Abstract
Background
Thyroid carcinoma is the most common malignant endocrine tumour, and its prevalence has been on the rise in recent years. However, mechanisms underlying the metastasis of thyroid carcinoma and candidate biomarkers remain elusive. In this study, we screened genes involved in the virulence and metastasis of papillary thyroid carcinoma (PTC).
Methods
Oxford Nanopore Technology full-length transcriptome sequencing and bioinformatic analyses were performed to analyse differentially expressed genes (DEGs) in PTC. We collected 15 cancerous and paracancerous tissue pairs from patients with PTC for RNA sequencing. The significance thresholds for DEGs were |log2(fold change)| ≥ 1 and false discovery rate (FDR) < 0.01. Gene Ontology and Kyoto Encyclopaedia Gene and Genome pathway enrichment analyses of the 50 most significant DEGs were performed. Immunohistochemistry was used to evaluate LAMB3 expression in tissue microarrays (58 pairs of PTC samples) and its correlation with clinicopathological parameters. Differences in gene expression between cancerous and paracancerous tissues were analysed using the Wilcoxon test. The correlation between gene expression and clinical variables was assessed using Fisher’s exact test.
Results
Transcriptomic analysis revealed the presence of 1,687 DEGs in PTC, and the expression of 804 and 883 genes was found to be upregulated and downregulated, respectively. LAMB3 expression was significantly elevated in cancerous tissues when compared to that in paracancerous tissues (p < 0.001). LAMB3 expression was significantly positively associated with PTC tumour tissue size (p = 0.001, r = 0.49) and T stage (p = 0.017, r = 0.339).
Conclusions
Our findings suggest that LAMB3 expression significantly increases in PTC and it is associated with tumor size and T stage.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-025-14916-0.
Keywords: Papillary thyroid carcinoma, Third-generation sequencing, LAMB3, Biomarker, Metastasis
Background
Thyroid carcinoma (TC) is the most common endocrine malignancy [1, 2]. In recent decades, its prevalence has rapidly increased to approximately 3.1% of all first-diagnosis cancers worldwide. Papillary thyroid carcinoma (PTC) is the most frequent TC, accounting for 80% of TC cases. Additionally, PTC has a 3-fold higher frequency in females than in males [3, 4]. PTC tends to metastasise to the lymph nodes; although the 5-year mortality rate of patients with PTC is approximately 98%, around 15% of patients experience recurrence and approximately 5% of patients experience a fatal outcome. Thyroid nodules are a prevalent clinical presentation and are found in around 20–76% of patients with PTC; however, only 7–15% of cases are malignant [5, 6]. Therefore, it is challenging to distinguish malignant tumours from thyroid nodules. Although fine needle aspiration cytology has become one of the most dependable diagnostic techniques for thyroid malignancies in recent years, its results are unsatisfactory in 10–15% of cases.
Currently, PTC is treated primarily with surgery and adjuvant thyroid hormone or radioiodine therapies [7], with typically good outcomes. However, 15% of patients experience postoperative recurrence. Therefore, better prognostic markers are required for patient prognosis and the selection of optimal treatment strategies. Clinical features including age, tissue type, tumour size, local invasion, and lymph node and distant metastases are commonly used as prognostic factors; however, these features cannot accurately predict the prognosis of individual patients. Identifying patients eligible for surgery, adjuvant therapies, or clinical follow-up requires an understanding of the molecular features of tumours that increase the risk of undesired outcomes.
Tumour aggression and progression is a major contributor to poor clinical outcomes in PTC cases, which are closely related to the extracellular matrix (ECM) and basement membrane destruction. which Laminin-332 is an ECM protein produced by human keratin-forming cells. Laminin-332 is encoded by LAMA3, lam3, and LAMC2 and is involved in the aggressiveness of several cancers [8–11]. In particular, LAMB3 participates in the infiltration and migration of pancreatic, lung, head and neck [12], prostate [13], and gastric [8] tumours. This gene affects cell adherence, multiplication, migration, and differentiation [14] and is associated with the clinicopathological characteristics of cancers [13, 15]. Upregulation of LAMB3 has been observed in pancreatic ductal adenocarcinoma wherein it regulates cell cycle arrest and apoptosis, thus altering tumour behaviour in terms of multiplication, aggression, and metastasis by modulating the PI3K/Akt signalling pathway [16]. In primary lung cancers, LAMB3 is upregulated; the high expression positively correlates with the grade of malignant differentiation, advanced tumour stage, lymphatic lung tumour metastasis, and the enhanced migration and infiltration of lung cancer cell lines [17]. Additionally, LAMB3 reportedly promotes gastric cancer via aberrant positive regulation of promoter demethylation [8].
The development of full-length transcriptome sequencing technology has allowed the analysis of DEGs to improve our understanding of PTC. Yet, there are a few applications of full-length transcriptome sequencing in PTC diagnosis and treatment. In this study, we employed full-length transcriptome analysis to screen genes that are closely associated with PTC occurrence and progression, with the aim of identifying biomarkers for PTC diagnosis and therapy.
Methods
Patients
Each study participant voluntarily provided informed consent. The criteria for inclusion were as follows: (i) 18 to 80 years of age with positive diagnosis of PTC using paraffin-embedded biopsy samples; (ii) no prior thyroidectomy; (iii) primary cancer without previously receiving chemotherapy, radiotherapy, targeted therapy, cytokine therapy, or immunotherapy; or (iv) no history of neck radiation. The criteria for exclusion were as follows: (i) prior radiation to the neck or a history of radiotherapy or chemotherapy; (ii) thyroid cancer surgery; or (iii) another primary malignancy.
Sample collection
Pairs of fresh tissues were obtained from 15 participants with PTC diagnosed from September 2021 to May 2022 at the Thyroid Surgery of The First Attached Hospital of Jinzhou Medical University. Cancerous and paracancerous (2 mm distal to the tumour site) tissues were obtained, instantly cryopreserved in liquid nitrogen, and shipped to Biomarker Biotechnologies Co. Ltd. (Beijing, China) for RNA sequencing.
RNA extraction and cDNA Preparation
TRIzol reagent (Takara Bio Inc., Kyoto, Japan) was used to extract the RNA. The mRNA portion of the total RNA was assessed using the Implen NanoPhotometer spectrophotometer (Implen, Westlake Village, CA, USA). RNA was reverse transcribed to cDNA and these cDNA libraries were built from total RNA using the cDNA-PCR Sequencing Kit (SQK-PCS109; Oxford Nanopore Technologies [ONT], Oxford, UK). Fourteen cycles of PCR amplification were conducted using LongAmp Tag DNA Polymerase (New England Biolabs, Ipswich, MA, USA). The final cDNA libraries were analysed using FLO-MIN109 flow cells (R9 Version, FLO-PRO002; ONT) and the PromethION48 platform (ONT).
Sequencing data and quality control
The initial format of the Nanopore sequencing data was second-generation FAST5. Guppy software (V6.4.6) was used to base-call the data. The fastq format was substituted for the original FAST5 format to analyse data quality. Clean data were checked against the database to further improve accuracy, and ribosomal RNA was filtered before further analysis. Comparison of whole-field sequences to reference genome sequences was performed using the min2 software (version 2.16; ONT), and consensus sequences were finally obtained using pinfish (v0.1.0; ONT). For each sample, concordant sequences were integrated and mapped to all reference genomes using minimap2, and the results were compared. Redundant sequences were removed, and sequences with an identity below 0.9 and coverage below 0.85 were removed. Results differing only at the 5′-end exon were integrated for variable splicing analyses. Redundant results from each sample were integrated to yield a nonredundant transcript sequence. The transcript structure was identified using whole-genome sequencing and compared with transcripts from the genome using GffCompare (version 0.9.8) to identify novel genes and transcripts.
Analysis of differentially expressed genes (DEGs)
Gene expression was determined as counts per million (CPM): CPM = reads assigned to transcripts/total reads mapped to samples. Gene expression was compared between cancerous and paracancerous tissues in terms of fold change (FC). Genes with an FDR < 0.01 and |log2(FC)| ≥ 1 found by DESeq2 (version 1.6.3) were assigned as differentially expressed.
Gene ontology (GO) analysis
GO analysis was employed to uncover significantly enriched terms considering biological processes, molecular functions, and cellular components; a p-value of < 0.05 was considered significant enrichment. R/clusterProfiler package was used for GO analysis.
Kyoto encyclopaedia of genes and genomes (KEGG) analysis
The q-value is the p-value after correcting for multiple hypothesis tests, and the lower the q-value, the higher the confidence of significant enrichment for DEGs in a given pathway. The KEGG database (http://www.genome.jp/kegg) [18] was used for pathway annotation of DEGs. A p value of < 0.05 indicated significant enrichment.
Immunohistochemistry staining of tissue microarrays
Tissue microarray analysis was approved by the Ethics Council of Shanghai Outdo Biotech Co., Ltd. (Ethics number: SHYJS-CP-1907007). Human tissue microarrays containing 58 pairs of cancerous and paracancerous tissues collected from patients with PTC were provided by Shanghai Outdo Biotech Co. Ltd. (Shanghai, China). The tissue chips were placed in an oven, maintained at 63 °C for 1 h, deparaffinised in xylene, and rehydrated in a graded ethanol series. After retrieving tissue antigens, the tissue sections were placed in distilled water at room temperature (20–26 °C) and allowed to cool for over 10 min. Subsequently, the tissue sections were rinsed thrice with phosphate-buffered saline with Tween for 1 min and then immersed in anti-LAMB3 primary antibody (SC133178, 1:50 dilution, Mouse Monoclonal; Santa Cruz Biotechnology, Dallas, TX, USA) at 4 °C overnight. The sections were rinsed thrice with phosphate-buffered saline for 1 min. Thereafter, the sections were incubated with the appropriate secondary antibody (K8002; Dako, Glostrup, Denmark) for 10 min. The sections were rinsed thrice with phosphate-buffered saline for 1 min. The stain was developed by treating the sections with horseradish peroxidase for 15 min. The sections were stained with diaminobenzidine and counterstained with haematoxylin, for 1 min each.
Interpretation of immunohistochemical staining
The scanner(Aperio XT, LEICA) was used for immunohistochemical analysis. Two experienced pathologists independently assessed the staining results. More than three fields of view with different staining intensities were selected, their positive rates were estimated, and the average positive rate was calculated. Staining intensity scores were as follows: 0 (negative), 0.5 (0.5+), 1 (1+), 2 (2+), and 3 (3+). A positive stain score was between 0% and 100%. The overall score was estimated as the product of “staining intensity score” and “stain positivity rate” (0–300%).
Statistical analysis
The Wilcoxon method was used to determine whether protein expression differed significantly between the cancerous and paracancerous tissues. Fisher’s exact test was used for analysing the relationship between protein expression and clinical parameters. p < 0.05 indicated significance. R software (version 4.2.0; R Foundation for Statistical Computing, Vienna, Austria) was used for data analysis. Additionally, we assessed the clinicopathological parameters (age, sex, lymph node migration, tumour size, and T and TNM stages).
Results
Analyses of DEGs
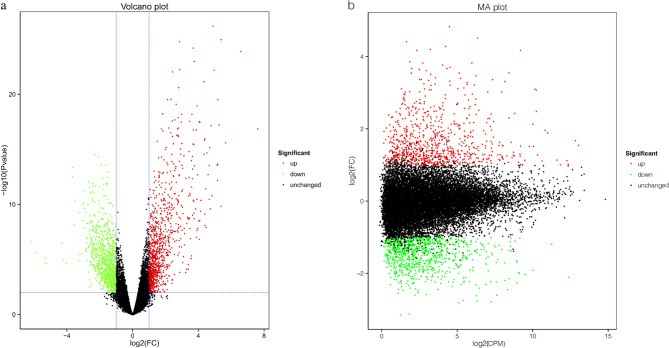
We identified 1,687 DEGs; among them, 804 were upregulated and 883 were downregulated (Table 1) (additional file 1). The volcano plot of DEGs between the cancerous and paracancerous tissue samples has been shown in Fig. 1a. The Bland–Altman plot of DEGs has been shown in Fig. 1b.
Table 1.
Number of DEGs between 15 pairs of cancerous and paracancerous tissues from patients with papillary thyroid carcinoma
| DEG set | Number of DEGs | Upregulated | Downregulated |
|---|---|---|---|
| N vs. C | 1687 | 804 | 883 |
| N1 vs. C1 | 929 | 427 | 502 |
| N2 vs. C2 | 897 | 522 | 375 |
| N3 vs. C3 | 474 | 360 | 114 |
| N4 vs. C4 | 882 | 397 | 485 |
| N5 vs. C5 | 639 | 382 | 257 |
| N6 vs. C6 | 990 | 497 | 493 |
| N7 vs. C7 | 490 | 114 | 376 |
| N8 vs. C8 | 875 | 485 | 390 |
| N9 vs. C9 | 1437 | 469 | 968 |
| N10 vs. C10 | 825 | 603 | 222 |
| N11 vs. C11 | 892 | 550 | 342 |
| N12 vs. C12 | 380 | 316 | 64 |
| N13 vs. C13 | 826 | 600 | 226 |
| N14 vs. C14 | 452 | 353 | 99 |
| N15 vs. C15 | 156 | 106 | 50 |
DEG Differentially expressed gene
Fig. 1.
Differential gene expression analysis of 15 paired papillary thyroid carcinoma (PTC) and paracancerous tissues. a Volcano plot of differentially expressed genes (DEGs). The abscissa represents a log2 fold change (FC) for each gene and the ordinate represents a p-value for log10 log-transformed analysis of significance. The upregulated DEGs are represented by red dots, downregulated DEGs by green dots, and genes with no change in expression by black dots. b Bland–Altman plot of DEGs. Each point in the DEG plot indicates one gene. The x-axis is the A value: log2 count per million (CPM), which is the log of the mean value of expression in the two specimens. The y-axis is the M value: log2(FC), that is, the log of the fold difference in gene expression between the specimens
Top 50 upregulated DEGs and selection of LAMB3
Figure 2 presents the 50 most significantly upregulated genes. By analyzing the up-regulated Top50 genes and Top20 enriched pathways(Fig. 3), we focused on ECM-recepor interaction in Top20 enriched pathways(Fig. 3). We identified the Top50-enriched gene LAMB3 enriched in the ECM-recepor interaction pathway as the candidated gene of the study (Fig. 5).
Fig. 2.
Clustering heat map of the top 50 upregulated DEGs in 15 pairs of PTC samples. The specimen names are represented by the x-axis, and the DEGs are represented by the y-axis. Upregulation is represented by red, downregulation by blue, and no difference in gene expression by white
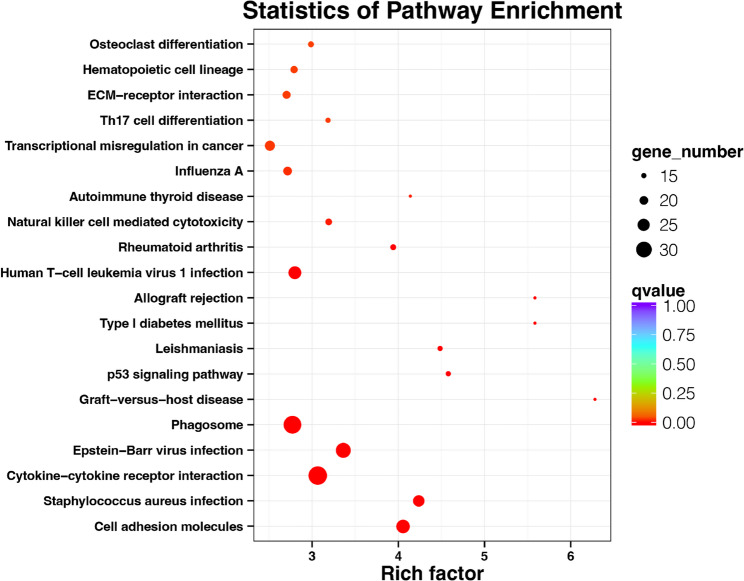
Fig. 3.
Top 20 Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathways enriched by DEGs. The plot magnitude represents the count of DEGs in that pathway, and the colour represents the degree of enrichment. The y-axis is the enriched factor, and the greater the value, the stronger the enrichment
Fig. 5.
KEGG pathway enrichment analysis of LAMB3. The lower the q-value, the more accurate the enrichment of LAMB3 for the given pathway
KEGG pathway enrichment analysis of the upregulated DEGs
The KEGG pathway results for the 804 upregulated genes revealed the top 20 enriched pathways were associated with cell adhesion molecules, Staphylococcus aureus infection, and Epstein–Barr virus infection (Fig. 3).
GO term enrichment analysis of the upregulated DEGs
Figure 4 presents the top 20 GO enriched terms for the 883 upregulated DEGs. Significantly enriched biological process terms included antigen processing and presentation, immune response, and inflammatory response. Significantly enriched cellular component terms included MHC class II protein complex, apical plasma membrane, and extracellular region. Molecular function terms included cytokine receptor binding, chemokine activity, and chemokine receptor binding.
Fig. 4.
Analysis of the top 20 Gene Ontology (GO) terms enriched by the upregulated DEGs. The vertical coordinate includes the names of the enriched terms, and the abscissa denotes the enrichment factor. The magnitude of the point denotes the quantity of enriched genes, that is, the bigger the point, the greater the genes are enriched for that item. The colour of the point indicates the q-value, which is the p-value after adjustment for multiple assumption testing. The lower the q-value, the more credible the enrichment of DEGs for the given term. A, biological process (BP); B, cellular component (CC); C, molecular function (MF)
GO term enrichment analysis of LAMB3
Table 2 reveals the enriched GO terms for LAMB3.
Table 2.
Enriched GO terms for LAMB3
| Category | ID | Description | p | q |
|---|---|---|---|---|
| BP | GO:0050873 | Brown fat cell differentiation | 0.002 | 0.30 |
| MF | GO:0044877 | Macromolecular complex binding | 0.061 | 0.47 |
| CC | GO:0005610 | Laminin-5 complex | 0.041 | 0.70 |
BP Biological process; CC Cellular component; MF Molecular function; GO Gene Ontology
KEGG pathway enrichment analysis of LAMB3
Figure 5 reveals the KEGG pathway enrichment analysis results for LAMB3. The enriched pathways included the ECM-recepor interaction pathway and toxoplasmosis. The KEGG analysis suggested that LAMB3 was mainly enriched in the ECM-recepor interaction pathway, and LAMB3 was selected from the 50 most significantly upregulated genes for further investigation.
LAMB3 expression in PTC tissue microarrays
Immunohistochemical staining revealed that the LAMB3 expression was substantially elevated in cancerous tissues relative to that in paracancerous tissues (p < 0.001; Fig. 6a–g). Figure 6 shows representative LAMB3 staining in PTC tissue microarrays. The images of the PTC tissue microarrays for LAMB3 immunohistochemical staining were shown in additional file 2. The formula for calculating the expression index when evaluating the results of immunohistochemistry was shown in additional file 3.
Fig. 6.
Representative images of different scores of immunohistochemical staining for LAMB3 in PTC tissue microarrays. Immunohistochemical score: Cancerous tissue: a 190%; c 112.5%; e 52.5%. Paracancerous tissue: b 75%; d 37.5%; f 7.5%.(magnification: ×200). Brown represents LAMB3 staining and blue represents haematoxylin staining. g LAMB3 expression in PTC cancerous and paracancerous tissues
Correlation between LAMB3 expression and clinicopathological parameters in PTC
LAMB3 expression in PTC significantly correlated with tumour size (p = 0.001, r = 0.49) and T-stage (p = 0.017, r = 0.339), but not with other clinicopathological factors (Table 3). LAMB3 expression was higher in cancerous tissues > 2 cm than in those ≤ 2 cm in size. Furthermore, its expression was higher in T2–T4 stages than in T1 stage.
Table 3.
Correlation between LAMB3 expression and clinicopathological characteristics
| Clinicopathological factor | Variable | LAMB3 expression | Total | p | r | |
|---|---|---|---|---|---|---|
| Low | High | |||||
| Sex | 0.55 | 0.104 | ||||
| Male | 17 | 5 | 22 | |||
| Female | 23 | 11 | 34 | |||
| Age (years) | 0.558 | 0.102 | ||||
| ≤ 48 | 22 | 7 | 29 | |||
| > 48 | 18 | 9 | 27 | |||
| Tumour size | 0.001 | 0.49 | ||||
| ≤ 2 cm | 31 | 4 | 35 | |||
| > 2 cm | 9 | 12 | 21 | |||
| Tumour number | 1 | −0.032 | ||||
| Single | 34 | 14 | 48 | |||
| Multiple | 6 | 2 | 8 | |||
| T-stage | 0.017 | 0.339 | ||||
| T1 | 25 | 4 | 29 | |||
| T2–T4 | 15 | 12 | 27 | |||
| N-stage | 1 | −0.022 | ||||
| N0 | 21 | 9 | 30 | |||
| N1 | 18 | 7 | 25 | |||
| TNM-stage | 0.763 | −0.059 | ||||
| I/II | 25 | 11 | 36 | |||
| III/IV | 15 | 5 | 20 | |||
T-stage Tumour stage; N-stage Node stage; TNM-stage Tumour node metastasis stage
Immunohistochemical score: High >142.5%; Low ≤ 142.5%
Discussion
In our study, we used full-length transcriptome sequencing to analyse DEGs in 15 pairs of cancerous and paracancerous PTC tissues. A total of 1,687 DEGs were identified, of those, 804 were upregulated and 883 were downregulated. Significant differences in LAMB3 expression were observed in cancer tissues compared with paracancerous tissues. According to the results of the differential expression of genes and KEGG analysis, we further investigated LAMB3 as a candidate biomarker among the upregulated genes. To our knowledge, this research was the first to examine, at the protein level, the correlation between LAMB3 expression and clinical features detected by immunohistochemistry. LAMB3 immunohistochemistry expression of PTC tissue microarrays was substantially elevated in cancerous tissues relative to that in paracancerous tissues, which corresponded with our RNA-sequencing data. These findings indicate the suitability of the considerable differential LAMB3 expression in cancerous tissues as a biomarker for PTC.
Laminins are an integral element of the basement membrane and are required for various critical biological processes [19], including wound repair, tissue formation, and pathological events, such as tumorigenesis [20]. LAMB3 belongs to the laminin large extracellular glycoprotein family. Our findings correspond with those of previous studies demonstrating the upregulated expression of LAMB3 in PTC [21]. Previous studies have also shown that LAMB3 contributes to PTC metastasis [21]; however, this differs from the present findings, and the difference in the results may be attributed to the smaller population of this study. Furthermore, higher LAMB3 expression was notably associated with larger and advanced tumour grade in this study, indicating a potential disruption of laminin expression in PTC, which maybe associated with disease invasiveness.
The current study did have certain limitations. We only preliminarily examined LAMB3 protein expression in tissue and its functional significance. We did not comprehensively assess the diagnostic and prognostic value of LAMB3 in PTC. In addition, the small sample size for immunohistochemistry leads to limited representativeness. Future research should focus on validating the current findings in larger samples and clinically accredited platform to assess the clinical significance of LAMB3 expression in PTC.
Conclusions
In this study, we revealed the expression profiles of PTC and the functional of differentially significantly expressed genes, and discovered potential biomarker LAMB3 for the diagnosis and treatment of PTC. And we validated LAMB3 upregulation via PTC tissue microarray analysis, where it was indeed associated with PTC tumor size and T stage. LAMB3 expression thus hold potential as a clinical biomarker for PTC disease severity assessment.
Supplementary Information
Acknowledgements
We are appreciative of Yvli Tang for her contribution to the initial work of the study.
Abbreviations
- DEG
Differentially expressed gene
- ECM
Extracellular matrix
- FC
Fold change
- FDR
False discovery rate
- GO
Gene Ontology
- KEGG
Kyoto Encyclopaedia of Genes and Genomes
- LAMB3
Laminin subunit beta-3
- PTC
Papillary thyroid cancer
- TNM
Tumour node metastasis
Author contributions
SL contributed to sample collection, manuscript preparation, data analysis, and experimental validation. YW contributed to the methodology, data interpretation, and supervision. FC contributed the collection of samples required for sequencing in this study. JZ, NZ, CZ, ZS, JZ and LF contributed to sample collection and material preparation. YX contributed to the resources and supervised the research. All researchers were involved in the preparation of the manuscript and agreed to its submission.
Funding
The research was financed by the Liaoning Nature Science Foundation (201702851).
Data availability
Our database is stored in NCBI(PRJNA1033723).
Declarations
Ethics approval and consent to participate
Our research was authorised by the Ethics Council of The Third Attached Hospital of Jinzhou Medical University (approval no. KX2021011). The tissue microarray was authorised by the Ethics Council of Shanghai Outdo Biotech Co., Ltd. (approval number SHYJS-CP-1907007). Each study participant voluntarily provided informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388:2783–95. 10.1016/s0140-6736(16)30172-6. [DOI] [PubMed] [Google Scholar]
- 2.Shen X, Zhu G, Liu R, Viola D, Elisei R, Puxeddu E, et al. Patient age-associated mortality risk is differentiated by BRAF V600E status in papillary thyroid cancer. J Clin Oncol. 2018;36:438–45. 10.1200/JCO.2017.74.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laha D, Nilubol N, Boufraqech M. New therapies for advanced thyroid cancer. Front Endocrinol (Lausanne). 2020;11:82. 10.3389/fendo.2020.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, et al. International patterns and trends in thyroid cancer incidence. 1973–2002. Cancer Causes Control. 2009;20:525–31. 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedüs L, et al. American association of clinical endocrinologists, associazione Medici endocrinologi, and European thyroid association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. J Endocrinol Investig. 2010;33:287–91. 10.1007/BF03346587. [DOI] [PubMed] [Google Scholar]
- 6.Hegedüs L. Clinical practice. The thyroid nodule. N Engl J Med. 2004;351(17):1764–71. 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 7.Al-Ammar Y, Al-Mansour B, Al-Rashood O, Tunio MA, Islam T, Al-Asiri M, et al. Impact of body mass index on survival outcome in patients with differentiated thyroid cancer. Braz J Otorhinolaryngol. 2018;84:220–6. 10.1016/j.bjorl.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon OH, Park JL, Kim M, Kim JH, Lee HC, Kim HJ, et al. Aberrant up-regulation of LAMB3 and LAMC2 by promoter demethylation in gastric cancer. Biochem Biophys Res Commun. 2011;406:539–45. 10.1016/j.bbrc.2011.02.082. [DOI] [PubMed]
- 9.Ii M, Yamamoto H, Taniguchi H, Adachi Y, Nakazawa M, Ohashi H, et al. Co-expression of laminin beta3 and gamma2 chains and epigenetic inactivation of laminin alpha3 chain in gastric cancer. Int J Oncol. 2011;39:593–9. 10.3892/ijo.2011.1048. [DOI] [PubMed] [Google Scholar]
- 10.Calaluce R, Kunkel MW, Watts GS, Schmelz M, Hao J, Barrera J, et al. Laminin-5 beta3A expression in LNCaP human prostate carcinoma cells increases cell migration and tumourigenicity. Neoplasia. 2004;6:468–79. 10.1593/neo.03499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinoshita T, Hanazawa T, Nohata N, Kikkawa N, Enokida H, Yoshino H, et al. Tumour suppressive microRNA-218 inhibits cancer cell migration and invasion through targeting laminin-332 in head and neck squamous cell carcinoma. Oncotarget. 2012;3:1386–400. 10.18632/oncotarget.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Jung SN, Oh C, Lee K, Won HR, Chang JW, et al. LAMB3 is associated with disease progression and cisplatin cytotoxic sensitivity in head and neck squamous cell carcinoma. Eur J Surg Oncol. 2019;45:359–65. 10.1016/j.ejso.2018.10.543. [DOI] [PubMed]
- 13.Reis ST, Timoszczuk LS, Pontes-Junior J, Viana N, Silva IA, Dip N, et al. The role of micro RNAs let7c, 100 and 218 expression and their target RAS, C-MYC, BUB1, RB, SMARCA5, LAMB3 and Ki-67 in prostate cancer. Clin (Sao Paulo). 2013;68:652–7. 10.6061/clinics/2013(05)12. [DOI] [PMC free article] [PubMed]
- 14.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–84. 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 15.Zhou X, Chen X, Hu L, Han S, Qiang F, Wu Y, et al. Polymorphisms involved in the miR-218- LAMB3 pathway and susceptibility of cervical cancer, a case-control study in Chinese women. Gynecol Oncol. 2010;117:287–90. 10.1016/j.ygyno.2010.01.020. [DOI] [PubMed]
- 16.Zhang H, Pan Y-Z, Cheung M, Cao M, Yu C, Chen L, et al. LAMB3 mediates apoptotic, proliferative, invasive, and metastatic behaviors in pancreatic cancer by regulating the PI3K/Akt signalling pathway. Cell Death Dis. 2019;10:230. 10.1038/s41419-019-1320-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang XM, Li J, Yan MX, Liu L, Jia DS, Geng Q, et al. Integrative analyses identify osteopontin, LAMB3 and ITGB1 as critical pro-metastatic genes for lung cancer. PLoS One. 2013;8:e55714. 10.1371/journal.pone.0055714. [DOI] [PMC free article] [PubMed] [Retracted]
- 18.Kanehisa M. The KEGG database. Chichester. UK: Wiley; 2002. 10.1002/0470857897.ch8. [Google Scholar]
- 19.Sasaki T, Fassler R, Hohenester E. Laminin: the crux of basement membrane assembly. J Cell Biol. 2004;164:959–63. 10.1083/jcb.200401058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marinkovich MP. Tumour microenvironment: laminin 332 in squamous-cell carcinoma. Nat Rev Cancer. 2007;7:370–80.10.1038/nrc2089. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Jin Y, Bhandari A, Yao Z, Yang F, Pan Y, et al. Upregulated LAMB3 increases proliferation and metastasis in thyroid cancer. Onco Targets Ther. 2018;11:37–46. 10.2147/OTT.S149613. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our database is stored in NCBI(PRJNA1033723).