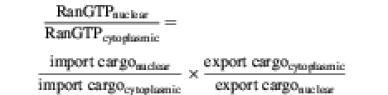Abstract
Importin β-related receptors mediate translocation through nuclear pore complexes. Co-operation with the RanGTPase system allows them to bind and subsequently release their substrates on opposite sides of the nuclear envelope, which in turn ensures a directed nucleocytoplasmic transport. Here we identify a novel family member from higher eukaryotes that functions primarily, but not exclusively, in import. It accounts for nuclear accumulation of the SUMO-1/sentrin-conjugating enzyme hUBC9 and mediates import of the RBM8 (Y14) protein, and is therefore referred to as importin 13 (Imp13). Unexpectedly, Imp13 also shows export activity towards the translation initiation factor eIF1A and is thus a case where a single importin β-like receptor transports different substrates in opposite directions. However, Imp13 operates differently from typical exportins in that the binding of eIF1A to Imp13 is only regulated indirectly by RanGTP, and the cytoplasmic release of eIF1A from Imp13 is triggered by the loading of import substrates onto Imp13.
Keywords: eIF1A/exportin/importin/UBC9/Y14
Introduction
Nucleocytoplasmic transport is an essential activity in eukaryotic cells. mRNAs, tRNAs and ribosomes, for example, are produced in the nuclear compartment and need to be exported to the cytoplasm where they function in translation. Conversely, all nuclear proteins are imported from the cytoplasm. Nuclear transport proceeds through nuclear pore complexes (NPCs), which allow passage in two modes: passive diffusion and facilitated translocation (for reviews see Mattaj and Englmeier, 1998; Görlich and Kutay, 1999; Nakielny and Dreyfuss, 1999). Passive diffusion is efficient for small molecules, but becomes slow and inefficient as the size approaches a limit of 20–40 kDa. In contrast, facilitated translocation allows passage of objects as large as 25 nm in diameter or several MDa in molecular weight. It is normally mediated by specific nuclear transport receptors and often coupled to an input of metabolic energy, which in turn allows cargo accumulation against a gradient of chemical activity (active transport).
Many, though not all, nuclear transport pathways are mediated by importin β-related transport receptors, which form a large protein superfamily (Fornerod et al., 1997b; Görlich et al., 1997) with 14 family members in the yeast Saccharomyces cerevisiae and probably >20 in human (Mattaj and Englmeier, 1998; Wozniak et al., 1998; Görlich and Kutay, 1999; Nakielny and Dreyfuss, 1999; this study; E.Hartmann and D.Görlich, unpublished). These receptors recognize cargo molecules, interact with NPCs and constantly circulate between nucleus and cytoplasm. Cargo binding and release are controlled by a RanGTP gradient across the nuclear envelope, with a high RanGTP concentration in the nucleus and low levels in the cytoplasm (Görlich et al., 1996; Izaurralde et al., 1997; Nachury and Weis, 1999). The gradient defines compartment identity and is sensed through the RanGTP-binding domains of the transport receptors.
The receptors can be classified as importins or exportins. Importins load cargoes at low RanGTP levels in the cytoplasm, translocate into the nucleus and release their cargo upon RanGTP binding (Rexach and Blobel, 1995; Chi et al., 1996; Görlich et al., 1996; Izaurralde et al., 1997; Schlenstedt et al., 1997; Siomi et al., 1997; Jäkel and Görlich, 1998). They finally return to the cytoplasm, where Ran is dissociated from the receptor and the Ran-bound GTP is hydrolysed (Bischoff and Görlich, 1997; Floer et al., 1997; Lounsbury and Macara, 1997). The importin can then bind and import another substrate molecule. The cytoplasmic GTP hydrolysis event constitutes the sole input of metabolic energy into these transport cycles (Schwoebel et al., 1998; Englmeier et al., 1999; Ribbeck et al., 1999).
Exportins control their substrate binding in an exactly converse manner to importins. They bind cargoes at high RanGTP concentrations in the nucleus, forming trimeric RanGTP–exportin–cargo complexes (Fornerod et al., 1997a; Kutay et al., 1997). Cytoplasmic cargo release is accomplished by GTP hydrolysis and the removal of Ran from the exportin (Bischoff and Görlich, 1997; Kutay et al., 1997). The empty exportin can then re-enter the nucleus and mediate another round of export. As importins and exportins constantly export RanGTP from the nucleus, the nuclear RanGTP pool must be replenished through NTF2-mediated import of RanGDP (Ribbeck et al., 1998; Smith et al., 1998), followed by a RanGEF-mediated re-charging of Ran with GTP (Bischoff and Ponstingl, 1991).
While characterizing a novel nuclear transport receptor, we identified a number of potential transport substrates including hUBC9, eIF1A, the RNA-binding motif protein 8 (RBM8) and MGN. eIF1A is a universally conserved translation factor which plays multiple roles in the initiation process and is required, for example, for scanning the mRNA for a start AUG codon (see Benne et al., 1978; Pestova et al., 1998, and references therein). UBC9 was identified originally in the yeast S.cerevisiae as an essential, predominantly nuclear protein related to ubiquitin-conjugating (E2) enzymes (Seufert et al., 1995). It later turned out not to use ubiquitin as a substrate, but rather a ubiquitin-related protein called SUMO-1 or sentrin (Matunis et al., 1996; Desterro et al., 1997; Gong et al., 1997; Johnson and Blobel, 1997; Mahajan et al., 1997). SUMO-1 is conjugated to a large number of usually nuclear localized proteins, such as PML, SP100 (Sternsdorf et al., 1997), p53 (Gostissa et al., 1999; Rodriguez et al., 1999) and DNA topoisomerases (Mao et al., 2000). In contrast to ubiquitin, SUMO-1 conjugation does not target proteins for proteasome-mediated degradation, and the direct physiological consequence of modifications with SUMO is in most cases still unclear. However, it is well established that SUMO-1 modification targets RanGAP to the cytoplasmic filaments of the NPC (Matunis et al., 1996; Mahajan et al., 1997).
MGN is the human homologue to the Drosophila mago nashi protein. Mago nashi plays multiple roles in early Drosophila embryogenesis; It is essential for germ cell formation, and is involved in polarizing the oocyte, axis formation and localization of oscar mRNA (Boswell et al., 1991; Newmark and Boswell, 1994; Micklem et al., 1997). It is highly conserved among higher eukaryotes and apparently expressed in all tissues (Zhao et al., 1998).
RBM8 (also called Y14) is an MGN-binding protein (Zhao et al., 2000). It is loaded onto mRNA as a result of the splicing reaction and has been suggested to promote selective export of spliced mRNAs by recruiting downstream export mediators such as TAP and REF (Kataoka et al., 2000; Le Hir et al., 2000).
Here we identify importin 13 (Imp13) as a novel importin β-related transport receptor that mediates nuclear import of the RBM8–MGN complex and of hUBC9. It is controlled by the RanGTPase system in a typical importin-like fashion, i.e. the import substrates can bind Imp13 at low RanGTP levels in the cytoplasm and become displaced upon RanGTP binding in the nucleus. Surprisingly, Imp13 also shows export activity towards eIF1A and thus helps to confine the translation machinery to the cytoplasm. In crude extracts, RanGTP is required for a stable binding of eIF1A to Imp13, just as expected for a bona fide exportin–cargo interaction. However, upon closer inspection, this regulation turned out to be more complex: the cytoplasmic displacement of eIF1A from Imp13 requires not only the transition of Imp13 from a RanGTP complex to the Ran-free form, but also the loading of import substrates onto the receptor. In a nuclear environment, RanGTP releases the import substrate, thereby allowing Imp13 to bind and export the next eIF1A molecule. Such a transport cycle can transport two molecules in opposite directions, while consuming only one GTP molecule. This lesser expenditure of energy as compared with more conventional transport cycles, however, also limits the extent of cargo accumulation against a gradient of chemical activity.
Results
Imp13—a putative nuclear transport receptor
By searching databases, we identified a human expressed sequence tag (EST; accession No. AB018267) with significant homology to known members of the importin β superfamily. The corresponding open reading frame codes for a 108 kDa protein that will be referred to as Imp13. Putative orthologues of Imp13 (detailed in Materials and methods) can be found in representative branches of eukaryotes, such as plants, insects, nematodes and fungi, suggesting that the protein evolved quite early in eukaryotic phylogenesis.
As an initial biochemical characterization, we established that recombinant human Imp13 specifically interacts with RanGTP (see below), indicating that the protein not only is sequence related to, but might also function like established Ran-regulated nuclear transport receptors.
Identification of Imp13-specific nuclear transport substrates
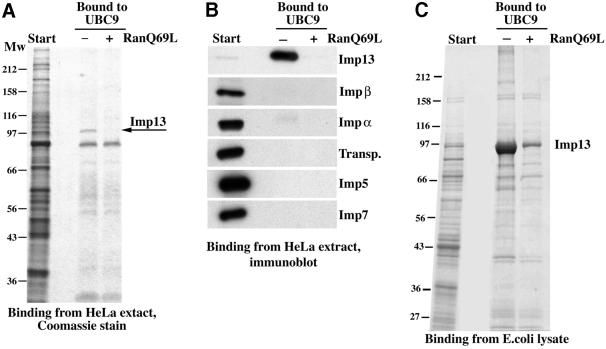
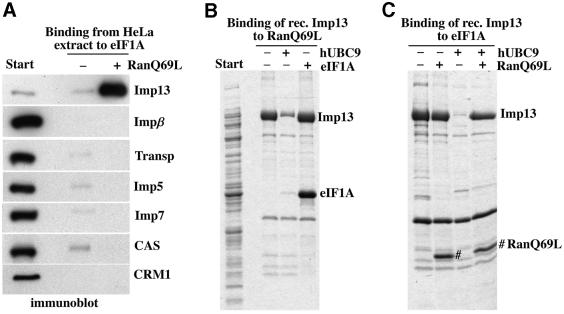
It was unclear initially whether Imp13 would mediate nuclear import or export, or indeed nuclear transport at all. To clarify this point, we used the immobilized receptor to retrieve potential Imp13-specific transport substrates from a HeLa cell extract. Specifically bound proteins were identified by mass spectrometry and are indicated in Figure 1. Most proteins recovered on the column could be displaced by RanGTP and, by this criterion, behaved like import substrates. These were: the ribosomal protein L5, the RBM8 protein (also called Y14), MGN, the NF-YB protein as well as the SUMO-conjugating enzyme hUBC9. The translation initiation factor eIF1A, however, bound efficiently only in the presence of RanGTP and thus constituted a potential Imp13-specific export substrate. Of these potential transport substrates, we chose hUBC9, RBM8, the MGN protein and eIF1A for further detailed analysis.
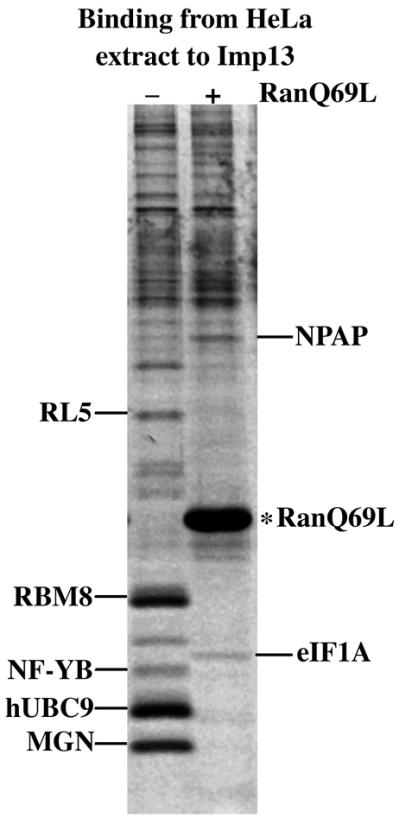
Fig. 1. Identification of potential Imp13-specific transport substrates. Immobilized Imp13 was used to fish interacting proteins from a cytosolic HeLa extract. Binding was performed in the absence or presence of RanQ69L GTP (5 µM) to identify potential import or export substrates, respectively. Bound proteins were separated by SDS–PAGE, visualized by Coomassie staining, digested with trypsin and finally identified by peptide sequencing and mass spectrometry. The following proteins were identified: RL5, ribosomal protein L5 (accession No. P46777); RBM8 (Y14), RNA-binding motif protein 8 (AAF37551); NF-YB-like protein (AAF67146); hUBC9 (AAH00427); MGN, Mago Nashi protein homologue (P50606); NPAP, nuclear pore-associated protein (AAD53401); eIF1A, eukaryotic translation initiation factor 1A (P47813).
Imp13 mediates nuclear import of the SUMO-conjugating enzyme hUBC9
hUBC9 is a predominantly nuclear protein whose import mechanism has so far remained elusive. An import by Imp13 therefore appeared an attractive possibility. To assess the specificity of the hUBC9–Imp13 interaction, we used immobilized hUBC9 to bind potential import receptors from a HeLa extract. The matrix retrieved a major ∼110 kDa band that was displaced by the GTPase-deficient RanQ69L mutant (Figure 2A). Immunoblotting confirmed the identity of this band as Imp13 and also demonstrated that other importins were not enriched in the hUBC9-bound fraction (Figure 2B).
Fig. 2. hUBC9 behaves like an Imp13-specific import substrate. (A) Immobilized hUBC9 was used to bind nuclear transport receptors from a cytoplasmic HeLa cell extract. Starting material and bound fractions were analysed by SDS–PAGE followed by Coomassie staining. Note, Imp13 was bound specifically to the matrix and this binding was abolished by the GTP-bound form of RanQ69L (5 µM) that was used to mimic a nuclear environment. (B) The samples from (A) were analysed by immunoblotting with various antibodies. Imp13 was highly enriched in the hUBC9-bound fraction, while binding of other transport receptors was insignificant. (C) Binding to hUBC9 was performed as in (A), except that an E.coli lysate with recombinant Imp13 was used as a source of nuclear transport receptors. The recombinant receptor behaved like the native protein from the HeLa extract (see A).
The binding of Imp13 to hUBC9 could have been direct or mediated through some other factor present in the HeLa extract. To discriminate between these possibilities, we repeated the experiment with recombinant proteins (Figure 2C). Again, Imp13 bound hUBC9 efficiently and in a RanGTP-sensitive manner. Thus, the Imp13–hUBC9 interaction is direct and stable under cytoplasmic conditions and becomes disrupted by a nuclear environment of a high RanGTP concentration.
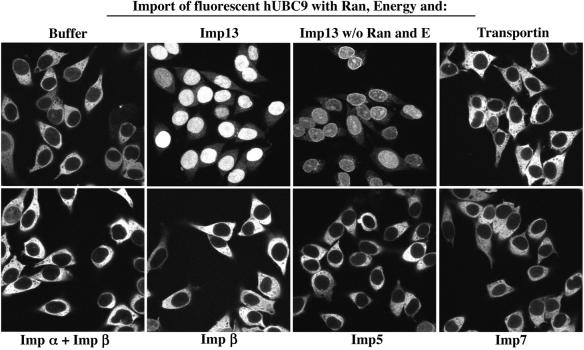
We next labelled hUBC9 with a fluorescent dye and studied its import into nuclei of permeabilized HeLa cells. Nuclear accumulation of hUBC9 was strictly Imp13 dependent and stimulated by the presence of Ran and an energy-regenerating system (Figure 3). Other importins, such as importin β, -5, -7, transportin or the importin α/β heterodimer, had no detectable effect. We can therefore conclude that Imp13 indeed functions as the import receptor for hUBC9.
Fig. 3. Imp13 mediates import of hUBC9. Import of fluorescently labelled GST–hUBC9 (1 µM) into nuclei of permeabilized cells was performed for 5 min with the indicated combinations of nuclear transport receptors (1 µM each). Panels show confocal sections of the hUBC9 distribution after import and fixation. Nuclear hUBC9 accumulation was dependent on the presence of Imp13, Ran mix and an energy-regenerating system (see Materials and methods).
Imp13 is an import receptor for the RBM8 (Y14)–MGN protein complex
The RNA-binding motif protein 8 and the MGN protein were further candidates for Imp13-specific import substrates (see Figure 1). RBM8 (Y14) is probably exported constantly to the cytoplasm while bound to RNA (Kataoka et al., 2000). Its predominantly nuclear localization should therefore require a very efficient import.
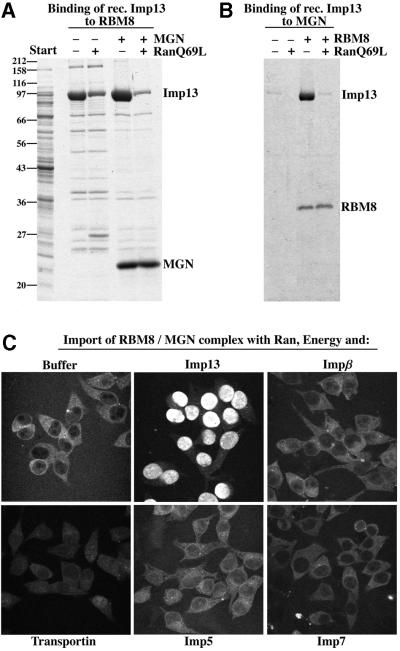
RBM8 is known to interact with the MGN protein (Zhao et al., 2000) and, indeed, the two recombinantly expressed proteins formed a stable complex upon mixing (Figure 4A and B). The MGN protein alone interacted only weakly with Imp13. However, RBM8 protein and its complex with MGN bound the receptor efficiently. The RBM8– MGN complex thus contacts Imp13 largely through its RBM8 subunit, while the MGN protein makes only a minor contribution. As expected for an Imp13-specific import substrate, the complex bound the receptor in a RanGTP-sensitive manner (Figure 4A and B).
Fig. 4. Imp13 mediates import of the RBM8–MGN complex. (A) An E.coli lysate with recombinant Imp13 was used as the starting material for the binding experiment. Imp13 bound very efficiently and in a RanGTP-sensitive manner to immobilized RBM8 and the RBM8–MGN complex. (B) Binding was performed as in (A), except that MGN was immobilized instead of RBM8. Imp13 bound only weakly to MGN alone, but efficiently to the MGN–RBM8 complex. (C) A pre-formed RBM8–MGN complex was fluorescently labelled and used at 1 µM as an import substrate as described in Figure 3. Import was efficient with Imp13 and hardly detectable with any other nuclear transport receptor (each used at 2 µM).
Consistent with the binding experiments, the RBM8– MGN complex was indeed imported efficiently with Imp13 (Figure 4C). Nuclear import was not observed when Imp13 was either omitted or replaced by transportin, importin β, importin 5 or importin 7. RBM8 previously has been reported to interact with importin 5 in a two-hybrid assay (Kataoka et al., 2000). The lack of importin 5-mediated import of RBM8 was therefore somewhat surprising, but might relate to our previous observation that not every cargo–import receptor complex is productive (Jäkel et al., 1999).
The RBM8–MGN complex is very stable (Zhao et al., 2000; see Figure 4A and B) and two further lines of evidence suggest that this complex represents a physiologically relevant species. First, the two subunits were recovered in a stoichiometric ratio on the Imp13 column (see Figure 1). Secondly, when RBM8 was imported alone, it accumulated in nucleoli (data not shown). In contrast, its steady-state localization in HeLa cells is nucleoplasmic (Kataoka et al., 2000) and a nucleoplasmic pattern could only be reproduced when RBM8 was imported as a complex with MGN (Figure 4C), suggesting that RBM8 normally occurs and probably also functions as such a complex.
Imp13 mediates nuclear export of eIF1A
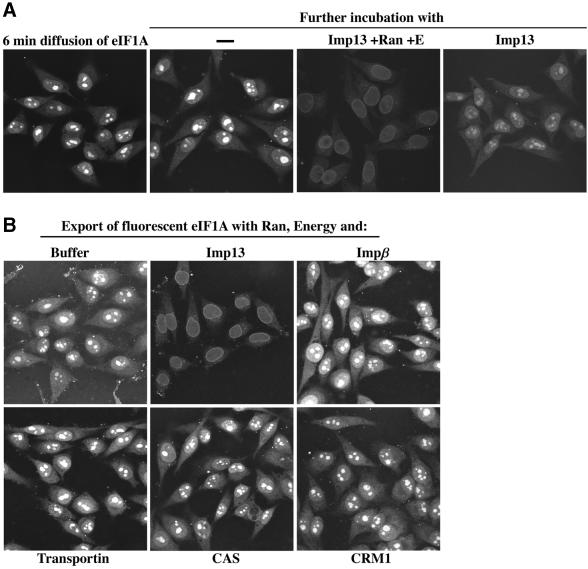
The translation initiation factor eIF1A functions in the cytoplasm, but should be small enough (18 kDa) to enter nuclei efficiently by passive diffusion. It therefore appeared reasonable to assume that its cytoplasmic localization is maintained by an active export mechanism, possibly through an Imp13-dependent export pathway. When incubated with permeabilized cells, fluorescently labelled eIF1A readily entered the nuclei and accumulated in bright nucleolar spots (Figure 5A). This accumulation occurred without exogenous transport factors and provided a convenient way to pre-load nuclei with eIF1A. When these nuclei were incubated with Imp13, Ran and an energy-regenerating system, eIF1A was exported efficiently and the nucleolar signal was lost. In addition, a significant fraction of eIF1A showed a typical NPC-staining pattern, which probably represents export intermediates at the stage of NPC passage. These effects were highly specific and export was not observed when Imp13 was either omitted or replaced by importin β, transportin, CAS or CRM1 (Figure 5B). Likewise, the export activity of Imp13 appears highly specific towards eIF1A and was not detectable towards importin α or snurportin (data not shown), which are exported by CAS and CRM1, respectively (Kutay et al., 1997; Paraskeva et al., 1999).
Fig. 5. Imp13 mediates nuclear export of eIF1A. (A) Fluorescent GST–eIF1A (1 µM) was allowed to diffuse for 6 min into nuclei. One sample was fixed immediately. The others were incubated with the indicated additions for a further 5 min before they were also fixed. The presence of Imp13 (2 µM), Ran and energy resulted in efficient eIF1A export and complete loss of the nucleolar signal. (B) A comparison of the indicated combinations of nuclear transport receptors (2 µM) in their export activity towards eIF1A. Export was performed as in (A).
Figure 5A also shows that the ommission of Ran and the energy-regenerating system clearly reduced, but did not completely abolish, Imp13-dependent export (discussed below).
The Imp13–eIF1A interaction is regulated differently from bona fide exportins
Substrate binding to previously characterized exportins is greatly enhanced by RanGTP (Fornerod et al., 1997a; Kutay et al., 1997, 1998; Arts et al., 1998; Kaffman et al., 1998; Lipowsky et al., 2000) and, indeed, binding of Imp13 from HeLa extract to immobilized eIF1A was efficient in the presence of RanQ69L GTP and hardly detectable without this addition (Figure 6A). Thus, under these conditions, formation of the trimeric RanGTP–Imp13–eIF1A complex is highly co-operative and, up to this point, consistent with the exportin paradigm. A surprise, however, came when the binding experiment was repeated with a bacterial lysate as a source of Imp13. Although, under these conditions, Imp13 could simultaneously bind Ran and eIF1A, the binding no longer showed any signs of co-operativity: binding of Imp13 to the RanGTP column was as efficient in the absence of eIF1A as in its presence (Figure 6B). Conversely, binding of Imp13 to immobilized eIF1A was not increased by the presence of RanGTP (Figure 6C). Thus, there is no direct cross-talk between the Ran- and eIF1A-binding sites in Imp13.
Fig. 6. Characterization of the eIF1A–Imp13 interaction. (A) Binding of nuclear transport receptors from a HeLa cell extract to immobilized eIF1A was analysed by immunoblotting with the indicated antibodies. Imp13 was highly enriched in the bound fraction, provided RanQ69L (5 µM) had been added to the incubation. (B) Imp13 was bound from an E.coli lysate to immobilized RanQ69L (GTP). This binding was antagonized efficiently by the import substrate GST–hUBC9. In contrast, GST–eIF1A did not compete the Imp13–Ran interaction and instead engaged in a trimeric eIF1A–Imp13–RanGTP complex. GST–hUBC9 and GST–eIF1A were each used at 10 µM (monomers) which compares with ∼1 µM Imp13 present in the extract. (C) Recombinant Imp13 was bound to immobilized eIF1A in the presence of the indicated combinations of RanQ69LGTP and GST–hUBC9. RanGTP engaged in trimeric eIF1A–Imp13–Ran complexes, but otherwiese had no effect on the Imp13–eIF1A interaction. hUBC9 efficiently competed Imp13 binding to eIF1A, but the presence of RanGTP fully abrogated this effect.
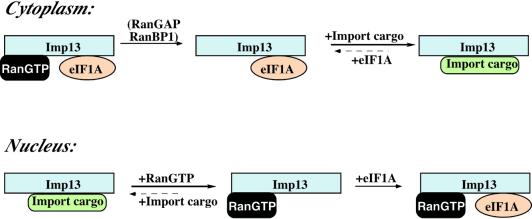
Amongst other possibilities, we reasoned that some additional factor from the HeLa extract might be required for the regulation. Indeed, the regulation could be restored when the binding was performed in the presence of import substrate. Figure 6C demonstrates that hUBC9 competes the eIF1A–Imp13 interaction. In the absence of RanGTP, hUBC9 clearly binds with higher affinity to Imp13 than does eIF1A, and is thus the ‘winner’ under cytoplasmic conditions. This situation becomes reversed in a nuclear environment: although RanGTP does not directly enhance Imp13’s affinity for its export substrate, it indirectly favours the Imp13–eIF1A interaction by displacing hUBC9, which antagonizes eIF1A binding. Such Ran-controlled competition of the eIF1A–Imp13 interaction was observed not only with hUBC9, but also for RBM8 and the RBM8–MGN complex (data not shown), and might thus apply to Imp13 import substrates in general. The scheme in Figure 7 summarizes these interactions.
Fig. 7. Scheme for the interactions of Imp13 with RanGTP, its export substrate eIF1A and an import cargo. Under cytoplasmic conditions, RanBP1/RanBP2 and RanGAP remove RanGTP from Imp13. This is insufficient to release eIF1A from Imp13, but allows a displacement of eIF1A by import substrates. The Imp13–import substrate complex can then enter nuclei. In a nuclear environment, the import substrate is displaced by RanGTP, which in turn favours the eIF1A–Imp13 interaction and thereby allows eIF1A export.
The question remains as to why the export reaction in Figure 5 was stimulated by Ran and GTP even though the eIF1A–Imp13 interaction is not regulated directly by RanGTP. We assume that upon nuclear entry, Imp13 becomes trapped by import substrates that are already present in the nucleus. Nuclear RanGTP would then be required to displace the receptor from these sites and make it available for eIF1A export. The trimeric eIF1A–Imp13–RanGTP complex can then form and be transferred to the cytoplasm. eIF1A now needs to be released from Imp13. Bona fide exportins release their substrates immediately upon hydrolysis of the Ran-bound GTP. This, however, is insufficient to displace eIF1A from Imp13. Instead, the displacement of eIF1A from Imp13 is brought about by the loading of import substrates. This peculiar feature implies that continued Imp13-dependent eIF1A export should depend on the availability of appropriate import substrates in the cytoplasm.
Discussion
Many of the transport events between nucleus and cytoplasm are mediated by receptors of the importin β type and it has therefore been a major goal in the field to obtain a complete and comprehensive description of these factors and the corresponding nuclear transport pathways. Mammals apparently are equipped with >20 such receptors; however, functions have been allocated so far to only seven human importins, namely importin β (Chi et al., 1995; Görlich et al., 1995; Imamoto et al., 1995; Radu et al., 1995), transportin 1 (Pollard et al., 1996), the importins 5 and 7 (Jäkel and Görlich, 1998), transportin SR (Kataoka et al., 1999), transportin SR2 (Lai et al., 2000) and importin 11 (Plafker and Macara, 2000), as well as to four exportins, namely CRM1 (Fornerod, 1997a), CAS (Kutay et al., 1997), exportin-t (Arts et al., 1998; Kutay et al., 1998) and exportin 4 (Lipowsky et al., 2000). Here we report on Imp13, a novel nuclear transport receptor that functions primarily, but not exclusively, in nuclear import. It behaves, on the one hand, like a prototypic importin and mediates nuclear accumulation of the SUMO-conjugating enzyme hUBC9, the RBM8– MGN complex and probably other substrates. On the other hand, it functions as an unconventional exportin for the translation initiation factor eIF1A. This might relate to a recent publication reporting that yeast Msn5p not only functions as a bona fide exportin (Kaffman et al., 1998), but also mediates import of the RPA complex (Yoshida and Blobel, 2001). Whether Msn5p coordinates import with export through a similar mechanism to that of Imp13 is still unclear.
UBC9 is a predominantly nuclear protein in the yeast S.cerevisiae (Seufert et al., 1995). The same applies to mammalian UBC9 and the SUMO-activating enzyme (Rodriguez et al., 2001). This would explain why most substrates for SUMO conjugation are nuclear proteins and, indeed, it has been demonstrated recently that nuclear localization of the substrates is a prerequisite for efficient SUMO conjugation (Rodriguez et al., 2001). The confinement of SUMO conjugation to the nuclear compartment therefore appears to be a major function of Imp13.
Vertebrate RanGAP is an exception amongst the hUBC9 substrates in that it is excluded from nuclei and requires SUMO conjugation for its anchoring to the cytoplasmic filaments of the NPC (Matunis et al., 1996; Mahajan et al., 1997). One could therefore assume that RanGAP relies on residual cytoplasmic or NPC-bound hUBC9 for SUMO modification (Lee et al., 1998). However, it is equally possible that RanGAP must pass transiently through nuclei in order to obtain this modification. We are testing this at present.
The nuclear envelope physically separates the processes of transcription and translation. One obvious reason for this compartmentation is the presence of introns in most eukaryotic genes, which implies that translation must not occur before splicing has been completed. The confinement of transcription and translation to distinct compartments can be considered as a perfect solution to this problem. However, nuclei contain many of the components required for translation: ribosomal subunits and mRNAs are produced there and nuclei even contain aminoacylated tRNA (Lund and Dahlberg, 1998), and so the question arises as to how nuclear translation is normally suppressed. One possible mechanism would be to deplete nuclei actively of at least some essential translation factors. Our data make this scenario probable and eIF1A is, to our knowledge, a first example of a bona fide translation factor that is exported from nuclei in a receptor-mediated manner.
A second benefit of eIF1A export might be that its untimely interaction with pre-ribosomes could interfere with ribosome biogenesis. It is therefore interesting to note that Imp13 not only promotes NPC passage of eIF1A out of nuclei, but also efficiently suppresses the retention of eIF1A at nucleolar binding sites (see Figure 5). Imp13 appears to recognize more than just a small export signal in eIF1A and instead requires the N-terminal RNA-binding domain together with the compactly folded central domain of eIF1A (Battiste et al., 2000) for efficient binding (J.-M.Mingot and D.Görlich, unpublished). Thus, Imp13 may be well designed to shield large parts of eIF1A against undesired nuclear interactions.
Energetic aspects of a bi-directional transport cycle
Imp13 circulates, like all nuclear transport receptors, between nucleus and cytoplasm. Primarily, it behaves like a standard importin. i.e. it binds import substrates, such as hUBC9, in the cytoplasmic compartment, and translocates into the nucleus, where the import cargo is released upon RanGTP binding. The Imp13–RanGTP complex then returns to the cytoplasm, where Ran is finally removed from the receptor and the Ran-bound GTP becomes hydrolysed. The receptor can then engage in another import cycle and these cycles can continue as long as the RanGTP gradient across the nuclear envelope remains stable and import substrates are available in the cytoplasm. A remarkable property of Imp13 is that it can also carry cargo, namely eIF1A, on its return to the cytoplasm, and this special feature clearly deserves more detailed reflection.
Ran-driven transport cycles can accumulate cargoes against a gradient of chemical activity, which is an energy-consuming task (for a detailed discussion see Görlich and Kutay, 1999). This energy is fed into the system by coupling cargo transport to RanGTP efflux from the nuclei. The transport thus occurs at the expense of the RanGTP gradient across the nuclear envelope. The Ran gradient, in turn, becomes replenished through other components of the RanGTPase system, which use Ran-dependent GTP hydrolysis as a primary energy source. Standard importin or exportin systems hydrolyse one GTP molecule for the active transfer of one cargo molecule across the nuclear envelope. Imp13-dependent transport cycles can achieve with one GTP hydrolysis event the transport of two cargo molecules. This appears a very economical mode of transport and poses the question of why we normally find specialized importins and exportins when single transport receptors could operate just as well in both directions. A plausible answer is that this lesser expenditure of energy as compared with conventional transport cycles severely limits the extent of potential cargo accumulation.
A conventional importin, for example, can be considered as an antiporter system. It can import one cargo molecule against a gradient by ‘paying’ with the export of one RanGTP molecule down the RanGTP gradient. The system reaches its equilibrium endpoint when the cargo gradient and the primary RanGTP gradient have reached identical chemical potentials:
A nuclear:cytoplasmic RanGTP ratio of 100, for example, would allow up to a 100-fold cargo accumulation in the nucleus, and this scenario also applies when Imp13 mediates only nuclear import.
In the case of the bi-directional Imp13-dependent transport cycles, the efflux of one RanGTP molecule must drive the transport of an import cargo and an export cargo against gradients of chemical activity. Applying the law of mass action to this situation, one finds:
For a nuclear:cytoplasmic RanGTP ratio of 100, the import and export cargoes could (on average) each become only 10-fold accumulated. This lesser expenditure of metabolic energy therefore has the clear drawback of limiting the endpoint of cargo accumulation.
Bi-directional transport by a single carrier might also be a feature of other transport receptors. However, due to compromised ‘accumulation power’, this mode of transport is probably an exception rather than the rule.
Materials and methods
Expression constructs
The coding regions of the respective proteins were first amplified from HeLa cell cDNA using specific primers pairs with appropriate restrictions sites. The various coding regions were cloned as follows: Imp13 as a BamHI–HindIII fragment into the BamHI–HindIII sites of pQE80 (N-His-tagged construct); hUBC9 as an NcoI–BamHI fragment into the NcoI–BamHI sites of pGEX60HRC (N-GST-tagged, C-His-tagged construct) and zz60 (N-zz-tagged, C-His-tagged); eIF1A as an NcoI– BamHI fragment into the NcoI–BglII sites of pGEX60HRC (N-GST-tagged, C-His-tagged construct) and the NcoI–BamHI sites of zzTev60 (N-zz-tagged, C-His-tagged); and RBM8 as a SphI–BamHI fragment into the SphI–BamHI sites of zzpQE70 (N-His-tagged construct). The MGN NcoI–BglII fragment was first subcloned into pQE60 and then transferred as an NcoI–HindIII fragment into pET30a (N- and C-terminal His tag). To obtain the zz-Tev Imp13 construct, the promoter region (including the His tag) of Imp13-pQE80 was removed as an EcoRI–BamHI fragment and replaced by a zz-Tev cassette with the same restriction sites. All constructs were verified by DNA sequencing.
Other constructs have been described previously (see Kutay et al., 1997, 1998; Jäkel and Görlich, 1998; Paraskeva et al., 1999).
Recombinant protein expression, purification and fluorescence labelling
Expression of nuclear transport factors other than Imp13 has been described previously (Görlich et al., 1996; Izaurralde et al., 1997; Kutay et al., 1997; Jäkel and Görlich, 1998; Paraskeva et al., 1999). N-His Imp13, GST-hUBC9-His, zz-hUBC9-His, GST-eIF1A-His, zz-eIF1A-His, His-tagged MGN and zz-RBM8-His were expressed in Escherichia coli and purified on nickel NTA–agarose, followed by chromatography on Superdex 200. The zzRBM8–MGN complex was obtained by mixing stoichiometric amounts of the subunits and purifying the resulting complex on Superdex 200.
Fluorescence labelling of the RBM8–MGN complex was with 5(6) carboxy fluorescein-N-hydroxy-succinimide ester (1:1 molar ratio). All other import or export substrates were labelled with Alexa 488 maleimide.
Import and export assays
HeLa cells were grown on 12 mm coverslips. Permeabilization was with 60 µg/ml digitonin for 1.5 min (Figures 3 and 4) or for 10 min (Figure 5). Import and export reactions were performed at room temperature essentially as described (Jäkel et al., 1998). Transport buffer was 20 mM HEPES–KOH pH 7.5, 110 mM potassium acetate, 5 mM magnesium acetate, 0.5 mM EGTA, 250 mM sucrose. The Ran mix contained 2 µM RanGDP, 0.2 µM NTF2, 0.2 µM RanBP1 and 0.2 µM RNA1p. The energy-regenerating system contained 5 mM creatine phosphate, 0.25 mM ATP and GTP, and 25 µg/ml creatine kinase. Fixation was with 2% paraformaldehyde.
Antibodies
Antibodies against Imp13 were raised against the 12 C-terminal residues of the protein. The other antibodies have been described previously (Jäkel and Görlich, 1998; Lipowsky et al., 2000).
Immobilization of proteins
Imp 13 (Figure 1), hUBC9 (Figure 2), RBM8 (Figure 4A), RanQ69L (Figure 6B) and eIF1A (Figure 6A and C) were immobilized to 1–2 µg/µl IgG–Sepharose matrix as zz-tagged proteins. The MGN protein was biotinylated and bound to streptavidin–agarose (Figure 4B).
Binding assays
A 20 µl aliquot of immobilized proteins was rotated for 3 h with the respective extracts. After four washes with binding buffer, bound proteins were eluted with 1.5 M MgCl2, precipitated by 95% isopropanol and analysed by SDS–PAGE followed by Coomassie staining or western blotting.
Binding from E.coli lysates was performed in 50 mM Tris–HCl pH 7.5, 50 mM NaCl, 3 mM MgCl2. Binding from HeLa cytosolic extract was performed in either 50 mM Tris–HCl pH 7.5, 200 mM NaCl, 3 mM MgCl2 (for binding to Imp13 or hUBC9) or 50 mM Tris–HCl pH 7.5, 80 mM potassium acetate, 3 mM MgCl2 (for binding to eIF1A).
Protein identification
Putative transport substrates eluted from immobilized Imp13 were separated by SDS–PAGE, Coomassie-stained bands were subjected to a tryptic digest and the resulting peptide mixture was analysed by mass spectrometry. Identified proteins are listed in Figure 1.
Homologies of Imp13
Imp13 belongs to the Mtr10/transportin SR subgroup of nuclear transport receptors. Putative Imp13 orthologues are present in Arabidopsis thaliana (C012187_17 protein, gbAAF78497), Drosophila melanogaster (CG7212 protein, gbAAF55502.1), Caenorhabditis elegans (T16G12.6 protein, emb|CAA82969.1) and Schizosaccharomyces pombe (C22G7.02 protein, emb|CAA91126.1). The most similar protein from S.cerevisiae is Pdr6p. Its identity with Imp13 is, however, too low to regard it as a clear orthologue.
DDBJ/EMBL/GenBank accession number
The nucleotide sequence of human Imp13 is listed under accession No. AF267987.
Acknowledgments
Acknowledgements
We wish to thank Petra Schwarzmaier and Petra Rübmann for excellent technical help, Stefan Jäkel for the pGEX60HRC expression vector, Markus Bohnsack for help with Figure 1, and Martin Pool as well as the members of our laboratory for critical reading of the manuscript and stimulating discussions. This work was supported by an EMBO long-term fellowship (to J.M.M.), the Fonds der Chemischen Industrie and grants from the DFG (SFB 352) and the HFSPO (RG0198/1998M).
References
- Arts G.J., Fornerod,M. and Mattaj,I.W. (1998) Identification of a nuclear export receptor for tRNA. Curr. Biol., 8, 305–314. [DOI] [PubMed] [Google Scholar]
- Battiste J.L., Pestova,T.V., Hellen,C.U. and Wagner,G. (2000) The eIF1A solution structure reveals a large RNA-binding surface important for scanning function. Mol. Cell, 5, 109–119. [DOI] [PubMed] [Google Scholar]
- Benne R., Brown-Luedi,M.L. and Hershey,J.W. (1978) Purification and characterization of protein synthesis initiation factors eIF-1, eIF-4C, eIF-4D and eIF-5 from rabbit reticulocytes. J. Biol. Chem., 253, 3070–3077. [PubMed] [Google Scholar]
- Bischoff F.R. and Görlich,D. (1997) RanBP1 is crucial for the release of RanGTP from importin β-related nuclear transport factors. FEBS Lett., 419, 249–254. [DOI] [PubMed] [Google Scholar]
- Bischoff F.R. and Ponstingl,H. (1991) Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature, 354, 80–82. [DOI] [PubMed] [Google Scholar]
- Boswell R.E., Prout,M.E. and Steichen,J.C. (1991) Mutations in a newly identified Drosophila melanogaster gene, mago nashi, disrupt germ cell formation and result in the formation of mirror-image symmetrical double abdomen embryos. Development, 113, 373–384. [DOI] [PubMed] [Google Scholar]
- Chi N.C., Adam,E.J. and Adam,S.A. (1995) Sequence and characterization of cytoplasmic nuclear protein import factor p97. J. Cell Biol., 130, 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi N.C., Adam,E.J.H., Visser,G.D. and Adam,S.A. (1996) RanBP1 stabilises the interaction of Ran with p97 in nuclear protein import. J. Cell Biol., 135, 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desterro J.M., Thomson,J. and Hay,R.T. (1997) Ubch9 conjugates SUMO but not ubiquitin. FEBS Lett., 417, 297–300. [DOI] [PubMed] [Google Scholar]
- Englmeier L., Olivo,J.C. and Mattaj,I.W. (1999) Receptor-mediated substrate translocation through the nuclear pore complex without nucleotide triphosphate hydrolysis. Curr. Biol., 9, 30–41. [DOI] [PubMed] [Google Scholar]
- Floer M., Blobel,G. and Rexach,M. (1997) Disassembly of RanGTP–karyopherin β complex, an intermediate in nuclear protein import. J. Biol. Chem., 272, 19538–19546. [DOI] [PubMed] [Google Scholar]
- Fornerod M., Ohno,M., Yoshida,M. and Mattaj,I.W. (1997a) Crm1 is an export receptor for leucine rich nuclear export signals. Cell, 90, 1051–1060. [DOI] [PubMed] [Google Scholar]
- Fornerod M., van Deursen,J., van Baal,S., Reynolds,A., Davis,D., Murti,K.G., Fransen,J. and Grosveld,G. (1997b) The human homologue of yeast CRM1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component Nup88. EMBO J., 16, 807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L., Kamitani,T., Fujise,K., Caskey,L.S. and Yeh,E.T. (1997) Preferential interaction of sentrin with a ubiquitin-conjugating enzyme, Ubc9. J. Biol. Chem., 272, 28198–28201. [DOI] [PubMed] [Google Scholar]
- Görlich D. and Kutay,U. (1999) Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell. Dev. Biol., 15, 607–660. [DOI] [PubMed] [Google Scholar]
- Görlich D., Kostka,S., Kraft,R., Dingwall,C., Laskey,R.A., Hartmann,E. and Prehn,S. (1995) Two different subunits of importin cooperate to recognize nuclear localization signals and bind them to the nuclear envelope. Curr. Biol., 5, 383–392. [DOI] [PubMed] [Google Scholar]
- Görlich D., Pante,N., Kutay,U., Aebi,U. and Bischoff,F.R. (1996) Identification of different roles for RanGDP and RanGTP in nuclear protein import. EMBO J., 15, 5584–5594. [PMC free article] [PubMed] [Google Scholar]
- Görlich D., Dabrowski,M., Bischoff,F.R., Kutay,U., Bork,P., Hartmann,E., Prehn,S. and Izaurralde,E. (1997) A novel class of RanGTP binding proteins. J. Cell Biol., 138, 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostissa M., Hengstermann,A., Fogal,V., Sandy,P., Schwarz,S.E., Scheffner,M. and Del Sal,G. (1999) Activation of p53 by conjugation to the ubiquitin-like protein SUMO-1. EMBO J., 18, 6462–6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoto N., Shimamoto,T., Takao,T., Tachibana,T., Kose,S., Matsubae,M., Sekimoto,T., Shimonishi,Y. and Yoneda,Y. (1995) In vivo evidence for involvement of a 58 kDa component of nuclear pore targeting complex in nuclear protein import. EMBO J., 14, 3617–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaurralde E., Kutay,U., von Kobbe,C., Mattaj,I.W. and Görlich,D. (1997) The asymmetric distribution of the constituents of the Ran system is essential for transport into and out of the nucleus. EMBO J., 16, 6535–6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäkel S. and Görlich,D. (1998) Importin β, transportin, RanBP5 and RanBP7 mediate nuclear import of ribosomal proteins in mammalian cells. EMBO J., 17, 4491–4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäkel S., Albig,W., Kutay,U., Bischoff,F.R., Schwamborn,K., Doenecke,D. and Görlich,D. (1999) The importin β/importin 7 heterodimer is a functional nuclear import receptor for histone H1. EMBO J., 18, 2411–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E.S. and Blobel,G. (1997) Ubc9p is the conjugating enzyme for the ubiquitin-like protein Smt3p. J. Biol. Chem., 272, 26799–26802. [DOI] [PubMed] [Google Scholar]
- Kaffman A., Rank,N.M., O’Neill,E.M., Huang,L.S. and O’Shea,E.K. (1998) The receptor Msn5 exports the phosphorylated transcription factor Pho4 out of the nucleus. Nature, 396, 482–486. [DOI] [PubMed] [Google Scholar]
- Kataoka N., Bachorik,J.L. and Dreyfuss,G. (1999) Transportin-SR, a nuclear import receptor for SR proteins. J. Cell Biol., 145, 1145–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka N., Yong,J., Kim,V.N., Velazquez,F., Perkinson,R.A., Wang,F. and Dreyfuss,G. (2000) Pre-mRNA splicing imprints mRNA in the nucleus with a novel RNA-binding protein that persists in the cytoplasm. Mol. Cell, 6, 673–682. [DOI] [PubMed] [Google Scholar]
- Kutay U., Bischoff,F.R., Kostka,S., Kraft,R. and Görlich,D. (1997) Export of importin α from the nucleus is mediated by a specific nuclear transport factor. Cell, 90, 1061–1071. [DOI] [PubMed] [Google Scholar]
- Kutay U., Lipowsky,G., Izaurralde,E., Bischoff,F.R., Schwarzmaier,P., Hartmann,E. and Görlich,D. (1998) Identification of a tRNA-specific nuclear export receptor. Mol. Cell, 1, 359–369. [DOI] [PubMed] [Google Scholar]
- Lai M.C., Lin,R.I., Huang,S.Y., Tsai,C.W. and Tarn,W.Y. (2000) A human importin-β family protein, transportin-SR2, interacts with the phosphorylated RS domain of SR proteins. J. Biol. Chem., 275, 7950–7957. [DOI] [PubMed] [Google Scholar]
- Lee G.W., Melchior,F., Matunis,M.J., Mahajan,R., Tian,Q. and Anderson,P. (1998) Modification of Ran GTPase-activating protein by the small ubiquitin-related modifier SUMO-1 requires Ubc9, an E2-type ubiquitin-conjugating enzyme homologue. J. Biol. Chem., 273, 6503–6507. [DOI] [PubMed] [Google Scholar]
- Le Hir H., Izaurralde,E., Maquat,L.E. and Moore,M.J. (2000) The spliceosome deposits multiple proteins 20–24 nucleotides upstream of mRNA exon–exon junctions. EMBO J., 19, 6860–6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipowsky G., Bischoff,F.R., Schwarzmaier,P., Kraft,R., Kostka,S., Hartmann,E., Kutay,U. and Görlich,D. (2000) Exportin 4: a mediator of a novel nuclear export pathway in higher eukaryotes. EMBO J., 19, 4362–4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounsbury K.M. and Macara,I.G. (1997) Ran-binding protein 1 (RanBP1) forms a ternary complex with Ran and karyopherin β and reduces Ran GTPase-activating protein (RanGAP) inhibition by karyopherin β. J. Biol. Chem., 272, 551–555. [DOI] [PubMed] [Google Scholar]
- Lund E. and Dahlberg,J.E. (1998) Proofreading and aminoacylation of tRNAs before export from the nucleus. Science, 282, 2082–2085. [DOI] [PubMed] [Google Scholar]
- Mahajan R., Delphin,C., Guan,T., Gerace,L. and Melchior,F. (1997) A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell, 88, 97–107. [DOI] [PubMed] [Google Scholar]
- Mao Y., Sun,M., Desai,S.D. and Liu,L.F. (2000) SUMO-1 conjugation to topoisomerase I: a possible repair response to topoisomerase-mediated DNA damage. Proc. Natl Acad. Sci. USA, 97, 4046–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I.W. and Englmeier,L. (1998) Nucleocytoplasmic transport: the soluble phase. Annu. Rev. Biochem., 67, 265–306. [DOI] [PubMed] [Google Scholar]
- Matunis M.J., Coutavas,E. and Blobel,G. (1996) A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J. Cell Biol., 135, 1457–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micklem D.R., Dasgupta,R., Elliott,H., Gergely,F., Davidson,C., Brand,A., Gonzalez-Reyes,A. and St Johnston,D. (1997) The mago nashi gene is required for the polarisation of the oocyte and the formation of perpendicular axes in Drosophila. Curr. Biol., 7, 468–478. [DOI] [PubMed] [Google Scholar]
- Nachury M.V. and Weis,K. (1999) The direction of transport through the nuclear pore can be inverted. Proc. Natl Acad. Sci. USA, 96, 9622–9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakielny S. and Dreyfuss,G. (1999) Transport of proteins and RNAs in and out of the nucleus. Cell, 99, 677–690. [DOI] [PubMed] [Google Scholar]
- Newmark P.A. and Boswell,R.E. (1994) The mago nashi locus encodes an essential product required for germ plasm assembly in Drosophila. Development, 120, 1303–1313. [DOI] [PubMed] [Google Scholar]
- Paraskeva E., Izaurralde,E., Bischoff,F.R., Huber,J., Kutay,U., Hartmann,E., Lührmann,R. and Görlich,D. (1999) CRM1-mediated recycling of snurportin 1 to the cytoplasm. J. Cell Biol., 145, 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova T.V., Borukhov,S.I. and Hellen,C.U. (1998) Eukaryotic ribosomes require initiation factors 1 and 1A to locate initiation codons. Nature, 394, 854–859. [DOI] [PubMed] [Google Scholar]
- Plafker S.M. and Macara,I.G. (2000) Importin-11, a nuclear import receptor for the ubiquitin-conjugating enzyme, UbcM2. EMBO J., 19, 5502–5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard V.W., Michael,W.M., Nakielny,S., Siomi,M.C., Wang,F. and Dreyfuss,G. (1996) A novel receptor-mediated nuclear protein import pathway. Cell, 86, 985–994. [DOI] [PubMed] [Google Scholar]
- Radu A., Blobel,G. and Moore,M.S. (1995) Identification of a protein complex that is required for nuclear protein import and mediates docking of import substrate to distinct nucleoporins. Proc. Natl Acad. Sci. USA, 92, 1769–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexach M. and Blobel,G. (1995) Protein import into nuclei: association and dissociation reactions involving transport substrate, transport factors and nucleoporins. Cell, 83, 683–692. [DOI] [PubMed] [Google Scholar]
- Ribbeck K., Lipowsky,G., Kent,H.M., Stewart,M. and Görlich,D. (1998) NTF2 mediates nuclear import of Ran. EMBO J., 17, 6587–6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbeck K., Kutay,U., Paraskeva,E. and Görlich,D. (1999) The translocation of transportin–cargo complexes through nuclear pores is independent of both Ran and energy. Curr. Biol., 9, 47–50. [DOI] [PubMed] [Google Scholar]
- Rodriguez M.S., Desterro,J.M., Lain,S., Midgley,C.A., Lane,D.P. and Hay,R.T. (1999) SUMO-1 modification activates the transcriptional response of p53. EMBO J., 18, 6455–6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M.S., Dargemont,C. and Hay,R.T. (2001) SUMO-1 conjugation in vivo requires both a consensus modification motif and nuclear targeting. J. Biol. Chem., 276, 12654–12659. [DOI] [PubMed] [Google Scholar]
- Schlenstedt G., Smirnova,E., Deane,R., Solsbacher,J., Kutay,U., Görlich,D., Ponstingl,H. and Bischoff,F.R. (1997) Yrb4p, a yeast ran-GTP-binding protein involved in import of ribosomal protein L25 into the nucleus. EMBO J., 16, 6237–6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwoebel E.D., Talcott,B., Cushman,I. and Moore,M.S. (1998) Ran-dependent signal-mediated nuclear import does not require GTP hydrolysis by Ran. J. Biol. Chem., 273, 35170–35175. [DOI] [PubMed] [Google Scholar]
- Seufert W., Futcher,B. and Jentsch,S. (1995) Role of a ubiquitin-conjugating enzyme in degradation of S- and M-phase cyclins. Nature, 373, 78–81. [DOI] [PubMed] [Google Scholar]
- Siomi M.C., Eder,P.S., Kataoka,N., Wan,L., Liu,Q. and Dreyfuss,G. (1997) Transportin-mediated nuclear import of heterogeneous nuclear RNP proteins. J. Cell Biol., 138, 1181–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., Brownawell,A. and Macara,I.G. (1998) Nuclear import of ran is mediated by the transport factor NTF2. Curr. Biol., 8, 1403–1406. [DOI] [PubMed] [Google Scholar]
- Sternsdorf T., Jensen,K. and Will,H. (1997) Evidence for covalent modification of the nuclear dot-associated proteins PML and Sp100 by PIC1/SUMO-1. J. Cell Biol., 139, 1621–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak R.W., Rout,M.P. and Aitchison,J.D. (1998) Karyopherins and kissing cousins. Trends Cell Biol., 8, 184–188. [DOI] [PubMed] [Google Scholar]
- Yoshida K. and Blobel,G. (2001) The karyopherin kap142p/msn5p mediates nuclear import and nuclear export of different cargo proteins. J. Cell Biol., 152, 729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.F., Colaizzo-Anas,T., Nowak,N.J., Shows,T.B., Elliott,R.W. and Aplan,P.D. (1998) The mammalian homologue of mago nashi encodes a serum-inducible protein. Genomics, 47, 319–322. [DOI] [PubMed] [Google Scholar]
- Zhao X.F., Nowak,N.J., Shows,T.B. and Aplan,P.D. (2000) MAGOH interacts with a novel RNA-binding protein. Genomics, 63, 145–148. [DOI] [PubMed] [Google Scholar]