Abstract
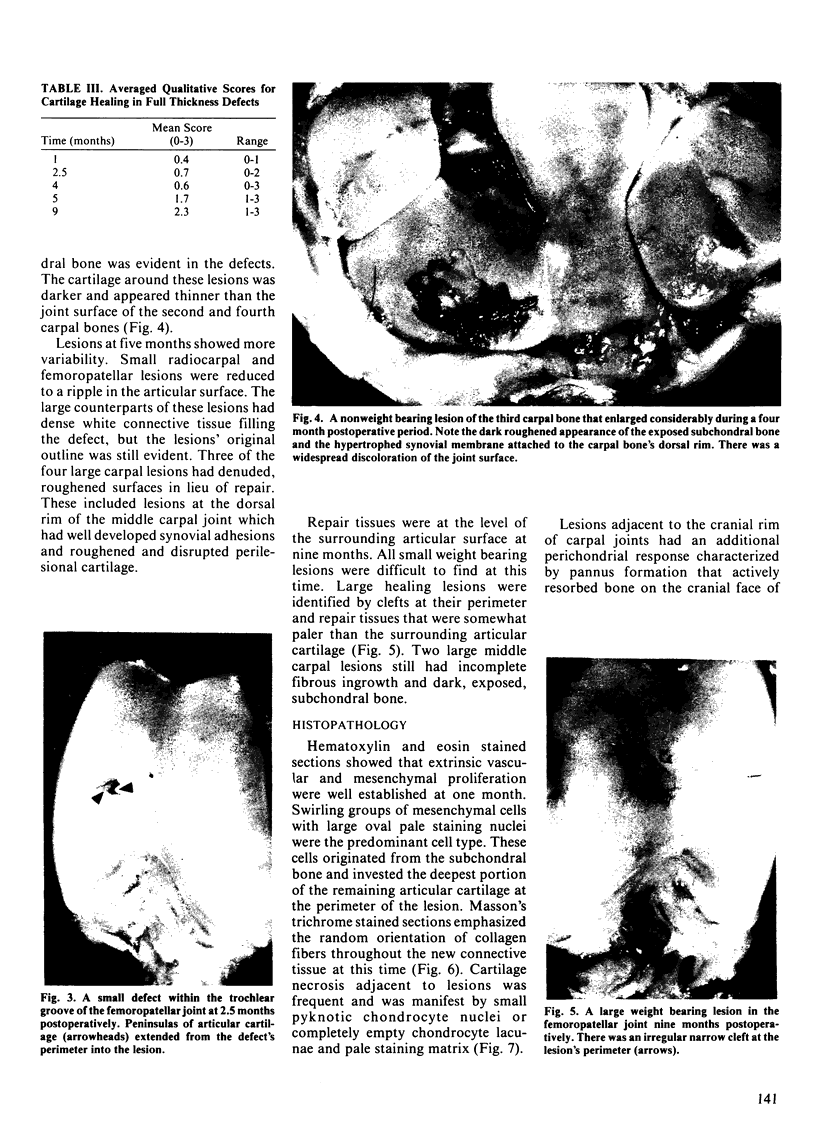
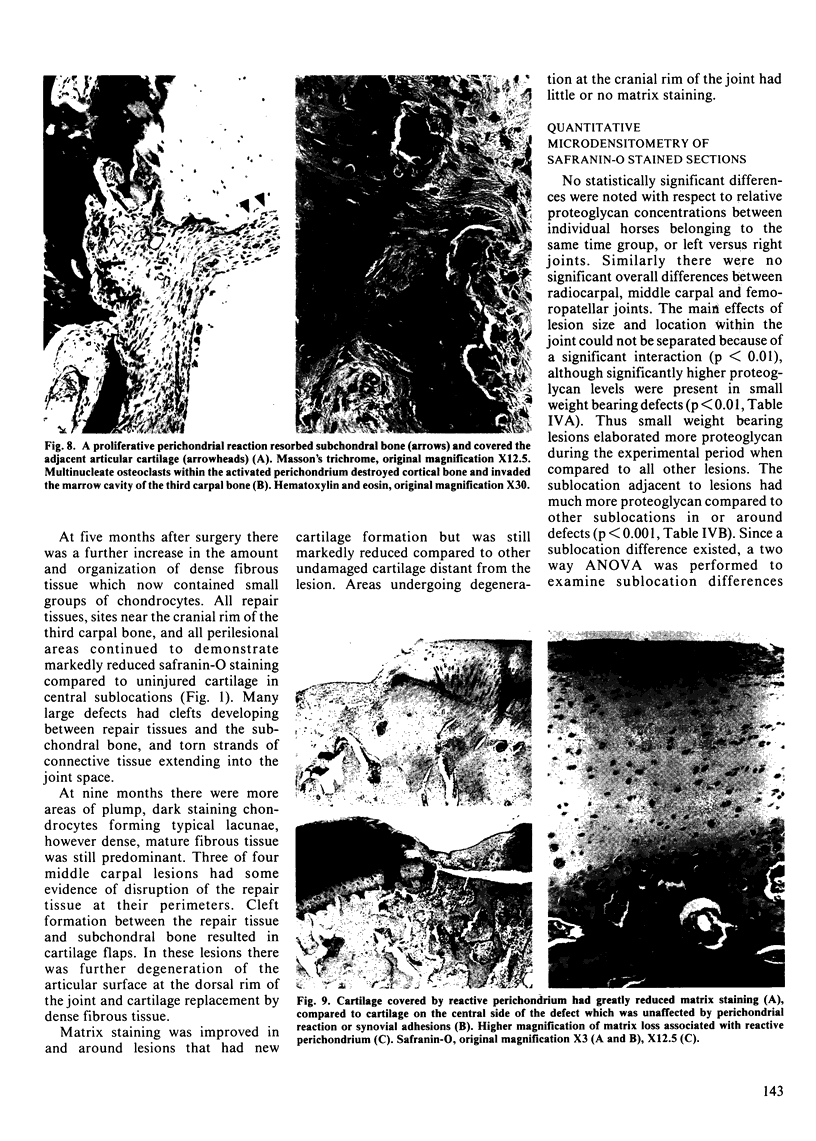
The mechanisms and completeness of equine articular cartilage repair were studied in ten horses over a nine month period. Large (15 mm square) and small (5 mm square) full-thickness lesions were made in weight bearing and nonweight bearing areas of the radiocarpal, middle carpal and femoropatellar joints. The horses were euthanized in groups of two 1, 2.5, 4, 5 and 9 months later. Gross pathology, microradiography, and histopathology were used to evaluate qualitative aspects of articular repair. Computer assisted microdensitometry of safranin-O stained cartilage sections was used to quantitate cartilage matrix proteoglycan levels. Structural repair had occurred in most small defects at the end of nine months by a combination of matrix flow and extrinsic repair mechanisms. Elaboration of matrix proteoglycans was not complete at this time. Statistically better healing occurred in small weight bearing lesions, compared to large or nonweight bearing lesions. Synovial and perichondrial pannus interfered with healing of osteochondral defects that were adjacent to the cranial rim of the third carpal bone. Clinical and experimental experience suggests that these lesions are unlikely to heal, whereas similar lesions in the radiocarpal and femoropatellar joints had satisfactory outcomes. Observations made in this study support the use of early postoperative ambulation, passive flexion of operated joints, and recuperative periods of up to a year for large cartilage defects.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker B., Becker R. O., Spadaro J. A study of electrochemical enhancement of articular cartilage repair. Clin Orthop Relat Res. 1974 Jul-Aug;(102):251–267. doi: 10.1097/00003086-197407000-00029. [DOI] [PubMed] [Google Scholar]
- Campbell C. J. The healing of cartilage defects. Clin Orthop Relat Res. 1969 May-Jun;64:45–63. [PubMed] [Google Scholar]
- Convery F. R., Akeson W. H., Keown G. H. The repair of large osteochondral defects. An experimental study in horses. Clin Orthop Relat Res. 1972 Jan-Feb;82:253–262. [PubMed] [Google Scholar]
- Evans C. H., Mazzocchi R. A., Nelson D. D., Rubash H. E. Experimental arthritis induced by intraarticular injection of allogenic cartilaginous particles into rabbit knees. Arthritis Rheum. 1984 Feb;27(2):200–207. doi: 10.1002/art.1780270212. [DOI] [PubMed] [Google Scholar]
- Fuller J. A., Ghadially F. N. Ultrastructural observations on surgically produced partial-thickness defects in articular cartilage. Clin Orthop Relat Res. 1972 Jul-Aug;86:193–205. doi: 10.1097/00003086-197207000-00031. [DOI] [PubMed] [Google Scholar]
- Jurvelin J., Kiviranta I., Tammi M., Helminen J. H. Softening of canine articular cartilage after immobilization of the knee joint. Clin Orthop Relat Res. 1986 Jun;(207):246–252. [PubMed] [Google Scholar]
- Kincaid S. A., Van Sickle D. C. Effects of exercise on the histochemical changes of articular chondrocytes in adult dogs. Am J Vet Res. 1982 Jul;43(7):1218–1226. [PubMed] [Google Scholar]
- Kiviranta I., Jurvelin J., Tammi M., Sämänen A. M., Helminen H. J. Microspectrophotometric quantitation of glycosaminoglycans in articular cartilage sections stained with Safranin O. Histochemistry. 1985;82(3):249–255. doi: 10.1007/BF00501401. [DOI] [PubMed] [Google Scholar]
- Kiviranta I., Tammi M., Jurvelin J., Sämänen A. M., Helminen H. J. Fixation, decalcification, and tissue processing effects on articular cartilage proteoglycans. Histochemistry. 1984;80(6):569–573. [PubMed] [Google Scholar]
- McIlwraith C. W., Fessler J. F., Blevins W. E., Page E. H., Rebar A. H., Van Sickle D. C., Coppoc G. L. Experimentally induced arthritis of the equine carpus: clinical determinations. Am J Vet Res. 1979 Jan;40(1):11–20. [PubMed] [Google Scholar]
- Mitchell N., Shepard N. The resurfacing of adult rabbit articular cartilage by multiple perforations through the subchondral bone. J Bone Joint Surg Am. 1976 Mar;58(2):230–233. [PubMed] [Google Scholar]
- Palmoski M. J., Colyer R. A., Brandt K. D. Joint motion in the absence of normal loading does not maintain normal articular cartilage. Arthritis Rheum. 1980 Mar;23(3):325–334. doi: 10.1002/art.1780230310. [DOI] [PubMed] [Google Scholar]
- Riddle W. E., Jr Healing of articular cartilage in the horse. J Am Vet Med Assoc. 1970 Dec 1;157(11):1471–1479. [PubMed] [Google Scholar]
- Salter R. B., Bell R. S., Keeley F. W. The protective effect of continuous passive motion in living articular cartilage in acute septic arthritis: an experimental investigation in the rabbit. Clin Orthop Relat Res. 1981 Sep;(159):223–247. [PubMed] [Google Scholar]
- Salter R. B., Simmonds D. F., Malcolm B. W., Rumble E. J., MacMichael D., Clements N. D. The biological effect of continuous passive motion on the healing of full-thickness defects in articular cartilage. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1980 Dec;62(8):1232–1251. [PubMed] [Google Scholar]