Abstract
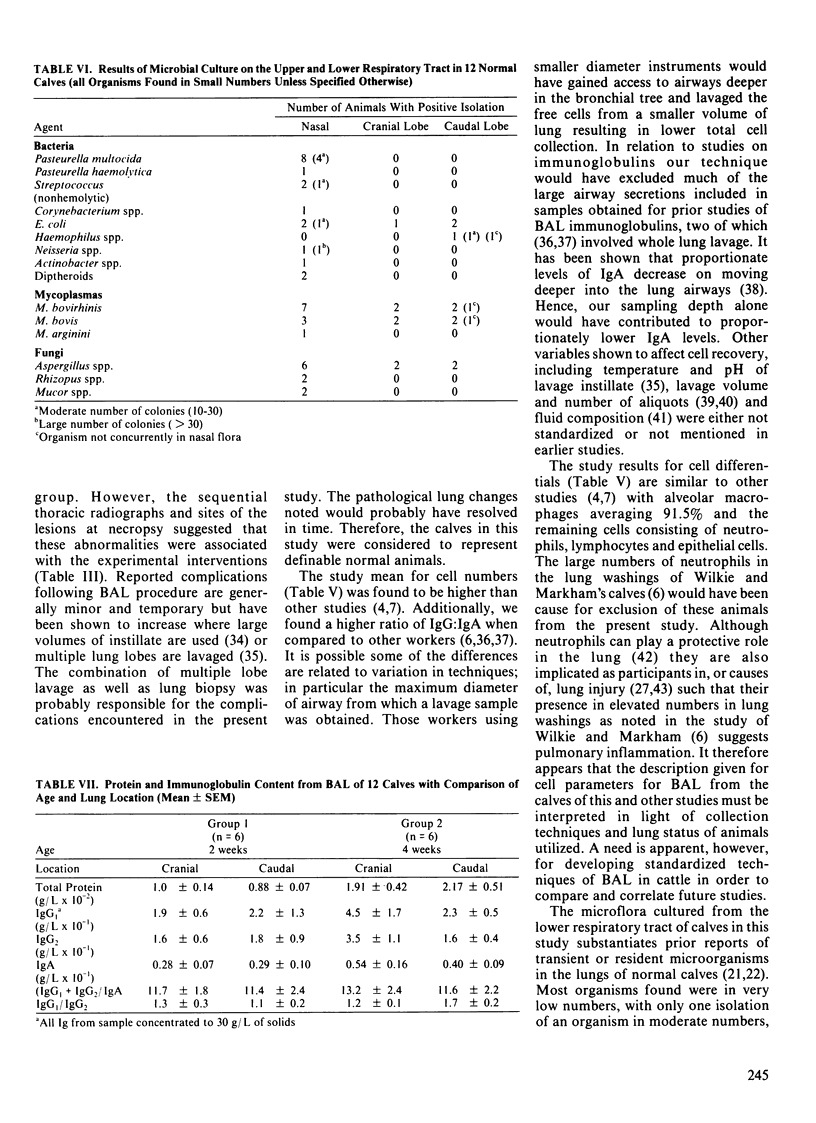
Of a group of 30 clinically normal male Holstein calves two to eight weeks of age, six two week old and six four week old calves met various radiographical and clinicopathological criteria for normality. Bronchoalveolar lavage was performed by fiberoptic bronchoscopy on cranial and caudal lung regions in all 30 calves and samples analyzed for free cells, microorganisms, and immunoglobulins. Lateral chest radiographs and lung biopsies were also conducted on each calf. Calves were euthanized and necropsied ten days after bronchoalveolar lavage was conducted. Reported in this paper are results from the 12 normal calves. Microorganisms were present in small numbers in the lower respiratory tract of some normal calves. There were no differences in the above parameters between cranial and caudal lobes. There were statistically significant changes in bronchoalveolar lavage cell proportions with age although there were no detectable differences in clinical signs. Four week old calves had a lower percentage of macrophages and a higher percentage of epithelial cells than two week old animals (p less than 0.05). There was also a trend toward an increased percentage of neutrophils in older calves but this was not significant (p greater than 0.05). Total bronchoalveolar lavage protein also appeared to increase with age (p less than 0.05). In both groups a higher proportion of IgG2 in bronchoalveolar lavage compared to serum was found, suggesting the presence of a local selective transfer mechanism into respiratory secretions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan E. M., Pirie H. M., Selman I. E., Wiseman A. Immunoglobulin containing cells in the bronchopulmonary system of non-pneumonic and pneumonic calves. Res Vet Sci. 1979 May;26(3):349–355. [PubMed] [Google Scholar]
- Anderson M. L., Moore P. F., Hyde D. M., Dungworth D. L. Bronchus associated lymphoid tissue in the lungs of cattle: relationship to age. Res Vet Sci. 1986 Sep;41(2):211–220. [PubMed] [Google Scholar]
- Brain J. D., Frank R. Alveolar macrophage adhesion: wash electrolyte composition and free cell yield. J Appl Physiol. 1973 Jan;34(1):75–80. doi: 10.1152/jappl.1973.34.1.75. [DOI] [PubMed] [Google Scholar]
- Butler J. E., Peterson L., McGivern P. L. A reliable method for the preparation of bovine secretory immunoglobulin A (SIgA) which circumvents problems posed by IgG1 dimers in colostrum. Mol Immunol. 1980 Jun;17(6):757–768. doi: 10.1016/0161-5890(80)90146-7. [DOI] [PubMed] [Google Scholar]
- COLLIER J. R., ROSSOW C. F. MICROFLORA OF APPARENTLY HEALTHY LUNG TISSUE OF CATTLE. Am J Vet Res. 1964 Mar;25:391–393. [PubMed] [Google Scholar]
- Dhillon D. P., Haslam P. L., Townsend P. J., Primett Z., Collins J. V., Turner-Warwick M. Bronchoalveolar lavage in patients with interstitial lung diseases: side effects and factors affecting fluid recovery. Eur J Respir Dis. 1986 May;68(5):342–350. [PubMed] [Google Scholar]
- Duncan J. R., Wilkie B. N., Hiestand F., Winter A. J. The serum and secretory immunoglobulins of cattle: characterization and quantitation. J Immunol. 1972 Apr;108(4):965–976. [PubMed] [Google Scholar]
- Ettensohn D. B., Lalor P. A., Roberts N. J., Jr Human alveolar macrophage regulation of lymphocyte proliferation. Am Rev Respir Dis. 1986 Jun;133(6):1091–1096. doi: 10.1164/arrd.1986.133.6.1091. [DOI] [PubMed] [Google Scholar]
- Forman A. J., Babiuk L. A., Baldwin F., Friend S. C. Effect of infectious bovine rhinotracheitis virus infection of calves on cell populations recovered by lung lavage. Am J Vet Res. 1982 Jul;43(7):1174–1179. [PubMed] [Google Scholar]
- Garcia J. G., Wolven R. G., Garcia P. L., Keogh B. A. Assessment of interlobar variation of bronchoalveolar lavage cellular differentials in interstitial lung diseases. Am Rev Respir Dis. 1986 Mar;133(3):444–449. doi: 10.1164/arrd.1986.133.3.444. [DOI] [PubMed] [Google Scholar]
- Grey C. L., Thomson R. G. Pasteurella haemolytica in the tracheal air of calves. Can J Comp Med. 1971 Apr;35(2):121–128. [PMC free article] [PubMed] [Google Scholar]
- HOERLEIN A. B., SAXENA S. P., MANSFIELD M. E. Studies on shipping fever of cattle. II. Prevalence of Pasteurella species in nasal secretions from normal calves and calves with shipping fever. Am J Vet Res. 1961 May;22:470–472. [PubMed] [Google Scholar]
- Hamdy A. H., Trapp A. L. Investigation of nasal microflora of feedlot calves before and after weaning. Am J Vet Res. 1967 Jul;28(125):1019–1025. [PubMed] [Google Scholar]
- Hocking W. G., Golde D. W. The pulmonary-alveolar macrophage (first of two parts). N Engl J Med. 1979 Sep 13;301(11):580–587. doi: 10.1056/NEJM197909133011104. [DOI] [PubMed] [Google Scholar]
- Kew R. R., Ghebrehiwet B., Janoff A. The role of complement in cigarette smoke-induced chemotactic activity of lung fluids. Am Rev Respir Dis. 1986 Mar;133(3):478–481. doi: 10.1164/arrd.1986.133.3.478. [DOI] [PubMed] [Google Scholar]
- Lam S., Leriche J. C., Kijek K., Phillips D. Effect of bronchial lavage volume on cellular and protein recovery. Chest. 1985 Dec;88(6):856–859. doi: 10.1378/chest.88.6.856. [DOI] [PubMed] [Google Scholar]
- Lopez A., Thomson R. G., Savan M. The pulmonary clearance of Pasteurella hemolytica in calves infected with bovine parainfluenza-3 virus. Can J Comp Med. 1976 Oct;40(4):385–391. [PMC free article] [PubMed] [Google Scholar]
- Lynn W. S. Control of cellular influx in lung and its role in pulmonary toxicology. Environ Health Perspect. 1984 Apr;55:307–311. doi: 10.1289/ehp.8455307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Martin T. R., Altman L. C., Albert R. K., Henderson W. R. Leukotriene B4 production by the human alveolar macrophage: a potential mechanism for amplifying inflammation in the lung. Am Rev Respir Dis. 1984 Jan;129(1):106–111. doi: 10.1164/arrd.1984.129.1.106. [DOI] [PubMed] [Google Scholar]
- Martínez-Burnes J., López A., Merino-Moncada M., Ochoa-Galván P., Mondragón I. Pulmonary recruitment of neutrophils and bacterial clearance in mice inoculated with aerosols of Pasteurella haemolytica or Staphylococcus aureus. Can J Comp Med. 1985 Jul;49(3):327–332. [PMC free article] [PubMed] [Google Scholar]
- Morgan K. L., Hussein A. M., Newby T. J., Bourne F. J. Quantification and origin of the immunoglobulins in porcine respiratory tract secretions. Immunology. 1980 Nov;41(3):729–736. [PMC free article] [PubMed] [Google Scholar]
- Muggenburg B. A., Mauderly J. L., Pickrell J. A., Chiffelle T. L., Jones R. K., Luft U. C., McClellan R. O., Pfleger R. C. Pathophysiologic sequelae of bronchopulmonary lavage in the dog. Am Rev Respir Dis. 1972 Aug;106(2):219–232. doi: 10.1164/arrd.1972.106.2.219. [DOI] [PubMed] [Google Scholar]
- Ogra P. L., Karzon D. T. Poliovirus antibody response in serum and nasal secretions following intranasal inoculation with inactivated poliovaccine. J Immunol. 1969 Jan;102(1):15–23. [PubMed] [Google Scholar]
- Osbaldiston G. W., Sullivan R. J. Cytochemical demonstration of esterases in peripheral blood leukocytes. Am J Vet Res. 1978 Apr;39(4):683–685. [PubMed] [Google Scholar]
- Pesce M. A., Strande C. S. A new micromethod for determination of protein in cerebrospinal fluid and urine. Clin Chem. 1973 Nov;19(11):1265–1267. [PubMed] [Google Scholar]
- Pfeiffer N. E., McGuire T. C., Bendel R. B., Weikel J. M. Quantitation of bovine immunoglobulins: comparison of single radial immunodiffusion, zinc sulfate turbidity, serum electrophoresis, and refractometer methods. Am J Vet Res. 1977 May;38(5):693–698. [PubMed] [Google Scholar]
- Pingleton S. K., Harrison G. F., Stechschulte D. J., Wesselius L. J., Kerby G. R., Ruth W. E. Effect of location, pH, and temperature of instillate in bronchoalveolar lavage in normal volunteers. Am Rev Respir Dis. 1983 Dec;128(6):1035–1037. doi: 10.1164/arrd.1983.128.6.1035. [DOI] [PubMed] [Google Scholar]
- Pinsker K. L., Norin A. J., Kamholz S. L., Montefusco C., Schreiber K., Hagstrom J. W., Veith F. J. Cell content in repetitive canine bronchoalveolar lavage. Acta Cytol. 1980 Nov-Dec;24(6):558–563. [PubMed] [Google Scholar]
- Quinn J. E. Delegate key functions to your staff for productivity. Dent Manage. 1983 Jun;23(6):34-5, 38, 40 passim. [PubMed] [Google Scholar]
- Rinaldo J. E., Dauber J. H., Christman J., Rogers R. M. Neutrophil alveolitis following endotoxemia. Enhancement by previous exposure to hyperoxia. Am Rev Respir Dis. 1984 Dec;130(6):1065–1071. doi: 10.1164/arrd.1984.130.6.1065. [DOI] [PubMed] [Google Scholar]
- Shiratori Y., Kino H., Honda N., Mori M., Sugimoto T. Paradoxical functions of alveolar macrophages from Calmette-Guérin bacillus-immunized rats. Respiration. 1985;47(4):285–292. doi: 10.1159/000194784. [DOI] [PubMed] [Google Scholar]
- Slocombe R. F., Malark J., Ingersoll R., Derksen F. J., Robinson N. E. Importance of neutrophils in the pathogenesis of acute pneumonic pasteurellosis in calves. Am J Vet Res. 1985 Nov;46(11):2253–2258. [PubMed] [Google Scholar]
- Sullivan A. L., Prendergast R. A., Antunes L. J., Silverstein A. M., Tomasi T. B., Jr Characterization of the serum and secretory immune systems of the cow and sheep. J Immunol. 1969 Aug;103(2):334–344. [PubMed] [Google Scholar]
- Tanskanen R. Colonisation pattern of the respiratory tract of calves by Mycoplasma dispar. Acta Vet Scand. 1984;25(4):577–592. doi: 10.1186/BF03546925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigo E., Liggitt H. D., Breeze R. G., Leid R. W., Silflow R. M. Bovine pulmonary alveolar macrophages: antemortem recovery and in vitro evaluation of bacterial phagocytosis and killing. Am J Vet Res. 1984 Sep;45(9):1842–1847. [PubMed] [Google Scholar]
- Walker R. D., Corstvet R. E., Lessley B. A., Panciera R. J. Study of bovine pulmonary response to Pasteurella haemolytica: specificity of immunoglobulins isolated from the bovine lung. Am J Vet Res. 1980 Jul;41(7):1015–1023. [PubMed] [Google Scholar]
- Walker R. D., Hopkins F. M., Schultz T. W., McCracken M. D., Moore R. N. Changes in leukocyte populations in pulmonary lavage fluids of calves after inhalation of Pasteurella haemolytica. Am J Vet Res. 1985 Dec;46(12):2429–2433. [PubMed] [Google Scholar]
- Wells P. W., Dawson A. M., Smith W. D., Smith B. S. Transfer of IgG from plasma to nasal secretions in newborn lambs. Vet Rec. 1975 Dec 6;97(23):455–455. [PubMed] [Google Scholar]
- Wilkie B. N., Markham R. J. Bronchoalveolar washing cells and immunoglobulins of clinically normal calves. Am J Vet Res. 1981 Feb;42(2):241–243. [PubMed] [Google Scholar]
- Wilkie B. N., Markham R. J. Sequential titration of bovine lung and serum antibodies after parenteral or pulmonary inoculation with Pasteurella haemolytica. Am J Vet Res. 1979 Dec;40(12):1690–1693. [PubMed] [Google Scholar]
- Wilkie B. N., Markham R. J., Shewen P. E. Response of calves to lung challenge exposure with Pasteurella haemolytica after parenteral or pulmonary immunization. Am J Vet Res. 1980 Nov;41(11):1773–1778. [PubMed] [Google Scholar]
- Williams M. R., Spooner R. L., Thomas L. H. Quantitative studies on bovine immunoglobulins. Vet Rec. 1975 Jan 25;96(4):81–84. doi: 10.1136/vr.96.4.81. [DOI] [PubMed] [Google Scholar]