfizer, Veracyte, Gilead, Daiichi Sankyo, and Roche, and support for attending meetings and/or travel from Gilead, Daiichi Sankyo, and Roche. All remaining authors have declared no conflicts of interest.
Funding
None declared.
Supplementary data
References
- 1.Harbeck N., Penault-Llorca F., Cortes J., et al. Breast cancer. Nat Rev Dis Primers. 2019;5:66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 2.Loibl S., André F., Bachelot T., et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2024;35:159–182. doi: 10.1016/j.annonc.2023.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 5.Korde L.A., Somerfield M.R., Carey L.A., et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol. 2021;39:1485–1505. doi: 10.1200/JCO.20.03399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Olmen J.P., Jacobs C.F., Bartels S.A.L., et al. Radiological, pathological and surgical outcomes after neoadjuvant endocrine treatment in patients with ER-positive/HER2-negative breast cancer with a clinical high risk and a low-risk 70-gene signature. Breast. 2024;75 doi: 10.1016/j.breast.2024.103726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sella T., Weiss A., Mittendorf E.A., et al. Neoadjuvant endocrine therapy in clinical practice. JAMA Oncol. 2021;7:1700. doi: 10.1001/jamaoncol.2021.2132. [DOI] [PubMed] [Google Scholar]
- 8.Ellis M.J., Tao Y., Luo J., et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst. 2008;100:1380–1388. doi: 10.1093/jnci/djn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gluz O, Christgen M, Nitz U, et al. Impact of age and ovarian function suppression (OFS) on endocrine response to short preoperative endocrine therapy (ET): results from the multicenter ADAPTcycle trial. Paper presented at the San Antonio Breast Cancer Conference. 5 December 2023 -9 December 2023; San Antonio, TX. Abstract LBO1-05.
- 10.Nitz U.A., Gluz O., Kümmel S., et al. Endocrine therapy response and 21-gene expression assay for therapy guidance in HR+/HER2– early breast cancer. J Clin Oncol. 2022;40:2557–2567. doi: 10.1200/JCO.21.02759. [DOI] [PubMed] [Google Scholar]
- 11.Nitz U., Gluz O., Graeser M., et al. De-escalated neoadjuvant pertuzumab plus trastuzumab therapy with or without weekly paclitaxel in HER2-positive, hormone receptor-negative, early breast cancer (WSG-ADAPT-HER2+/HR–): survival outcomes from a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2022;23:625–635. doi: 10.1016/S1470-2045(22)00159-0. [DOI] [PubMed] [Google Scholar]
- 12.Smith I., Robertson J., Kilburn L., et al. Long-term outcome and prognostic value of Ki67 after perioperative endocrine therapy in postmenopausal women with hormone-sensitive early breast cancer (POETIC): an open-label, multicentre, parallel-group, randomised, phase 3 trial. Lancet Oncol. 2020;21:1443–1454. doi: 10.1016/S1470-2045(20)30458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston S., Puhalla S., Wheatley D., et al. Randomized phase II study evaluating palbociclib in addition to letrozole as neoadjuvant therapy in estrogen receptor-positive early breast cancer: PALLET trial. J Clin Oncol. 2019;37 doi: 10.1200/JCO.18.01624. [DOI] [PubMed] [Google Scholar]
- 14.Wolff A.C., Hammond M.E.H., Allison K.H., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 15.Allison K.H., Hammond M.E.H., Dowsett M., et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38:1346–1366. doi: 10.1200/JCO.19.02309. [DOI] [PubMed] [Google Scholar]
- 16.Prat A., Cheang M.C., Martín M., et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol. 2013;31:203–209. doi: 10.1200/JCO.2012.43.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallden B., Storhoff J., Nielsen T., et al. Development and verification of the PAM50-based Prosigna breast cancer gene signature assay. BMC Med Genomics. 2015;8:54. doi: 10.1186/s12920-015-0129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schettini F., Nucera S., Brasó-Maristany F., et al. Unraveling the clinicopathological and molecular changes induced by neoadjuvant chemotherapy and endocrine therapy in hormone receptor-positive/HER2-low and HER2-0 breast cancer. ESMO Open. 2024;9 doi: 10.1016/j.esmoop.2024.103619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schettini F., Brasó-Maristany F., Pascual T., et al. Identifying predictors of treatment response and molecular changes induced by neoadjuvant chemotherapy and endocrine therapy in hormone receptor-positive/HER2-negative breast cancer: the NEOENDO translational study. ESMO Open. 2024;9 doi: 10.1016/j.esmoop.2024.103989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pascual T., Fernandez-Martinez A., Agrawal Y., et al. Cell-cycle inhibition and immune microenvironment in breast cancer treated with ribociclib and letrozole or chemotherapy. NPJ Breast Cancer. 2024;10:20. doi: 10.1038/s41523-024-00625-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novartis Pharmaceuticals A Study of Ribociclib and Letrozole in Hormone Receptor-Positive, HER2-Negative Early Breast Cancer (RIBOLARIS). Preprint at 2022. https://clinicaltrials.gov/study/NCT05296746 Available at. Accessed April 11, 2025.
- 22.Dowsett M., lburn L., Rimawi M.F., et al. Biomarkers of response and resistance to palbociclib plus letrozole in patients with ER+/HER2− breast cancer. Clin Cancer Res. 2022;28:163–174. doi: 10.1158/1078-0432.CCR-21-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurvitz S.A., Martin M., Press M.F., et al. Potent cell-cycle inhibition and upregulation of immune response with abemaciclib and anastrozole in neoMONARCH, phase II neoadjuvant study in HR+/HER2− breast cancer. Clin Cancer Res. 2020;26:566–580. doi: 10.1158/1078-0432.CCR-19-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kudo R., Safonov A., Jones C., et al. Long-term breast cancer response to CDK4/6 inhibition defined by TP53-mediated geroconversion. Cancer Cell. 2024;42:1919–1935.e9. doi: 10.1016/j.ccell.2024.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.