Abstract
Cytokinesis in eukaryotic organisms is under the control of small GTP-binding proteins, although the underlying molecular mechanisms are not fully understood. Cortexillins are actin-binding pro teins whose activity is crucial for cytokinesis in Dictyostelium. Here we show that the IQGAP-related and Rac1-binding protein DGAP1 specifically interacts with the C-terminal, actin-bundling domain of cortexillin I. Like cortexillin I, DGAP1 is enriched in the cortex of interphase cells and translocates to the cleavage furrow during cytokinesis. The activated form of the small GTPase Rac1A recruits DGAP1 into a quaternary complex with cortexillin I and II. In DGAP1– mutants, a complex can still be formed with a second IQGAP-related protein, GAPA. The simultaneous elimination of DGAP1 and GAPA, however, prevents complex formation and localization of the cortexillins to the cleavage furrow. This leads to a severe defect in cytokinesis, which is similar to that found in cortexillin I/II double-null mutants. Our observations define a novel and functionally significant signaling pathway that is required for cytokinesis.
Keywords: cortexillin/cytokinesis/IQGAP/Rac1
Introduction
Cortexillins of Dictyostelium discoideum are actin-bundling proteins that organize actin filaments preferentially into anti-parallel bundles and associate them into three-dimensional meshworks (Faix et al., 1996). Cortexillins are enriched in the cortex of interphase cells, translocate to the equatorial region of dividing cells and are required for the correct positioning of the cleavage furrow (Weber et al., 1999, 2000). Mutants lacking both isoforms, cortexillin I (CI) and II (CII), are severely impaired in cytokinesis and thus form large, multinucleate cells. In mutants lacking only CI, the defect in cytokinesis is less severe, but still substantial, whereas the elimination of CII has only a moderate effect (Faix et al., 1996).
The N-terminal halves of each cortexillin subunit encompass a conserved actin-binding domain of the α-actinin/spectrin type (Faix et al., 1996). A central coiled-coil domain is responsible for the assembly of the cortexillins into parallel dimers (Steinmetz et al., 1998). The C-terminal region of CI is pivotal for the targeting and biological activity of the molecule. It harbors the strongest actin-bundling activity and a phosphatidylinositol (4,5)bisphosphate (PIP2)-binding site (Stock et al., 1999). Studies with green fluorescent protein (GFP) fusions have shown that the C-terminal fragment of CI is sufficient for cortical localization, recruitment to the cleavage furrow and for the complete rescue of cytokinesis in CI– mutants (Weber et al., 1999).
The small GTP-binding proteins Rho, Rac and Cdc42 play an important role in cytokinesis of different eukaryotic organisms (Glotzer, 1997; Prokopenko et al., 2000; Robinson and Spudich, 2000a). Both Rho and Cdc42 are required for cytokinesis in Xenopus and mammalian cells, whereas only Rho has been implicated in cytokinesis in Caenorhabditis elegans and Drosophila (Prokopenko et al., 1999, 2000). No Cdc42 or Rho homologs have been identified in D.discoideum. In this organism, the functions of Cdc42/Rho are assumed by a large number of Rac proteins (Bush et al., 1993; Larochelle et al., 1996; Faix et al., 1998; Rivero et al., 1999; Dumontier et al., 2000). Defects in cytokinesis have been reported for mutants deficient in RacE (Larochelle et al., 1996), for all three Rac1 members, Rac1A, Rac1B and Rac1C (Dumontier et al., 2000; Palmieri et al., 2000), and also for one Ras protein, RasG (Tuxworth et al., 1997). The Rac1 GTPases of D.discoideum are >90% identical to each other and are closely related to human Rac1 (Bush et al., 1993). Each activated Rac1 protein binds strongly to the IQGAP-related protein DGAP1 (Dumontier et al., 2000).
IQGAPs are a conserved family of Rho family GTPase-binding proteins implicated in cytokinesis (Brill et al., 1996; Hart et al., 1996; Kuroda et al., 1996; Machesky, 1998). In Schizosaccharomyces pombe, the IQGAP-related protein Rng2 is a component of the acto-myosin ring and the spindle pole body necessary for F-actin organization during cytokinesis (Eng et al., 1998). In Saccharomyces cerevisiae, the recruitment of actin filaments and myosin II to the contractile ring requires Cyk1p/Iqg1p (Epp and Chant, 1997; Lippincott and Li, 1998; Shannon and Li, 1999). Dictyostelium discoideum has two IQGAP-related proteins that are ∼50% identical and both DGAP1 and GAPA are involved in cytokinesis. GAPA– cells can initiate the formation of a cleavage furrow, but often fail to complete cytokinesis (Adachi et al., 1997). The elimination of DGAP1 alters cytoskeletal architecture, resulting in increased cell motility and abnormal development, and even a moderate overexpression of DGAP1 leads to a defect in cytokinesis (Faix and Dittrich, 1996; Faix et al., 1998). Despite their homology to GTPase-activating proteins (GAPs), IQGAP-related proteins do not posses GAP activity (Weissbach et al., 1994; Brill et al., 1996; Hart et al., 1996; Faix et al., 1998). The goal of this study was to establish the function and mechanism of IQGAP-related proteins in cytokinesis. We have identified and characterized a protein complex composed of activated Rac1A, the IQGAP-related proteins DGAP1 or GAPA, and CI and CII, which is required for cytokinesis.
Results
The C-terminal region of CI interacts with DGAP1 in vivo
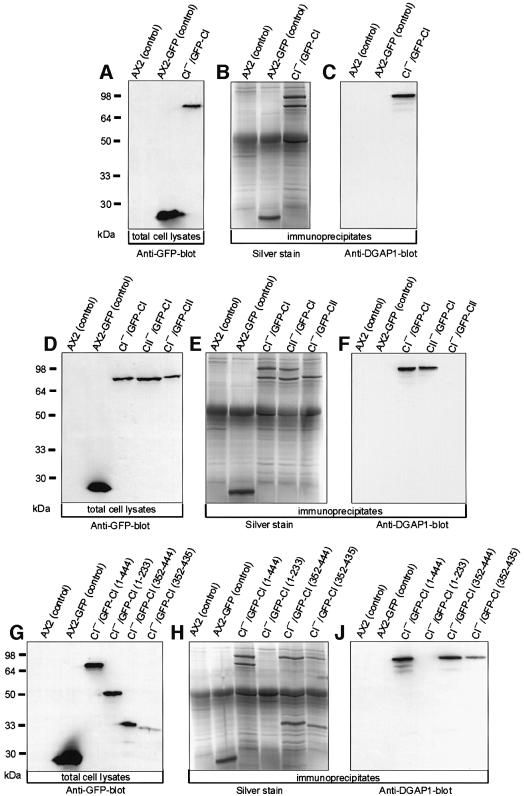
To identify cortexillin-binding proteins involved in cytokinesis, CI was immunoprecipitated with affinity-purified anti-GFP polyclonal antibodies from mutants in which CI was replaced by GFP–CI (CI–/GFP–CI). The precipitate was analyzed by SDS–PAGE and silver staining, and compared with control precipitates obtained from AX2 wild-type and AX2 cells expressing GFP alone (Figure 1A). In addition to the prominent 76 kDa band of GFP–CI, a second major protein with an apparent molecular mass of ∼95 kDa was immunoprecipitated from CI–/GFP–CI cells (Figure 1B). The interaction of CI and the 95 kDa protein was specific, as this protein was not detected in the AX2 and AX2–GFP control precipitates. The co-precipitated protein was identified, by matrix-assisted laser desorption–ionization mass spectrometry (Maldi-MS) and peptide microsequencing, as the IQGAP-related protein DGAP1 (Faix and Dittrich, 1996). This finding was confirmed by western blotting of the precipitates with DGAP1-specific mAb 216-394-1 (Figure 1C).
Fig. 1. DGAP1 interacts with the C-terminus of CI. (A–C) DGAP1 specifically co-immunoprecipitates with CI. (A) Western blot of total cellular proteins from the cell lines used for the immunoprecipitation labeled with anti-GFP mAb 264-449-2. (B) After immunoprecipitation with anti-GFP polyclonal antibodies, bound proteins were resolved by SDS–PAGE and stained with silver. The 95 kDa protein specifically co-immunoprecipitated with GFP–CI was identified as DGAP1. (C) Western blotting of the immunoprecipitates with anti-DGAP1 mAb 216-394-1. (D–F) DGAP1 interacts with the CI isoform. (D) Western blot of total cellular proteins from the cell lines used for the immunoprecipitation labeled with anti-GFP mAb 264-449-2. (E) Silver-stained gel of the immunoprecipitates obtained with anti-GFP polyclonal antibodies. (F) Immunoblot of the immunoprecipitates labeled with anti-DGAP1 mAb 216-394-1. DGAP1 was only co-immunoprecipitated from lysates of cell lines expressing either endogenous or GFP-tagged cortexillin I. (G–I) Mapping of the DGAP1-binding region on CI. (G) Western blot of AX2 and AX2-GFP control cells and of CI– cells expressing full-length (1–444) CI, the N-terminal actin-binding site (1–233), the complete C-terminal domain (352–444), and the C-terminal domain without the PIP2-binding site (352–435) fused to GFP. The blot was labeled with anti-GFP mAb 264-449-2. (H) Silver-stained gel of the proteins precipitated with anti-GFP polyclonal antibodies. (J) Western blot of the immunoprecipitates labeled with anti-DGAP1 mAb 216-394-1.
To determine whether DGAP1 binds to only one or both cortexillin isoforms, we used two additional cell lines for immunoprecipitation (Figure 1D). The first one is derived from a CII-null mutant and expresses wild-type and GFP-tagged CI (CII–/GFP–CI). The second cell line is derived from a CI– mutant and expresses wild-type and GFP-tagged CII (CI–/GFP–CII). DGAP1 was co-immunoprecipitated from the lysates of CI–/GFP–CI cells and of CII–/GFP–CI cells, but not from the lysates of CI–/GFP–CII cells that do not express CI (Figure 1E and F). This finding identifies specifically the CI isoform as the major DGAP1-interacting protein.
To define the DGAP1-binding region on the CI molecule, we immunoprecipitated GFP fusion proteins with anti-GFP antibodies from cell lines that express truncated versions of GFP-tagged CI in a CI– background (Figure 1G). DGAP1 co-immunoprecipitated with C-terminal constructs of CI that either encompass (residues 352–444) or lack (residues 352–435) the PIP2-binding site of CI (Figure 1H and I). These results show that the DGAP1-binding site is located in the C-terminal domain of CI, and that the PIP2-binding site is not required for the interaction.
DGAP1 accumulates in the cleavage furrow of dividing cells
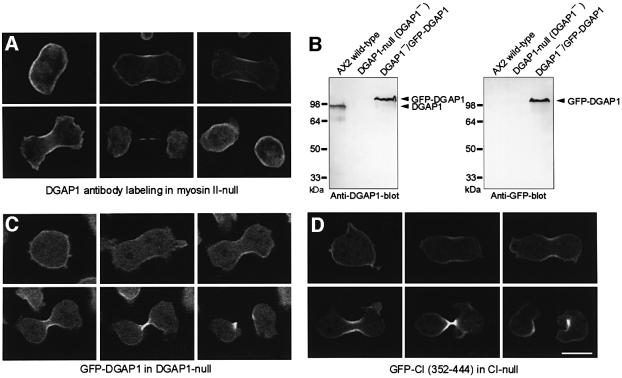
CI is enriched in the cell cortex of interphase cells and strongly accumulates in the cleavage furrow of dividing cells. The interaction of DGAP1 with CI prompted us to analyze the cellular distribution of DGAP1 during cytokinesis. As the accumulation of CI in the equatorial zone is more pronounced in myosin II-null cells (Weber et al., 1999), we first fixed and labeled these mutant cells with anti-DGAP1 antibodies (Figure 2A). The micrographs show that endogenous DGAP1 was uniformly enriched in the cortical region of interphase cells. Prior to ingression of the cleavage furrow in mitotic cells, DGAP1 accumulated in the equatorial region and remained there until cleavage was completed. To monitor the redistribution of DGAP1 in living cells, and to show that this redistribution is not restricted to myosin II-null cells, we expressed GFP-tagged DGAP1 in DGAP1– cells (Figure 2B). Confocal microscopy of mitotic DGAP1–/GFP–DGAP1 cells confirmed that GFP–DGAP1 is initially localized uniformly in the cell cortex and then translocates to the cleavage furrow, in accord with the antibody-labeling pattern observed in myosin II-null cells (Figure 2C). In conclusion, GFP–DGAP1 displayed a temporal and spatial dynamics during cytokinesis similar to full-length CI or its C-terminal domain fused to GFP (Figure 2C and D).
Fig. 2. DGAP1 translocates to the cleavage furrow. (A) Localization of endogenous DGAP1 in myosin II-null cells. Cells of this mutant were fixed and labeled with anti-DGAP1 antibody mAb 216-394-1. Confocal sections of a representative interphase cell and of five cells in successive stages of cytokinesis are shown. (B) Western blots of total cellular proteins of AX2 cells, of the DGAP1-null mutant and of DGAP1-null cells expressing GFP–DGAP1 labeled with anti-DGAP1 antibody mAb 216-394-1 (left), or with anti-GFP-antibody 264-449-2 (right). (C) Mitotic recruitment of GFP–DGAP1 in living DGAP1-null cells. Confocal image series show uniform cortical localization of GFP–DGAP1 before ingression of the cleavage furrow and rapid relocalization of the fusion protein to the furrow region in dividing cells. (D) For comparison, a confocal image series of a typical CI– cell expressing the GFP-tagged C-terminal domain of CI is shown. The C-terminal domain shows a cellular localization similar to that of GFP–DGAP1. Bar: 10 µm.
DGAP1 and CI form a complex with activated Rac1A in vitro
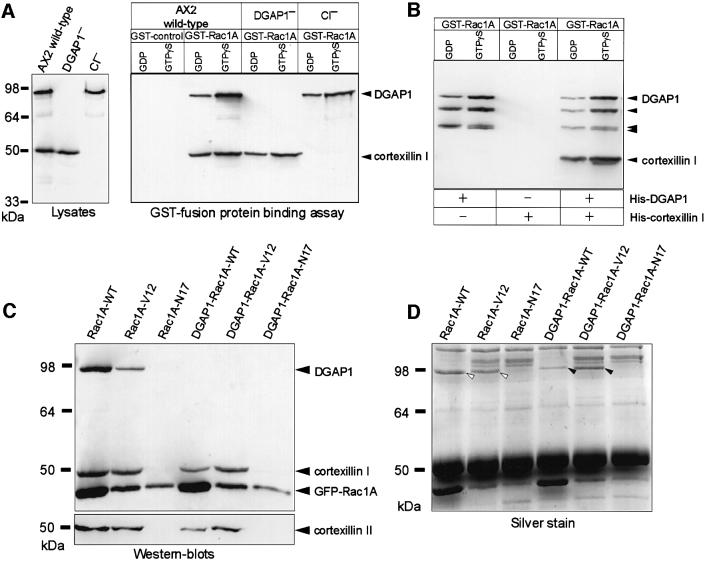
All three members of the Dictyostelium Rac1 group interact equally and preferentially in their activated GTP-bound form with DGAP1 (Dumontier et al., 2000), and one member, Rac1A, was shown previously to interact directly with recombinant DGAP1 (Faix et al., 1998). Binding assays were performed in order to determine whether DGAP1, CI and Rac1A form a molecular complex. Glutathione S-transferase (GST)-bound Rac1A was charged with either GDP or GTPγS [guanosine 5′-O-(3-thio)triphosphate], and incubated with Dictyostelium lysates prepared from AX2 cells, or from mutant cells lacking either DGAP1 or CI (Figure 3A, left). After repeated washing of the beads, the presence of bound proteins was analyzed in western blots using antibodies against DGAP1 or CI. Using lysates from AX2 cells, both DGAP1 and CI co-sedimented with GST–Rac1 (Figure 3A, right). No binding of either protein was detected in the GST control, indicating that the binding is specific for Rac1A. The binding of DGAP1 and CI to Rac1A was most prominent when GTPγS was bound to the GTPase, suggesting that the complex was preferentially formed with the activated, GTP-bound form of Rac1A.
Fig. 3. DGAP1 or GAPA link CI into a quarternary complex with activated Rac1A. (A) DGAP1 and CI form a complex preferentially with activated Rac1A in vitro. Western blot of lysates prepared from AX2 cells or from mutants lacking either DGAP1 or CI labeled with anti-DGAP1 mAb 216-394-1 and anti-CI mAb 241-438-1 (left). The glutathione–Sepharose bound GST-control or GST–Rac1A fusion proteins were loaded with either GDP or GTPγS and were incubated with the lysates indicated. Bound proteins were eluted with SDS sample buffer, and aliquots analyzed by western blotting using the same antibodies. (B) DGAP1 mediates complex formation with recombinant Rac1A and CI. GDP or GTPγS-bound Rac1A (250 nM each) was tested in the GST fusion protein-binding assay to determine whether it interacts with purified His-tagged CI and His-tagged DGAP1 (40 nM each). After extensive washing of the beads, DGAP1 and CI binding was determined by western blotting using anti-DGAP1 and anti-cortexillin antibodies. CI bound to GST–Rac1A only in the presence of DGAP1. The complex was preferentially formed with the activated form of the GTPase. The three bands that appear below full length DGAP1 are previously described proteolytic degradation products of DGAP1, which retained the ability to interact with Rac1A (Faix et al., 1998). (C) Western blot of immunoprecipitates obtained from AX2 and DGAP1-null-derived transformants that express wild-type (Rac1A-WT), constitutively activated (Rac1A-V12) or constitutively inactivated (Rac1A-N17) Rac1A, N-terminally fused to GFP. The GFP fusion proteins were immunoprecipitated with polyclonal anti-GFP antibodies, and the precipitates analyzed by triple labeling with anti-GFP, anti-DGAP1 and anti-CI monoclonal antibodies (top). A parallel blot was labeled for CII (bottom). DGAP1 and both cortexillins were exclusively co-immunoprecipitated with Rac1A-WT and Rac1A-V12, demonstrating that the complex is only formed with the activated form of the GTPase in vivo. A similar complex containing activated Rac1A, both cortexillins and another protein was formed in DGAP1-null-derived transformants. (D) To visualize the protein required for complex formation in DGAP1-null mutants, a parallel gel was developed with silver. White arrowheads indicate the position of DGAP1, and black arrowheads denote the position of a 100 kDa protein isolated from DGAP1-null cells, which displayed similar binding properties as DGAP1. This protein was identified by peptide fingerprinting as GAPA.
As expected from the direct interaction of DGAP1 and Rac1A in vitro, DGAP1 precipitated from the lysate of CI– cells. It was surprising, however, that CI co-sedimented with Rac1A even when the DGAP1– lysate was used, suggesting that either Rac1A and CI are capable of directly interacting with each other, or that in the absence of DGAP1, yet another protein is capable of linking Rac1A and CI into a complex. To distinguish between these possibilities, we purified CI and DGAP1 as His-tagged proteins from Escherichia coli and tested their ability to interact separately or in combination with GDP or GTPγS-bound Rac1A. CI alone was not able to bind to Rac1A in the GST fusion protein-binding assay, but co-sedimented with the GTPase in the presence of DGAP1 (Figure 3B). The strongest association of CI and DGAP1 was detected with the activated form of Rac1A. These results provide conclusive evidence that GTP-bound Rac1A, DGAP1 and CI form a complex in vitro. They further imply that, in the absence of DGAP1, another protein is required to link Rac1A to CI.
GAPA, a second IQGAP-like protein, links activated Rac1A to CI in the absence of DGAP1
To confirm that the complex is also formed in vivo, we used recently described transformants, which express N-terminally GFP-tagged wild-type (WT), constitutively activated (V12) or constitutively inactivated (N17) forms of Rac1A (Dumontier et al., 2000). The Rac1A fusion proteins from these cell lines were immunoprecipitated with anti-GFP polyclonal antibodies, and the precipitated proteins analyzed by western blotting for the presence of Rac1A, DGAP1, CI and CII. DGAP1 and both cortexillin isoforms were co-precipitated with Rac1A-WT and Rac1A-V12, but not with Rac1A-N17 (Figure 3C). As the wild-type GTPase is able to cycle between the GDP- and GTP-bound state, immunoprecipitated Rac1A-WT contained a mixture of activated and inactivated Rac1A. We conclude from these results that the complex containing DGAP1, CI and CII is formed exclusively with the activated, GTP-bound form of Rac1A in vivo.
To identify the protein that mediates complex formation in the absence of DGAP1, lysates of DGAP1–-derived transformants were used for immunoprecipitation. CI and CII again co-precipitated only with Rac1A-WT and Rac1A-V12, but not with Rac1A-N17 (Figure 3C). This finding is in line with the previous GST fusion protein-binding assays and suggests that the other protein had to have DGAP1-like properties in order to link Rac1A, CI and CII into a complex. In order to visualize this protein, a parallel gel loaded with immunoprecipitates was stained with silver and analyzed for proteins that solely precipitated with Rac1A-WT and Rac1A-V12. A single protein with an apparent molecular mass of ∼100 kDa was detected in this screen (Figure 3D). After tryptic cleavage, this protein was identified by peptide mass fingerprinting as GAPA, the second IQGAP-related protein of D.discoideum (Adachi et al., 1997). These results suggest that activated Rac1A and both cortexillins can be linked into a complex with either DGAP1 or GAPA.
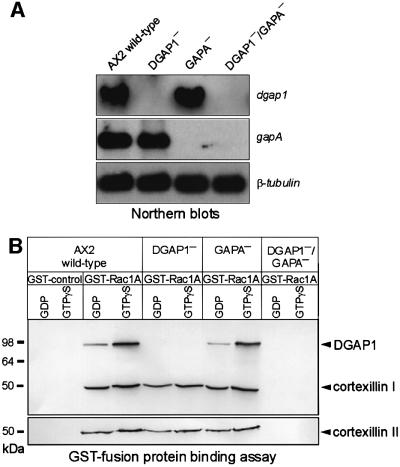
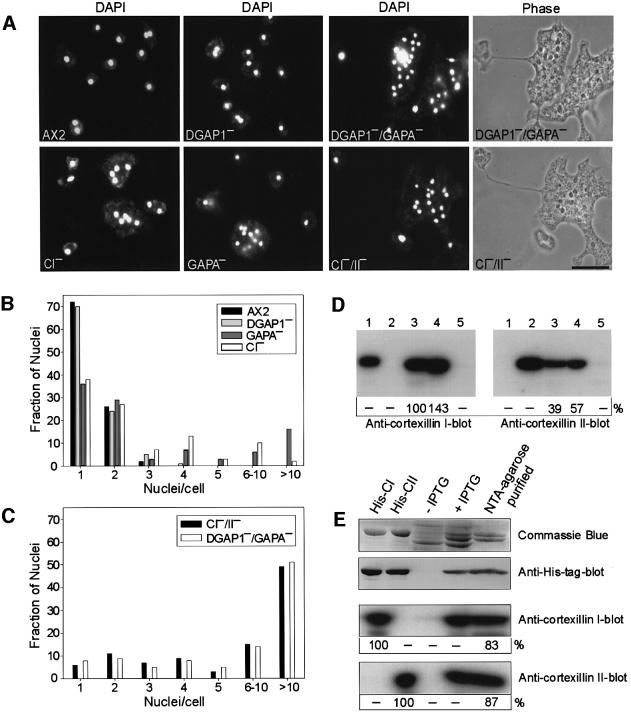
To test this hypothesis, we disrupted the gapA gene by gene replacement in DGAP1-null cells to obtain DGAP1/GAPA double-knockout mutants, and also in AX2 to obtain mutants lacking only GAPA (Figure 4A). Lysates of these mutants were tested for complex formation in the binding assay using GST–Rac1A. Both cortexillins were co-precipitated from AX2 and the single mutant cells lacking either DGAP1 or GAPA, but not from cells of the DGAP1–/GAPA– double mutant (Figure 4B). This finding demonstrates that at least one of the IQGAP homologs needs to be present for formation of the quaternary complex containing Rac1A, CI and CII.
Fig. 4. Simultaneous elimination of DGAP1 and GAPA prevents complex formation of CI and CII with Rac1A. (A) Northern blots of total RNA demonstrating the presence or absence of dgap1 or gapA transcripts in wild-type and mutants strains. (Top) Blot probed with a dgap1 DNA fragment. (Middle) Parallel northern blot probed with a gapA DNA fragment. (Bottom) Third blot probed with a β-tubulin DNA fragment as an internal control. (B) Complex formation requires either DGAP1 or GAPA. GDP and GTPγS-charged GST–Rac1A were immobilized on glutathione–Sepharose beads and tested for their ability to precipitate CI and CII from lysates of AX2 and the mutant cell lines indicated. Binding of the cortexillins and DGAP1 to the beads was monitored by western blotting as described in Figure 3A. CI and CII co-sedimented with Rac1A in the absence of either DGAP1 or GAPA, but not in the absence of both proteins.
DGAP1/GAPA double mutants show severe defects in cytokinesis
To test whether the complexes formed by DGAP1 and GAPA are essential for normal cytokinesis, we analyzed DGAP1 and GAPA mutants by phase-contrast and fluorescence microscopy and compared them with cortexillin mutants (Figure 5A–C). Consistent with previous studies, DGAP1– cells showed no cytokinesis defect (Dumontier et al., 2000), whereas GAPA– cells displayed a significant impairment of mitotic cell division (Adachi et al., 1997), as quantified by the ratio of mono- to multinucleate cells (Figure 5B). A striking feature of the DGAP1–/GAPA– double-mutant cells was that the cells were extremely large, flat, multinucleate and phenotypically very similar to the CI–/CII– double-null mutants (Figure 5A). This finding was further substantiated by counting DAPI-stained nuclei (Figure 5C), indicating that the simultaneous elimination of DGAP1 and GAPA mimics the loss of both cortexillins. As only the CI isoform was found to interact with DGAP1 or GAPA in the immunoprecipitation studies shown in Figure 1E and F, this result suggests that CI and II associate into heterodimers in vivo.
Fig. 5. Analysis of cytokinesis defects. (A) The indicated cell lines were cultivated in nutrient medium on glass coverslips for 3 days, fixed, and the nuclei were stained with DAPI. The phase-contrast images of the double mutants DGAP1–/GAPA– and CI–/II– are shown to illustrate similarity of their phenotypes. Thin extended cytoplasmatic bridges frequently connected portions of these large, multinucleate cells, as shown previously for CI–/CII– double mutants (Faix et al., 1996). Bar: 10 µm. (B) Histogram showing distributions of the number of nuclei per cell in AX2 and in single mutant cells. (C) Histogram showing distributions of the number of nuclei per cell in DGAP1–/GAPA– and CI–/II– cells. Between 400 and 600 nuclei were counted for each strain in (B) and (C). (D) CI and CII form heterodimers in vivo. CI was immunoprecipitated with CI-specific mAb 241-36-2 from AX2 cells, and the precipitate and controls analyzed in two parallel western blots. One was labeled for CI with iodinated anti-CI-specific mAb 241-438-1 (left), and the other was labeled for CII with iodinated anti-CII-specific mAb 232-238-10 (left). Numbers below the blots indicate radioactivity of the antibody label relative to immunoprecipitated CI. Lanes 1 and 2, recombinant CI and CII to demonstrate specificity of the antibodies; lane 3, immunoprecipitate with CI-specific mAb 241-36-2; lane 4, 10 µl of AX2 lysate corresponding to total cellular proteins of 2 × 105 wild-type cells; lane 5, mock immunoprecipitate obtained without mAb 241-36-2. The quantification of labeled bands showed that the same amount of CII was co-precipitated with CI, when the signals in the precipitates were standardized to the corresponding signals in the AX2 homogenate. (E) Recombinant cortexillins copurify as heterodimers. Recombinant His-tagged CII was co-expressed together with untagged CI in E.coli. After IPTG induction (lane 4) and purification of His-tagged CII (lane 5), comparable amounts of CI were eluted from the column, as indicated by Coomassie Blue staining and quantification of 125I-labeled bands. In anti-CI and CII blots, the amounts of purified His-CII and CI (lane 5) were quantified relative to recombinant His-CI and His-CII (lanes 1 and 2; 1 µg each), which were set to 100%. The anti-His-tag blot shows that in the heterodimer only CII carried the His-tag.
To test for heterodimer formation, CI was immunoprecipitated from AX2 cells with CI-specific mAb 241-36-2, and the precipitate analyzed by western blotting with antibodies specific for either CI or CII (Figure 5D). Quantification of labeled bands showed that, with CI, approximately the same amount of CII was co-precipitated (Figure 5D), indicating that the cortexillins form heterodimers in vivo. To exclude the possibility that in Dictyostelium cells the association of CI with CII is mediated by other proteins, we also co-expressed recombinant His-tagged CII together with untagged CI. After purification of His-tagged CII from bacterial lysates by Ni-NTA–agarose chromatography, equimolar amounts of CI were eluted from the column, confirming that the cortexillins form a heterodimer (Figure 5E). Taken together, our results suggest that the two IQGAP-related proteins can control the localization of the cortexillin heterodimer through interaction with CI.
DGAP1/GAPA control cortexillin localization to the cleavage furrow
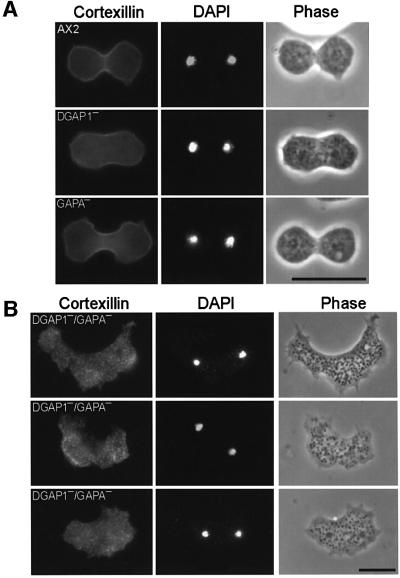
To examine whether the severe cytokinesis defect in DGAP1–/GAPA– cells is brought about by an improper localization of cortexillin to the cleavage furrow region, the cellular distribution of the cortexillin heterodimer was visualized by immunofluorescence labeling. As outlined in Figure 6A, cortexillin was enriched in the cleavage furrows of dividing AX2 cells and also in the single mutant cells lacking either DGAP1 or GAPA. In sharp contrast, cortexillin in the DGAP1–/GAPA– cells cortexillin was distributed in a punctuate pattern throughout the cells and was also not enriched in the cleavage furrow region (Figure 6B). These double-mutant cells displayed none of the changes in cell shape typical of mitotic cell division in wild-type cells or single mutants and were incapable forming normal cleavage furrows (Figure 6A). In spite of the severe irregularities in cleavage furrow formation, mitotic double-mutant cells were identified by choosing only those cells having small nuclei containing chromatin condensed into chromosomes. We conclude from this analysis that the accumulation of cortexillin in the region of the incipient cleavage furrow is abolished in DGAP1–/GAPA– cells, and that this deficiency in cortexillin recruitment is primarily responsible for the severe impairment of cytokinesis.
Fig. 6. Cortexillin localization and recruitment to the cleavage furrow in AX2 and DGAP1–/GAPA– mutant cells. (A) Recruitment of CI and CII to the cleavage furrow in dividing AX2 and in DGAP1– or GAPA– single-mutant cells. Cells from the indicated cell lines were fixed and labeled for cortexillin with mAb 241-71-3 that recognizes both cortexillin isoforms. Corresponding fluorescence images of condensed nuclei labeled with DAPI and phase-contrast images were recorded in parallel to capture cells undergoing cytokinesis. CI and CII accumulated in the cleavage furrow in AX2 and single-mutant cells. (B) Loss of cortical localization and lack of recruitment of cortexillin to the cleavage furrow in the DGAP1–/GAPA– double mutant. DGAP1–/GAPA– cells were fixed, and labeled as in (A). All analyzed mutant cells formed strongly irregular and asymmetric cleavage furrows and cortexillin did not accumulate in these regions (n = 21). Bars: 20 µm.
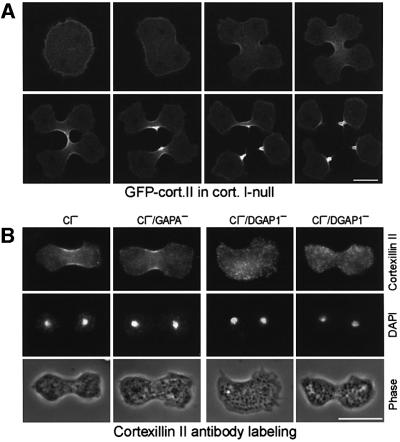
Although the cortexillins form heterodimers, our previous results suggest that only the CI isoforms couples to DGAP1 or GAPA. It was therefore puzzling that only the CI–/CII– cells display such a severe cytokinesis defects compared with the CI– cells (Figure 5). To clarify this point, we analyzed the distribution of GFP-tagged CII in CI–/GFP–CII cells during cytokinesis. Unexpectedly, in the CI– background, CII still localized to the cell cortex and accumulated in the cleavage furrow (Figure 7A). As neither CI nor CII localize to the cell cortex and cleavage furrow in DGAP1–/GAPA– cells (Figure 6B), this finding indicates that in the absence of CI, CII can interact with either DGAP1 or GAPA. To distinguish between these possibilities, we analyzed the distribution of CII during cytokinesis in two additional double mutants, CI–/DGAP1– and CI–/GAPA–. As in CI– cells, CII still localized to the cleavage furrow in CI–/GAPA–, but not in CI–/DGAP1– cells (Figure 7B). The CI–/DGAP1– cells were very flat and severely impaired in cytokinesis (a detailed analysis of the mutants will be described elsewhere). Although no evidence for an interaction of DGAP1 with CII was found by immunoprecipitation or GST fusion protein-binding assays (Figures 1D–F and 3), these localization studies show that in CI– cells CII is recruited to the cleavage furrow by DGAP1. Furthermore, these results explain the less severe cytokinesis defect of the CI– mutant.
Fig. 7. CII interacts with DGAP1 in the absence of CI. (A) GFP–CII translocates to the cleavage furrow in a multinucleate CI–/GFP–CII cell. Confocal image series shows uniform cortical localization of GFP–CII before ingression of the cleavage furrow and accumulation of the fusion protein in the midzone during cytokinesis. (B) Endogenous CII accumulates in the cleavage furrow. Cells of the indicated mutants were fixed and labeled for the CII isoform with mAb 241-71-3. In CI– and CI–/GAPA– cells regular cleavage furrows formed that were enriched in for CII. In contrast, in CI–/DGAP1– cells, CII was distributed in a punctuate pattern throughout the cells, with a complete lack of cortexillin recruitment into the cleavage furrow region similar to DGAP1–/GAPA– cells (see Figure 6B). Moreover, 16 out of 18 analyzed dividing cells showed strong defects in cleavage furrow formation (third column) and only two cells formed regular cleavage furrows (fourth column). Bars: 10 µm.
Discussion
The objective of this study was to establish the signaling pathway that controls the activity of cortexillins during mitotic cell division. Using an immunological approach in combination with Maldi-MS analysis, we show that CI interacts with the IQGAP-related protein DGAP1, a protein we identified previously as a target of activated Rac1A (Faix et al., 1998). Consistent with this result, CI and DGAP1 were originally isolated from the same actin–myosin complex (Faix et al., 1996; Faix and Dittrich, 1996). According to the immunoprecipitation studies, the interaction with DGAP1 with the cortexillin heterodimer (from here on referred to as cortexillin) is mainly restricted to the CI isoform (Figure 1). However, in CI– cells, proper localization of CII was strictly dependent on the presence of DGAP1 (Figure 7B), indicating that DGAP1 binds to CII with a different affinity than to CI. The affinity between CII and DGAP1 is presumably too low to allow for co-immunoprecipitation of both proteins under our conditions, but is clearly sufficient, to ensure proper localization of CII within CI–cells. The DGAP1-binding site was mapped to the C-terminal domain of CI between residues 352 and 435 (Figure 1), which previously was shown to be sufficient for targeting to the cleavage furrow and rescue of cytokinesis (Weber et al., 1999).
The interaction between DGAP1 and CI is consistent with their cellular localization: both proteins are evenly distributed in the cell cortex of interphase cells and become recruited to the cleavage furrow during mitotic cell division (Figure 2). As proposed for the yeast IQGAP-related protein Iqg1p by Osman and Cerione (1998), DGAP1 and GAPA appear to act as scaffolds that recruit and localize a protein complex required for signal relay from a Rho-type GTPase onto the actin cytoskeleton.
The fact that elimination of DGAP1 does not impair cytokinesis can be explained by the presence of GAPA, which is also able to form a complex with activated Rac1A and cortexillin. On the other hand, GAPA-null cells have a significant cytokinesis defect (Adachi et al., 1997), despite the fact that a complex of activated Rac1A, DGAP1, CI and CII is formed in these cells (Figure 4B), and cortexillin still accumulates in the cleavage furrow during cytokinesis (Figure 6A). Although DGAP1 and GAPA have similar molecular masses (Figure 4C), we also did not detect any peptide mass fragment of GAPA in the GFP–CI immunoprecipitates obtained from AX2 cells (Figure 1). It appears, therefore, that in wild-type cells, the complex between Rac1A and cortexillin is exclusively formed with DGAP1, and that GAPA can substitute DGAP1 only after its elimination by gene replacement. Thus, the primary function of GAPA in AX2 cells is most likely to mediate signal transduction through another small GTPase.
The elimination of both DGAP1 and GAPA by gene replacement interfered with cytokinesis to a similar extent as the simultaneous elimination of both cortexillin isoforms. Both double mutants formed extremely flat, giant cells containing multiple nuclei (Figure 5A and C). The presence of cortexillin in the cell cortex strongly contributes to its mechanical properties, as indicated by the findings that cells deficient for either CI or CII have a reduced cortical bending stiffness (Simson et al., 1998). There is evidence for a number of other proteins that act in concert with cortexillin in regulating cortical activities. Dynacortin was recently isolated by complementation of cytokinesis mutants as a suppressor of CI deficiency, and was proposed to play a role in equatorial contractility and the control of the cell shape (Robinson and Spudich, 2000b). Members of the myosin superfamily, myosin I and II, influence the cortical tension (Egelhoff et al., 1996; Dai et al., 1999). Loss of RacE causes a reduction in cortical tension and leads to a strong cytokinesis defect in cells grown in suspension (Gerald et al., 1998). Rho GTPases in other systems also modulate the mechanical strength of the cortex, and some of the effects of Rho are relayed through Rho-activated kinases (O’Connell et al., 1999; Prokopenko et al., 2000; Robinson and Spudich, 2000a). Rho-activated mouse citron kinase regulates cleavage furrow contractility (Madaule et al., 1998), and bovine Rho-activated kinase ROCK controls contractility by inactivation of myosin II light-chain phosphatase, which in turn inhibits myosin II filament assembly in the cleavage furrow (Kawano et al., 1999).
Other Rho signal transduction pathways that connect to the actin cytoskeleton include the formin homology proteins Drosophila diaphanous and its mouse homolog p140Dia (Castrillon and Wasserman, 1994; Watanabe et al., 1997). These Rho effectors bind to and regulate the G-actin-sequestering protein profilin, which is required for cytokinesis in different organisms (Haugwitz et al., 1994; Giansanti et al., 1998; Suetsugu et al., 1999). The C-terminal domain of N-WASP, an effector of activated Cdc42, binds to the Arp2/3 complex (Machesky and Gould, 1999) and dramatically stimulates its ability to nucleate actin polymerization (Symons et al., 1996; Rohatgi et al., 1999). Activated Rac1 mediates the translocation of the putative actin-cross-linking protein cortactin from the cytoplasm to the cell periphery in PDGF-stimulated Swiss 3T3 and 10T1/2 fibroblasts (Huang et al., 1997; Weed et al., 1998). However, no direct interaction between Rac1 and cortactin was detected in these studies. Taken together, these examples indicate that complex signal transduction pathways controlling actin-binding proteins by Rho-GTPases are involved in the regulation of actin cytoskeleton dynamics in general, and of cytokinesis in particular. As no cortexillin homologs have been described in animal cells as yet, it remains to be determined whether their role in cytokinesis is carried out by functional homologs of cortexillin in these cells, or is taken over by a different class of proteins.
To this end our observations define a novel signaling pathway in which activated Rac1A and its IQGAP-related effector protein form a quaternary complex with its downstream target cortexillin. Assembly of this complex is necessary for the recruitment of cortexillin to the midzone of a dividing cell and, consequently, for the formation of a cleavage furrow. This is, to our knowledge, the first example of a direct link between a small Rho-type GTPase and an actin-bundling protein during cytokinesis.
Materials and methods
Transformation vectors
For the expression of GFP–CII in CI-null cells, the entire coding region of the cII gene was amplified by PCR as a 1.5 kb SalI–SalI fragment, the fragment digested with SalI, and cloned in sense orientation into the SalI site of pDGFP-MCS (Weber et al., 1999). For the expression of GFP–CI in CII-null cells, the coding region of the cI gene was excised with SalI from plasmid pDGFP-MCS-CI (Weber et al., 1999), and the fragment cloned in sense orientation into the SalI site of pDGFP-MCS-Neo (Dumontier et al., 2000). For the expression of GFP–DGAP1 in DGAP1-null cells, the entire coding region of the dgap1 gene was amplified by PCR as a 2.6 kb EcoRI–EcoRI fragment, the fragment digested with EcoRI, and cloned in sense orientation into the EcoRI site of pDGFP-MCS-Neo. For construction of the gapA targeting vector, a 600 bp 5′ BglII–HindIII fragment and a 600 bp 3′ KpnI–KpnI fragment of the gapA gene were amplified from genomic AX2 wild-type DNA by PCR. The oligonucleotide primers used for the 5′ fragment were 5′-GCGAGATCTAAAATGGAAGGACTAGAAATTGAA-3′ and 5′-GCGCAAGCTTACTTACTTTAATATTTGATCTAT-3′, and the primers for the 3′ fragment were 5′-CGCGGTACCGAGATGATTTAATATCAATACTTC-3′ and 5′-CGCGGTACCGGATCCGTGTCATATTAACATCTAGAACAA-3′. Both fragments were gel-purified after cleavage with BglII–HindIII and KpnI, and the 5′ fragment cloned into the BglII–HindIII sites of pIC20R. The resulting vector pIC20R5’GAPA was digested with BamHI and KpnI, the ends dephosphorylated, and the vector ligated with the 2.0 kb BamHI–KpnI G418 cassette from plasmid pDCEV4 (Faix et al., 1990). The resulting vector was cleaved with KpnI, the ends dephosphorylated, and the vector ligated in sense orientation with the 600 bp 3′ fragment of the gapA gene. The 3.2 kb fragment containing the G418 resistance cassette flanked by gapA sequences was excised with EcoRI, the ends dephosphorylated, and the linearized fragment used to disrupt the gapA gene in the AX2 wild-type to obtain GAPA-null mutants, and in DGAP1-null mutant G10- (Faix and Dittrich, 1996) to obtain DGAP1/GAPA double mutants.
Cell culture, cell lines and transformation of D.discoideum cells
Cells of D.discoideum AX2 wild-type strain and of transformants were cultivated in axenic medium at 23°C on polystyrene culture dishes (Claviez et al., 1982). Cells were transformed by electroporation as described (Weber et al., 1999).
The following cell lines of D.discoideum were used in this study. Wild-type AX2-214, AX2-derived myosin II– strain HS2205 (Manstein et al., 1989), AX2-derived DGAP1– strain G10- (Faix and Dittrich, 1996), AX2-derived GAPA– strain (this work), GAPA–-derived CI–/GAPA– double-null strain (this work), DGAP1–-derived DGAP1–/GAPA– and CI–/DGAP1– double-null strains (this work), AX2-derived CI–, CII– and CI–/CII– strains (Faix et al., 1996), AX2-derived control cells expressing GFP (Dumontier et al., 2000), CI– cells expressing full length or fragments of CI fused to GFP (Stock et al., 1999; Weber et al., 1999), CI– cells expressing GFP–CII (this work), CII– cells expressing GFP–CI (this work), DGAP1– cells expressing GFP–DGAP1 (this work), AX2 cells expressing wild-type (Rac1A-WT), constitutively activated (Rac1A-V12) and constitutively inactivated (Rac1A-N17) Rac1A fused to GFP, and DGAP1– cells expressing wild-type (DGAP1-Rac1A-WT), constitutively activated (DGAP1-Rac1A-V12) and constitutively inactivated (DGAP1-Rac1A-N17) Rac1A fused to GFP (Dumontier et al., 2000).
Miscellaneous
The expression and purification of N-terminally His-tagged CI and DGAP1 have been described (Faix et al., 1996, 1998). For the expression of His-tagged CII, the entire cII cDNA was cloned into the BamHI and SalI sites of pQE32 (Qiagen) giving rise to pQE32-Cort2. For the co-expression of His-tagged CII together with untagged CI, the cI cDNA was cloned into the SalI site of pQE32-Cort2. The expression and purification of GST and GST–Rac1A, and the subsequent GST fusion protein-binding assays with Dictyostelium lysates or with purified proteins, were performed essentially as described (Faix et al., 1998; Dumontier et al., 2000). Northern blot analysis was performed as described (Faix et al., 1990).
Antibodies, immunoprecipitation and western blotting
Polyclonal antibodies raised against GFP were obtained by immunizing female white New Zealand rabbits with recombinant GFP together with complete Freund’s adjuvant (Sigma). Polyclonal antisera were affinity-purified using purified GFP covalently coupled to cyanogen bromide activated agarose (Sigma).
For immunoprecipitations, the cells were harvested by centrifugation at 1200 g for 3 min, washed twice and resuspended at a density of 1 × 108 cells/ml in cold 25 mM HEPES buffer pH 7.4. One milliliter of the cell suspension was sedimented for 2 min at 2000 g and the pellets incubated with lysis buffer containing 25 mM HEPES pH 7.4, 50 mM NaCl, 1 mM EGTA, 2 mM benzamidine, and bestatin, pepstatin, antipain and leupeptin (each 1 µg/ml) 2 mM dithiothreitol (DTT), 5% glycerol, 1% n-octylpolyoxyethylene (Bachem). The crude lysates were spun for 10 min at 15 000 g and the clear lysates were each supplemented with 100 µg of affinity-purified anti-GFP polyclonal antibodies together with 150 µl of protein A–Sepharose CL-4B (Sigma) slurry previously equilibrated in lysis buffer. After 2 h of incubation at 4°C, the beads were sedimented, washed seven times with 1 ml lysis buffer, and bound proteins eluted with SDS sample buffer. For immunoprecipitations of CI from AX2 cells, mAb 241-36-2 was used (Faix et al., 1996).
Immunoblotting was performed by standard procedures using DGAP1-specific mAb 216-394-1 (Faix and Dittrich, 1996), GFP-specific mAb 264-449-2 (Weber et al., 1999), CI-specific mAb 241-438-1, CII-specific mAb 232-238-10, and His-tag-specific mAb 232-470-5 (Faix et al., 1996). Primary antibodies were visualized with phosphatase-coupled anti-mouse IgG (Dianova). Iodination of antibodies was performed according to the chloramine-T method and bound antibodies were quantified by phosphoimaging as described (Weber et al., 1999).
Fluorescence microscopy
Microscopy was performed essentially as described (Weber et al., 1999). DGAP1 was labeled with mAb 216-394-1 and CI and CII were labeled with mAb 241-71-3 (Weber et al., 1999). Primary antibodies were visualized with tetramethylrhodamine isothiocyanate (TRITC)-conjugated anti-mouse IgG (Sigma). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). The specimens were either analyzed by confocal scanning microscopy using a LSM 410 (Zeiss), or by conventional fluorescence microscopy using an Axiophot 2 microscope (Zeiss).
Peptide mass fingerprinting and amino acid sequence analysis
The sample containing the 95 kDa protein that co-precipitated with GFP–CI (see Figure 1B) was run on a 8% SDS gel, stained with Coomassie Brilliant Blue, and the 95 kDa band excised. Cleavage in the gel was performed essentially according to the method described in Eckerskorn and Lottspeich (1989). After extensive washing in water, the gel pieces were lyophylized and re-soaked with buffer containing endoproteinase LysC (Boehringer, Mannheim). The resulting peptide fragments were eluted with formic acid/acetonitrile/water (5:50:45). Peptide mass fingerprint on a Reflex III MALDI mass spectrometer (Bruker, Bremen) identified the protein as DGAP1. This was further confirmed by peptide sequencing after separation of the peptide mixture by reversed phase HPLC on a Microcart 150-1 Purospher C18 5 µm column (Merck). Eluents in the HPLC were solvent A (0.1% trifluoroacetic acid in water) and solvent B (0.1% trifluoroacetic acid in acetonitrile). The gradient was developed from 0 to 60% of the solvent B in 60 min at a flow rate of 60 µl/min. Eluted peptides were detected at 206 nm, collected manually and sequenced in a amino acid sequencer (PE-Biosystems, Weiterstadt), according to the instructions of the manufacturer. The sequences obtained were XMANIATVGDFLK and KLEEYNLTTSADNYS, which both fit perfectly into the amino acid sequence of DGAP1 protein (DDBJ/EMBL/GenBank accession number L75794). The 100 kDa protein that co-immunoprecipitated from DGAP1-Rac1A-V12 cells, as shown in Figure 3D, was identified after tryptic cleavage in gel by peptide mass fingerprinting as GAPA (DDBJ/EMBL/GenBank accession number D88027).
Acknowledgments
Acknowledgements
We thank Günther Gerisch for discussions and reading of the manuscript. This work was supported by set-up funds provided by the University of Wisconsin to G.M., and by a grant to G.G. from the Deutsche Forschungsgemeinschaft (SFB 266/D7).
References
- Adachi H., Takahashi,Y., Hasebe,T., Shirouzu,M., Yokoyama,S. and Sutoh,K. (1997) Dictyostelium IQGAP-related protein specifically involved in the completion of cytokinesis. J. Cell Biol., 137, 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill S., Li,S., Lyman,C.W., Church,D.M., Wasmuth,J.J., Weissbach,L., Bernards,A. and Snijders,A.J. (1996) The Ras GTPase-activating-protein-related human protein IQGAP2 harbors a potential actin-binding domain and interacts with calmodulin and Rho family GTPases. Mol. Cell. Biol., 16, 4869–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush J., Franek,K. and Cardelli,J. (1993) Cloning and characterization of seven novel Dictyostelium discoideum rac-related genes belonging to the rho family of GTPases. Gene, 136, 61–68. [DOI] [PubMed] [Google Scholar]
- Castrillon D.H. and Wasserman,S.A. (1994) Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development, 120, 3367–3377. [DOI] [PubMed] [Google Scholar]
- Claviez M., Pagh,K., Maruta,H., Baltes,W., Fisher,P. and Gerisch,G. (1982) Electron microscopic mapping of monoclonal antibodies on the tail region of Dictyostelium myosin. EMBO J., 1, 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Ting-Beall,H.P., Hochmuth,R.M., Sheetz,M.P. and Titus,M.A. (1999) Myosin I contributes to the generation of resting cortical tension. Biophys. J., 77, 1168–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontier M., Höcht,P. Mintert,U. and Faix,J. (2000) Rac1 GTPases control filopodia formation, cell motility, endocytosis, cytokinesis and development in Dictyostelium. J. Cell Sci., 113, 2253–2265. [DOI] [PubMed] [Google Scholar]
- Eckerskorn C. and Lottspeich,F. (1989) Internal amino acid sequence analysis of proteins separated by gel electrophoresis after tryptic digestion in polyacrylamide matrix. Chromatographia, 28, 92–94. [Google Scholar]
- Egelhoff T.T., Naismith,T.V. and Brozovich,F.V. (1996) Myosin-based cortical tension in Dictyostelium resolved into heavy and light chain-regulated components. J. Muscle Res. Cell Motil., 17, 269–274. [DOI] [PubMed] [Google Scholar]
- Eng K., Naqvi,N.I., Wong,K.C. and Balasubramanian,M.K. (1998). Rng2p, a protein required for cytokinesis in fission yeast, is a component of the actomyosin ring and the spindle pole body. Curr. Biol., 8, 611–621. [DOI] [PubMed] [Google Scholar]
- Epp J.A. and Chant,J. (1997) An IQGAP-related protein controls actin-ring formation and cytokinesis in yeast. Curr. Biol., 7, 921–929. [DOI] [PubMed] [Google Scholar]
- Faix J. and Dittrich,W. (1996) DGAP1, a homologue of rasGTPase-activating proteins that controls growth, cytokinesis, and development in Dictyostelium discoideum. FEBS Lett., 394, 251–257. [DOI] [PubMed] [Google Scholar]
- Faix J., Gerisch,G. and Noegel,A.A. (1990) Constitutive over-expression of the contact site A glycoprotein enables growth-phase cells of Dictyostelium discoideum to aggregate. EMBO J., 9, 2709–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faix J. et al. (1996) Cortexillins, major determinants of cell shape and size, are actin-bundling proteins with a parallel coiled-coil tail. Cell, 86, 631–642. [DOI] [PubMed] [Google Scholar]
- Faix J., Clougherty,C., Konzok,A., Mintert,U., Murphy,J., Albrecht,R., Mühlbauer,B. and Kuhlmann,J. (1998) The IQGAP-related protein DGAP1 interacts with Rac and is involved in the modulation of the F-actin cytoskeleton and control of cell motility. J. Cell Sci., 111, 3059–3071. [DOI] [PubMed] [Google Scholar]
- Gerald N., Dai,J., Ting-Beall,H.P. and De Lozanne,A. (1998) A role for Dictyostelium racE in cortical tension and cleavage furrow progression. J. Cell Biol., 141, 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giansanti M.G., Bonaccorsi,S., Williams,B., Williams,E.V., Santolamazza,C., Goldberg,M.L. and Gatti,M. (1998) Cooperative interactions between the central spindle and the contractile ring during Drosophila cytokinesis. Genes Dev., 12, 396–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M. (1997) The mechanism and control of cytokinesis. Curr. Opin. Cell Biol., 9, 815–823. [DOI] [PubMed] [Google Scholar]
- Hart M.J., Callow,M.G., Souza,B. and Polakis,P. (1996) IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for Cdc42Hs. EMBO J., 15, 2997–3005. [PMC free article] [PubMed] [Google Scholar]
- Haugwitz M., Noegel,A.A., Karakesisoglou,J. and Schleicher,M. (1994) Dictyostelium amoebae that lack G-actin-sequestering profilins show defects in F-actin content, cytokinesis, and development. Cell, 79, 303–314. [DOI] [PubMed] [Google Scholar]
- Huang C., Ni,Y., Wang,T., Gao,Y., Haudenschild,C.C. and Zhan,X. (1997) Down-regulation of the filamentous actin cross-linking activity of cortactin by Src-mediated tyrosine phosphorylation. J. Biol. Chem., 272, 13911–13915. [DOI] [PubMed] [Google Scholar]
- Kawano Y., Fukata,Y., Oshiro,N., Amano,M., Nakamura,T., Ito,M., Matsumura,F., Inagaki,M. and Kaibuchi,K. (1999) Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J. Cell Biol., 147, 1023–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S., Fukata,M., Kobayashi,K., Nakafuku,M., Nomura,N., Iwamatsu,A. and Kaibuchi,K. (1996) Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J. Biol. Chem., 271, 23363–23367. [DOI] [PubMed] [Google Scholar]
- Larochelle D.A., Vithalani,K.K. and De Lozanne,A. (1996) A novel member of the rho Family of small GTP-binding proteins is specifically required for cytokinesis. J. Cell Biol., 133, 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott J. and Li,R. (1998) Sequential assembly of myosin II, an IQGAP-like protein, and filamentous actin to a ring structure involved in budding yeast cytokinesis. J. Cell Biol., 140, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky L.M. (1998) IQGAPs find a function. Curr. Biol., 8, 202–205. [DOI] [PubMed] [Google Scholar]
- Machesky L.M. and Gould,K.L. (1999) The Arp2/3 complex: a multifunctional actin organizer. Curr. Opin. Cell Biol., 11, 117–121. [DOI] [PubMed] [Google Scholar]
- Madaule P., Eda,M., Watanabe,N., Fujisawa,K., Matsuoka,T., Bito,H., Ishizaki,T. and Narumiya,S. (1998) Role of citron kinase as a target of the small GTPase Rho in cytokinesis. Nature, 394, 491–494. [DOI] [PubMed] [Google Scholar]
- Manstein D.J., Titus,M.A., De Lozanne,A. and Spudich,J.A. (1989) Gene replacement in Dictyostelium: generation of myosin-null mutants. EMBO J., 8, 923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell C.B., Wheatley,S.P., Ahmed,S. and Wang,Y.L. (1999) The small GTP-binding protein rho regulates cortical activities in cultured cells during division. J. Cell Biol., 144, 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman M.A. and Cerione,R.A. (1998) Iqg1p, a yeast homologue of the mammalian IQGAPs, mediates cdc42p effects on the actin cytoskeleton. J. Cell Biol., 142, 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri S.J. et al. (2000) Mutant Rac1B expression in Dictyostelium: effects on morphology, growth, endocytosis, development, and the actin cytoskeleton. Cell Mot. Cytoskel., 46, 285–304. [DOI] [PubMed] [Google Scholar]
- Prokopenko S.N., Brumby,A., O’Keefe,L., Prior,L., He,Y., Saint,R. and Bellen,H.J. (1999) A putative exchange factor for Rho1 GTPase is required for initiation of cytokinesis in Drosophila. Genes Dev., 13, 2301–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopenko S.N., Saint,R. and Bellen,H.J. (2000) Untying the Gordian knot of cytokinesis: role of small G proteins and their regulators. J. Cell Biol., 148, 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero F., Albrecht,R., Dislich,H., Bracco,E., Graciotti,L., Bozzaro,S. and Noegel,A.A. (1999) RacF1, a novel member of the rho protein family in Dictyostelium discoideum, associates transiently with cell contact areas, macropinosomes, and phagosomes. Mol. Biol. Cell, 10, 1205–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D.N. and Spudich,J.A. (2000a) Towards a molecular understanding of cytokinesis. Trends Cell Biol., 10, 228–237. [DOI] [PubMed] [Google Scholar]
- Robinson D.N. and Spudich,J.A. (2000b) Dynacortin, a genetic link between equatorial contractility and global shape control discovered by library complementation of a Dictyostelium discoideum cytokinesis mutant. J. Cell Biol., 150, 823–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R., Ma,L., Miki,H., Lopez,M., Kirchhausen,T., Takenawa,T. and Kirschner,M.W. (1999) The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell, 97, 221–231. [DOI] [PubMed] [Google Scholar]
- Shannon K.B. and Li,R. (1999) The multiple roles of Cyk1p in the assembly and function of the actomyosin ring in budding yeast. Mol. Biol. Cell., 10, 283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simson R., Wallraff,E., Faix,J., Niewöhner,J., Gerisch,G. and Sackmann,E. (1998) Membrane bending modulus and adhesion energy of wild-type and mutant cells of Dictyostelium lacking talin or cortexillins. Biophys. J., 74, 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M.O., Stock,A., Schulthess,T., Landwehr,R., Lustig,A., Faix,J., Gerisch,G., Aebi,U. and Kammerer,R.A. (1998) A distinct 14-residue site triggers coiled-coil formation in cortexillin I. EMBO J., 17, 1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock A., Steinmetz,M.O., Janmey,P., Aebi,U., Gerisch,G., Kammerer, R.A., Weber,I. and Faix,J. (1999) Domain analysis of cortexillin I: actin-bundling, PIP2-binding and the rescue of cytokinesis. EMBO J., 18, 5274–5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu S., Miki,H. and Takenawa,T. (1999) Distinct roles of profilin in cell morphological changes: microspikes, membrane ruffles, stress fibers, and cytokinesis. FEBS Lett., 457, 470–474. [DOI] [PubMed] [Google Scholar]
- Symons M., Derry,J.M., Karlak,B., Jiang,S., Lemahieu,V., Mccormick,F., Francke,U. and Abo,A. (1996) Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell, 84, 723–734. [DOI] [PubMed] [Google Scholar]
- Tuxworth R.I., Cheetham,J.L., Machesky,L.M., Spiegelmann,G.B., Weeks,G. and Insall,R.H. (1997) Dictyostelium RasG is required for normal motility and cytokinesis, but not growth. J. Cell Biol., 138, 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N. et al. (1997) p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J., 16, 3044–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber I., Gerisch,G., Heizer,C., Murphy,J., Badelt,K., Stock,A., Schwartz,J.M. and Faix,J. (1999) Cytokinesis mediated through the recruitment of cortexillins into the cleavage furrow. EMBO J., 18, 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber I., Neujahr,R., Du,A., Köhler,J., Faix,J. and Gerisch,G. (2000) Two-step positioning of a cleavage furrow by cortexillin and myosin II. Curr. Biol., 10, 501–506. [DOI] [PubMed] [Google Scholar]
- Weed S.A., Du,Y. and Parsons,J.T. (1998) Translocation of cortactin to the cell periphery is mediated by the small GTPase Rac1. J. Cell Sci., 111, 2433–2443. [DOI] [PubMed] [Google Scholar]
- Weissbach L., Settleman,J., Kalady,M.F., Snijders,A.J., Murthy,A.E., Yan,Y.-X. and Bernards,A. (1994) Identification of a human rasGAP-related protein containing calmodulin-binding motifs. J. Biol. Chem., 269, 20517–20521. [PubMed] [Google Scholar]