Abstract
Background
Greater precision is required for arrhythmic risk stratification of patients with nonischemic cardiomyopathy (NICM). We sought to evaluate whether fibrosis entropy, a measure of scar texture heterogeneity derived from late gadolinium enhancement cardiovascular magnetic resonance, has incremental utility to fibrosis presence for arrhythmic risk prediction in NICM.
Methods
In this prospective observational cohort study, fibrosis entropy was calculated for patients with NICM and fibrosis (late gadolinium enhancement positive, LGE+), including regions of core fibrosis, gray zone fibrosis and combined core and gray zone fibrosis. Patients with NICM and no fibrosis (LGE‐) were included as a comparator group. Adjudicated follow‐up for life‐threatening arrhythmia included sudden cardiac death, aborted sudden cardiac death, or sustained ventricular tachycardia.
Results
Of 291 patients with LGE+ NICM, 38 (13.1%) experienced life‐threatening arrhythmia over a median follow‐up of 6.3 years. Core fibrosis entropy (per‐SD hazard ratio [HR], 1.77 [95% CI, 1.25–2.52]; P=0.001), gray zone fibrosis entropy (HR, 1.97 [95% CI, 1.20–2.54]; P=0.004), and combined fibrosis entropy (HR, 1.98 [95% CI, 1.30–3.02]; P=0.004) were each associated with life‐threatening arrhythmia after adjustment for variables used to determine implantable cardioverter‐defibrillator candidacy in clinical practice (left ventricular ejection fraction ≤35% and New York Heart Association class >1) and remained associated after accounting for core and gray zone fibrosis mass. Left ventricular ejection fraction ≤35% was not associated with life‐threatening arrhythmia (HR, 1.45 [95% CI, 0.77–2.74]; P=0.250). Integration of fibrosis presence with fibrosis entropy classified patients into low‐, intermediate‐, and high‐arrhythmic‐risk groups.
Conclusions
Deeper phenotypic characterization of scar using fibrosis entropy offers incremental utility to left ventricular ejection fraction and fibrosis presence for arrhythmic risk stratification in NICM.
Keywords: arrhythmic risk stratification, entropy, fibrosis, nonischemic cardiomyopathy
Subject Categories: Cardiomyopathy, Magnetic Resonance Imaging (MRI), Prognosis, Sudden Cardiac Death, Arrhythmias

Nonstandard Abbreviations and Acronyms
- DANISH
The Danish Study to Assess the Efficacy of ICDs in Patients With Nonischemic Systolic Heart Failure on Mortality
- DCM
dilated cardiomyopathy
- LTA
life‐threatening arrhythmia
- NICM
nonischemic cardiomyopathy
- NYHA
New York Heart Association
- SCD
sudden cardiac death
Clinical Perspective.
What Is New?
Fibrosis entropy, a measure of scar texture heterogeneity calculated from late gadolinium enhancement cardiovascular magnetic resonance, was independently associated with life‐threatening arrhythmia in patients with nonischemic cardiomyopathy and midwall/subepicardial myocardial fibrosis; this association was independent of variables used to determine implantable cardioverter‐defibrillator candidacy in clinical practice and incremental to contemporary risk stratification approaches.
Left ventricular ejection fraction was not associated with arrhythmic events.
What Are the Clinical Implications?
These findings support a transition toward risk stratification based on direct characterization of the underlying arrhythmic substrate rather than left ventricular ejection fraction in patients with nonischemic cardiomyopathy.
Life‐threatening arrhythmia (LTA) is an important cause of morbidity and mortality in patients with nonischemic cardiomyopathy (NICM). While implantable cardioverter‐defibrillators (ICDs) offer a degree of protection, identification of those likely to derive prognostic benefit from implantation is challenging and the subject of ongoing commentary, especially in light of results from the DANISH (The Danish Study to Assess the Efficacy of ICDs in Patients With Nonischemic Systolic Heart Failure on Mortality) study demonstrating that ICDs did not improve survival in this population. 1 , 2 , 3 Myocardial replacement fibrosis, detected by late gadolinium enhancement (LGE) cardiovascular magnetic resonance (CMR), is established as the major substrate for ventricular arrhythmia 4 ; however, left ventricular ejection fraction (LVEF) remains the cornerstone of guidelines determining primary prevention ICD candidacy. 5 , 6 We have previously shown that LVEF is an imprecise predictor of LTA across the full spectrum of phenotypic severity, highlighting the need for more precise diagnostic tools and risk stratification algorithms. 7 , 8 Whether deeper phenotypic characterization of scar features can achieve this goal is unknown and has been identified by the European Society of Cardiology as an important evidence gap. 9 To this end, we have previously identified fibrosis mass, position, and interface area as important determinants of LTA 7 , 10 , 11 but are yet to examine the potentially important role of scar texture. Entropy is a mathematical concept that describes an inherent level of uncertainty in information theory, 12 , 13 for which many applications exist. Applied to LGE CMR images, entropy can be used to measure signal intensity heterogeneity in regions of scar. Prior studies into the association between scar/myocardial entropy and LTA have been restricted to either small cohorts of patients with ICDs, patients with prior ventricular arrhythmia, or cohorts of mixed disease etiology. 13 , 14 , 15 , 16 In this study, we evaluated the association between core and/or gray zone (GZ) fibrosis entropy and incident LTA in a cohort of patients with NICM with midwall/subepicardial fibrosis on CMR, to determine whether this method of substrate characterization offers incremental predictive value above existing approaches.
Methods
Study Population
Consecutive patients referred for a CMR scan between 2009 and 2017 from our clinical service and a network of surrounding district general hospitals were prospectively enrolled into the Royal Brompton Hospital Cardiovascular Research Centre Biobank. The study complied with the Declaration of Helsinki and was approved by the National Research Ethics Service (South Central Hampshire B Research Ethics Committee; Reference 19/SC/0257). All participants provided written consent. The major inclusion criterion was a diagnosis of NICM with midwall/subepicardial fibrosis on CMR (LGE positive, LGE+). This included patients with dilated cardiomyopathy (DCM; reduced LVEF and increased indexed left ventricular (LV) end‐diastolic volume with respect to age‐ and sex‐specific reference values) 17 or nondilated left ventricular cardiomyopathy (reduced LVEF or increased LV end‐diastolic volume index). 18 , 19 Exclusion criteria were ischemic heart disease, defined as a stenosis of >50% in a major epicardial coronary artery, inducible ischemia on functional testing, subendocardial LGE on CMR indicating prior myocardial infarction, or prior coronary revascularization; further exclusion criteria were abnormal loading conditions (uncontrolled hypertension or severe primary valve disease), congenital heart disease, active myocarditis, or an alternative cardiomyopathic processes. A second cohort of patients with NICM (reduced LVEF and/or increased LV end‐diastolic volume index) without midwall/subepicardial fibrosis on CMR (LGE negative, LGE‐) was included as a comparator group in the survival analysis, as it was anticipated that this would constitute a low‐risk group. It is important to note that it is not possible to compute fibrosis entropy for LGE‐ patients. Requests to access the data set and methods from qualified researchers may be made on reasonable request to the corresponding author.
CMR Acquisition
All patients underwent a CMR scan on a 1.5 Tesla scanner (Sonata/Avanto, Siemens, Erlangen, Germany) using standardized protocol. Breath‐hold steady‐state free precession sequences were performed to produce cine images. Gadopentetate dimeglumine or gadobutrol (0.1 mmol/kg) was intravenously injected and an inversion recovery gradient echo sequence was undertaken to acquire the LGE images at 10 minutes. The LGE images were acquired in the long‐axis planes and consecutive short‐axis slices (8 mm slice thickness with 2 mm gap) covering the left ventricle from base to apex. Inversion times were optimized to ensure adequate nulling of normal myocardium.
CMR Analysis
Left and right ventricular volumes and LV mass were measured using a thresholding technique in CMRtools (Cardiovascular Imaging Solutions, UK) and indexed to body surface area. LGE presence was determined by 2 expert CMR readers, with a third adjudicating cases of disagreement. LGE was defined as an area of enhancement within the intramyocardial and/or subepicardial layers and considered present when seen in both long‐ and short‐axis planes, in 2 orthogonal views, extending beyond the LV/right ventricular insertion points. Endocardial and epicardial borders were semi‐automatically contoured using CVI42 (Circle Cardiovascular Imaging Inc, Calgary, Canada) and optimized by a single expert CMR reader blinded to clinical outcomes. Core fibrosis was classified using a full‐width half‐maximum method (any LV region with signal intensity ≥50% maximal signal intensity) and GZ fibrosis as regions with ≥35% but <50% maximal signal intensity. 20 , 21 Using LGE CMR images and corresponding quantification masks, 2‐dimensional fibrosis texture features were extracted using proprietary software, as described previously. 11 , 22 Fibrosis entropy (computed as standard Shannon entropy) was calculated for core fibrosis, GZ, and combined core+GZ fibrosis signal intensities (see Data S1 for full description of fibrosis entropy computation). 23 Entropy measures per slice were aggregated across the short‐axis stack to represent LGE topology throughout the left ventricle (Figure 1).
Figure 1. Fibrosis entropy quantification.

Quantification of myocardial fibrosis entropy from late gadolinium enhancement cardiovascular magnetic resonance. A, LGE CMR short‐axis stack; (B) single LGE short‐axis slice; (C) classification of core fibrosis (red) and gray zone fibrosis (pink); (D) visual representation of pixel signal intensity for region of core fibrosis with corresponding signal intensity histogram. CMR indicates cardiovascular magnetic resonance; and LGE, late gadolinium enhancement.
Follow‐Up and End Points
Patients were followed up using postal questionnaires and medical information from primary care and hospital records. Follow‐up duration was measured from the date of CMR and truncated after 10 years. All events were adjudicated by a panel of experienced cardiologists using medical information, death certificates, autopsy reports, and ICD reports. All potential arrhythmic events were adjudicated by a cardiologist with expertise in implantable cardiac devices; ICD electrograms were reviewed where necessary. Adjudicators were blinded to CMR data throughout. The primary end point was LTA (composite of sudden cardiac death [SCD], aborted SCD, or sustained ventricular tachycardia). Secondary end points were (1) major heart failure (HF) events (composite of HF death, heart transplant, LV assist device implantation, or HF hospitalization); and (2) cardiovascular death (see Data S1 for full end point definitions).
Statistical Analysis
Patient characteristics are presented as frequency (percentage) for categorical variables and median (interquartile range) for continuous variables. Categorical variables were compared using Fisher's exact test. Continuous variables were compared using the Mann–Whitney U test or Kruskal–Wallis test. Ordinal values were compared using Cochran–Armitage test for trend. Cumulative incidence curves were fitted for end points stratified by median values and compared using log‐rank test. The Youden J statistic was calculated from receiver operating characteristic analyses to assess whether optimized thresholds could be derived for entropy values. The association between fibrosis entropy and clinical end points were assessed using univariable and multivariable Cox proportional hazard modeling. For the primary end point, 3 a priori selected multivariable models were used. Model 1 was aligned to criteria recommended by international guidelines to determine primary prevention ICD implantation and thus adjusted for LVEF ≤35% and New York Heart Association (NYHA) class >1. Multivariable model 2 adjusted for LVEF ≤35% and NYHA class >1, with the addition of core fibrosis mass. Multivariable model 3 adjusted for LVEF ≤35% and NYHA class >1, with the addition of GZ mass. Models 2 and 3 were selected as sensitivity analyses to demonstrate independence of any association between entropy and LTA from core fibrosis and GZ mass, respectively. For the secondary end points, a single multivariable model was used adjusting for age, sex, LVEF (as a continuous variable), and NYHA class, selected on the basis of established associations between these variables and progressive HF and cardiovascular death. 24 Model performance was assessed using Harrell's C‐statistic. The incremental predictive value of fibrosis entropy was assessed using categorical net reclassification indices. A 2‐tailed P value of <0.05 was considered significant. Statistical analysis was performed using Python version 3.7.4 (Python Software Foundation, Wilmington, DE).
Results
Cohort
Of 939 patients assessed for eligibility, the final LGE+ NICM cohort comprised 291 patients (Figure 2). Of these, 250 (85.9%) had DCM and 41 (14.1%) had nondilated LV cardiomyopathy (Table S1). Most patients (73.9%) had been referred for a CMR to further characterize LV dysfunction and/or dilatation; followed by a smaller proportion to investigate arrhythmia (10.0%) or for family screening (5.5%). The remaining patients (10.7%) had undergone CMR for other indications. Coronary artery disease was excluded with invasive angiography in 72.9% of patients, by computed tomography coronary angiography in 5.2%, and by functional testing demonstrating no inducible ischemia in 12.0%. The remaining 9.9% (29 patients) had a low clinical probability of coronary artery disease and did not undergo investigation to formally exclude: 48.3% were aged ≤40 years, none had prior angina, and none required coronary revascularization or experienced an acute coronary syndrome during follow‐up. The comparator group comprised 574 patients with LGE‐ NICM (Table S2).
Figure 2. Study cohort.

Flowchart illustrating the assembly of the study cohort. CMR indicates cardiovascular magnetic resonance; LGE, late gadolinium enhancement; LVEDVi, indexed left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; and NICM, nonischemic cardiomyopathy.
The median age of the primary cohort of patients with LGE+ NICM was 57.0 (interquartile range, 49.0–66.0) years and the majority were men (75.3%) and White individuals (85.9%) (Table 1). A high proportion of patients were prescribed prognostic HF drugs. The median LVEF was 39.0% (interquartile range, 26.5%–50.0%). Following enrollment, 106 patients (36.4%) underwent ICD implantation, of whom 65 (22.3%) received cardiac resynchronization. The indication for ICD implantation was for primary prevention in all except 4, for whom ICDs were implanted following resuscitated cardiac arrests that occurred during follow‐up. No patients had an indication for an ICD for secondary prevention at the point of enrollment. An additional 10 patients received cardiac resynchronization therapy–pacemaker devices, and 4 patients received single‐chamber or dual‐chamber pacemakers during follow‐up.
Table 1.
Patient Baseline and Cardiovascular Magnetic Resonance Characteristics Stratified by the Primary End Point
| Total cohort (N=291) | Event‐free (N=253) | Event (N=38) | P value | |
|---|---|---|---|---|
| Age, y | 57.0 (49.0–66.0) | 58.0 (48.0–66.0) | 52.5 (48.25–60.75) | 0.197 |
| Male sex, n (%) | 219 (75.3) | 189 (74.7) | 30 (78.9) | 0.689 |
| White race, n (%) | 250 (85.9) | 213 (84.2) | 37 (97.4) | 0.054 |
| Body surface area, m2 | 2.03 (1.84–2.17) | 2.04 (1.83–2.18) | 2.0 (1.87–2.16) | 0.933 |
| Heart rate, beats/min | 70.0 (62.0–82.0) | 70.0 (62.0–81.5) | 72.0 (58.2–82.5) | 0.565 |
| Systolic blood pressure, mm Hg | 120.5 (107.0–137.0) | 123.0 (107.8–138.0) | 115.5 (104.5–126.8) | 0.063 |
| Diastolic blood pressure, mm Hg | 71.0 (63.0–83.0) | 71.0 (63.0–84.0) | 72.0 (63.5–79.0) | 0.612 |
| Diabetes, n (%) | 43 (14.8) | 35 (13.8) | 8 (21.1) | 0.355 |
| Hypertension, n (%) | 115 (39.5) | 102 (40.3) | 13 (34.2) | 0.594 |
| LBBB, n (%) | 64 (22.0) | 55 (21.7) | 9 (23.7) | 0.952 |
| Family history of dilated cardiomyopathy, n (%) | 47 (16.2) | 43 (17.0) | 4 (10.5) | 0.439 |
| Family history of sudden cardiac death, n (%) | 38 (13.1) | 33 (13.0) | 5 (13.2) | 1.000 |
| NYHA functional class, n (%) | 0.070 | |||
| I | 121 (41.6) | 102 (40.3) | 19 (50.0) | |
| II | 125 (43 | 115 (45.5) | 10 (26.3) | |
| III/IV | 45 (15.5) | 36 (14.2) | 9 (23.7) | |
| Medications, n (%) | ||||
| ACE inhibitor/ARB | 251 (86.3) | 216 (85.4) | 35 (92.1) | 0.384 |
| β Blocker | 221 (75.9) | 189 (74.7) | 32 (84.2) | 0.282 |
| Mineralocorticoid receptor antagonists | 124 (42.6) | 108 (42.7) | 16 (42.1) | 1.000 |
| Loop diuretic | 152 (52.2) | 132 (52.2) | 20 (52.6) | 1.000 |
| CMR measurements | ||||
| LV end‐diastolic volume index, mL/m2 | 120.0 (102.5–152.5) | 120.0 (101.0–149.0) | 122.5 (105.2–172.0) | 0.466 |
| LV end‐systolic volume index, mL/m2 | 72.0 (54.0–107.0) | 70.0 (53.0–107.0) | 82.0 (54.5–128.0) | 0.308 |
| LV ejection fraction, % | 39.0 (26.5–50.0) | 39.0 (28.0–50.0) | 38.5 (25.0–50.5) | 0.446 |
| LV mass index, g/m2 | 90.0 (74.5–112.0) | 88.0 (74.0–111.0) | 97.5 (84.25–118.75) | 0.033* |
| RV end‐diastolic volume index, mL/m2 | 85.0 (70.0–103.0) | 86.0 (70.0–103.0) | 83.0 (67.0–103.0) | 0.546 |
| RV end‐systolic volume index, mL/m2 | 39.0 (28.5–53.0) | 38.0 (28.0–53.0) | 42.0 (33.0–56.5) | 0.371 |
| RV ejection fraction, % | 54.0 (45.0–61.0) | 54.0 (45.0–61.0) | 51.0 (41.0–57.0) | 0.087 |
| Left atrial volume index, mL/m2 | 58.3 (45.8–73.6) | 57.5 (45.3–73.0) | 59.6 (53.2–75.1) | 0.231 |
| Core fibrosis mass, g | 6.5 (3.5–10.3) | 6.01 (3.4–9.5) | 9.3 (7.3–14.4) | 0.018* |
| Gray zone fibrosis mass, g | 6.7 (3.7–11.2) | 6.5 (3.2–10.5) | 10.4 (5.3–14.1) | 0.023* |
| Combined fibrosis mass, g | 13.4 (6.9–22.2) | 12.7 (6.4–20.1) | 20.7 (12.6–28.0) | 0.017* |
| Core fibrosis entropy | 15.1 (11.5–19.3) | 14.3 (11.5–19.0) | 16.9 (13.5–20.3) | 0.031* |
| Gray zone fibrosis entropy | 10.3 (7.3–14.2) | 10.0 (7.2–14.0) | 12.0 (10.1–14.5) | 0.017* |
| Combined fibrosis entropy | 16.5 (12.7–21.5) | 16.0 (12.6–21.3) | 18.2 (14.7–22.4) | 0.034* |
Patient characteristics are presented as frequency (percentage) for categorical variables and median (interquartile range) for continuous variables. ACE indicates angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; CMR, cardiovascular magnetic resonance; LBBB, left bundle branch block; LV, left ventricular; NYHA, New York Heart Association; and RV, right ventricular.
P <0.05.
Fibrosis entropy was computed for all patients with LGE+ NICM. There was no difference in fibrosis mass or entropy values between scans performed using different contrast agents (gadopentetate dimeglumine versus gadobutrol), in patients with atrial fibrillation compared with sinus rhythm, or in patients with severe HF symptoms (NYHA class IV) compared with those with less severe HF symptoms (NYHA class I–III) (Tables S3 through S5). Measures of fibrosis mass and entropy were moderate to highly correlated (Figure S1).
Primary End Point
Over a median follow‐up of 6.3 years (interquartile range, 4.6–9.1), 38 of 291 (13.1%) patients with LGE+ NICM met the primary end point. Of these, 7 (18.4%) were adjudicated as SCDs (5 of whom had autopsy reports available to adjudicators), 18 (47.4%) as aborted SCD events (14 with an appropriate ICD shock, 4 due to resuscitated ventricular fibrillation/ventricular tachycardia cardiac arrest requiring electrical cardioversion/defibrillation); and 13 (31.6%) patients were adjudicated to have had sustained ventricular tachycardia. Of note, there was no significant difference in LVEF between patients who did and did not meet the primary end point (38.5% [25.0%–50.5%] versus 39.0% [28.0%–50.0%]; P=0.446). Importantly, 19 of 38 patients (50%) with LGE+ NICM who experienced LTA had an LVEF >35%. There was no difference in LTA event rate between patients with DCM and nondilated LV cardiomyopathy (P=0.594; Table S6). Patients who met the primary end point had a higher LV mass index (P=0.033), higher core fibrosis mass (P=0.018), higher GZ fibrosis mass (P=0.023), and higher combined fibrosis mass (P=0.017) compared with those who did not experience LTA. Patients who met the primary end point also had higher core fibrosis entropy (P=0.031), higher GZ fibrosis entropy (P=0.017), and higher combined fibrosis entropy (P=0.034) than patients who did not experience LTA. Of the comparator cohort of patients with LGE‐ NICM, only 14 of 574 (2.4%) met the primary end point during follow‐up.
Among patients with LGE+ NICM, core fibrosis entropy (log‐rank P=0.03), GZ fibrosis entropy (log‐rank P=0.003) and combined fibrosis entropy (log‐rank P=0.002) values above median were associated with a higher cumulative incidence of the primary end point. By contrast, there was no difference in the cumulative incidence of LTA between patients with LVEF ≤35% compared with those with LVEF >35% (log‐rank P=0.25) (Figure 3; Figures S2 and S3). When these findings were considered alongside the comparator group of patients with LGE‐ NICM, integration of LGE presence with fibrosis entropy delineated tiered arrhythmic risk groups. Namely, patients with LGE‐ NICM represent a low‐risk group (event rate per 100 patient‐years, 0.29), while fibrosis entropy classified patients with LGE+ NICM into intermediate risk (LGE+ with core/GZ/combined fibrosis entropy less than or equal to median, event rate, 1.08–1.10) and high‐risk (LGE+ with core/GZ/combined fibrosis entropy greater than median, event rate, 2.39–2.44) groups (Tables S7 through S9). In contrast with classifying patients on the basis of median entropy values, dichotomizing by values above and below the Youden J index–derived thresholds was not associated with a difference in the cumulative incidence of the primary end point (combined entropy, log rank P=0.19; Figure S4).
Figure 3. Fibrosis entropy and LVEF in relation to the primary end point.
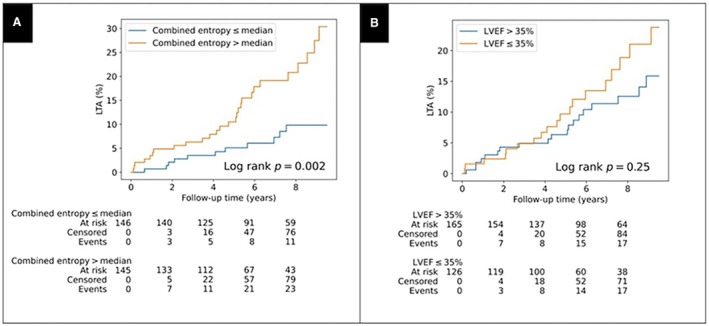
Cumulative incidence curves for life‐threatening arrhythmia stratified by (A) combined fibrosis entropy above median; and (B) LVEF ≤35%. Combined fibrosis entropy greater than median was associated with increased cumulative incidence of life‐threatening arrhythmia; LVEF ≤35% was not associated with increased cumulative incidence of LTA. LTA indicates life‐threatening arrhythmia; and LVEF, left ventricular ejection fraction.
Among patients with LGE+ NICM, core fibrosis entropy (per‐SD hazard ratio [HR], 1.75 [95% CI, 1.25–2.44]; P=0.001), GZ fibrosis entropy (HR, 1.77 [95% CI, 1.24–2.54]; P=0.002), and combined fibrosis entropy (HR, 1.88 [95% CI, 1.27–2.78]; P=0.002) were associated with the primary end point in univariable analysis (Table 2). On multivariable analysis using model 1 (adjusted for LVEF ≤35% and NYHA class >1), core fibrosis entropy (HR, 1.77 [95% CI, 1.25–2.52]; P=0.001), GZ fibrosis entropy (HR, 1.97 [95% CI, 1.20–2.54]; P=0.004) and combined fibrosis entropy (HR, 1.98 [95% CI, 1.30–3.02]; P=0.004) remained independently associated with LTA (Table 2, Table S10). The baseline C‐statistic for model 1 was 0.49, and the addition of core fibrosis entropy, GZ fibrosis entropy, and combined fibrosis entropy individually as continuous variables resulted in an increment in C‐statistic to 0.59, 0.62, and 0.62, respectively. When core fibrosis entropy, GZ fibrosis entropy, and combined fibrosis entropy were considered as dichotomous variables stratified by median and added to model 1, the C‐statistic values were 0.57, 0.57, and 0.58, respectively. The net reclassification indices for fibrosis entropy, GZ fibrosis entropy, and combined fibrosis entropy (each dichotomized by median value) were 0.23 (P=0.08), 0.29 (P=0.02), and 0.32 (P=0.01), respectively, compared with LVEF (≤35% versus >35%) (Tables S11 through S13). For example, use of combined fibrosis entropy correctly reclassified 8 more patients as high risk for LTA and 27 more patients as low risk for LTA, compared with LVEF.
Table 2.
Univariable and Multivariable Associations Between Fibrosis Entropy and the Primary End Point
| Univariable | Multivariable model 1* | Multivariable model 2† | Multivariable model 3‡ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| Core fibrosis entropy | 1.75 | 1.25–2.44 | 0.001δ | 1.77 | 1.25–2.52 | 0.001δ | 1.70 | 1.02–2.82 | 0.042δ | 1.79 | 1.09–2.96 | 0.022δ |
| Gray zone fibrosis entropy | 1.77 | 1.24–2.54 | 0.002δ | 1.97 | 1.20–2.54 | 0.004δ | 1.71 | 1.06–2.75 | 0.027δ | 1.59 | 1.06–2.37 | 0.024δ |
| Combined fibrosis entropy | 1.88 | 1.27–2.78 | 0.002δ | 1.98 | 1.30–3.02 | 0.004δ | 1.57 | 0.93–2.64 | 0.093 | 1.76 | 1.07–2.91 | 0.027δ |
HR indicates hazard ratio; LVEF, left ventricular ejection fraction; and NYHA, New York Heart Association.
Model 1 adjusted for LVEF ≤35%, NYHA class >1.
Model 2 adjusted for LVEF ≤35%, NYHA class >1, core fibrosis mass.
Model 3 adjusted for LVEF ≤35%, NYHA class >1, gray zone mass.
P <0.05.
LVEF was not associated with the primary end point on univariable analysis, either as a dichotomous variable (LVEF ≤35% versus >35%: HR, 1.45 [95% CI, 0.77–2.74]; P=0.254), or continuous variable (LVEF per 10% increase: HR, 0.80 [95% CI, 0.63–1.01]; P=0.057).
Sensitivity Analyses
On sensitivity analysis using multivariable model 2 (adjusting for LVEF ≤35%, NYHA class >1, and core fibrosis mass), core fibrosis entropy remained associated with the primary end point (HR, 1.70 [95% CI, 1.02–2.82]; P=0.042) despite the inclusion of core fibrosis mass in the model (Table 2, Table S14). The baseline C‐statistic for model 2 was 0.58, and the addition of core fibrosis entropy resulted in a small step up to 0.60. On sensitivity analysis using multivariable model 3 (LVEF ≤35%, NYHA class >1, and GZ fibrosis mass), GZ fibrosis entropy remained associated with the primary end point (HR, 1.59 [95% CI, 1.06–2.37]; P=0.024), despite the inclusion of GZ fibrosis mass in the model (Table 2, Table S15). The baseline C‐statistic for model 3 was 0.62, and the addition of GZ fibrosis entropy resulted in a small step up to 0.65. In additional post hoc analyses, core fibrosis entropy, GZ fibrosis entropy, and combined fibrosis entropy each remained associated with the primary end point in multivariable models that accounted for other potentially relevant scar characteristics, including fibrosis position (septal versus free‐wall versus both) and fibrosis pattern (midwall versus subepicardial versus focal versus multiple patterns) (Table S16). Core fibrosis entropy and combined fibrosis entropy remained associated with the primary end point when the fibrosis interface area was included in the multivariable model (Table S16). In a further sensitivity analysis, we tested whether adjusting for NYHA class >2, rather than NYHA class >1 in multivariable model 1, affected the association between entropy parameters and the primary outcome. Each entropy parameter remained associated with the primary end point when adjusting for NYHA class >2 and LVEF <35% (Table S17).
Secondary End Points
Major HF Events
In total, 61 of 291 (21.0%) patients with LGE+ NICM met the composite HF end point during follow‐up. The HF event rate was higher in patients with DCM than nondilated LV cardiomyopathy (P<0.001; Table S6). On univariable analysis, there was no association between core fibrosis entropy (HR, 1.13 [95% CI, 0.88–1.45]; P=0.340), GZ fibrosis entropy (HR, 1.24 [95% CI, 0.96–1.6]; P=0.107) or combined fibrosis entropy (HR, 1.20 [95% CI, 0.92–1.56]; P=0.175) and major HF events. On multivariable analysis (adjusted for age, sex, LVEF, and NYHA class), there remained no association between core fibrosis entropy (HR, 1.04 [95% CI, 0.8–1.36]; P=0.759), GZ fibrosis entropy (HR, 1.11 [95% CI, 0.84–1.46]; P=0.452), or combined fibrosis entropy (HR, 1.08 [95% CI, 0.82–1.41]; P=0.592) and major HF events.
Cardiovascular Death
During follow‐up, 38 of 291 (13.1%) patients with LGE+ NICM met the cardiovascular death end point, including 24 (8.2%) who died from HF, 7 (2.4%) from SCD, and 7 (2.4%) from other cardiovascular causes. The cardiovascular mortality rate was higher in patients with DCM than nondilated LV cardiomyopathy (P=0.004; Table S6). On univariable analysis, GZ fibrosis entropy (HR, 1.48 [95% CI, 1.05–2.08]; P=0.027), and combined fibrosis entropy (HR, 1.45 [95% CI, 1.02–2.08]; P=0.039) were associated with cardiovascular death, whereas core fibrosis entropy (HR, 1.33 [95% CI, 0.96–1.84]; P=0.085) was not. On multivariable analysis (adjusted for age, sex, LVEF, and NYHA class), neither core fibrosis entropy (HR, 1.28 [95% CI, 0.92–1.77]; P=0.145), GZ fibrosis entropy (HR, 1.26 [95% CI, 0.88–1.79]; P=0.202), nor combined fibrosis entropy (HR, 1.39 [95% CI, 0.97–1.99]; P=0.070) remained associated with cardiovascular death.
Discussion
Fibrosis Entropy Is Independently Associated With LTA in NICM
We demonstrate that fibrosis entropy, a measure of LGE CMR scar texture heterogeneity, is associated with LTA in patients with LGE+ NICM. This association is independent of benchmark variables used in clinical practice to determine ICD implantation and independent of core fibrosis and GZ mass. The addition of fibrosis entropy to an arrhythmic risk model on the basis of current clinical guidelines enhanced precision. Moreover, fibrosis entropy can be used to further classify patients with LGE+ NICM as either intermediate‐risk (LGE+ with low fibrosis entropy) or high‐risk (LGE+ with high fibrosis entropy) with respect to incident LTA. Importantly, the absence of association between fibrosis entropy and major HF events means that fibrosis entropy measurements may have utility in identifying patients with a higher proportional risk of death from LTA, without a concurrently enhanced risk of death from HF, and thus may aid in identifying patients who are most likely to derive a survival benefit from ICD implantation.
The Relative Contribution of Core Fibrosis Entropy Versus GZ Fibrosis Entropy to the Association With Arrhythmia
Both core fibrosis entropy and GZ fibrosis entropy were independently associated with LTA in this population. Considering multivariable model 1, the increment in C‐statistic was similar for each measure of fibrosis/GZ entropy. The finding that combined fibrosis entropy was not associated with a greater increment in C‐statistic than the entropy of either of its components alone indicates that the effect of core fibrosis entropy and GZ entropy are not additive to each other and is likely related to correlation between these variables (Figure S1). The effect of fibrosis entropy on arrhythmic risk was independent of core fibrosis and GZ mass, as illustrated by the sensitivity analyses.
In patients with ischemic cardiomyopathy, the histological composition of the GZ consists of viable cardiac myocytes interspersed with fibrotic collagen deposits, which causes spatial heterogeneity and anisotropy. 25 Conduction through critical isthmuses of viable myocardium combined with fixed and/or functional electrical conduction block, propagate reentry circuits leading to ventricular arrhythmia. 26 While there are limited data describing the histological composition of the GZ in NICM, there are emerging data indicating an association between GZ fibrosis and arrhythmia. 7 , 27 Our data support the notion that greater tissue heterogeneity within the GZ is associated with a higher risk of arrhythmia. Although the electrophysiological basis of the association between core fibrosis entropy and arrhythmia is unclear, it is also feasible that this may relate to a degree of interspersion of viable cardiac myocytes within these regions of nonischemic scar, which is typically less uniform than ischemic scar. However, such a hypothesis requires further in‐depth investigation via histological studies or biophysically detailed computational simulation studies. We have previously demonstrated that high fibrosis entropy is associated with transmural conduction block in simulated programmed electrical simulation models. 22 Further computational modeling experiments have similarly demonstrated an association between fibrosis heterogeneity and arrhythmogenesis. 28
LVEF and Risk of LTA in NICM
A further important finding is the lack of association found between LVEF and LTA in patients with LGE+ NICM. Half of the patients with LGE+ NICM who experienced LTA had LVEF >35% and would not have met the criteria for primary prevention ICD implantation. The poor performance of the guideline‐derived baseline clinical model based on LVEF and NYHA class (C‐statistic, 0.49) highlights the inadequacy of the current paradigm. These observations, coupled with the increment in C‐statistic from the addition of fibrosis entropy to the model, provides further impetus for transition toward risk stratification methods that directly evaluate the underlying arrhythmic substrate in favor of continued dependence on LVEF.
Fibrosis Entropy Is Not Associated With Major HF Events or Cardiovascular Death in NICM
No association was found between fibrosis entropy and either major HF events or cardiovascular death after accounting for important covariates. This observation is unsurprising, as there is no known or hypothetical mechanistic link between the degree of fibrosis heterogeneity and progressive HF. While a plausible mechanistic basis for an association between fibrosis entropy and SCD does exist as outlined, it is important to note that SCD occurred in only 7 patients in this cohort, thus contributing only 18.4% of cardiovascular deaths, whereas the majority of decedents (63.2%) died from HF. The low event rate in the setting of current advances in therapy highlights the need for more precise algorithms of patient selection for device therapy.
Prior Analyses of Entropy in NICM
An association has previously been demonstrated between entropy of the entire myocardium and major arrhythmia in a cohort of patients with DCM referred for ICD implantation. However, it is unclear whether this association was driven by heterogeneity within areas of myocardial fibrosis or was simply the result of greater tissue heterogeneity caused by the presence alone of fibrosis in some patients and not others. 14 As myocardial fibrosis is recognized as the major arrhythmic substrate in NICM, we focused our analysis specifically on characterization of tissue heterogeneity of regions of fibrosis, including core, GZ, and combined core and GZ fibrosis. This approach was adopted to pinpoint the site of arrhythmogenesis in NICM and provides novelty to prior studies. We take this forward by integrating fibrosis presence with fibrosis entropy to delineate tiered risk groups in NICM, enabling more precise risk stratification. This study also includes the full spectrum of NICM, including nondilated LV cardiomyopathy, and additionally was not enriched for patients with an existing indication for an ICD. We have previously evaluated fibrosis entropy in 156 patients with LGE+ DCM, the majority of whom are presented in this cohort, albeit here with longer follow‐up duration. 11 In this earlier analysis, we reported a nonsignificant trend between core fibrosis entropy and LTA. 11 In the study presented, we adopt a revised methodology for entropy quantification that accounts for both core and GZ fibrosis, in a larger cohort with extended follow‐up.
Limitations
The study was conducted in a single‐center tertiary UK hospital; the authors acknowledge a potential referral bias introduced by this design. However, many patients had been referred from a network of surrounding regional hospitals and continued to receive clinical care in other institutions after enrollment. A high proportion of the cohort were White individuals (86%), which may limit applicability of the findings. One technical limitation relates to lack of consensus regarding the optimal method for classifying core and GZ fibrosis. While fibrosis entropy values were similar among patients who received different contrast agents, it remains unknown whether scanner vendor, field strength, or LGE sequence may affect these parameters. We further acknowledge that the proprietary software used for LGE analysis may vary from other analysis software that is routinely available for measuring fibrosis mass; the automated pipeline we used did not permit reproducibility analysis. We acknowledge the potential challenges that exist in analyzing LGE in patients with NICM and thinned LV walls; however, the mean wall thickness in this cohort was 8 mm. The lack of T1 parametric mapping data is a limitation, and testing the incremental value of fibrosis entropy above T1 mapping data represents an area for future work.
Conclusions
Fibrosis entropy, a measure of scar heterogeneity, is an important imaging biomarker in NICM that is independently associated with LTA and enhances arrhythmic risk prediction. By contrast, LVEF is a poor discriminator of LTA in patients with LGE+ NICM. Fibrosis entropy is not associated with major HF events or cardiovascular death and hence may have utility in identifying patients with LGE+ NICM with a high proportional risk of LTA and a lower competing risk of HF death.
Sources of Funding
This work was supported by: NHLF Royston Centre for Cardiomyopathy grant awarded to S.K.P., D.J.H., R.E.J., U.T., and B.P.H.; The British Heart Foundation (FS/ICRF/21/26019; RE/18/4/34215; SP/17/11/32885); EPSRC 2018/19 DTP—EP/R513064/1 grant; the National Institute for Health Research Biomedical Research Centre at Guy's and St. Thomas’ Trust and King's College, the Centre of Excellence in Medical Engineering funded by the Wellcome Trust and Engineering and Physical Sciences; Research Council (EPSRC; WT088641/Z/09/Z); Rosetrees Trust; Alexander Jansons Myocarditis UK Foundation; Royston Centre for Cardiomyopathy Research; Sir Jules Thorn Charitable Trust (21JTA); National Institute for Health Research Royal Brompton Cardiovascular Biomedical Research Unit; National Institute for Health Research Imperial College Biomedical Research Centre; P.L. holds a Wellcome Trust Senior Research Fellowship (209 450/Z/17/Z); H.Z. acknowledges EPSRC 2018/19 DTP‐EP/R513064/1 grant; M.J.B., S.K.P., and B.P.H. acknowledge the BHF through project grant PG/22/11159. The views expressed in this work are those of the authors and not necessarily those of the funders.
Disclosures
R.B. has received honoraria from AstraZeneca, Vifor, and Medtronic. K.G. has received honoraria from Bayer, Pfizer, Novartis, AstraZeneca, and Servier Laboratories; a previous unrestricted educational grant from Biotronik; and previous travel assistance from Abbott Laboratories, Medtronic, Biotronik, and Boston Scientific. F.L. is a consultant with and has received research funding from Medtronic Inc., Boston Scientific, Abbott, Microport, and Biotronik. J.S.W. has acted as a consultant for MyoKardia, Foresite Labs, Pfizer, Health Lumen, and Tenaya Therapeutics. D.J.P. has received research funding from Siemens. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S17
Figures S1–S4
Acknowledgments
The authors thank the Royal Brompton and Harefield Cardiovascular Research Centre nurses and support staff, led by Geraldine Sloane, and Won Yoon, Suprateeka Talukder, and Rohin Reddy, who assisted with follow‐up data collection.
M. J. Bishop and S. K. Prasad are co‐senior authors.
This manuscript was sent to Daniel E. Clark, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.124.040517
For Sources of Funding and Disclosures, see page 10 .
References
- 1. Hammersley DJ, Halliday BP. Sudden cardiac death prediction in non‐ischemic dilated cardiomyopathy: a multiparametric and dynamic approach. Curr Cardiol Rep. 2020;22:85. doi: 10.1007/s11886-020-01343-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hammersley DJ, Jones RE, Mach L, Halliday BP, Prasad SK. Cardiovascular magnetic resonance in heritable cardiomyopathies. Heart Fail Clin. 2021;17:25–39. doi: 10.1016/j.hfc.2020.08.004 [DOI] [PubMed] [Google Scholar]
- 3. Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–1230. doi: 10.1056/NEJMoa1608029 [DOI] [PubMed] [Google Scholar]
- 4. Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TDH, Ismail N, Dweck MR, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. J Am Med Assoc. 2013;309:896–908. doi: 10.1001/jama.2013.1363 [DOI] [PubMed] [Google Scholar]
- 5. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, et al. 2022 AHA/ACC/HFSA guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 6. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Celutkiene J, Chioncel O, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 7. Hammersley DJ, Zegard A, Androulakis E, Jones RE, Okafor O, Hatipoglu S, Mach L, Lota AS, Khalique Z, De Marvao A, et al. Arrhythmic risk stratification by cardiovascular magnetic resonance imaging in patients with nonischemic cardiomyopathy. J Am Coll Cardiol. 2024;84:1407–1420. doi: 10.1016/j.jacc.2024.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hammersley DJ, Mukhopadhyay S, Chen X, Jones RE, Ragavan A, Javed S, Rajabali H, Androulakis E, Curran L, Mach L, et al. Precision prediction of heart failure events in patients with dilated cardiomyopathy and mildly reduced ejection fraction using multi‐parametric cardiovascular magnetic resonance. Eur J Heart Fail. 2024;26:ejhf.3425. doi: 10.1002/ejhf.3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeppenfeld K, Tfelt‐Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, Charron P, Corrado D, Dagres N, de Chillou C, et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022;43:3997–4126. doi: 10.1093/eurheartj/ehac262 [DOI] [PubMed] [Google Scholar]
- 10. Halliday BP, Baksi AJ, Gulati A, Ali A, Newsome S, Izgi C, Arzanauskaite M, Lota A, Tayal U, Vassiliou VS, et al. Outcome in dilated cardiomyopathy related to the extent, location, and pattern of late gadolinium enhancement. JACC Cardiovasc Imaging. 2019;12:1645–1655. doi: 10.1016/j.jcmg.2018.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Balaban G, Halliday BP, Porter B, Bai W, Nygåard S, Owen R, Hatipoglu S, Ferreira ND, Izgi C, Tayal U, et al. Late‐gadolinium enhancement Interface area and electrophysiological simulations predict arrhythmic events in patients with nonischemic dilated cardiomyopathy. JACC Clin Electrophysiol. 2021;7:238–249. doi: 10.1016/j.jacep.2020.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shannon CE. A mathematical theory of communication. Bell Syst Tech J. 1948;27:379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x [DOI] [Google Scholar]
- 13. Androulakis AFA, Zeppenfeld K, Paiman EHM, Piers SRD, Wijnmaalen AP, Siebelink HMJ, Sramko M, Lamb HJ, van der Geest RJ, de Riva M, et al. Entropy as a novel measure of myocardial tissue heterogeneity for prediction of ventricular arrhythmias and mortality in post‐infarct patients. JACC Clin Electrophysiol. 2019;5:480–489. doi: 10.1016/j.jacep.2018.12.005 [DOI] [PubMed] [Google Scholar]
- 14. Muthalaly RG, Kwong RY, John RM, van der Geest RJ, Tao Q, Schaeffer B, Tanigawa S, Nakamura T, Kaneko K, Tedrow UB, et al. Left ventricular entropy is a novel predictor of arrhythmic events in patients with dilated cardiomyopathy receiving defibrillators for primary prevention. JACC Cardiovasc Imaging. 2019;12:1177–1184. doi: 10.1016/j.jcmg.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 15. Gould J, Porter B, Claridge S, Chen Z, Sieniewicz BJ, Sidhu BS, Niederer S, Bishop MJ, Murgatroyd F, Ganeshan B, et al. Mean entropy predicts implantable cardioverter‐defibrillator therapy using cardiac magnetic resonance texture analysis of scar heterogeneity. Heart Rhythm. 2019;16:1242–1250. doi: 10.1016/j.hrthm.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 16. Antiochos P, Ge Y, van der Geest RJ, Madamanchi C, Qamar I, Seno A, Jerosch‐Herold M, Tedrow UB, Stevenson WG, Kwong RY. Entropy as a measure of myocardial tissue heterogeneity in patients with ventricular arrhythmias. JACC Cardiovasc Imaging. 2022;15:783–792. doi: 10.1016/j.jcmg.2021.12.003 [DOI] [PubMed] [Google Scholar]
- 17. Maceira AM, Prasad SK, Khan M, Pennell DJ. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2006;8:417–426. doi: 10.1080/10976640600572889 [DOI] [PubMed] [Google Scholar]
- 18. Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales‐Villa R, Basso C, Bezzina CR, Biagini E, Blom NA, de Boer RA, et al. 2023 ESC guidelines for the management of cardiomyopathies. Eur Heart J. 2023;44:3503–3626. doi: 10.1093/eurheartj/ehad194 [DOI] [PubMed] [Google Scholar]
- 19. Hammersley DJ, Jones RE, Owen R, Mach L, Lota AS, Khalique Z, De Marvao A, Androulakis E, Hatipoglu S, Gulati A, et al. Phenotype, outcomes and natural history of early‐stage non‐ischaemic cardiomyopathy. Eur J Heart Fail. 2023;25:2050–2059. doi: 10.1002/ejhf.3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roes SD, Borleffs CJW, Van Der Geest RJ, Westenberg JJM, Marsan NA, Kaandorp TAM, Reiber JHC, Zeppenfeld K, Lamb HJ, De Roos A, et al. Infarct tissue heterogeneity assessed with contrast‐enhanced mri predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter‐defibrillator. Circ Cardiovasc Imaging. 2009;2:183–190. doi: 10.1161/CIRCIMAGING.108.826529 [DOI] [PubMed] [Google Scholar]
- 21. Jones RE, Zaidi HA, Hammersley DJ, Hatipoglu S, Owen R, Balaban G, de Marvao A, Simard F, Lota AS, Mahon C, et al. Comprehensive phenotypic characterization of late gadolinium enhancement predicts sudden cardiac death in coronary artery disease. JACC Cardiovasc Imaging. 2023;16:628–638. doi: 10.1016/j.jcmg.2022.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Balaban G, Halliday BP, Bai W, Porter B, Malvuccio C, Lamata P, Rinaldi CA, Plank G, Rueckert D, Prasad SK, et al. Scar shape analysis and simulated electrical instabilities in a non‐ischemic dilated cardiomyopathy patient cohort. PLoS Comput Biol. 2019;15:1–18. doi: 10.1371/journal.pcbi.1007421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zaidi HA, Jones RE, Hammersley DJ, Hatipoglu S, Balaban G, Mach L, Halliday BP, Lamata P, Prasad SK, Bishop MJ. Machine learning analysis of complex late gadolinium enhancement patterns to improve risk prediction of major arrhythmic events. Front Cardiovasc Med. 2023;10:1082778. doi: 10.3389/fcvm.2023.1082778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hammersley DJ, Mukhopadhyay S, Chen X, Cheng L, Jones RE, Mach L, Curran L, Yazdani M, Iacob A, Lota AS, et al. Comparative prognostic importance of measures of left atrial structure and function in non‐ischaemic dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2024;25:1566–1574. doi: 10.1093/ehjci/jeae080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dillon SM, Allessie MA, Ursell PC, Wit AL. Influences of anisotropic tissue structure on reentrant circuits in the epicardial border zone of subacute canine infarcts. Circ Res. 1988;63:182–206. doi: 10.1161/01.RES.63.1.182 [DOI] [PubMed] [Google Scholar]
- 26. Wu KC. Sudden cardiac death substrate imaged by magnetic resonance imaging: from investigational tool to clinical applications. Circ Cardiovasc Imaging. 2017;10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leyva F, Zegard A, Okafor O, Foley P, Umar F, Taylor RJ, Marshall H, Stegemann B, Moody W, Steeds RP, et al. Myocardial fibrosis predicts ventricular arrhythmias and sudden death after cardiac electronic device implantation. J Am Coll Cardiol. 2022;79:665–678. doi: 10.1016/j.jacc.2021.11.050 [DOI] [PubMed] [Google Scholar]
- 28. Kazbanov IV, Ten Tusscher KHWJ, Panfilov AV. Effects of heterogeneous diffuse fibrosis on arrhythmia dynamics and mechanism. Sci Rep. 2016;6:20835. doi: 10.1038/srep20835 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S17
Figures S1–S4


