Abstract
A pneumopathic strain of bovine viral diarrhea virus was grown in cell culture and purified. Genomic ribonucleic acid was extracted, polyadenylated at the 3' end, and copied into complementary DNA after oligo-dT priming. Complementary DNA was male double stranded and cloned into the pUC9 plasmid. Approximately 200 complementary DNA clones varying in length from 0.5 to 2.5 kilobases were obtained. Hybridization assays indicated that the sequences isolated were specific for bovine viral diarrhea virus and that at least 5.5 kilobases of bovine viral diarrhea virus genome was represented in the library of complementary DNA clones, the majority of which may have originated from the 3' end of the virus genome. One cloned complementary DNA sequence was used as a 32P-labelled hybridization probe for bovine viral diarrhea virus detection. The probe hybridized with all cytopathic and noncytopathic strains of bovine viral diarrhea virus tested and was 100 times more sensitive than infectivity assays for the detection of bovine viral diarrhea virus. Hybridization did not occur with nucleic acids from bovine coronavirus, bluetongue virus, bovine adenovirus or uninfected cell cultures. Native plasmid DNA sequences, labelled with 32P, did not hybridize with bovine viral diarrhea virus ribonucleic acid.
Full text
PDF


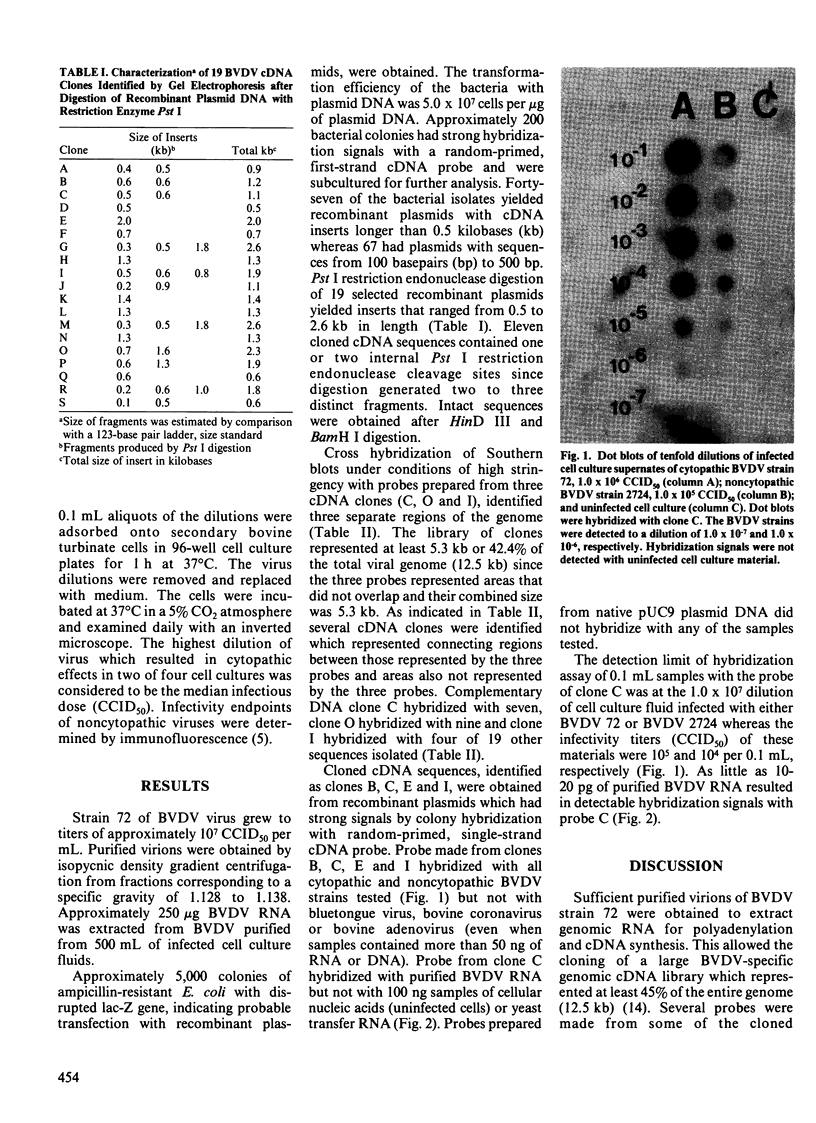

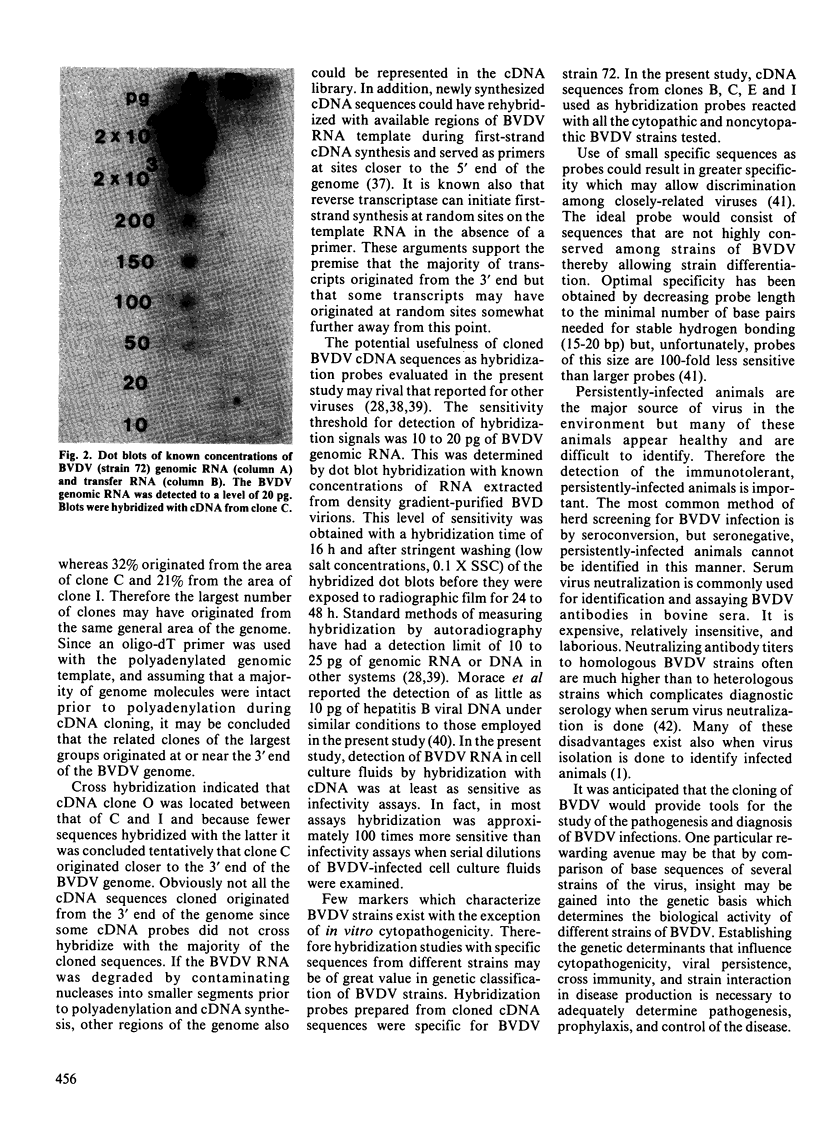

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. C. Bovine viral diarrhea virus: a review. J Am Vet Med Assoc. 1987 Jun 1;190(11):1449–1458. [PubMed] [Google Scholar]
- Bielefeldt Ohmann H., Babiuk L. A. Viral infections in domestic animals as models for studies of viral immunology and pathogenesis. J Gen Virol. 1986 Jan;67(Pt 1):1–25. doi: 10.1099/0022-1317-67-1-1. [DOI] [PubMed] [Google Scholar]
- Binns M. M., Boursnell M. E., Foulds I. J., Brown T. D. The use of a random priming procedure to generate cDNA libraries of infectious bronchitis virus, a large RNA virus. J Virol Methods. 1985 Jul;11(3):265–269. doi: 10.1016/0166-0934(85)90116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin S. R., McClurkin A. W., Cutlip R. C., Coria M. F. Response of cattle persistently infected with noncytopathic bovine viral diarrhea virus to vaccination for bovine viral diarrhea and to subsequent challenge exposure with cytopathic bovine viral diarrhea virus. Am J Vet Res. 1985 Dec;46(12):2467–2470. [PubMed] [Google Scholar]
- Bolin S. R., McClurkin A. W., Cutlip R. C., Coria M. F. Severe clinical disease induced in cattle persistently infected with noncytopathic bovine viral diarrhea virus by superinfection with cytopathic bovine viral diarrhea virus. Am J Vet Res. 1985 Mar;46(3):573–576. [PubMed] [Google Scholar]
- Castrucci G., Avellini G., Cilli V., Pedini B., McKercher D. G., Valente C. A study of immunologic relationships among serologically heterologous strains of bovine viral diarrhea virus by cross immunity tests. Cornell Vet. 1975 Jan;65(1):65–72. [PubMed] [Google Scholar]
- Chu H. J., Zee Y. C. Morphology of bovine viral diarrhea virus. Am J Vet Res. 1984 May;45(5):845–850. [PubMed] [Google Scholar]
- Coria M. F., Schmerr M. J., McClurkin A. W., Bolin S. R. Differentiation of cytopathic and noncytopathic isolates of bovine viral diarrhea virus by virus neutralization. Am J Vet Res. 1984 Oct;45(10):2129–2131. [PubMed] [Google Scholar]
- Coria M. F., Schmerr M. J., McClurkin A. W. Characterization of the major structural proteins of purified bovine viral diarrhea virus. Arch Virol. 1983;76(4):335–339. doi: 10.1007/BF01311200. [DOI] [PubMed] [Google Scholar]
- Duffell S. J., Harkness J. W. Bovine virus diarrhoea-mucosal disease infection in cattle. Vet Rec. 1985 Sep 7;117(10):240–245. doi: 10.1136/vr.117.10.240. [DOI] [PubMed] [Google Scholar]
- Duffell S. J., Sharp M. W., Winkler C. E., Terlecki S., Richardson C., Done J. T., Roeder P. L., Hebert C. N. Bovine virus diarrhoea-mucosal disease virus-induced fetopathy in cattle: Efficacy of prophylactic maternal pre-exposure. Vet Rec. 1984 Jun 9;114(23):558–561. doi: 10.1136/vr.114.23.558. [DOI] [PubMed] [Google Scholar]
- Friedman E. Y., Rosbash M. The syntheiss of high yields of full-length reverse transcripts of globin mRNA. Nucleic Acids Res. 1977 Oct;4(10):3455–3471. doi: 10.1093/nar/4.10.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Harkness J. W. The control of bovine viral diarrhoea virus infection. Ann Rech Vet. 1987;18(2):167–174. [PubMed] [Google Scholar]
- Horzinek M. C., Van Berlo M. F. The pestiviruses: where do they belong? Ann Rech Vet. 1987;18(2):115–119. [PubMed] [Google Scholar]
- Howard C. J., Clarke M. C., Brownlie J. An enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies to bovine viral diarrhoea virus (BVDV) in cattle sera. Vet Microbiol. 1985 Jun;10(4):359–369. doi: 10.1016/0378-1135(85)90006-9. [DOI] [PubMed] [Google Scholar]
- Hyypiä T., Stålhandske P., Vainionpä R., Pettersson U. Detection of enteroviruses by spot hybridization. J Clin Microbiol. 1984 Mar;19(3):436–438. doi: 10.1128/jcm.19.3.436-438.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapps W., Hogue B. G., Brian D. A. Sequence analysis of the bovine coronavirus nucleocapsid and matrix protein genes. Virology. 1987 Mar;157(1):47–57. doi: 10.1016/0042-6822(87)90312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laude H. Nonarbo-togaviridae: comparative hydrodynamic properties of the pestivirus genus. Brief report. Arch Virol. 1979;62(4):347–352. doi: 10.1007/BF01318109. [DOI] [PubMed] [Google Scholar]
- Lin M., Imai M., Bellamy A. R., Ikegami N., Furuichi Y., Summers D., Nuss D. L., Deibel R. Diagnosis of rotavirus infection with cloned cDNA copies of viral genome segments. J Virol. 1985 Aug;55(2):509–512. doi: 10.1128/jvi.55.2.509-512.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure M. A., Perrault J. RNA virus genomes hybridize to cellular rRNAs and to each other. J Virol. 1986 Mar;57(3):917–921. doi: 10.1128/jvi.57.3.917-921.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebus C. A., Stair E. L., Rhodes M. B., Twiehaus M. J. Neonatal calf diarrhea: propagation, attenuation, and characteristics of a coronavirus-like agent. Am J Vet Res. 1973 Feb;34(2):145–150. [PubMed] [Google Scholar]
- Morace G., von der Helm K., Jilg W., Deinhardt F. Detection of hepatitis B virus DNA in serum by a rapid filtration-hybridization assay. J Virol Methods. 1985 Dec;12(3-4):235–242. doi: 10.1016/0166-0934(85)90134-x. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez J., Kristiansen T., Kårsnas P. Bovine viral diarrhea virus: affinity chromatography on Crotalaria juncea lectin. J Virol Methods. 1981 Apr;2(5):293–300. doi: 10.1016/0166-0934(81)90028-8. [DOI] [PubMed] [Google Scholar]
- Nagele M. J. Outbreak of mucosal disease among apparently immunotolerant heifers. Vet Rec. 1984 Nov 10;115(19):496–499. doi: 10.1136/vr.115.19.496. [DOI] [PubMed] [Google Scholar]
- Parks J. B., Pritchett R. F., Zee Y. C. Buoyant density of bovine viral diarrhea virus. Proc Soc Exp Biol Med. 1972 Jun;140(2):595–598. doi: 10.3181/00379727-140-36511. [DOI] [PubMed] [Google Scholar]
- Peterson E. M., Aarnaes S. L., Bryan R. N., Ruth J. L., de la Maza L. M. Typing of herpes simplex virus with synthetic DNA probes. J Infect Dis. 1986 Apr;153(4):757–762. doi: 10.1093/infdis/153.4.757. [DOI] [PubMed] [Google Scholar]
- Potgieter L. N., McCracken M. D., Hopkins F. M., Guy J. S. Comparison of the pneumopathogenicity of two strains of bovine viral diarrhea virus. Am J Vet Res. 1985 Jan;46(1):151–153. [PubMed] [Google Scholar]
- Potgieter L. N., McCracken M. D., Hopkins F. M., Walker R. D. Effect of bovine viral diarrhea virus infection on the distribution of infectious bovine rhinotracheitis virus in calves. Am J Vet Res. 1984 Apr;45(4):687–690. [PubMed] [Google Scholar]
- Potgieter L. N., McCracken M. D., Hopkins F. M., Walker R. D., Guy J. S. Experimental production of bovine respiratory tract disease with bovine viral diarrhea virus. Am J Vet Res. 1984 Aug;45(8):1582–1585. [PubMed] [Google Scholar]
- Pritchett R., Manning J. S., Zee Y. C. Characterization of bovine viral diarrhea virus RNA. J Virol. 1975 Jun;15(6):1342–1347. doi: 10.1128/jvi.15.6.1342-1347.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Larson R., Collett M. S. Characterization of virus-specific RNA synthesized in bovine cells infected with bovine viral diarrhea virus. J Virol. 1983 Oct;48(1):320–324. doi: 10.1128/jvi.48.1.320-324.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Larson R., Torborg L. L., Collett M. S. Cell-free translation of bovine viral diarrhea virus RNA. J Virol. 1984 Dec;52(3):973–975. doi: 10.1128/jvi.52.3.973-975.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard A., Guiot C., Schmetz D., Dagenais L., Pastoret P. P., Dina D., Martial J. A. Molecular cloning of bovine viral diarrhea viral sequences. DNA. 1985 Dec;4(6):429–438. doi: 10.1089/dna.1985.4.429. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Wu R. Terminal transferase-catalyzed addition of nucleotides to the 3' termini of DNA. Methods Enzymol. 1980;65(1):43–62. doi: 10.1016/s0076-6879(80)65009-5. [DOI] [PubMed] [Google Scholar]




