Abstract
Cyclin-dependent kinase (CDK)7–cyclin H, the CDK-activating kinase (CAK) and TFIIH-associated kinase in metazoans can be activated in vitro through T-loop phosphorylation or binding to the RING finger protein MAT1. Although the two mechanisms can operate independently, we show that in a physiological setting, MAT1 binding and T-loop phosphorylation cooperate to stabilize the CAK complex of Drosophila. CDK7 forms a stable complex with cyclin H and MAT1 in vivo only when phosphorylated on either one of two residues (Ser164 or Thr170) in its T-loop. Mutation of both phosphorylation sites causes temperature-dependent dissociation of CDK7 complexes and lethality. Furthermore, phosphorylation of Thr170 greatly stimulates the activity of the CDK7– cyclin H–MAT1 complex towards the C-terminal domain of RNA polymerase II without significantly affecting activity towards CDK2. Remarkably, the substrate-specific increase in activity caused by T-loop phosphorylation is due entirely to accelerated enzyme turnover. Thus phosphorylation on Thr170 could provide a mechanism to augment CTD phosphorylation by TFIIH-associated CDK7, and thereby regulate transcription.
Keywords: CAK/cell cycle/cyclin-dependent kinase/Drosophila/TFIIH
Introduction
Two events are essential for the activation of the cyclin-dependent kinases (CDKs) that drive the cell cycle: binding to a cyclin partner and phosphorylation on the activation segment or T-loop by a CDK-activating kinase (CAK) (Morgan, 1995). CDK7 was first purified from metazoan sources as the catalytic subunit of CAK, and later shown to be required for CAK activity in vivo in Drosophila melanogaster (Harper and Elledge, 1998; Larochelle et al., 1998). CDK7 also plays a central role in the regulation of transcription as the kinase subunit of the general transcription factor IIH (TFIIH). In that context, CDK7 phosphorylates the C-terminal domain (CTD) of RNA polymerase II (RNA pol II) to facilitate promoter clearance (Dahmus, 1996). The dual role of CDK7 has not been universally conserved, however, because the budding yeast Saccharomyces cerevisiae maintains distinct enzymes for the two functions (Kaldis, 1999).
To form a stable binary complex with its activating partner, cyclin H, in vitro, CDK7 must be phosphorylated on a conserved threonine (Thr170) in its own T-loop (Fisher et al., 1995; Martinez et al., 1997). CDK7 has an additional phosphorylated serine (Ser164) within the T-loop, but it is not required for binding cyclin H or for activating CDK7 complexes in vitro (Fisher et al., 1995). Remarkably, the requirement for T-loop phosphorylation can be bypassed in vitro altogether by the association of CDK7 and cyclin H with the RING finger protein, MAT1 (Devault et al., 1995; Fisher et al., 1995; Tassan et al., 1995; Martinez et al., 1997; Garrett et al., 2001).
Although bulk CAK activity and levels of CDK7, cyclin H and MAT1 proteins do not appear to fluctuate during the cell cycle (Brown et al., 1994; Poon et al., 1994; Tassan et al., 1994), CDK7 could be regulated by differential association with other proteins, or by other post-translational modifications. For example, it has been reported that TFIIH-bound CDK7 phosphorylates the CTD more efficiently than it does CDK2 (Rossignol et al., 1997). In addition, TFIIH binding appears to confer sensitivity to UV irradiation on CDK7 activity in vivo (Adamczewski et al., 1996). Within the trimeric complex, MAT1 has been proposed to increase the activity of CDK7 towards the CTD at the expense of CAK activity (Yankulov and Bentley, 1997). Finally, TFIIH-associated kinase activity appears to decrease at mitosis (Long et al., 1998), and a recent study suggested that changes in the levels of Ser164 phosphorylation are responsible for that repression (Akoulitchev and Reinberg, 1998).
To address the functional significance of CDK7 T-loop phosphorylation in vivo, we have combined genetics in Drosophila with biochemical analysis of purified mammalian components. Drosophila CDK7 is phosphorylated on two sites, Ser164 and Thr170, within the T-loop, as is its mammalian counterpart. These phosphorylations are important determinants of CDK7–cyclin H–MAT1 complex stability; the trimeric CAK complex dissociates in vivo and in vitro in the absence of T-loop phosphorylation. In vitro, Thr170 phosphorylation specifically stimulates the CTD kinase activity of the CDK7 trimeric complex by increasing enzyme turnover ∼20-fold, but has little effect on CAK activity. Ser164 phosphorylation by itself, in contrast, has only minimal stimulatory effects on both CAK and CTD kinase activities.
Therefore, T-loop phosphorylation and binding to MAT1 are cooperating rather than alternative modes of CDK7 activation in vivo, with modifications at Ser164 and Thr170 playing redundant roles in the stabilization of the trimeric CAK complex. The phosphorylation state of TFIIH-bound CDK7, moreover, could be an important determinant of CTD phosphorylation rates during the transcription cycle.
Results
Drosophila CAK complexes
The only component of CAK described to date in Drosophila is the catalytic subunit, CDK7 (Larochelle et al., 1998). We identified in the database Drosophila genes coding for proteins homologous to the known partners of vertebrate CDK7, cyclin H and MAT1, and isolated corresponding cDNAs from an embryonic library. The putative Drosophila cyclin H is 42% identical to human cyclin H, and the candidate Drosophila MAT1 protein shares 52% amino acid identity with human MAT1 (not shown). To determine the composition of physiological Drosophila CAK complexes, we immunoprecipitated CDK7 from embryonic extracts and identified the associated proteins by mass spectrometry of tryptic peptide fragments. We confirmed that CDK7 complexes contain the products of the cycH and MAT1 cDNAs we identified (Figure 1A). Therefore, Drosophila CAK, like its vertebrate counterpart, contains the three subunits: CDK7, cyclin H and MAT1. A fraction of CDK7 is also bound to XPD (Figure 1A), which is found along with CAK in TFIIH. A quaternary complex composed of CDK7, cyclin H, MAT1 and XPD has also been described in mammalian cell extracts (Drapkin et al., 1996; Reardon et al., 1996; Rossignol et al., 1997).
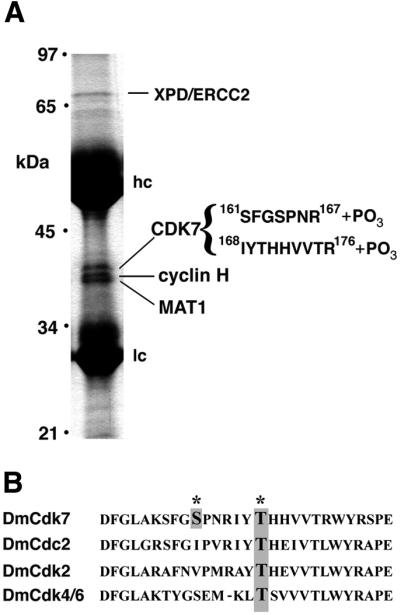
Fig. 1. DmCAK contains cyclin H, MAT1 and XPD. (A) Immuno precipitations were carried out on 0–16 h embryonic extracts, and the isolated proteins were subjected to mass spectrometry. The identities of the four major proteins present in the immunoprecipitates are indicated on the right. Complete Drosophila cyclin H (AF024618) and MAT1 (AF071227) sequences can be obtained from GenBank. DmXPD has been described previously (Reynaud et al., 1999). (B) T-loop sequences and phosphorylation sites of Drosophila CDKs. In addition to the conserved threonine at position 170, Ser164 within the T-loop of CDK7 is also a target of phosphorylation.
Mass spectrometric analysis of the Drosophila CAK peptides (Supplementary data are available at The EMBO Journal Online) indicated that both Ser164 and Thr170 are phosphorylated in vivo, as are the corresponding residues in vertebrate CDK7 (Labbé et al., 1994). CDK7 is the only member of the CDK family with two documented phosphorylations within the T-loop (Figure 1B).
Abrogation of CDK7 phosphorylation in vivo
We mutated Ser164 and Thr170, individually (S164A, T170A) and in combination (S164A/T170A), to alanine. A third mutation, Ser180 to alanine (S180A), was a control. Ser180 is part of the conserved WYR(A/S)PE motif of protein kinases and is an alanine in most other CDKs, including mammalian CDK7. The activity of CDK7S180A is identical to that of wild-type CDK7 (not shown).
We assessed the ability of a given allele of cdk7 to rescue the lethality associated with the cdk7null mutation by crossing males carrying the mutant transgene on the third chromosome (yw/Y; +/+; Pw+[cdk7mutant]/+) to balanced cdk7null females (cdk7null/FM7c; +/+; +/+). The presence of any males carrying the cdk7null chromosome in the progeny from this cross indicates that the transgene rescued the lack of cdk7. All mutations tested were able to rescue the lethality of the null mutation at 18°C (Table I). Although relative viability varied somewhat among individual transgenic lines, stocks of each line could be established and maintained at 18°C (cdk7null/cdk7null; +/+; Pw+ [cdk7mutant]/Pw+[cdk7mutant]). Thus, CDK7 T-loop phosphorylation is not absolutely essential in vivo. However, the cdk7S164A/T170A double mutant transgene was unable to rescue viability at 25°C, and the T170A transgene, when present as a single copy, could only rescue viability consistently at temperatures below 29°C (Table I).
Table I. Temperature-sensitive phenotypes associated with mutations of CDK7 phosphorylation sites.
| Mutation | Rescue cdk7null |
||
|---|---|---|---|
| 18°C | 25°C | 29°C | |
| S180A | yes | yes | yes |
| S164A | yes | yes | yes |
| T170A | yes | yes | noa |
| S164A/T170A | yes | noa | nob |
aDie as pharate adults.
bDie as embryos.
Our results are in contrast to a recent report suggesting that the T170A mutation causes CDK7 to behave in a dominant-negative fashion (Leclerc et al., 2000). The fact that CDK7T170A expressed at or near endogenous levels in a cdk7null background can fully rescue viability (Table I) argues that the dominant effects observed by Leclerc et al. were probably secondary to overexpression. Our data also indicate that CDK7T170A is less active than wild-type CDK7 towards at least one substrate (see below), possibly explaining why CDK7T170A failed to rescue viability of the null mutation when expressed at levels much lower than that of the endogenous protein (Leclerc et al., 2000). We therefore conclude that cdk7T170A behaves genetically as a weak loss-of-function, rather than a dominant-negative, mutation at expression levels near that of wild-type cdk7.
In contrast to the effects of the previously described conditional allele of cdk7 (cdk7P140S; Larochelle et al., 1998), the temperature sensitivity of the cdk7S164A/T170A allele is expressed almost immediately upon transfer to the restrictive temperature, resulting in a rapid arrest of egg laying by adults, and in embryonic lethality at 29°C. Furthermore, the cdk7S164A/T170A adult flies die after 48–72 h at 29°C, also in contrast to the cdk7P140S mutant, in which viability at high temperatures was not compromised after animals reached adulthood (Larochelle et al., 1998). S164A/T170A larvae, moreover, do not survive a 60 min heat shock at 37°C, probably due to a failure to induce a normal heat-shock response. This suggests a more complete loss of CDK7 activity in vivo upon temperature shift when the T-loop cannot be phosphorylated.
The steady-state levels of CDK7 T-loop phosphorylation change during development
The various CDK7 phospho-isoforms observed in ovaries of mutant animals are shown in Figure 2A. Phosphoryl ation of CDK7 on the T-loop increases electrophoretic mobility, as has been observed for other CDKs. We can resolve at least three phospho-isoforms under optimal conditions. In wild-type (or S180A) adults, the fastest migrating, doubly phosphorylated isoform predominates, but we also observe significant amounts of the slowest migrating, unphosphorylated form. In the S164A mutant animals, the doubly phosphorylated form disappears, and an isoform with intermediate electrophoretic mobility, presumably representing CDK7 singly phosphorylated on Thr170, appears.
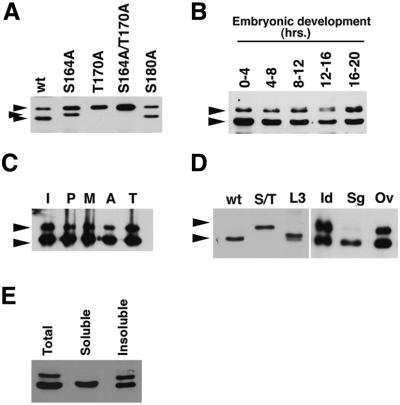
Fig. 2. Phospho-isoforms of Drosophila CDK7 detected by electro phoretic mobility shift. (A) CDK7 protein from ovaries of wild-type and mutant animals is shown. Dual T-loop phosphorylation (bottom arrowhead) results in a large increase in mobility compared with the unphosphorylated isoform (top arrowhead). Phosphorylation on Thr170 alone in the S164A mutant causes an intermediate shift (middle arrow). In this analysis, we could not distinguish the form singly phosphorylated on Ser164 from the completely unphosphorylated form. (B) The relative abundance of CDK7 protein and its state of T-loop phosphorylation vary little during embryonic development. (C) The state of CDK7 T-loop phosphorylation does not change appreciably during the embryonic cell cycles. Embryos were selected individually (embryonic cycle 9–13) after methanol fixation and staining for DNA, and identified by microscopic inspection as being in interphase (I), prophase (P) metaphase (M), anaphase (A) or telophase (T). (D) The state of CDK7 T-loop phosphorylation varies among different tissues: ovaries from wild-type (wt) and the double-mutant (S/T) animals, third instar larvae (L3), imaginal discs (Id), salivary glands (Sg) and ovaries (Ov). Note that the difference in isoform representation between the first and last lanes, which both contain extracts of wild-type ovaries, reflects differences in the age of the animals. We typically see more unphosphorylated CDK7 in more mature ovaries. Proteins were extracted by directly boiling the tissues in SDS sample buffer. (E) The unphosphorylated form of CDK7 exhibits low solubility. Embryos were homogenized in SDS sample buffer (Total) or HoB buffer. Following centrifugation, the supernatant (soluble) and pellet (insoluble) were boiled in SDS sample buffer. The unphosphorylated form of CDK7 is extracted efficiently only in the presence of SDS (lanes 1 and 3).
We asked whether the T-loop phosphorylation state of CDK7 changes in a number of physiological contexts. During embryonic development, the distribution of CDK7 between a predominant, doubly phosphorylated form and a minor, unphosphorylated form appears to be relatively constant (Figure 2B). Likewise, CDK7 isoforms do not fluctuate appreciably in early embryos fractionated into interphase (I), prophase (P), metaphase (M), anaphase (A) and telophase (T) populations (Figure 2C). In contrast, we observe variations when we compare different developmental stages and different tissues (Figure 2D). In third instar larvae (L3), the unphosphorylated isoform is virtually absent. Instead, we see a doublet probably corresponding to doubly and singly phosphorylated CDK7, with the singly, presumably Thr170-phosphorylated, form usually predominating. In contrast, the unphosphorylated form is a major one in imaginal disc, and is also abundant in ovaries. All of the extracts for the analysis shown in Figure 2D were prepared under denaturing conditions (boiling in SDS sample buffer); extraction under non-denaturing conditions solubilized little or no unphosphorylated CDK7 (Figure 2E), precluding meaningful comparison of CDK7-associated kinase activity in different tissues. Although we do not yet understand their physiological significance, these tissue-specific differences suggest that CDK7 T-loop phosphorylation in vivo could modulate kinase activity in response to developmental or environmental signals.
T-loop phosphorylation protects trimeric CAK from thermal inactivation in vitro
To understand the temperature-sensitive phenotype in cdk7 mutant animals, we tested whether the mutant proteins could be inactivated by a temperature shift in vitro. We measured the activity of CDK7 immunoprecipitated from embryos or adult flies raised at 18°C towards both CDK2 and CTD after incubation at either room temperature or 33°C. Remarkably, mutation of Thr170 to alanine differentially affected activity towards the two different substrates, revealing a previously unsuspected role for this residue in determining substrate specificity (see also Figure 7). In addition, both the CAK and CTD kinase activities of all T-loop mutant forms of CDK7 were reduced after a short incubation at 33°C in vitro (Figure 3A and B). Interestingly, the activity associated with CDK7 in the S164A/T170A mutant is <5% (CAK) or 1% (CTD kinase) that of wild-type CDK7 (Figure 3), although the animals are viable. Thus, wild-type CDK7 activity vastly exceeds the level required to sustain its essential function or, alternatively, compensatory mechanisms can act to rescue a drastic drop in CAK and CTD kinase activity.
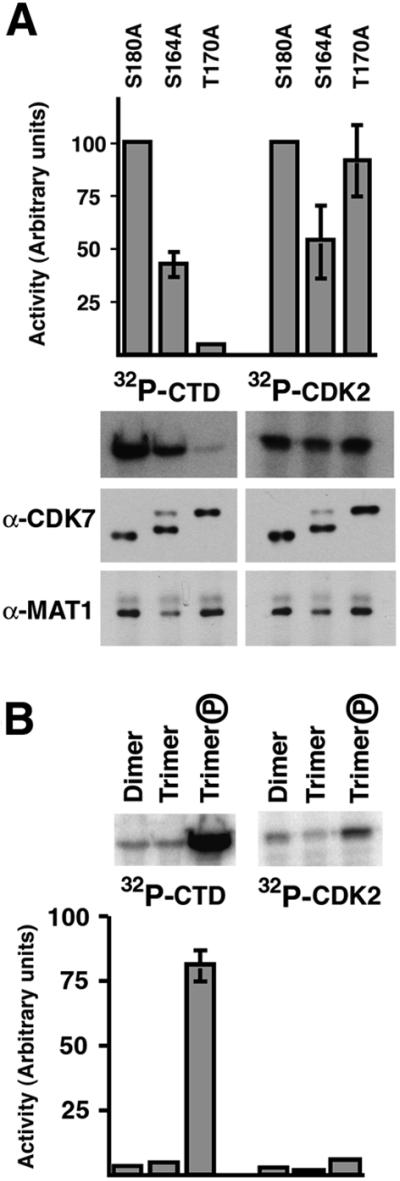
Fig. 7. T-loop phosphorylation of CDK7 regulates activity in a substrate-dependent manner. (A) CDK7 was immunoprecipitated from S180A, S164A and T170A mutants. Each sample was divided in half and tested for kinase activity towards either GST–CTD or CDK2–cyclin A (top panel). Incorporation was quantified and expressed as a percentage of incorporation by the wild-type (S180A) control immunoprecipitate. The T170A mutation results in a loss of activity that is specific to the CTD substrate. Both wild-type and T170A immunoprecipitates contain similar amounts of MAT1 protein, indicating that the substrate-specific effect is caused by the lack of phosphorylation rather than by loss of MAT1 from the complex (bottom panel). Some of the loss of activity by the S164A mutant with both substrates probably stems from the fact that S164A immuno precipitates contain significant amounts of completely dephosphoryl ated (monomeric) CDK7, which is paralleled by a reduced amount of MAT1 in S164A immunoprecipitates as compared with both wild-type and T170A. (B) The purified mammalian phosphorylated trimer exhibits increased activity (25- to 50-fold) towards the CTD substrate when compared with either phosphorylated dimer or unphosphorylated trimer, but the effect of phosphorylation on CAK activity is modest (∼2-fold).
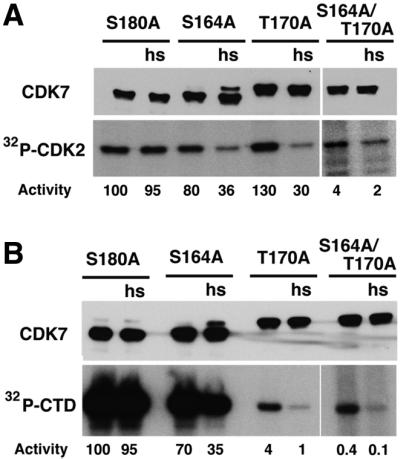
Fig. 3. T-loop mutant forms of Drosophila CDK7 are temperature sensitive in vitro. CDK7 was immunoprecipitated from the various mutants; the immunoprecipitates were divided in half and either left on ice or incubated at 33°C for 30 min (hs) prior to kinase assays with either CDK2D145N–cyclin A (A) or GST–CTD (B) as substrates. Note that the exposure times for experiments with CDK7S164A/T170A were longer than for the wild-type and single mutants. For both substrates, incorporation was quantified by PhosphorImager and expressed as a percentage of incorporation by wild-type CDK7 not subjected to heat shock (defined as 100%). Measured values are indicated below each lane in (A) and (B). Top panel, anti-CDK7 immunoblot; bottom panel, 32P incorporation into substrates.
T-loop phosphorylation appears to protect CDK7 from thermal inactivation. It remained possible, however, that increased thermal lability was due to disruption of protein conformation because alanine cannot substitute adequately for unphosphorylated serine or threonine. To address this possibility, we compared the activity and stability of phosphorylated and unphosphorylated forms of trimeric wild-type CDK7, by using mammalian CDK7, cyclin H and MAT1 proteins expressed with baculoviruses. When we co-infect insect cells with CDK7 and cyclin H viruses only, the dimeric complex that we purify contains CDK7 that is almost quantitatively phosphorylated on both Ser164 and Thr170 (Figure 4A). In contrast, co-infection with CDK7, cyclin H and MAT1 baculoviruses results in a trimeric complex containing completely unphosphorylated CDK7 (Figure 4A). We compared the thermal stability of the three forms of CDK7—phosphorylated dimer, unphosphorylated trimer and phosphorylated trimer—by incubating the different complexes for various times at 42°C before assaying their activity (Figure 4B and C). Both dimer and unphosphorylated trimer rapidly lost activity at 42°C, whereas the phosphorylated trimer, reconstituted by addition of pure MAT1 to the dimer, was resistant to thermal inactivation. We therefore conclude that T-loop phosphorylation is an important contributor to the thermal stability of physiological CDK7 complexes, even when they contain MAT1.
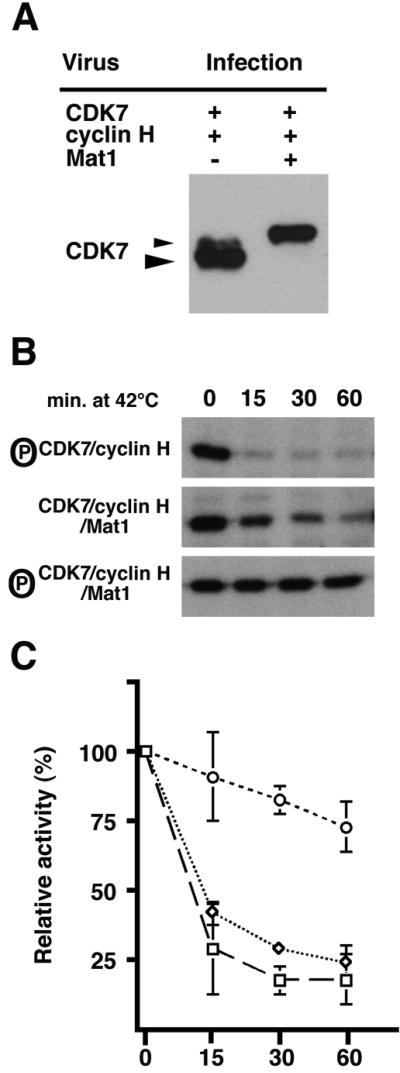
Fig. 4. T-loop phosphorylation protects mammalian CDK7 from thermal inactivation. (A) Anti-CDK7 immunoblots of recombinant CAK purified from Sf9 cells infected with baculoviruses encoding the mammalian CAK subunits. Double infections with CDK7 and cyclin H viruses produce CDK7 protein that is doubly phosphorylated on the T-loop (left: large arrowhead). The small arrowhead indicates a less abundant, probably singly phosphorylated CDK7 isoform. Triple infections with CDK7, cyclin H and MAT1 result in the isolation of CDK7 protein that is entirely unphosphorylated (right). (B) Time course of thermal inactivation of the different forms of CAK in vitro. We incubated the three complexes at 42°C for the indicated periods of time before we measured activity towards a CDK2–cyclin A substrate. (C) Relative thermal stability of the CAK dimer, unphosphorylated trimer and phosphorylated trimer quantified on the basis of triplicate experiments (represented by B). The phosphorylated trimer (circle) is resistant to heat inactivation compared with either the unphosphoryl ated trimer (diamond) or the phosphorylated dimer (square).
CDK7 T-loop phosphorylation is required for efficient assembly of the CAK trimer
In vertebrates, CDK7 exists in two major complexes that can be separated by gel filtration: a >600 kDa complex corresponding to TFIIH; and an ∼100 kDa heterotrimeric complex comprising CDK7, cyclin H and MAT1, which migrates aberrantly with an apparent size of ∼240 kDa (Devault et al., 1995; Fisher et al., 1995). We fractionated Drosophila embryonic extracts to determine the apparent size of the CDK7-containing complexes (Figure 5A). As controls, we analyzed mammalian CDK7–cyclin H dimer and CDK7–cyclin H–MAT1 trimer on the same column (Figure 5A). Most endogenous Drosophila CDK7 chromatographs with the same apparent size as the mammalian trimer. Therefore, soluble CDK7 in embryos is predominantly in the form of free CAK trimer, most of which is phosphorylated on the T-loop. This is consistent with the apparently stoichiometric amounts of CDK7, cyclin H and MAT1 we typically recover in anti-CDK7 immunoprecipitates from embryonic extracts (Figure 1A). We cannot exclude, however, the presence of minor CDK7 complexes lacking MAT1 or containing other subunits, such as XPD, which might not affect chromatographic behavior. After chromatography, we immunoprecipitated the fractions with an anti-CDK7 antibody, and measured kinase activity towards a recombinant CTD substrate (Figure 5A). We consistently observed a minor peak of both immunoreactivity (Figure 5B) and CTD kinase activity (Figure 5A) in fraction 18, which probably corresponds to TFIIH. Thus Drosophila CDK7 forms most or all of the same complexes as does vertebrate CDK7.
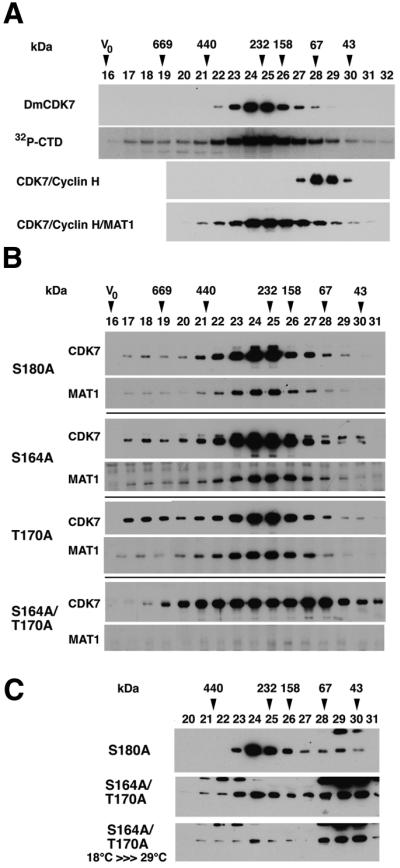
Fig. 5. T-loop phosphorylation is required for the formation of a stable CDK7–cyclin H–MAT1 complex. (A) Embryonic extracts were fractionated on a Superdex 200 gel filtration column; each fraction was subjected to immunoprecipitation with anti-CDK7 antibodies and analyzed by immunoblot or assayed for phosphorylation of GST–CTD in vitro. Pure mammalian dimeric and trimeric CAK complexes were used as size standards in chromatography, and detected by immuno blotting with anti-CDK7 antibodies for the dimer and with anti-MAT1 antibodies for the trimer. (B) Size distribution of CDK7 protein in the various T-loop mutants. Embryonic extracts from the indicated mutants were fractionated as in (A); the fractions were immunoprecipitated with anti-CDK7 antibodies and subjected to SDS–PAGE followed by immunoblotting for CDK7 and MAT1 proteins. Due to the fragility of the complex in the total absence of CDK7 T-loop phosphorylation, no MAT1 could be detected in immunoprecipitates from the S164A/T170A mutant. (C) Decreased thermal stability of unphosphorylated CDK7-containing complexes. Embryonic extracts were fractionated as in (A) and analyzed directly (without immunoprecipitation) by SDS–PAGE and immunoblotting with anti-CDK7 antibodies. When S164A/T170A embryos are shifted from 18 to 29°C, the recovery of the complex is diminished further (bottom panel).
We next looked at the size distribution of the CDK7 proteins with T-loop mutations (Figure 5B). When we analyzed extracts from either cdk7S164A or cdk7T170A embryos, the majority of CDK7 remained in fractions corresponding to the trimeric form. In both cases, however, detectable amounts of CDK7 protein appeared in the smaller size fractions, possibly corresponding to free CDK7 monomer. Interestingly, the unphosphorylated CDK7 isoform was enriched in the monomer-sized fractions of the S164A lysate (Figure 5B, fractions 29 and 30). In cdk7S164A/T170A lysates, the redistribution of CDK7 protein to low molecular weight forms was even more pronounced (Figure 5B), indicating a defect in complex formation when CDK7 cannot be phosphorylated. Consistent with this interpretation, we could detect little or no MAT1 in immunoprecipitates of fractions of the S164A/T170A lysate, although it was detected readily in wild-type and both single mutants (Figure 5B). Because the cdk7S164A/T170A mutation caused lethality at high temperature and altered the distribution of CDK7 between different complexes, we asked whether the basis for temperature sensitivity might be an impaired ability to interact with cyclin H and MAT1. Indeed, after cdk7S164A/T170A embryos were shifted from 18 to 29°C, CDK7 complexes dissociated almost completely (Figure 5C). This correlated well with the inactivation of mutant CDK7 complexes in vitro (Figure 3), suggesting that the basis for thermal instability in the absence of T-loop phosphorylation is due, at least in part, to decreased affinity of CDK7 for its positive regulators, cyclin H and MAT1.
Disappearance of the trimer did not correlate perfectly with the amount of apparent monomer recovered (Figure 5C). In fact, we also reproducibly observed increased immunoreactivity in higher molecular weight fractions and in fractions intermediate in size between trimers and monomers (Figure 5B), possibly representing aggregated or misfolded mutant CDK7 protein, partially dissociated complexes or disruption of higher order complexes not extracted efficiently from wild-type embryos. To confirm the release of CDK7 protein from active complexes, we measured the relative amounts of dissociated CDK7 in the various mutants by immunoprecipitation with antibodies that distinguish between free and complexed CDK7. Monoclonal antibody (mAb) 4A7 recognizes an epitope that is inaccessible when CDK7 is in a functional complex, but is fully accessible when complexes are dissociated in vitro (Figure 6A). Although mAb 4A7 precipitated very little CDK7 from wild-type, S164A and T170A homogenates, it readily precipitated CDK7 protein from the S164A/T170A lysates (Figure 6B, right). mAb 20H5, which recognizes an epitope available in both the complex and the monomeric forms, precipitated similar amounts of CDK7 proteins from all samples (Figure 6B, left). These data indicate that the CDK7S164A/T170A protein dissociated from cyclin H and MAT1 even with minimal handling of the samples in vitro, and is probably dissociated extensively in vivo.

Fig. 6. Dephosphorylation of the T-loop destabilizes CDK7 complexes. (A) Anti-Cdk7 4A7 is specific for the monomeric form of CDK7, whereas the 20H5 antibody recognizes both complex and monomeric forms of CDK7. Antibody 20H5 can precipitate Cdk7 from an embryonic lysate whereas 4A7 cannot (Left). Following immuno precipitation with 20H5 and denaturation by boiling in SDS, 4A7 and 20H5 precipitate Cdk7 with equal efficiency (right). (B) The 4A7 antibody immunoprecipitates large amounts of protein only from the S164A/T170A mutant, and a small amount from the S164A mutant, whereas the 20H5 antibody precipitates similar amounts from all samples. Top panel, anti-CDK7 immunolot of total lysates prior to immunoprecipitation; second panel, anti-CDK7 immunoblot of immunoprecipitates; third panel, anti-CDK7 immunoblot of post-immunoprecipitation supernatants; bottom panel, anti-MAT1 immunoblot on the immunoprecipitated material. (C) Similar to (B) after 15 h incubation at 4°C. Detectable amounts of CDK7 can be immunoprecipitated from all samples with the 4A7 antibody. Both single mutants (S164A and T170A) dissociate to a much greater extent than does the wild-type (S180A) control. In each case, however, only the slow migrating (unphosphorylated) form can be precipitated, indicating that dephosphorylation of the T-loop is required to destabilize complexes. Dephosphorylation of the S164A protein correlates with the loss of MAT1 from the complex (bottom panel).
After several hours of incubation at 4°C, CDK7S164A and CDK7T170A proteins can also be immunoprecipitated readily with mAb 4A7 (Figure 6C, right), consistent with spontaneous dissociation of the complex in crude extracts. Only a barely detectable amount of wild-type (S180A) CDK7 can be precipitated under similar conditions. Interestingly, only the slow migrating (unphosphorylated) isoform of CDK7 can be precipitated from the wild-type and S164A lysates with mAb 4A7 (Figure 6C), arguing that the inability to form a stable complex is strictly a function of phosphorylation, not of misfolding caused by serine/threonine to alanine substitutions. We conclude that T-loop phosphorylation of CDK7 is required to form a stable CDK7–cyclin H–MAT1 complex in vitro and in vivo. Phosphorylation of either Ser164 or Thr170 is sufficient to stabilize the complex, because only unphosphorylated CDK7 can be precipitated by the monomer-specific 4A7 antibody. Indeed, the CAK complexes from either cdk7T170A or cdk7S164A mutant animals initially are relatively stable (Figure 6B), but decay upon prolonged incubation at 4°C as shown by the loss of MAT1 in parallel with dephosphorylation of the T-loop of CDK7 (Figure 6C).
T-loop phosphorylation stimulates kinase activity of trimeric CDK7 in a substrate-specific manner
In the absence of heat treatment, we observed little difference between wild-type Drosophila CDK7 and the single phosphorylation site mutants in activity towards a CDK2 substrate (Figure 3A). However, the T170A mutant protein had dramatically reduced activity towards CTD, compared with wild-type (Figure 3B). To measure the relative effects of Ser164 and Thr170 phosphorylation on CDK7 activity towards CDK2 and the CTD, anti-CDK7 immunoprecipitates were divided in half and assayed with both substrates. The immunoprecipitations were done under conditions that minimized CDK7 complex dissociation in vitro (similar to Figure 6B). The CDK7S164A protein is ∼50% as active as wild-type CDK7 with either substrate (Figure 7A), possibly because it is more prone than wild-type and T170A proteins to dephosphorylation and consequent destabilization of the complex. Indeed, the CDK7S164A immunoprecipitate shows a reduced amount of MAT1 relative to the wild-type, which correlates with the presence of completely unphosphorylated CDK7 (Figure 7A). The CDK7T170A protein, in contrast, is nearly identical to wild-type CDK7 in activity towards CDK2, but only 4% as active towards the CTD (Figure 7A). Moreover, complexes containing CDK7T170A remain intact throughout the immunoprecipitation and the kinase assays, as judged by the stable association of MAT1 (Figure 7A). Thus, phosphorylation of Thr170 stimulates CTD kinase activity ∼25-fold under these assay conditions without significantly affecting CAK activity. To confirm these observations, we carried out similar experiments with purified wild-type mammalian components in both the phosphorylated and unphosphorylated states (Figure 7B). The phosphorylated trimer is ∼20-fold more active towards the GST–CTD substrate, but only slighly more active towards CDK2. Although the relative contributions of phosphorylations on Ser164 and Thr170 can only be measured in Drosophila, the results with either the fly or mammalian enzymes are in remarkably good agreement: phosphorylation of trimeric CDK7 on Thr170 specifically and dramatically (∼20-fold) stimulates activity towards the CTD without significantly affecting CAK activity. Phosphorylation on Ser164, in contrast, causes only a modest stimulation of activity without substrate preference.
Phosphorylation of the CDK7 T-loop could boost CTD kinase activity in either of two ways: an increase in catalytic efficiency specific to the Tyr-Ser-Pro-Thr-Ser-Pro-Ser target sequence of the CTD, or a change in the enzymatic mechanism from a distributive to a processive one. Because the full-length CTD has 52 potential sites of phosphorylation by CDK7, an increase in processivity could well account for the apparent increase in activity when CDK7 is doubly phosphorylated on the T-loop. To test this idea, we compared the activity of CDK7 immunoprecipitated from wild-type, S164A and T170A animals towards a CTD peptide containing only two heptad repeats. The results obtained with the peptide substrate were identical to those obtained with the full-length CTD (data not shown), suggesting an increased catalytic efficiency rather than processivity. In addition, kinetic analysis of the three mammalian CAK complexes (phosphorylated dimer, unphosphorylated trimer and doubly phosphorylated trimer) shows that the substrate-specific increase in catalytic efficiency is due entirely to accelerated enzyme turnover (Table II). Thr170 phosphorylation could therefore be a key step in regulating the rate of CTD phosphorylation by TFIIH during transcription.
Table II. Apparent kinetic parameters of the diferent CAK complexes with the CTD and CDK2–cyclin A substrates.
| Parameter | CTD |
CDK2D145N–cyclin A |
||||
|---|---|---|---|---|---|---|
| Dimer | Trimer | P-Trimer | Dimer | Trimer | P-Trimer | |
| Km (µM) | 7.5 ± 2.5 | 3.8 ± 1.6 | 4.3 ± 1.4 | 0.26 ± 0.08 | 0.39 ± 0.07 | 0.23 ± 0.05 |
| Vmax (pmol Pi/min) | 1.6 ± 0.3 | 1.6 ± 0.2 | 26.6 ± 3.3 | 0.094 ± 0.010 | 0.054 ± 0.010 | 0.19 ± 0.02 |
| kcat (/s) | 0.23 | 0.22 | 3.69 | 0.013 | 0.008 | 0.026 |
| kcat/Km | 0.03 | 0.06 | 0.86 | 0.04 | 0.02 | 0.11 |
A defect in CTD hyperphosphorylation after heat shock in cdk7T170A mutant larvae
The effect of Thr170 phosphorylation on the kinetics of CTD kinase activity in vitro suggests that its abrogation might compromise normal RNA pol II phosphorylation in vivo. The fact that cdk7T170A flies are viable under most conditions tested, however, argues against a constitutive, global disruption of CTD phosphorylation. It is therefore not surprising that we saw no major difference in the steady-state levels of CTD phosphorylation, on either Ser2 or Ser5, among the cdk7 mutants raised at different temperatures (Figure 8; data not shown). In contrast, when we subjected larvae of the different mutant backgrounds to a brief heat shock, phosphorylation of the CTD reproducibly increased above basal levels in both the wild-type and S164A mutant, whereas the response was blunted in the T170A mutant animals (Figure 8). Although the mechanisms underlying these fluctuations are likely to be complex, our data suggest that a kinetically defective, CDK7T170A mutant enzyme is unable to support the increased rate of CTD phosphorylation that normally occurs after an acute thermal stress.
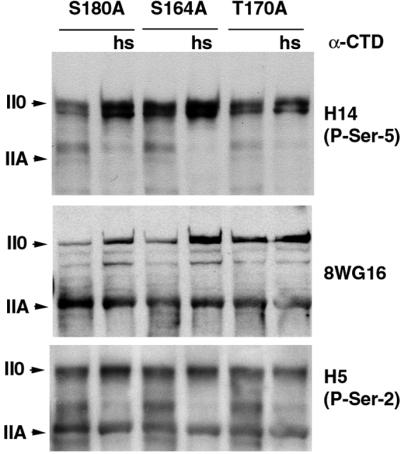
Fig. 8. RNA polymerase II phosphorylation in CDK7 mutants. Third instar wandering larvae were homogenized directly in SDS sample buffer either prior to, or after a 30 min heat shock at 37°C (hs). Each sample was immunoblotted with antibodies to the CTD of the large subunit of RNA pol II: 8WG16, H5 (specific for phosphorylated Ser2 of the CTD heptad repeat) and H14 (specific for phosphorylated Ser5 of the CTD heptad repeat). Although the 8WG16 antibody preferentially recognizes unphosphorylated repeats (enriched in form IIA), it cross-reacts to some extent with form II0 that is not completely phosphorylated.
Discussion
T-loop phosphorylation: a stabilizer of the CDK7–cyclin H–MAT1 trimeric complex
In the best studied cases of CDKs that drive the cell cycle, T-loop phosphorylation is essential for physiological function (Morgan, 1997). Although that requirement has been demonstrated for Cdc2 in fission yeast (Gould et al., 1991), Cdc28 in budding yeast (Lim et al., 1996; Cross and Levine, 1998) and Cdc2 in Drosophila (Larochelle et al., 1998), its basis can only be inferred from structural studies of the human CDK2–cyclin A complex (De Bondt et al., 1993; Jeffrey et al., 1995; Russo et al., 1996). Phos phorylation of Thr160 in the T-loop of CDK2 stimulated the rate of catalysis ∼300-fold, but also strengthened the association between the catalytic subunit and the cyclin (Russo et al., 1996). T-loop phosphorylation is also required to stabilize complexes of CDC2 and cyclin A (Ducommun et al., 1991; Desai et al., 1995; Larochelle et al., 1998). Here we demonstrate that a major role in vivo for T-loop phosphorylation of CDK7 is the stabilization of the predominant physiological form of the kinase: the CDK7–cyclin H–MAT1 trimer.
Our results suggest that the two mechanisms for CDK7 complex stabilization and activation—MAT1 addition and T-loop phosphorylation—which can operate independently in vitro, actually cooperate under physiological conditions to maintain complex integrity. With prolonged exposure to elevated temperature, dissociation to monomeric subunits occurs in vivo when CDK7 is dephosphorylated, even in the presence of MAT1.
Might there be physiological situations in which the trimeric CAK complex needs to be destabilized, for example to facilitate subunit rearrangements or redistribution of CAK between free and TFIIH-bound forms? Interestingly, we have observed spontaneous dephosphorylation, and dissociation of Drosophila CDK7 in vitro, either after prolonged incubation on ice or when immunoprecipitated complexes are subjected to a brief heat treatment. In the latter case, we may infer the presence, in complex with CDK7, of a phosphatase capable of dephosphorylating Thr170. Under both conditions, CDK7S164A appears especially susceptible to dephosphorylation and dissociation, whereas the doubly phosphorylated, wild-type form appears largely resistant. Thus the dual phosphorylation might be part of a regulatory circuit controlling CDK7 function at the level of complex stability.
CDK7 T-loop phosphorylation: separate controls for cell cycle and transcription?
Since its discovery as a component of both CAK and TFIIH in metazoans, CDK7 has been studied as a possible link between the cell cycle and transcriptional machinery. Those investigations have uncovered several potential regulatory mechanisms, but no clear evidence for their usefulness in vivo. T-loop phosphorylation is an example of such a mechanism in search of a biological context. Our studies have uncovered two important functions of CDK7 phosphorylation: stable complex assembly and modulation of CTD kinase activity. Whereas neither function is absolutely essential, impairment of either may cause temperature-sensitive loss of viability.
The ability to bypass T-loop phosphorylation of CDK7 in vitro (Devault et al., 1995; Fisher et al., 1995; Tassan et al., 1995) appeared to obviate its requirement in vivo. Kin28, the budding yeast ortholog of CDK7, is phosphorylated uniquely on Thr162 by Cak1, which also activates Cdc28 (Espinoza et al., 1998; Kimmelman et al., 1999). Although phosphorylation stimulates the activity of the Kin28 complex towards the CTD (Espinoza et al., 1998; Kimmelman et al., 1999), strains containing Kin28 lacking Thr162 are viable, with no obvious transcriptional defects or thermal sensitivity. The kin28T162A allele is, however, synthetically lethal with mutations in the gene encoding Tfb3, the budding yeast ortholog of MAT1 (Kimmelman et al., 1999). Thus T-loop phosphorylation and the RING subunit reinforce each other in yeast as they do in metazoans, but either one may suffice to ensure adequate kinase function. Both appear to be necessary in Drosophila, which die even at moderate temperatures when T-loop phosphorylation is blocked, despite having a full complement of wild-type MAT1.
No CDK7-activating kinase has been identified conclusively in a metazoan species, but candidates have emerged from studies of CDK activation in vitro as well as in yeast. Vertebrate CDC2 and CDK2 activate CDK7 in vitro (Fisher et al., 1995; Martinez et al., 1997), and are able to phosphorylate both Ser164 and Thr170 with equal efficiency (Garrett et al., 2001). Budding yeast Cak1 and fission yeast Csk1, both monomeric kinases distantly related to CDKs, activate the CDK7 orthologs, Kin28 and Mcs6, respectively, in vivo (Molz and Beach, 1993; Espinoza et al., 1998; Hermand et al., 1998; Kimmelman et al., 1999; Lee et al., 1999). Thus, the T-loop of CDK7 may be a critical regulatory target, possibly helping to coordinate patterns of gene expression with cell cycle progression.
T-loop phosphorylation: a regulator of CTD phosphorylation and transcription rates?
It has been reported that the addition of MAT1 to the CDK7–cyclin H complex alters its substrate specificity, favoring CTD phosphorylation at the expense of CAK activity (Yankulov and Bentley, 1997). The phosphorylation state of the CDK7 catalytic subunit was not determined and the effects of MAT1 addition were also quite modest: an ∼4-fold increase in the CTD:CDK2 phosphorylation ratio (Yankulov and Bentley, 1997). In contrast, we consistently observe an ∼20-fold stimulation of the CTD kinase activity of trimeric CDK7–cyclin H–MAT1 when Thr170 is phosphorylated, with no loss (or gain) of CAK activity, under conditions where neither substrate is in limiting concentration. MAT1 is required for this effect; the phosphorylated dimeric complex is no more active than the unphosphorylated trimer. Indeed, the modest lowering of the Km for CTD when MAT1 joins the complex (Table II) could explain the apparent stimulation observed previously (Yankulov and Bentley, 1997). We suggest, however, that MAT1 merely serves to facilitate substrate-specific stimulation by Thr170 phosphorylation, and that cycles of phosphorylation and dephosphorylation of the T-loop are more likely to regulate the function of CDK7 in vivo than are association and dissociation of MAT1.
The CTD of RNA pol II undergoes a cycle of phosphorylation and dephosphorylation during the process of transcription. RNA pol II with a hypophosphorylated CTD initiates transcription, the CTD becomes phosphorylated as the enzyme proceeds from initiation to elongation and, finally, the CTD is dephosphorylated as it completes the transcription cycle (Dahmus, 1996). Phosphorylation of Thr170 uniquely regulates the activity of CDK7 towards the CTD. The mechanism is direct acceleration of the catalytic rate of the enzyme, and so would provide a way to increase CTD phosphorylation rates and thereby favor promotor clearance, perhaps in opposition to dephosphorylation by a CTD phosphatase (Cho et al., 1999). Whether this modulation is critical to regulation of gene expression has yet to be tested thoroughly. However, our studies raise the intriguing possibility that a kinase cascade or network regulates transcription through changes in the state of CDK7 T-loop phosphorylation. The failure to observe any changes in the steady-state levels of CTD phosphorylation in our cdk7 mutants may reflect the complex network of kinases and phosphatases that act in concert on the CTD. Regulation of CDK7 T-loop phosphorylation may be critical, however, when rapid changes in gene expression are induced, for example by heat shock.
The dual function of metazoan CDK7 in control of cell cycle and transcription programs remains a puzzle. Although the notion that CDK7 coordinates gene expression with cell division in some fashion is intriguing, it has received little experimental support, and so the question of why two seemingly disparate functions are combined in one enzyme is still unanswered. We have more insight into how CDK7 can phosphorylate both the T-loops of CDKs and the CTD of RNA pol II, despite the complete lack of sequence homology between its two physiological substrates, by adopting different strategies for substrate recognition (Garrett et al., 2001). Moreover, in this report, we have demonstrated how the CTD kinase activity of CDK7 can be regulated, by Thr170 phosphorylation, independently of CAK activity. Strikingly, Thr170 phosphorylation of trimeric CDK7 enables the enzyme to catalyze CTD phosphorylation at ∼100 times the maximal rate for CDK2 phosphorylation (Table II). Because the CTD contains many (∼52) target sites for CDK7-mediated phosphorylation, whereas CDK2 contains only one, this rate enhancement could allow the major physiological form of CDK7, the phosphorylated trimer, to catalyze CDK activation and CTD hyperphosphorylation at very similar rates.
Materials and methods
Drosophila stocks and growth conditions
Unless otherwise noted, adult flies and embryos were kept at 18°C. The different mutant stocks were obtained by crossing balanced cdk7null females [wDf(1)JB254, Pw+(snf+,dhd+)/FM7c; +/+; +/+] (Larochelle et al., 1998) to the different lines carrying the cdk7 mutant transgene on the third chromosome. Stocks were: [wDf(1)JB254, Pw+(snf+,dhd+)/wDf(1)JB254, Pw+(snf+,dhd+); Pw+(cdk7m)/Pw+ (cdk7m); +/+] or [wDf(1)JB254, Pw+(snf+,dhd+)/wDf(1)JB254, Pw+(snf+,dhd+); +/+; Pw+(cdk7m)/Pw+ (cdk7m)]. Embryos were collected at the indicated temperatures on apple juice–agarose plates, dechorionated by treatment with 50% bleach, washed thoroughly, flash frozen in liquid nitrogen and stored dry at –80°C until use.
Size exclusion chromatography
Embryos were homogenized on ice in HoB [25 mM HEPES pH 7.4, 150 mM NaCl, 20 mM NaF, 20 mM β-glycerophosphate, 1 mM EDTA, 1 mM dithiothreitol (DTT), 0.1% Triton X-100] + protease inhibitors. We typically used 250 mg dry weight of embryos per ml of buffer, resulting in a total protein concentration of ∼15 mg/ml after 30 min centrifugation at 100 000 g. We loaded 0.2–0.5 ml over a Superdex 200 10/30 HR gel filtration column (Amersham Pharmacia Biotech) equilibrated with 25 mM HEPES pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.5 mM DTT, 10% glycerol. Fractions (0.5 ml) were collected and 10 µl of each was used for immunoblots. For immunoprecipitations, Triton X-100 was added to each fraction to a final concentration of 0.1%.
Antibodies
Monoclonal antibodies specific for Drosophila CDK7 were raised against the full-length protein as described previously (Larochelle et al., 1998). The monoclonal antibody detecting the N-terminal part of MAT1 (1G6) was a kind gift of Jean-Marc Egly. Antibodies directed against the CTD of RNA pol II (8WG16, H5 and H14) were obtained from Covance, Inc.
Electrophoresis and immunoblotting
In order to separate the various phospho-isoforms of CDK7 reliably, SDS–PAGE was carried out with piperazine di-acrylamide instead of bis-acrylamide as the cross-linker (Kumagai and Dunphy, 1995), and the pH of the resolving gel was increased to 9.2. Separation of the different CDK7 phospho-isoforms increased with distance migrated through the gel, and may not be obvious in shorter runs. Immunoblots to detect Drosophila CDK7 were carried out with a mixture of monoclonal antibodies (4A7, 4D12 and 20H5) in the form of hybridoma culture supernatant at a dilution of 1:20–1:50 (∼0.5 µg/ml). Affinity-purified polyclonal antibodies against human CDK7 and MAT1 were used at 1:1000 (∼0.2 µg/ml). For pol II immunoblots, 10 third instar wandering larvae of each genotype were either collected at 25°C or heat shocked in a 1.5 ml microtube immersed in a 37°C water bath for 30 min. The samples were frozen in liquid nitrogen, and later homogenized directly in SDS sample buffer.
Immunoprecipitation and kinase assays
For measurements of activity in CDK7 immunoprecipitates (IPs), embryos or adult flies were homogenized in HoB as described above. For each IP, 150 µl of mAb supernatant (4A7, 4D12 or 20H5) and 15 µl of protein G–agarose were mixed with ∼2 mg of total protein for 2 h at 4°C. The IPs were washed three times with HoB and three times with kinase buffer (25 mM HEPES pH 7.4, 150 mM NaCl, 10 mM MgCl2, 0.5 mM DTT) and tested for kinase activity towards 7 µg of recombinant GST–CTD, or 2 µg of catalytically inactive CDK2D145N–cyclin A complex, in a volume of 30 µl of kinase buffer + 0.1 mM ATP + 2.5 µCi of [γ-32P]ATP. Thermal inactivation experiments for both immunoprecipitated Drosophila CDK7 and pure mammalian CDK7 complexes were carried out in kinase buffer. Mammalian CDK7–cyclin H and CDK7– cyclin H–MAT1 complexes were purified as described previously (Fisher, 1997) from insect Sf9 cells infected doubly or triply, respectively, with the appropriate recombinant baculoviruses. Briefly, lysates were subjected to sequential chromatography with HiTrap Q, ATP-agarose and Superose 12 columns. The phosphorylated trimeric complex was generated by mixing the purified dimer (phosphorylated in vivo during infection) with purified MAT1 in an equimolar ratio. The mixture was incubated on ice for 1 h prior to kinase assays. Assays with purified proteins were carried out using 2–50 ng of enzyme in a final volume of 20–50 µl.
Measurements of kinetic parameters
Kinase assays with the purified mammalian complexes were carried out using a final CAK concentration of 6 nM in a volume of 20 µl for CTD and 10 µl for CDK2 kinase assays. Each assay was carried out in triplicate in 25 mM HEPES pH 7.4, 150 mM NaCl, 10 mM MgCl2, 0.5 mM DTT, 200 µM ATP, 5 µCi of [γ-32P]ATP for 5 min at 23°C. The concentrations of substrate were: for the CTD, 0.35, 0.875, 1.70, 3.40, 8.75, 14 and 17.5 µM; and for CDK2D145N–cyclin A, 0.05, 0.125, 0.25, 0.5; 1.25 and 2.5 µM. The reactions were stopped by adding two volumes of 2× sample buffer and boiling. Samples were separated by SDS–PAGE and incorporation was quantified by scanning dried gels with a STORM 840 phosphorimager using the ImageQuant software. Incorporation was determined using various dilutions of [γ-32P]ATP spotted on paper as calibration standards. Apparent Km and Vmax values were calculated by fitting the data to the Michaelis–Menten equation using the GraphPad Prism software for Macintosh.
Supplementary data
Supplementary data for this paper are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Leah Bernstein for technical assistance, Arpi Nazarian and Anita Grewal for expert help with mass spectrometric analysis, Jean-Marc Egly for MAT1 antibody, Andrew Koff, Karen Lee and Stewart Shuman for critical reading of the manuscript, Kathryn Anderson and Mary Baylies for access to Drosophila rearing facilities, and Sabino Guzman for preparing fly food. S.L. is especially grateful to Julia Saiz, Karen Lee, Bill Barton, Jack Liao, Christophe Rachez, Andrew Swan and Reiko Cyr for stimulating discussions and support during the course of this work. This work is supported by grants to R.P.F. from the NIH and the American Cancer Society, and to B.S. from the National Cancer Institute of Canada with funds from the Canadian Cancer Society. Also supported by NCI Cancer Center grant P30 CA08748. J.C. was the recipient of a studentship from the Fonds pour la formation de chercheurs et l’aide à la recherche (FCAR Quebec). S.L. is a research fellow of the National Cancer Institute of Canada, supported with funds provided by the Terry Fox run.
References
- Adamczewski J.P., Rossignol,M., Tassan,J.-P., Nigg,E.A., Moncollin,V. and Egly,J.-M. (1996) MAT1, cdk7 and cyclin H form a kinase complex which is UV light-sensitive upon association with TFIIH. EMBO J., 15, 1877–1884. [PMC free article] [PubMed] [Google Scholar]
- Akoulitchev S. and Reinberg,D. (1998) The molecular mechanism of mitotic inhibition of TFIIH is mediated by phosphorylation of CDK7. Genes Dev., 12, 3541–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.J., Jones,T. and Shuttleworth,J. (1994) Expression and activity of p40MO15, the catalytic subunit of cdk-activating kinase, during Xenopus oogenesis and embryogenesis. Mol. Biol. Cell, 5, 921–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H., Kim,T.K., Mancebo,H., Lane,W.S., Flores,O. and Reinberg,D. (1999) A protein phosphatase functions to recycle RNA polymerase II. Genes Dev., 13, 1540–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F.R. and Levine,K. (1998) Molecular evolution allows bypass of the requirement for activation loop phosphorylation of the Cdc28 cyclin-dependent kinase. Mol. Cell. Biol., 18, 2923–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmus M.E. (1996) Reversible phosphorylation of the C-terminal domain of RNA polymerase II. J. Biol. Chem., 271, 19009–19012. [DOI] [PubMed] [Google Scholar]
- De Bondt H.L., Rosenblatt,J., Jancarik,J., Jones,H.D., Morgan,D.O. and Kim,S.-H. (1993) Crystal structure of cyclin-dependent kinase 2. Nature, 363, 595–602. [DOI] [PubMed] [Google Scholar]
- Desai D., Wessling,H.C., Fisher,R.P. and Morgan,D.O. (1995) The effect of phosphorylation by CAK on cyclin binding by CDC2 and CDK2. Mol. Cell. Biol., 15, 345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devault A., Martinez,A.-M., Fesquet,D., Labbé,J.-C., Morin,N., Cavadore,J.-C. and Dorée,M. (1995) MAT1 (‘ménage à trois’) a new RING finger protein subunit stabilizing cyclin H–cdk7 complexes in starfish and Xenopus CAK. EMBO J., 14, 5027–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapkin R., Le Roy,G., Cho,H., Akoulitchev,S. and Reinberg,D. (1996) Human cyclin-dependent kinase-activating kinase exists in three distinct complexes. Proc. Natl Acad. Sci. USA, 93, 6488–6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducommun B., Brambilla,P., Felix,M.-A., Franza,B.R., Karsenti,E. and Draetta,G. (1991) cdc2 phosphorylation is required for its interaction with cyclin. EMBO J., 10, 3311–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza F.H.E., Farrell,A., Nourse,J.L., Chamberlin,H.M., Gileadi,O. and Morgan,D.O. (1998) Cak1 is required for Kin28 phosphorylation and activation in vivo. Mol. Cell. Biol., 18, 6365–6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R.P. (1997) Reconstitution of mammalian CDK-activating kinase. Methods Enzymol., 283, 256–270. [DOI] [PubMed] [Google Scholar]
- Fisher R.P., Jin,P., Chamberlin,H.M. and Morgan,D.O. (1995) Alternative mechanisms of CAK assembly require an assembly factor or an activating kinase. Cell, 83, 47–57. [DOI] [PubMed] [Google Scholar]
- Garrett S., Barton,B.A., Knights,R., Jin,P., Morgan,D.O. and Fisher,R.P. (2001) Reciprocal activation by cyclin-dependent kinases 2 and 7 is directed by substrate specificity determinants outside the T-loop. Mol. Cell. Biol., 21, 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K.L., Moreno,S., Owen,D.J., Sazer,S. and Nurse,P. (1991) Phosphorylation at Thr167 is required for Schizosaccharomyces pombe p34cdc2 function. EMBO J., 10, 3297–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J.W. and Elledge,S.J. (1998) The role of Cdk7 in CAK function, a retro-retrospective. Genes Dev., 12, 285–289. [DOI] [PubMed] [Google Scholar]
- Hermand D., Pihlak,A., Westerling,T., Damagnez,V., Vandenhaute,J., Cottarel,G. and Mäkelä,T.P. (1998) Fission yeast Csk1 is a CAK-activating kinase (CAKAK). EMBO J., 17, 7230–7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey P.D., Russo,A.A., Polyak,K., Gibbs,E., Hurwitz,J., Massagué,J. and Pavletich,N.P. (1995) Mechanism of CDK activation revealed by the structure of a cyclin A–CDK2 complex. Nature, 376, 313–320. [DOI] [PubMed] [Google Scholar]
- Kaldis P. (1999) The cdk-activating kinase (CAK): from yeast to mammals. Cell Mol. Life Sci., 55, 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmelman J., Kaldis,P., Hengartner,C.J., Laff,G.M., Koh,S.S., Young,R.A. and Solomon,M.J. (1999) Activating phosphorylation of the kin28p subunit of yeast TFIIH by cak1p. Mol. Cell. Biol., 19, 4774–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A. and Dunphy,W.G. (1995) Control of the Cdc2/cyclin B complex in Xenopus egg extracts arrested at a G2/M checkpoint with DNA synthesis inhibitors. Mol. Biol. Cell, 6, 199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé J.-C. et al. (1994) p40MO15 associates with a p36 subunit and requires both nuclear translocation and Thr176 phosphorylation to generate cdk-activating kinase activity in Xenopus oocytes. EMBO J., 13, 5155–5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle S., Pandur,J., Fisher,R.P., Salz,H.K. and Suter,B. (1998) Cdk7 is essential for mitosis and for in vivo Cdk-activating kinase activity. Genes Dev., 12, 370–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc V., Raisin,S. and Léopold,P. (2000) Dominant-negative mutants reveal a role for the Cdk7 kinase at the mid-blastula transition in Drosophila embryos. EMBO J., 19, 1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.M., Saiz,J.E., Barton,W.A. and Fisher,R.P. (1999) Cdc2 activation in fission yeast depends on Mcs6 and Csk1, two partially redundant Cdk-activating kinases CAKs). Curr. Biol., 9, 441–444. [DOI] [PubMed] [Google Scholar]
- Lim H.H., Loy,C.J., Zaman,S. and Surana,U. (1996) Dephosphorylation of threonine 169 of Cdc28 is not required for exit from mitosis but may be necessary for Start in Saccharomyces cerevisiae. Mol. Cell. Biol., 16, 4573–4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J.J., Leresche,A., Kriwacki,R.W. and Gottesfeld,J.M. (1998) Repression of TFIIH transcriptional activity and TFIIH-associated cdk7 kinase activity at mitosis. Mol. Cell. Biol., 18, 1467–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A.-M., Afshar,M., Martin,F., Cavadore,J.-C., Labbé,J.-C. and Dorée,M. (1997) Dual phosphorylation of the T-loop in cdk7: its role in controlling cyclin H binding and CAK activity. EMBO J., 16, 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molz L. and Beach,D. (1993) Characterization of the fission yeast mcs2 cyclin and its associated protein kinase activity. EMBO J., 12, 1723–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D.O. (1995) Principles of CDK regulation. Nature, 374, 131–134. [DOI] [PubMed] [Google Scholar]
- Morgan D.O. (1997) Cyclin-dependent kinases: engines, clocks and microprocessors. Annu. Rev. Cell Dev. Biol., 13, 261–291. [DOI] [PubMed] [Google Scholar]
- Poon R.Y.C., Yamashita,K., Howell,M., Ershler,M.A., Belyavsky,A. and Hunt,T. (1994) Cell cycle regulation of the p34cdc2/p33cdk2-activating kinase p40MO15. J. Cell Sci., 107, 2789–2799. [DOI] [PubMed] [Google Scholar]
- Reardon J.T., Ge,H., Gibbs,E., Sancar,A., Hurwitz,J. and Pan,Z.-Q. (1996) Isolation and characterization of two human transcription factor IIH (TFIIH)-related complexes: ERCC2/CAK and TFIIH*. Proc. Natl Acad. Sci. USA, 93, 6482–6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud E., Lomelí,H., Vázquez,M. and Zurita,M. (1999) The Drosophila melanogaster homologue of the xeroderma pigmentosum D gene product is located in euchromatic regions and has a dynamic response to UV light-induced lesions in polytene chromosomes. Mol. Biol. Cell, 10, 1191–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol M., Kolb-Cheynel,I. and Egly,J.-M. (1997) Substrate specificity of the cdk-activating kinase (CAK) is altered upon association with TFIIH. EMBO J., 16, 1628–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A.A., Jeffrey,P.D. and Pavletich,N.P. (1996) Structural basis of cyclin-dependent kinase activation by phosphorylation. Nature Struct. Biol., 3, 696–700. [DOI] [PubMed] [Google Scholar]
- Tassan J.-P., Schultz,S.J., Bartek,J. and Nigg,E.A. (1994) Cell cycle analysis of the activity, subcellular localization and subunit composition of human CAK (CDK-activating kinase). J. Cell Biol., 127, 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassan J.-P., Jaquenod,M., Fry,A.M., Frutiger,S., Hughes,G. and Nigg,E.A. (1995) In vitro assembly of a functional human cdk7/cyclin H complex requires MAT1, a novel 36 kDa RING finger protein. EMBO J., 14, 5608–5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankulov K.Y. and Bentley,D.L. (1997) Regulation of CDK7 substrate specificity by MAT1 and TFIIH. EMBO J., 16, 1638–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]