Introduction
Embryogenesis transforms the fertilized egg cell into a multicellular organism. In higher animals, the mature embryo is a miniature variant of the adult animal, and whatever changes may take place during post-embryonic development, they occur within the confines of the body organization established during embryogenesis. By contrast, higher plant embryogenesis generates a juvenile form, the seedling, which lacks most species-specific features of the adult plant. Post-embryonic development originates from two primary meristems, stem-cell systems that occupy opposite ends of the main body axis. The primary shoot meristem at the top end is the source of cells for new organs, such as leaves, and secondary shoot meristems, including flowers. The primary root meristem at the bottom end produces cells for extension growth of the primary root. In addition, root branches are initiated from specific cell groups within the primary root. These primordia recapitulate radial patterning and root meristem establishment as occurs in embryogenesis (Malamy and Benfey, 1997). Thus, the considerable increase in architectural complexity during post-embryonic development is contingent upon the basic body organization laid down during embryogenesis.
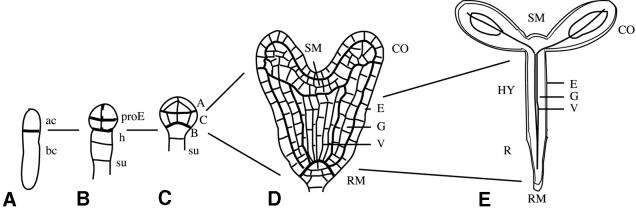
Formally, the seedling body organization can be viewed as a superimposition of two patterns: an apical–basal pattern along the main axis of polarity and a radial pattern across the axis (Figure 1). Apical–basal pattern elements are, from top to bottom: shoot meristem, one or two cotyledons (embryonic leaves), hypocotyl (embryonic stem), radicle (embryonic root) and root meristem. The radial pattern consists of tissue layers that are arranged concentrically from the periphery to the centre: epidermis, cortex and endodermis (both derived from ground tissue), pericycle and vascular tissue (xylem and phloem). The origin of seedling structures can be traced back to cell groups in the young embryo, due to the highly invariant pattern of cell division in Arabidopsis early embryogenesis (Figure 1; for a description of embryogenesis, see Jürgens and Mayer, 1994). This particularity of Arabidopsis and related species facilitates the analysis of pattern formation, but does not imply that cell ancestry is instrumental in setting up the pattern. Rather, cell–cell communication plays a major role, as discussed below. This review focuses on specific aspects of apical–basal pattern formation that link embryogenesis with post-embryonic development: establishment of the axis of polarity and the origin of the primary meristems.
Fig. 1. Development of the apical–basal pattern during Arabidopsis embryogenesis. (A) One-cell stage. The zygote has divided asymmetrically into an apical (ac) and a basal (bc) daughter cell. (B) Octant stage. The proembryo (proE) derived from the apical cell consists of two tiers each of four cells. The basal cell has produced a file of cells, including the hypophysis (h) and the suspensor (su). (C) Dermatogen stage. Three embryo regions are indicated: A, apical; C, central; B, basal. (D) Heart stage. The basic body organization is in place. SM, shoot meristem; CO, cotyledon primordia; RM, root meristem; E, epidermis; G, ground tissue; V, vascular primordium. (E) Seedling. HY, hypocotyl; R, root. Lines indicate the origin of seedling structures from early embryo regions.
Establishment of the apical–basal axis of polarity in early embryogenesis
Following fertilization, the embryo develops within an embryo sac, which is surrounded by maternal diploid tissue of the ovule. The Arabidopsis zygote initially measures only ∼20 µm in diameter, but expands ∼3-fold in the apical–basal axis of the embryo before dividing asymmetrically (Figure 1A). The apical and basal daughter cells of the zygote differ in several features. The apical cell is small, cytoplasm rich, and partitioned into eight proembryo cells by two rounds of vertical divisions followed by one round of horizontal division (Figure 1B). By contrast, the basal cell is large, contains a vacuole and divides repeatedly horizontally, giving a single file of 7–9 cells. All of these cells are initially extra-embryonic and, with the exception of the uppermost derivative, form the extra-embryonic suspensor that attaches the developing embryo to the wall of the embryo sac. The uppermost cell of the file joins the adjacent proembryo to adopt an embryonic fate, giving rise to part of the root meristem. The two daughter cells of the zygote can also be distinguished by differential gene expression. Only the apical, but not the basal, cell expresses the homeobox gene Arabidopsis thaliana MERISTEM LAYER 1 (AtML1), whose expression is later confined to the epidermis primordium of the embryo (Lu et al., 1996; Sessions et al., 1999). Thus, by all available criteria, the asymmetric division of the zygote establishes two cells of different fate, presaging the apical–basal polarity of the embryo.
The octant stage proembryo consists of two tiers, each of four cells (Figure 1B). The upper tier gives rise to the apical region of the embryo from which the shoot meristem and (most of) the cotyledons originate, whereas the lower tier produces the central region of the embryo, which generates the remainder (shoulder region) of the cotyledons, the hypocotyl, the root and the upper tier of root meristem stem cells (Figure 1C–E). The uppermost derivative of the basal daughter cell of the zygote joins the proembryo to become the hypophysis, or founder of the basal region of the embryo. The basal region gives rise to the remainder of the root meristem, the quiescent centre and the lower tier of stem cells. Thus, the apical–basal axis of the young embryo is partitioned into three main regions: apical, central and basal. Each of these regions develops differently, as indicated by their distinct cell division patterns and differential gene expression. For example, the inner cells within the apical region of the 16-cell embryo express the homeobox gene WUSCHEL (WUS), which plays a role in shoot meristem development (Mayer et al., 1998). By contrast, the central region undergoes a series of periclinal (tangential) cell divisions that generate the radial pattern of tissue layers. In addition, inner cells within the central region display apical–basal cell polarity at the transition from 16- to 32-cell stage, as is evident by the accumulation of the putative auxin efflux carrier PIN-FORMED 1 (PIN1) in their basal plasma membranes (Steinmann et al., 1999). The basal region is initiated from a single cell, the hypophysis, which undergoes a stereotypic series of cell divisions. Although the early embryo regions can be clearly defined, they do not bear any clonal relationship with the apical–basal pattern elements of the seedling, thus pointing to the role of cell–cell communication in patterning.
When is the apical–basal axis of polarity first established? The short answer is that we do not know. The axis of the embryo is aligned with the axis of polarity of the ovule, suggesting a maternal influence in orienting the embryo axis. However, somatic embryos initiated from isolated leaf protoplasts can mimic the cell division pattern of zygotic early embryos in the absence of maternal cues (Luo and Koop, 1997). Furthermore, suspensor-derived secondary embryos that result from disrupting the communication between the embryo and the basally attached suspensor display the same or opposite polarity to the primary embryo (Schwartz et al., 1994; Vernon and Meinke, 1994). Thus, embryo polarity may result from the relative position of embryonic and non-embryonic cells that normally derive from the apical and basal daughter cells of the zygote, respectively.
Mechanisms underlying embryo axis formation are not understood. So far, only mutations in a single gene, GNOM (GN), have been shown to interfere with the stable fixation of the apical–basal axis of the Arabidopsis embryo, as evidenced by the variable expression of an apical marker, LIPID TRANSFER PROTEIN (LTP) (Vroemen et al., 1996), and the failure to establish a coordinated polar localization of PIN1 (Steinmann et al., 1999). The earliest defect of gn mutant embryos was observed in a variable, rather than asymmetrical, division of the zygote, followed by abnormal cell division patterns in the young embryo (Mayer et al., 1993). The GN gene encodes a brefeldin A (BFA)-sensitive guanine-nucleotide exchange factor (GEF) for small GTP-binding proteins of the ARF family (Steinmann et al., 1999). Since BFA also affects the polar localization of the putative auxin efflux carrier PIN1, one aspect of GN action may involve polar transport of the phytohormone auxin. The gn mutant phenotype can be mimicked by altering auxin transport or response in the experimentally accessible early embryo of the closely related species Brassica juncea (Hadfi et al., 1998). In Arabidopsis, the polar localization of PIN1 is established later than the earliest defect observed in gn mutant embryos. However, mutations in two other genes, MONOPTEROS (MP) and BODENLOS (BDL), both of which are involved in auxin response (see below), alter the division plane of the apical daughter cell of the zygote (Hamann et al., 1999). Thus, auxin may play a direct role in establishing embryo polarity.
Origin of the primary root meristem
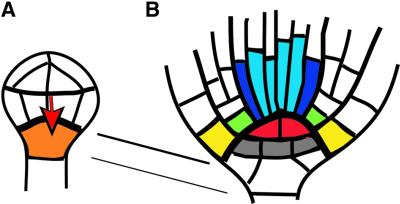
The root meristem is a stem-cell system with a layered organization at the bottom end of the seedling axis (Figures 1D, E and 2B). Its core is the quiescent centre comprised of four mitotically inactive cells that are situated between two tiers of stem cells. The upper tier gives off daughter cells that extend the cell files of the embryonic root tissues. These stem cells appear not to have any intrinsic information about the cell types to be produced. Rather, the apically adjacent differentiated root tissues seem to determine the fate of newly formed cells (Van den Berg et al., 1995). The lower tier stem cells add new cell layers to the central root cap as the old ones are sloughed off. The quiescent centre cells maintain, by local interaction, the undifferentiated state of the adjacent stem cells, as suggested by ablation of individual quiescent centre cells (Van den Berg et al., 1997). However, if completely abolished by laser ablation, the quiescent centre is regenerated through cell fate change of the apically adjacent vascular stem cells (Van den Berg et al., 1995). Recently, the phytohormone auxin has been implicated in this regeneration (Sabatini et al., 1999).
Fig. 2. Origin of the primary root meristem during embryogenesis. (A) Dermatogen stage. A signal from the proembryo (red arrow) is presumed to induce the cell fate of the hypophysis (orange). (B) Bottom end of heart-stage embryo. The quiescent centre (red) presumably induces stem-cell fate of surrounding cells. The upper tier of stem cells (colour coded) will produce root tissue cells; the lower tier of stem cells (grey) will form central root cap cells. The connecting lines indicate descent of the quiescent centre and the lower tier stem cells from the hypophysis.
Although acting as a functional unit, the root meristem comes from two clonally distinct cell populations. The upper tier of stem cells is derived, via the proembryo, from the apical daughter cell of the zygote, whereas the quiescent centre and the lower tier of stem cells originate, via the hypophysis, from the basal daughter cell of the zygote (Jürgens and Mayer, 1994; Scheres et al., 1994). Thus, cell–cell communication across a clonal boundary seems to be instrumental in setting up the primary root meristem during embryogenesis. Formally, two inductive steps can be distinguished (Figure 2). (i) At the octant or dermatogen stage, the proembryo induces a potentially extra-embryonic cell to become the hypophysis, or founder cell of the basal region of the embryo (Figure 2A). The hypophysis divides asymmetrically, giving a large basal daughter cell from which the lower tier of root meristem stem cells are derived, and a lens-shaped apical daughter cell that produces the four mitotically inactive cells of the quiescent centre. (ii) At the heart stage, the quiescent centre induces the surrounding cells to become stem cells of the root meristem, presumably by preventing their differentiation (Figure 2B). It is at this stage that the stem cells start to generate daughter cells. What is the experimental evidence for the two postulated inductive steps in primary root meristem formation?
Mutations in three genes, MONOPTEROS (MP), BODENLOS (BDL) and AUXIN RESISTENT 6 (AXR6), give seedlings that lack a primary root but are capable of forming roots post-embryonically (Berleth and Jürgens, 1993; Hamann et al., 1999; Hobbie et al., 2000). Thus, these genes are required for organizing root formation within the context of embryogenesis, but not for root formation per se. In both mp and bdl mutant embryos, the proembryo is abnormal before any defect can be discerned in the presumptive hypophysis (see above; Berleth and Jürgens, 1993; Hamann et al., 1999). These observations are consistent with a defect in signalling between the proembryo and the hypophysis (Figure 2A). This idea of a non-autonomous effect could be tested, for example, by expressing the MP gene specifically in the proembryo of a mp mutant. Mutations in the AXR6 gene also cause abnormal cell divisions in early embryogenesis (Hobbie et al., 2000). As a consequence, the hypophysis fails to undergo the asymmetrical division to give rise to the quiescent centre. The MP gene encodes an auxin-response transcription factor (Hardtke and Berleth, 1998) and the bdl mutation confers auxin insensitivity (Hamann et al., 1999). Thus, an auxin-dependent process appears to mediate cell fate of the hypophysis, although it is not known whether auxin itself is the signal.
A different class of mutants, including hobbit (hbt) and other ‘hypophyseal cell group’ mutants, are incapable of forming a root meristem not only in the embryo, but also during lateral root development (Scheres et al., 1996). Mutations in the HBT gene primarily affect the precursor of the hypophysis, resulting in a failure to form the quiescent centre (Willemsen et al., 1998). A possible explanation for this defect could be that the cell in place of the hypophysis does not respond properly to the presumed signal(s) from the proembryo. Although hbt and bdl mutant embryos differ in their earliest defect, they display a similar heart-stage phenotype. Cells adjacent to the progeny of the abnormal hypophysis behave like differentiating daughters of root meristem stem cells (Willemsen et al., 1998; Hamann et al., 1999). This defect resembles the differentiation of stem cells due to laser ablation of a quiescent centre cell in the seedling root meristem, as mentioned above (Van den Berg et al., 1997). It is therefore likely that the quiescent centre not only maintains stem-cell fate in the functional root meristem of the seedling, but also recruits adjacent cells as stem cells during root meristem formation in the embryo.
Origin of the primary shoot meristem within the apical region of the embryo
The primary shoot meristem is a stem-cell system at the top end of the seedling axis (Figures 1E and 3). It is the ultimate source of cells for all aerial parts of the plant, which include the stem as well as lateral organs, such as leaves, and secondary shoot meristems, such as axillary and flower meristems. Lateral primordia are initiated at the flank of the primary shoot meristem in a specific spatial pattern termed phyllotaxis (see below). Although the shoot meristem continually gives off cells for the formation of shoot structures, it maintains its approximate size. These multiple activities require interaction both within the shoot meristem and with existing primordia.
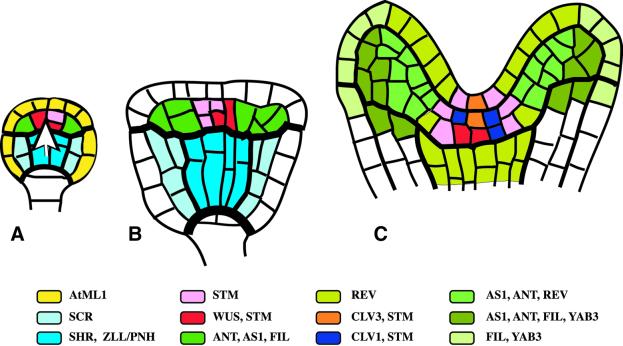
Fig. 3. Origin of the primary shoot meristem and the shoot apical organization. Approximate domains of gene expression are colour coded. (A) Globular embryo. The epidermis-specific AtML1 gene is expressed in both the central and the apical region (yellow; not shown in B and C). The white arrow indicates a cue from the central region in positioning of the shoot meristem primordium. (B) Transition-stage embryo displaying essentially the same expression pattern as the globular embryo. (C) Top end of heart-stage embryo. Expression domains of radial patterning genes, SCR and SHR, and of ZLL/PNH are not shown. In the shoot meristem primordium, the expression domains of CLV3 and CLV1 overlap. Note adaxial (REV) and abaxial (FIL, YAB3) gene expression domains in the cotyledon primordia. REV is also expressed in the vascular primordium.
The primary shoot meristem is organized into three zones with different properties and functions. The central zone harbours slowly dividing stem cells at the summit and an organizing centre underneath that maintains the stem cells by expressing the homeobox gene WUS (Mayer et al., 1998). The stem cells, in turn, express CLAVATA 3 (CLV3) (Fletcher et al., 1999), a small protein ligand that activates the membrane receptor serine/threonine kinase CLAVATA 1 (CLV1) and thus represses the WUS gene (Brand et al., 2000; Schoof et al., 2000; Trotochaud et al., 2000). In this way, size regulation of the stem-cell population is achieved. Daughters of stem cells that are displaced from the summit enter the flanking ring-shaped peripheral zone, where cells divide faster and primordia are initiated (Laufs et al., 1998). Cells that are not recruited into primordia become incorporated into tissues of the stem. The rib zone underneath the organizing centre consists of a population of faster dividing cells that contribute to inner tissues of the stem. Shoot meristem cells express the homeobox gene SHOOT MERISTEM LESS (STM) (Long et al., 1996). STM expression is switched off at sites of organ primordium initiation, which then express the myb-domain transcription factor ASYMMETRIC LEAVES 1 (AS1) (Byrne et al., 2000). The shoot-meristem defect of stm mutants is suppressed in stm as1 double mutants, indicating that STM maintains the undifferentiated state of meristematic cells by repressing the primordia-promoting AS1 gene.
How are primordia initiated? The cells in the peripheral zone are competent to become founder cells. However, primordia are only initiated at specific sites such that a spiral phyllotactic pattern of primordia is established. Floral meristem initiation sites, for example, are presaged by the expression of REVOLUTA (REV), which encodes a putative transcription factor involved in secondary shoot meristem formation (Otsuga et al., 2001). Similarly, AINTEGUMENTA (ANT) expression within the peripheral zone indicates the site of organ primordium initiation (Elliott et al., 1996). The ANT gene encodes a transcription factor that maintains the proliferative cell state during organ development (Mizukami and Fischer, 2000). It has long been known that the positioning of primordium initiation sites is affected by existing primordia. Recent studies on the pin1 mutant have provided evidence for a role of auxin in phyllotaxis since local application of auxin initiates the formation of a primordium within the peripheral zone (Reinhardt et al., 2000). Moreover, PIN1 is expressed at the site of primordium initiation, and pin1 mutant shoot meristems fail to establish the local expression domain of ANT (Vernoux et al., 2000). Together, these observations suggest that primordia are initiated at sites of auxin accumulation within the peripheral zone.
Developing primordia also appear to promote shoot meristem activity. Leaf primordia are subdivided into adaxial (upper) and abaxial (lower) domains soon after their initiation, presumably under influence from the shoot meristem (Siegfried et al., 1999). FILAMENTOUS FLOWER (FIL) and other YABBY (YAB) genes encoding putative transcription factors are expressed in the abaxial domain (Sawa et al., 1999; Siegfried et al., 1999). Ectopic expression of FIL or YAB3 not only changes cell fate from adaxial to abaxial within the primordium, but also results in termination of the shoot meristem, suggesting that adaxial cells promote shoot meristem activity. The same effect was noted for mutations in the PINHEAD (PNH) gene [also named ZWILLE (ZLL)], which is expressed in the adaxial domain of leaf primordia (Lynn et al., 1999). Conversely, the dominant mutant phabulosa (phb-1d) has adaxialized leaves that ectopically form axillary shoot meristems, as evidenced by STM expression (McConnell and Barton, 1998).
In summary, the primary shoot meristem is part of a complex organization of the shoot apex. Whereas stem cells are maintained by local interaction with the organizing centre, primordium initiation is influenced by existing primordia, which also promote shoot meristem activity. How is this intricate system of interactions within the shoot apex set up during embryogenesis?
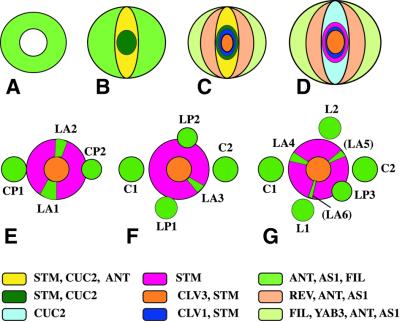
The primary shoot meristem originates, together with the flanking cotyledon primordia, from the apical region of the embryo (Figures 3 and 4A–D). In contrast to true leaves, the cotyledon primordia are not derived from cells that previously expressed the meristem-specific STM gene (Long et al., 1996; Long and Barton, 1998). Instead, the apical region of the globular embryo is partitioned into two domains: a peripheral domain of ANT-expressing cells and a central domain of cells that do not express the primordia-specific ANT gene (Figure 4A; Elliott et al., 1996). STM expression comes on in a peripheral cell and then spreads across the central domain to the opposite side, resulting in an expression stripe that overlaps the ring-shaped ANT domain at two peripheral sites (Figure 4B; Long and Barton, 1998). Bilateral symmetry becomes evident with the formation of two separate cotyledon primordia at the heart stage, although the domains of ANT and STM expression still overlap in the periphery (Figures 3C and 4C). No functional shoot meristem is established in stm mutant embryos, and the bases of the cotyledon primordia are fused (Barton and Poethig, 1993). This defect has been attributed to ectopic expression of the primordia-promoting AS1 gene in the absence of its repressor STM (Byrne et al., 2000). Two other functionally redundant genes, CUP-SHAPED COTYLEDON 1 (CUC1) and CUC2, are also required for the separation of the cotyledon primordia and for the formation of a functional shoot meristem (Aida et al., 1997). The CUC2 expression domain is similar to that of STM during the globular and heart stages of embryogenesis (Figure 4B and C; Aida et al., 1999). However, the two expression patterns become mutually exclusive during the walking-stick stage, such that STM expression is confined to the centrally located primary shoot meristem and surrounded by a CUC2 expression domain that marks the boundary region between the shoot meristem and the bases of the cotyledon primordia (Figure 4D). In cuc1 cuc2 mutant embryos, STM is not expressed, suggesting that the former genes act upstream of STM. Conversely, the initial stripe of CUC2 expression is normal in stm mutant embryos, but CUC2 expression becomes abnormal at the walking-stick stage, being confined to small groups of cells at the periphery of the apical region (Aida et al., 1999).
Fig. 4. Origin of phyllotaxis in the shoot apex. (A–D) Schematic cross-section through the apical region of the embryo. (A) Globular stage, with ring-shaped expression domain of ANT, AS1, FIL in the periphery. (B) Transition stage, showing expression stripe of STM and CUC2 across the apex. (C) Heart stage. CLV1 and CLV3 are expressed in the centre, and the periphery is subdivided into adaxial (REV) and abaxial (FIL) expression domains. (D) Walking-stick stage. CUC2 and STM expression domains become mutually exclusive. (E–G) Gradual transition from embryonic to post-embryonic phyllotaxis. The angles separating successive primordia are approximately: 180° (C1, C2), 100° (C2, L1), 175° (L1, L2), 110° (L2, L3), 160° (L3, L4), 130° (L4, L5) and 140° (L5, L6). Leaf primordium initiation sites (green sectors) within the peripheral zone of the shoot meristem are highlighted by expression of PIN1, AN, AS1, REV and FIL and by absence of STM expression (see text). C, cotyledon; CP, cotyledon primordium; L, leaf; LP, leaf primordium; LA, leaf anlage (founder cells at initiation site). Young primordia bordering on the peripheral zone have inhibitory effects on the initiation of the next primordium.
Bilateral symmetry of the embryo implies that the two cotyledon primordia originate simultaneously, which poses the problem of how the spiral phyllotaxis originates during post-embryonic development (Figure 4). However, a recent study of the embryonic shoot fate map suggests that the two cotyledon primordia arise sequentially, as do the shoot meristem-derived primordia of leaves 1 and 2 (Woodrick et al., 2000). The latter form a nearly straight line across the shoot apex and approximately at a right angle to the cotyledons (Figure 4E). Owing to the inhibiting influence of the existing leaf primordia, the next leaf primordium starts the spiral phyllotaxis of the shoot organs (Figure 4F and G). Measurements of the angles that separate the positions of successive cotyledon and leaf primordia indicate that the angle oscillates initially between ∼180 and 90°, but from leaf primordium 3, it is gradually dampened to the value of 137.5° characteristic of spiral phyllotaxis (Hamada et al., 2000). Thus, primordium initiation sites may be positioned by a single mechanism both in the embryo and during post-embryonic development. Indeed, the phytohormone auxin provides the missing link. Local accumulation of auxin within the peripheral zone of the functional shoot meristem induces primordium initiation, as discussed above (Reinhardt et al., 2000; Vernoux et al., 2000). Similarly, the application of auxin transport inhibitors in Brassica embryos (Liu et al., 1993; Hadfi et al., 1998) mimics the fused cotyledon or ‘cotyledon collar’ phenotype of Arabidopsis pin1, gn and bdl mp mutant embryos, which are defective in auxin transport or response (Liu et al., 1993; Mayer et al., 1993; Hamann et al., 1999). In addition, seedlings mutant for PIN1 or the auxin response-related protein kinase PINOID (PID) show a variable number or positioning of cotyledons (Christensen et al., 2000; Vernoux et al., 2000). Furthermore, both PIN1 protein and PID mRNA accumulate in the presumptive cotyledon primordia of the globular embryo (Steinmann et al., 1999; Christensen et al., 2000). In summary, local auxin accumulation appears to initiate organ primordia both in the embryo apical region and in the post-embryonic shoot, with the respective sites being influenced by the existing primordia.
The primary shoot meristem originates in the centre of the apical region of the embryo and becomes morphologically recognizable past the heart stage (Barton and Poethig, 1993). However, the elements of the WUS/CLV feedback loop for size regulation of the shoot meristem are already expressed at the heart stage (Figures 3C and 4C; Long and Barton, 1998; Fletcher et al., 1999; Schoof et al., 2000). In stm mutant embryos, CLV1 and WUS expression is initiated normally, but not maintained, and conversely, STM expression is not maintained in wus mutant embryos (Long and Barton, 1998; Mayer et al., 1998). Thus, the expression patterns of key regulators of shoot meristem development, such as WUS and STM, are established independently of each other during embryogenesis, but their subsequent expression is mutually dependent. So how is the expression pattern set up?
WUS expression is highly dynamic during early embryogenesis, starting in the inner cells of the apical region at the 16-cell stage (Mayer et al., 1998). During subsequent cell divisions, WUS expression continues only in the daughter cells that are close to the vascular primordium, which is established as the innermost element of the radial pattern within the subjacent central region of the embryo (Figure 3A and B). The vascular primordium may, thus, link the origin of the shoot meristem primordium with radial patterning. Within the central region, the inner cells are initially partitioned into a vascular primordium and surrounding ground tissue cells. The ground tissue is subdivided into an outer layer of cortex cells and an inner layer of endodermis cells by asymmetric cell divisions that are affected by mutations in two genes: SCARECROW (SCR) and SHORT ROOT (SHR) (Scheres et al., 1995). These genes are expressed, from the globular stage, in the ground tissue and vascular primordium, respectively (Figure 3A and B; Di Laurenzio et al., 1996; Heliariutta et al., 2000). SHR expression in the vascular primordium is required for SCR expression and, if ectopically expressed, SHR induces supernumerary cell layers expressing the SCR gene (Heliariutta et al., 2000). Thus, the centrally located vascular primordium appears to organize subepidermal radial patterning.
Several observations suggest that the vascular primordium also influences the origin of the shoot meristem. For example, fackel (fk) mutant embryos that are deficient in phytosterol biosynthesis show the earliest defect in the vascular primordium and subsequently express the shoot meristem marker STM in variable patterns (Schrick et al., 2000). As a result, fk mutant seedlings display multiple shoot meristems and multiple cotyledons. Furthermore, the STM gene is not expressed in embryos that are mutant for both the ZLL/PNH gene and the ARGONAUTE 1 (AGO1) gene (Lynn et al., 1999). ZLL/PNH and AGO1 encode similar proteins related to the eukaryotic translation initiation factor eIF2C, with ZLL/PNH being expressed in the vascular primordium of the globular embryo and later in the adaxial region of the developing cotyledon primordia (Figure 3A and B; Moussian et al., 1998; Lynn et al., 1999). Most zll/pnh embryos fail to establish a functional primary shoot meristem and instead differentiate a leaf-like organ in its place, which suggests that the failure to establish or maintain STM expression results in a change from meristemic to organ cell fate (Moussian et al., 1998; Lynn et al., 1999).
It is not clear whether the early expression of ZLL/PNH in the vascular primordium is required for shoot meristem primordium initiation or whether its later expression in the adaxial region of the developing cotyledon primordia is necessary for shoot meristem maintenance. The REV gene required for secondary shoot meristem initiation during post-embryonic development is also expressed in the vascular primordium of the heart-stage embryo and in the adaxial region of the cotyledon primordia (Figures 3C, 4C and D; Otsuga et al., 2001). In addition, AS1 and FIL are expressed in the presumptive cotyledon primordia from the late-globular stage (Figures 3, 4A and D; Siegfried et al., 1999; Byrne et al., 2000). Later, FIL and other members of the YABBY gene family are expressed in the abaxial region of the cotyledon primordia (Figures 3C, 4C and D; Siegfried et al., 1999), suggesting a similar subdivision to that of leaf primordia, which makes it likely that cotyledon primordia also promote shoot meristem maintenance. Thus, by all accounts, the functional organization of the post-embryonic shoot apex, with its complex interactions between shoot meristem and lateral primordia, is established during embryogenesis.
Concluding remarks
To what extent can we extrapolate from Arabidopsis to distantly related plant species, notably monocots such as maize? Although maize embryos display no regular cell division patterns, develop only one cotyledon and consist of many more cells at maturity, there may be differences in detail rather than in overall patterning processes. For example, the epidermal cell layer is established much later in maize than in Arabidopsis embryogenesis. Nonetheless, a putative homologue of AtML1, the maize OCL1 gene, is not only related by sequence, but also expressed in a similar manner to AtML1 (Ingram et al., 1999). Similarly, the maize KNOTTED gene, a putative homologue of STM, is expressed in a complementary domain to rough sheath2, the homologue of the Arabidopsis leaf-initiation gene AS1, which is negatively regulated by the shoot meristem-promoting STM protein (Jackson et al., 1994; Kerstetter et al., 1997; Timmermans et al., 1999; Tsiantis et al., 1999). Additional similarities are likely to be discovered as more genes are being analysed in maize.
Plant embryogenesis establishes a basic body organization, including two stem-cell systems at opposite ends of the main axis of polarity. These stem-cell systems are organized around small cell groups named the organizing centre or quiescent centre, which maintain the undifferentiated stem cells by local signals. However, in order to generate new structures of the shoot or to extend the tissue organization of the root, the naive stem cells require information from existing organs or tissues. Thus, the body organization of the embryo serves as a reference for post-embryonic development. In this view, plant development may be more similar to animal development than previously thought. In other regards, however, plant embryogenesis appears to be very different from animal development. So far, there is no evidence in Arabidopsis for an extensive maternal control of early embryo patterning, as is prevalent in Drosophila. On the other hand, cell–cell communication involving diffusible substances is likely to play a comparable role in Arabidopsis embryogenesis as in Drosophila imaginal disk patterning. However, the kinds of molecules involved may be very different. In addition, plant cells can exchange proteins with their neighbours through cytoplasmic connections, which is generally not the case in multicellular animal development.
Mechanisms underlying pattern formation during plant embryogenesis are still unknown. A large number of genes are expressed in distinct spatial patterns, indicating that they respond to positional information. Local signalling plays a role in some patterning processes, whereas others may make use of long-range signals, such as the phyto hormone auxin, which has recently surfaced as a candidate for a pattern-generating substance in embryogenesis. Auxin appears to influence the apical–basal axis of polarity, the initiation of the primary root meristem and the phyllotaxis of the shoot apex. However, the distribution of auxin within the developing embryo remains to be visualized and molecular mechanisms of auxin action in embryo patterning have yet to be identified. With the Arabidopsis genome sequence in hand (The Arabidopsis Genome Initiative, 2000), functional analysis of genes by large-scale insertion mutagenesis (Young et al., 2001) can be expected to give further insight into mechanisms underlying pattern formation in Arabidopsis embryogenesis.
Acknowledgments
Acknowledgements
I thank Niko Geldner, Thorsten Hamann, Ulrike Mayer and Kathrin Schrick for helpful comments.
References
- Aida M., Ishida,T., Fukaki,H., Fujisawa,H. and Tasaka,M. (1997) Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. Plant Cell, 9, 841–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aida M., Ishida,T. and Tasaka M. (1999) Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: interaction among the CUP-SHAPED COTYLEDON and SHOOT MERI STEMLESS genes. Development, 126, 1563–1570. [DOI] [PubMed] [Google Scholar]
- Barton M.K. and Poethig,R.S. (1993) Formation of the shoot apical meristem in Arabidopsis thaliana: an analysis of development in the wild type and in the shoot meristemless mutant. Development, 119, 823–831. [Google Scholar]
- Berleth T. and Jürgens,G. (1993) The role of the monopteros gene in organising the basal body region of the Arabidopsis embryo. Development, 118, 575–587. [Google Scholar]
- Brand U., Fletcher,J.C., Hobe,M., Meyerowitz,E.M. and Simon,R. (2000) Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science, 289, 817–819. [DOI] [PubMed] [Google Scholar]
- Byrne M.E., Barley,R., Curtis,M., Arroyo,J.M., Dunham,M., Hudson,A. and Martienssen,R.A. (2000) Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature, 408, 967–971. [DOI] [PubMed] [Google Scholar]
- Christensen S.K., Dagenais,N., Chory,J. and Weigel,D. (2000) Regulation of auxin response by the protein kinase PINOID. Cell, 100, 469–478. [DOI] [PubMed] [Google Scholar]
- Di Laurenzio L., Wysocka-Diller,J., Malamy,J.E., Pysh,L., Helariutta,Y., Freshour,G., Hahn,M.G., Feldmann,K.A. and Benfey,P.N. (1996) The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell, 86, 423–433. [DOI] [PubMed] [Google Scholar]
- Elliott R.C., Betzner,A.S., Huttner,E., Oakes,M.P., Tucker,W.Q., Gerentes,D., Perez,P. and Smyth,D.R. (1996) AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell, 8, 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J.C., Brand,U., Running,M.P., Simon,R. and Meyerowitz,E.M. (1999) Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science, 283, 1911–1914. [DOI] [PubMed] [Google Scholar]
- Hadfi K., Speth,V. and Neuhaus,G. (1998) Auxin-induced develop mental patterns in Brassica juncea embryos. Development, 125, 879–887. [DOI] [PubMed] [Google Scholar]
- Hamada S., Onouchi,H., Tanaka,H., Kudo,M., Liu,Y.-G., Shibata,D., Machida,C. and Machida,Y. (2000) Mutations in the WUSCHEL gene of Arabidopsis thaliana result in the development of shoots without juvenile leaves. Plant J., 24, 91–101. [DOI] [PubMed] [Google Scholar]
- Hamann T., Mayer,U. and Jürgens,G. (1999) The auxin-insensitive bodenlos mutation affects primary root formation and apical–basal patterning in the Arabidopsis embryo. Development, 126, 1387–1395. [DOI] [PubMed] [Google Scholar]
- Hardtke C.S. and Berleth,T. (1998) The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J., 17, 1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helariutta Y., Fukaki,H., Wysocka-Diller,J., Nakajima,K., Jung,J., Sena,G., Hauser,M.T. and Benfey,P.N. (2000) The SHORT ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell, 101, 555–567. [DOI] [PubMed] [Google Scholar]
- Hobbie L., McGovern,M., Hurwitz,L.R., Pierro,A., Liu,N.Y., Bandyopadhyay,A. and Estelle,M. (2000) The axr6 mutants of Arabidopsis thaliana define a gene involved in auxin response and early development. Development, 127, 23–32. [DOI] [PubMed] [Google Scholar]
- Ingram G.C., Magnard,J.L., Vergne,P., Dumas,C. and Rogowsky,P.M. (1999) ZmOCL1, an HDGL2 family homeobox gene, is expressed in the outer cell layer throughout maize development. Plant Mol. Biol., 40, 343–354. [DOI] [PubMed] [Google Scholar]
- Jackson D., Veit,B. and Hake,S. (1994) Expression of maize KNOTTED1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development, 120, 405–413. [Google Scholar]
- Jürgens G. and Mayer,U. (1994) Arabidopsis. In Bard,J.B.L. (ed.), Embryos. Colour Atlas of Development. Wolfe Publishing, London, UK, pp. 7–21.
- Kerstetter R.A., Laudencia-Chingcuanco,D., Smith,L.G. and Hake,S. (1997) Loss-of-function mutations in the maize homeobox gene, knotted1, are defective in shoot meristem maintenance. Development, 124, 3045–3054. [DOI] [PubMed] [Google Scholar]
- Laufs P., Grandjean,O., Jonk,C., Kien,K. and Traas,J. (1998) Cellular parameters of the shoot apical meristem in Arabidopsis. Plant Cell, 10, 1375–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.-M., Xu,Z.-H. and Chua,N.-H. (1993) Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell, 5, 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J.A. and Barton,M.K. (1998) The development of apical embryonic pattern in Arabidopsis. Development, 125, 3027–3035. [DOI] [PubMed] [Google Scholar]
- Long J.A., Moan,E.I., Medford,J.I. and Barton,M.K. (1996) A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature, 379, 66–69. [DOI] [PubMed] [Google Scholar]
- Lu P., Porat,R., Nadeau,J.A. and O’Neill,S.D. (1996) Identification of a meristem L1 layer-specific gene in Arabidopsis that is expressed during embryonic pattern formation and defines a new class of homeobox genes. Plant Cell, 8, 2155–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y. and Koop,H.U. (1997) Somatic embryogenesis in cultured immature zygotic embryos and leaf protoplasts of Arabidopsis thaliana ecotypes. Planta, 202, 387–396. [DOI] [PubMed] [Google Scholar]
- Lynn K., Fernandez,A., Aida,M., Sedbrook,J., Tasaka,M., Masson,P. and Barton,M.K. (1999) The PINHEAD/ZWILLE gene acts pleiotropically in Arabidopsis development and has overlapping functions with the ARGONAUTE1 gene. Development, 126, 469–481. [DOI] [PubMed] [Google Scholar]
- Malamy J.E. and Benfey,P.N. (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development, 124, 33–44. [DOI] [PubMed] [Google Scholar]
- Mayer K.F., Schoof,H., Haecker,A., Lenhard,M., Jürgens,G. and Laux,T. (1998) Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell, 95, 805–815. [DOI] [PubMed] [Google Scholar]
- Mayer U., Büttner,G. and Jürgens,G. (1993) Apical–basal pattern formation in the Arabidopsis embryo: studies on the role of the gnom gene. Development, 117, 149–162. [Google Scholar]
- McConnell J.R. and Barton,M.K. (1998) Leaf polarity and meristem formation in Arabidopsis. Development, 125, 2935–2942. [DOI] [PubMed] [Google Scholar]
- Mizukami Y. and Fischer,R.L. (2000) Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proc. Natl Acad. Sci. USA, 97, 942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussian B., Schoof,H., Haecker,A., Jürgens,G. and Laux,T. (1998) Role of the ZWILLE gene in the regulation of central shoot meristem cell fate during Arabidopsis embryogenesis. EMBO J., 17, 1799–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuga D., DeGuzman,B., Prigge,M.J., Drews,G.N. and Clark,S.E. (2001) REVOLUTA regulates meristem initiation at lateral positions. Plant J., 25, 223–236. [DOI] [PubMed] [Google Scholar]
- Reinhardt D., Mandel,T. and Kuhlemeier,C. (2000) Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell, 12, 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S. et al. (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell, 99, 463–472. [DOI] [PubMed] [Google Scholar]
- Sawa S., Watanabe,K., Goto,K., Kanaya,E., Morita,E.H. and Okada,K. (1999) FILAMENTOUS FLOWER, a meristem and organ identity gene of Arabidopsis, encodes a protein with a zinc finger and HMG-related domains. Genes Dev., 13, 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres B., Wolkenfelt,H., Willemsen,V., Terlouw,M., Lawson,E., Dean,C. and Weisbeek,P. (1994) Embryonic origin of the Arabidopsis primary root and root meristem initials. Development, 120, 2475–2487. [Google Scholar]
- Scheres B., Di Laurenzio,L., Willemsen,V., Hauser,M., Janmaat,K., Weisbeek,P. and Benfey,P.N. (1995) Mutations affecting the radial organisation of the Arabidopsis root display specific defects throughout the embryonic axis. Development, 121, 53–62. [Google Scholar]
- Scheres B., McKhann,H.I. and van den Berg,C. (1996) Roots redefined: anatomical and genetical analysis of root development. Plant Physiol., 111, 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof H., Lenhard,M., Haecker,A., Mayer,K.F.X., Jürgens,G. and Laux,T. (2000) The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell, 100, 635–644. [DOI] [PubMed] [Google Scholar]
- Schrick K., Mayer,U., Horrichs,A., Kuhnt,C., Bellini,C., Dangl,J. Schmidt,J. and Jürgens,G. (2000) FACKEL is a sterol C-14 reductase required for organized cell division and expansion in Arabidopsis embryogenesis. Genes Dev., 14, 1471–1484. [PMC free article] [PubMed] [Google Scholar]
- Schwartz B.W., Yeung,E.C. and Meinke,D.W. (1994) Disruption of morphogenesis and transformation of the suspensor in abnormal suspensor mutants of Arabidopsis. Development, 120, 3235–3245. [DOI] [PubMed] [Google Scholar]
- Sessions A., Weigel,D. and Yanofsky,M.F. (1999) The Arabidopsis thaliana MERISTEM LAYER 1 promoter specifies epidermal expression in meristems and young primordia. Plant J., 20, 259–263. [DOI] [PubMed] [Google Scholar]
- Siegfried K.R., Eshed,Y., Baum,S.F., Otsuga,D., Drews,G.N. and Bowman,J.L. (1999) Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development, 126, 4117–4128. [DOI] [PubMed] [Google Scholar]
- Steinmann T., Geldner,N., Grebe,M., Mangold,S., Jackson,C.L., Paris,S., Gälweiler,L., Palme,K. and Jürgens,G. (1999) Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science, 286, 316–318. [DOI] [PubMed] [Google Scholar]
- The Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature, 408, 796–815. [DOI] [PubMed] [Google Scholar]
- Timmermans M.C.P., Hudson,A., Becraft,P.W. and Nelson,T. (1999) ROUGH SHEATH2: a Myb protein that represses knox homeobox genes in maize lateral organ primordia. Science, 284, 151–153. [DOI] [PubMed] [Google Scholar]
- Trotochaud A.E., Jeong,S. and Clark,S.E. (2000) CLAVATA3, a multimeric ligand for the CLAVATA1 receptor-kinase. Science, 289, 613–617. [DOI] [PubMed] [Google Scholar]
- Tsiantis M., Schneeberger,R., Golz,J.F., Freeling,M. and Langdale,J.A. (1999) The maize rough sheath2 gene and leaf development programs in monocot and dicot plants. Science, 284, 154–156. [DOI] [PubMed] [Google Scholar]
- Van den Berg C., Willemsen,V., Hage,W., Weisbeek,P. and Scheres,B. (1995) Cell fate in the Arabidopsis root meristem determined by directional signalling. Nature, 378, 62–65. [DOI] [PubMed] [Google Scholar]
- Van den Berg C., Willemsen,V., Hendriks,G., Weisbeek,P. and Scheres,B. (1997) Short-range control of cell differentiation in the Arabidopsis root meristem. Nature, 390, 287–289. [DOI] [PubMed] [Google Scholar]
- Vernon D.M. and Meinke,D.W. (1994) Embryogenic transformation of the suspensor in twin, a polyembryonic mutant of Arabidopsis. Dev. Biol., 165, 566–573. [DOI] [PubMed] [Google Scholar]
- Vernoux T., Kronenberger,J., Grandjean,O., Laufs,P. and Traas,J. (2000) PIN-FORMED 1 regulates cell fate at the periphery of the shoot apical meristem. Development, 127, 5157–5165. [DOI] [PubMed] [Google Scholar]
- Vroemen C.W., Langeveld,S., Mayer,U., Ripper,G., Jürgens,G., Van Kammen,A. and De Vries,S.C. (1996) Pattern formation in the Arabidopsis embryo revealed by position-specific lipid transfer protein gene expression. Plant Cell, 8, 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen V., Wolkenfelt,H., de Vrieze,G., Weisbeek,P. and Scheres,B. (1998) The HOBBIT gene is required for formation of the root meristem in the Arabidopsis embryo. Development, 125, 521–531. [DOI] [PubMed] [Google Scholar]
- Woodrick R., Martin,P.R., Birman,I. and Pickett,F.B. (2000) The Arabidopsis embryonic shoot fate map. Development, 127, 813–820. [DOI] [PubMed] [Google Scholar]
- Young J.C., Krysan,P.J. and Sussman,M.R. (2001) Efficient screening of Arabidopsis T-DNA insertion lines using degenerate primers. Plant Physiol., 125, 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]