Highlights
-
•
SARS-CoV-2 serology test results influenced COVID-19 vaccination behavior.
-
•
A negative test result was associated with increased receipt of COVID-19 vaccination.
-
•
Perceived susceptibility to COVID-19 infection influences vaccination behavior.
-
•
SARS-CoV-2 serology test results appear to cause risk compensation.
-
•
Future research could explore using perceived susceptibility to boost vaccine uptake.
Keywords: SARS-CoV-2 serology, vaccination, COVID-19 vaccine, vaccine hesitancy, vaccine acceptance, vaccine behavior
Abstract
Introduction
Although the utility of SARS-CoV-2 serology testing is limited, individuals may have used serology test results to inform COVID-19 vaccination decisions during the pandemic. Serology test results may have changed individuals’ perceived susceptibility to SARS-CoV2 infection, leading to risk compensation behavior. Understanding how serology results as a measure of perceived susceptibility influence COVID-19 vaccination decisions could inform future interventions to increase adult vaccine acceptance. This study examined the association between SARS-CoV-2 serology (antibody) test results and COVID-19 vaccination uptake.
Methods
This retrospective cohort study included adults who received ≥1 serology test between January 1, 2021, and February 28, 2023. The date of the first serology test represented Day 0, and vaccination uptake was followed for 30 days. Individuals could have multiple serology tests, and person-time follow-up was enumerated across tests. The main exposure was the SARS-CoV-2 serology test result. Receipt of COVID-19 vaccination (BNT162b2 or mRNA-1273) was analyzed using Cox proportional hazards regression, controlling for age, sex, race/ethnicity, tobacco use, and comorbidities.
Results
Within a cohort of 28,610 adults, there were 28,820 serology test results. Approximately 38% (n=10,802) of the cohort had at least 1 positive serology result. Among negative tests, the COVID-19 vaccination rate was 1,489 per 1,000 person-years of follow-up; among positive tests, the rate was 829 vaccinations per 1,000 person-years. A negative serology test result was positively associated with COVID-19 vaccination (adjusted hazard ratio=1.58; 95% CI=1.45, 1.72).
Conclusions
SARS-CoV-2 serology results appear to influence vaccination behavior. Future interventions could target perceived illness susceptibility to increase vaccine acceptance.
INTRODUCTION
In the spring of 2020, the U.S. Food and Drug Administration (FDA) granted emergency use authorization for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) serology (antibody) tests to aid the national response to the pandemic.1, 2, 3 Serology testing, which measures antibody levels indicative of past infection or vaccination, is a valuable public health tool for epidemiologic surveillance.4, 5, 6 Although SARS-CoV-2 antibody tests were indicated for certain clinical scenarios, much of the demand for serologic testing was driven by individual consumers who were seeking evidence of immunity, either from prior infection or vaccination.7, 8, 9 However, throughout the pandemic, the FDA discouraged the use of antibody tests to gauge immune status owing to the absence of validated antibody threshold levels indicating protective immunity.9, 10, 11, 12
Despite recommendations, individuals may have sought antibody testing early in the pandemic as a potential release from social distancing.13 After coronavirus disease 2019 (COVID-19) vaccines were introduced, people who were vaccine hesitant may have used antibody results to support decisions to seek or avoid vaccination. These behaviors represent risk compensation, a theory that suggests that people alter their behavior in response to their perceived level of risk.14,15 Perceived risk (susceptibility to infection) is also a construct of the Health Belief Model, a behavioral theory used to explain vaccination intentions and behaviors.16 Survey studies have shown that individuals are more likely to be vaccine hesitant if they have a low perceived susceptibility to COVID-19 infection or other vaccine-preventable diseases.17,18 Receiving a positive SARS-CoV-2 antibody test may have given some individuals an exaggerated sense of security, reducing their intentions to vaccinate. Conversely, receiving a negative antibody test may have increased some individuals’ perceived susceptibility to COVID-19 infection and influenced them to seek vaccination. These theoretically driven hypotheses have not been tested using real-world serologic and vaccination data. Understanding how antibody results as a measure of perceived susceptibility influence actual vaccine behavior could inform future interventions to increase vaccine acceptance as well as serologic testing policies during a future pandemic.
The authors conducted a retrospective cohort study of individuals in 2 large healthcare systems who received a SARS-CoV-2 antibody test and assessed whether the results influenced subsequent COVID-19 vaccination receipt. The authors hypothesized that a negative antibody result would be associated with an increased likelihood of receiving a COVID-19 vaccine.
METHODS
Study Population
This retrospective cohort study was conducted with electronic health record data from 2 integrated health insurance plans and healthcare delivery systems: Kaiser Permanente Colorado (KPCO) and Kaiser Permanente Southern California (KPSC). Together, these systems serve approximately 5.3 million members. The authors identified a cohort of adults who received a commercially available serology test for SARS-CoV2 antibodies between January 1, 2021, and February 28, 2023. Individuals had to be aged ≥18 years on the date of the test and have at least 12 months of continuous health plan enrollment prior to the test. The end of the study period was March 30, 2023.
The objective was to compare the incidence of COVID-19 vaccination between individuals with positive and negative SARS-CoV-2 serology results. Serology testing was not routinely recommended at either health system; rather, testing was generally conducted at a patient’s request. Four antibody tests were used at KPCO over the study period: Abbott Architect SARS-CoV-2 nucleocapsid IgG test (COV-2 test), Siemens ADVIA Centaur SARS-CoV-2 (COV2T) Spike Total IgG/IgM, Siemens ADVIA Centaur Spike IgG COV2G, and Siemens ADVIA Centaur sCOVG Spike IgG.19,20 Antinucleocapsid antibodies are a marker of natural infection, whereas antispike protein receptor binding domain antibodies are a marker of natural infection or immunization.21,22 Over the study period, KPSC used the Abbott antinucleocapsid IgG assay from roughly January 1, 2021 to July 1, 2021 and then the Roche antinucleocapsid total Ig assay. Patients were informed of a positive or negative test result; actual antibody titer results were not provided.
The date of the first serology test represented Day 0 of follow-up, and individuals were followed forward for a maximum of 120 days from Day 0 to assess vaccination status. On the basis of stimulus response theory,23 the authors expected the effect of the stimuli (antibody test result) on the response (vaccination behavior) to wane over time. Therefore, the authors assessed the association between antibody test result and vaccine receipt within intervals of 30, 60, 90, and 120 days after Day 0. The authors treated the 30-day follow-up as the primary analysis. Individuals who received a documented COVD-19 vaccination before Day 0 were excluded. A negative test result was considered exposed, and a positive test result was considered unexposed. Exposure status was assessed as a time-varying variable; after Day 0, exposure changed on the basis of the serology test result. For example, if an individual received a negative test result at Day 0 and then a positive result at Day 20 and did not receive a vaccination during follow-up, this person would have 20 days of exposed time and 10 days of unexposed time. Individuals with only a single negative test at Day 0 had all of their follow-up as exposed time, and individuals with only a single positive test at Day 0 had all of their follow-up as unexposed time. Covariates (potential confounders) were measured during a baseline window of 365 days before Day 0. The KPSC IRB approved the study and waived the requirement for written informed consent owing to minimal risk to the study participants.
Measures
The main outcome was COVID-19 vaccination status. Over the study period, 3 vaccines (Pfizer BioNTech BNT162b2, Moderna mRNA-1273, and Johnson & Johnson Janssen Ad26. COV2. S) and 2 boosters (BNT162b2 and mRNA-1273) became available. The study sites captured vaccination date and manufacturer of each vaccine dose administered both within and outside their healthcare systems. The sites also captured COVID-19 vaccinations recorded in their respective state immunization registries.24 The authors analyzed vaccination status as a dichotomous variable (yes/no).
Demographic and clinical covariates were measured 365 days prior to Day 0. Covariates included age, sex, race/ethnicity, tobacco use, and Elixhauser comorbidity score.25,26 Age was categorized into 7 groups: 18–29, 30–39, 40–49, 50–64, 65–74, 75–84, and ≥85 years. Using ICD-10 codes, the authors identified immunosuppressive conditions that may be associated with an impaired serologic response and the propensity to receive a COVID-19 vaccination (Table 1).27, 28, 29, 30
Table 1.
ICD-10-CM Codes Used to Identify Underlying Medical Conditions.
| Category | Condition | ICD-10-CM Codes |
|---|---|---|
| Overweight or obesity | E66x, Z68.25–Z68.29, Z68.3, Z68.4 | |
| Diabetes mellitus | E10x, E11x, E13x, Z79.4, Z79.84 | |
| Immunosuppression | HIV | B20x |
| Cancer | Cxx | |
| Solid organ or hematopoietic stem cell transplantation | T86x, Z94.0–Z94.4, Z94.81, Z94.84, Z94.89, Z94.9 | |
| Receipt of systemic corticosteroids, chemotherapy, radiotherapy, or other immunosuppressive therapy | Z79.52, Z92.21, Z92.241, Z92.25, Z92.85, Z92.86 | |
| Immunodeficiency | D70x, D71x, D80x, D81x, D82x, D83x, D84x, D89x |
The authors assessed COVID-19 infection during the 30-day follow-up, as measured by viral testing. At KPCO, SARS-CoV-2 viral testing results were mostly from the transcription-mediated amplification test for the qualitative detection of SARS-CoV-2 by Aptima on the Panther (Hologic, Inc., San Diego, CA). KPSC primarily used polymerase chain reaction assays made by Roche (Rotkreuz, Switzerland) and Thermo Fisher Scientific (Waltham, MA).
Statistical Analysis
Baseline characteristics were compared between individuals with at least 1 positive antibody test and individuals with only 1 or more negative tests using 2-sided chi-square tests for categorical variables and means and the Kruskal–Wallis tests for continuous variables. To examine the association between serology test result and COVID-19 vaccination, the authors used Cox proportional hazards regression for counting processes to model the serology test result as a time-varying exposure. The authors stratified by year and month of the serology test result date and study site to control for temporal and site-specific outcome variation. Individuals were censored at the first dose of COVID-19 vaccine, disenrollment from the health plan, death, 30 days after Day 0, or the end of the study period, whichever came first. Unadjusted and adjusted hazard ratios (aHRs) and 95% CIs were estimated, representing the relative incidence of vaccination between the 2 serology exposure groups. For age, 18–29 years was the referent group; for race/ethnicity, non-Hispanic White was the referent; and for immunosuppression, no immunosuppression was the referent. The authors conducted 2-sided statistical tests with a p<0.05 cutoff for statistical significance. Interactions between serology result and age, sex, and race/ethnicity were tested. The proportional hazards assumption for time-constant covariates was evaluated with scaled Schoenfeld residuals plots and tests of Schoenfeld residuals.
As a secondary analysis, the authors assessed the impact of a test result for COVD-19 infection after Day 0. For example, an individual with a negative serology result at Day 0 may intend to get vaccinated but then test positive for SARS-CoV-2 before getting the vaccine and subsequently decide to forgo vaccination, assuming that they developed natural immunity. If a negative serology result is associated with receipt of vaccination, this behavior would bias the results toward the null hypothesis. For this secondary analysis, the authors excluded negative serology tests that were followed by a positive SARS-CoV-2 viral test.
RESULTS
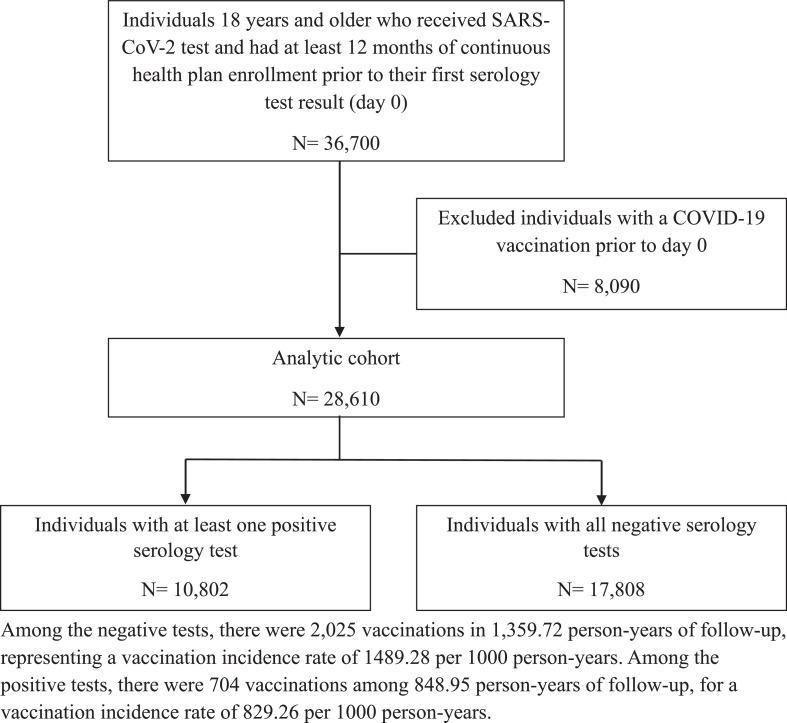
Between January 1, 2021, and February 28, 2023, 36,700 adults received a SARS-CoV-2 test and had at least 12 months of continuous health plan enrollment prior to their first serology test result. The authors excluded 8,090 (22.0%) individuals with a COVID-19 vaccination prior to Day 0. Among the remaining 28,610 individuals in the cohort, there were 28,820 serology tests during the follow-up (Figure 1). The mean age of the cohort was 49.98 (SD=15.19) years; 60.3% were female; and 30.9% were classified as Hispanic, 5.6% were classified as Black, and 6.4% were classified as Asian in the electronic health record. Approximately 82% of the cohort were aged <65 years. There were 10,802 (37.8%) individuals with at least 1 positive serology result and 17,808 (62.2%) individuals with all negative test results. Those with positive and negative results differed significantly by age, sex, race/ethnicity, tobacco use, immunosuppressive conditions, and Elixhauser score (Table 2).
Figure 1.
Study flow chart diagram.
Table 2.
Characteristics of the Study Cohort, by SARS CoV-2 Serology Test Result.
| Characteristics | Overall cohort (N=28,610) | Individuals with at least 1 positive test (n=10,802) | Individuals with all tests negative (n=17,808) |
|---|---|---|---|
| Age, years, n (%)a | |||
| 18–29 | 2,391 (8.36) | 907 (8.40) | 1,484 (8.33) |
| 30–39 | 5,511 (19.26) | 1,971 (18.25) | 3,540 (19.88) |
| 40–49 | 6376 (22.29) | 2,396 (22.18) | 3,980 (22.35) |
| 50–64 | 9,297 (32.50) | 3,598 (33.31) | 5,699 (32.00) |
| 65–74 | 3,413 (11.93) | 1,291 (11.95) | 2,122 (11.92) |
| 75–84 | 1,346 (4.70) | 528 (4.89) | 818 (4.59) |
| ≥85 | 276 (0.96) | 111 (1.03) | 165 (0.93) |
| Female, n (%)a | 17,249 (60.29) | 6,606 (61.16) | 10,643 (59.77) |
| Race/ethnicity, n (%)a | |||
| Hispanic | 8,840 (30.90) | 4,055 (37.54) | 4,785 (26.87) |
| Non-Hispanic White | 14,519 (50.75) | 4,844 (44.84) | 9,675 (54.33) |
| Non-Hispanic Black | 1,588 (5.55) | 597 (5.53) | 991 (5.56) |
| Non-Hispanic Asian | 1,832 (6.40) | 608 (5.63) | 1,224 (6.87) |
| Native American/Pacific Islander | 187 (0.65) | 77 (0.71) | 110 (0.62) |
| Other/mixed | 483 (1.69) | 178 (1.65) | 305 (1.71) |
| Unknown | 1,161 (4.06) | 443 (4.10) | 718 (4.03) |
| Tobacco use, n (%)a | 1,375 (4.81) | 398 (3.68) | 977 (5.49) |
| Immunosuppressive conditions, n (%)a | 1,683 (5.88) | 606 (5.61) | 1,077 (6.05) |
| Elixhauser score, mean (SD)a | 1.41 (1.99) | 1.52 (2.06) | 1.34 (1.94) |
| Serology tests, mean (SD)a | 1.01 (0.09) | 1.01 (0.11) | 1.0 (0.07) |
p<0.05.
Among the negative tests, there were 2,025 vaccinations in 1,359.72 person-years of follow-up, representing a vaccination incidence rate of 1489.28 per 1,000 person-years. Among the positive tests, there were 704 vaccinations among 848.95 person-years of follow-up, for a vaccination incidence rate of 829.26 per 1,000 person-years. The difference was statistically significant (p<0.0001).
In the multivariable Cox regression analysis, a negative serology result was positively associated with vaccination, and the magnitude of the effect decreased over time. For the 30-, 60-, 90-, and 120-day follow-up periods, the aHRs were 1.58 (95% CI=1.45, 1.72), 1.30 (95% CI=1.22, 1.39), 1.23 (95% CI=1.16, 1.30), and 1.19 (95% CI=1.13, 1.25), respectively. For the primary analyses, individuals in age groups between 50 and 84 years were more likely to receive a COVID-19 vaccine (Table 3) than in the 18–29-year referent group. The greatest association was in the 65–74 age group (aHR=1.47; 95% CI=1.24, 1.75) and the 75–84 age group (aHR=1.47; 95% CI=1.17, 1.84). Race/ethnicity was significantly associated with vaccination (Table 3): the non-Hispanic Black group was more likely to receive vaccinations than the non-Hispanic White group (aHR=1.32; 95% CI=1.13, 1.55). Tobacco use was negatively associated with vaccination (aHR=0.79; 95% CI=0.65, 0.96). The proportional hazards assumption for the model did not appear to be violated on the basis of the Schoenfeld residual plots and tests of Schoenfeld residuals (p>0.05). In the secondary analysis excluding negative serology tests followed by a positive SARS-CoV-2 viral test, the association between serology result and vaccination was similar to the main results (aHR=1.60; 95% CI=1.47, 1.75).
Table 3.
The Association of SARS-CoV-2 Serology Test Result With COVID-19 Vaccination.
| Variable | Univariate HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|
| Negative serology | 1.57 (1.44, 1.71) | 1.58 (1.45, 1.72) |
| Age, years | ||
| 18–29 | ref | ref |
| 30–39 | 1.09 (0.92, 1.28) | 1.07 (0.91, 1.26) |
| 40–49 | 1.17 (1.00, 1.36) | 1.16 (0.99, 1.36) |
| 50–64 | 1.23 (1.06, 1.43) | 1.25 (1.08, 1.45) |
| 65–74 | 1.43 (1.20, 1.69) | 1.47 (1.24, 1.75) |
| 75–84 | 1.37 (1.10, 1.70) | 1.47 (1.17, 1.84) |
| ≥85 | 1.26 (0.84, 1.87) | 1.34 (0.89, 2.02) |
| Sex | ||
| Male | ref | ref |
| Female | 0.99 (0.92, 1.07) | 1.01 (0.94, 1.09) |
| Race/ethnicity | ||
| Non-Hispanic White | ref | ref |
| Hispanic | 0.85 (0.77, 0.94) | 0.94 (0.86, 1.04) |
| Non-Hispanic Black | 1.26 (1.07, 1.47) | 1.32 (1.13, 1.54) |
| Other/mixed | 1.01 (0.89, 1.16) | 1.07 (0.93, 1.23) |
| Unknown | 1.00 (0.83, 1.21) | 1.05 (0.87, 1.27) |
| Tobacco use | ||
| No | ref | ref |
| Yes | 0.82 (0.68, 0.99) | 0.79 (0.66, 0.96) |
| Immunosuppressive conditions | ||
| No | ref | ref |
| Yes | 1.08 (0.93, 1.27) | 1.04 (0.88, 1.23) |
| Elixhauser score | 1.00 (0.98, 1.02) | 0.98 (0.96, 1.01) |
HR, hazard ratio.
DISCUSSION
In this multisite cohort study, a negative SARS-CoV-2 serology test result was associated with increased receipt of COVID-19 vaccination. These findings suggest that risk compensation occurs and that perceived susceptibility to COVID-19 infection influences vaccination behavior. The results have implications for future interventions to increase vaccine acceptance and policies regarding the use of serology testing for individual decision making during a future pandemic.
Other interventions for which there was speculation about the potential for risk compensation include seat belts, bicycle helmets, human papillomavirus vaccination, pre-exposure prophylaxis for preventing HIV, and naloxone for reversing opioid overdose.31, 32, 33, 34, 35, 36, 37, 38 However, there is little empirical evidence that exposure to these interventions increases risk-taking behaviors. Although there was some evidence that mandatory seat belt laws may lead to riskier driving behaviors, such behaviors have been offset by the net benefit of the laws on reductions in motor vehicle fatalities.31,38, 39, 40
Assessments of risk compensation during the COVID-19 pandemic have been mixed. An ecologic study in the U.S. observed an association between face mask mandates and increased time in public places measured with mobile device data, suggestive of risk compensation behavior.41 In contrast, a study in which U.S. college students were randomized to receive SARS-CoV-2 serology results at either a baseline or delayed time point did not find evidence that serology results influenced self-reported risk behaviors, including avoiding social events, staying at home from work or school, wearing a face mask in public, and physical distancing in public.42 However, that study was conducted before COVID-19 vaccines were available, and only 47 (4.4%) of the 1,076 students tested positive for SARS-CoV-2 antibodies. This study was conducted in an observational, real-world setting after COVID-19 vaccines were introduced and comprised a cohort of 28,610 adults who sought serology testing, 10,802 (37.8%) of whom tested positive for COVID-19 antibodies. In this setting, it appears that some individuals intended to use serology results to inform their vaccination behaviors.
Shortly after the FDA approved an emergency use authorization for serology tests early in the pandemic, an influx of poor-quality tests flooded the U.S. market.10 This proved to be a costly policy owing to the large demand for testing among individuals seeking immunity passports before COVID-19 vaccines became available.43 The FDA and Centers for Disease Control and Prevention have since issued strong statements discouraging the use of such tests for individual decision making and have established a new, more rigorous process for developing, evaluating, and approving valid serology tests in the future.10 The results of this study, which suggest that some individuals used the tests to guide their vaccine decisions, emphasize the importance of carefully devising recommendations and policies for serology testing during a pandemic.
It has been suggested that the Health Belief Model can guide behavioral interventions to address vaccine hesitancy.17,43,44 Survey studies have demonstrated that the perceived susceptibility domain of the Health Belief Model is a predictor of vaccine intentions, both for childhood and COVID-19 vaccination.17,44, 45, 46 This longitudinal study supports this association, using serology results as a behavioral proxy of perceived susceptibility (immunity) to COVID-19 infection and COVID-19 vaccination receipt rather than intentions. These results suggest that interventions should focus, at least in part, on perceived illness susceptibility to increase adult vaccine uptake.47
Limitations
This study has some important limitations. The authors used an observational study design that could be impacted by selection bias and confounding, if factors associated with serology testing and vaccination were unmeasured. The authors attempted to mitigate this potential for bias by restricting the population to individuals who received a serology test and analyzing test results as the main exposure, so that differences between tested and untested individuals could not affect the results. In addition, by using electronic health records to longitudinally measure the exposure and outcome, the authors minimized the potential for recall bias and measurement error resulting from self-reported vaccination status. The cohort was a large insured population from 2 integrated healthcare systems, which may have limited the study’s generalizability. For example, the non-Hispanic Black population in this study had higher vaccination rates than the non-Hispanic White population, which does not align with published studies of COVID-19 vaccination uptake.48 However, it should be noted that both health system membership populations are demographically representative of their respective geographic regions.49,50 In addition, tobacco use was associated with decreased uptake in this study, aligning with the literature.51
CONCLUSIONS
The findings suggest that SARS-CoV-2 serology results can lead to risk compensation and affect vaccination behavior, which may have impacted COVID-19 vaccine uptake early in the pandemic. Future interventional research could focus on how to leverage individuals’ perceived susceptibility to infection to increase adult vaccine acceptance.
Acknowledgments
The authors thank their collaborators at Kaiser Permanente Southern California who assisted with data curation and grant administration: Jessica Lam, MPH; Cecilia Portugal, MPH; and John Chang, MPH.
Funding: This study was funded by Garfield Memorial Research Fund and the Kaiser Permanente Community Health Fund.
Declaration of interest: IAB received royalties from UpToDate (Wolters Kluwer) for unrelated educational content on the heatlh of incarcerated persons. KJB has received funding from Moderna, Pfizer, Dynavax, Gilead, and GlaxoSmithKline for other research. No other financial disclosures were reported.
CRediT AUTHOR STATEMENT
Jason M. Glanz: Conceptualization, Methodology, Supervision, Validation, Writing - original draft, Writing - review & editing. Darryl E. Palmer-Toy: Conceptualization, Writing - review & editing, Funding acquisition, Supervision, Writing - review & editing. Komal J. Narwaney: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Writing - review & editing. Jennifer C. Barrow: Project administration, Investigation, Writing - review & editing. Katia J. Bruxvoort: Writing - review & editing. Courtney R. Kraus: Investigation, Visualization, Writing - review & editing. Jason A. Lyons: Data curation, Software, Writing - review & editing. Ingrid A. Binswanger: Conceptualization, Methodology, Supervision, Validation, Writing - original draft, Writing - review & editing.
REFERENCES
- 1.Zuckerman DM. Emergency use authorizations (EUAs) versus FDA approval: implications for COVID-19 and public health. Am J Public Health. 2021;111(6):1065–1069. doi: 10.2105/AJPH.2021.306273. https://DOI.ORG/10.2105/AJPH.2021.306273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronavirus (COVID-19) update: daily roundup May 4, 2020. Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-daily-roundup-may-4-2020. Accessed August 2, 2024.
- 3.In vitro diagnostics emergency use authorizations (EUAs)-serology and other adaptive immune response tests for SARS-CoV-2.https://www.fda.gov/medical-devices/covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-emergency-use-authorizations-euas-serology-and-other-adaptive-immune-response. Updated 2020. Accessed August 15, 2024.
- 4.Jones J.M., Manrique I.M., Stone M.S., et al. Estimates of SARS-CoV-2 seroprevalence and incidence of primary SARS-CoV-2 infections among blood donors, by COVID-19 vaccination status—United States, April 2021–September 2022. MMWR Morb Mortal Wkly Rep. 2023;72(22):601–605. doi: 10.15585/mmwr.mm7222a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones J.M., Stone M., Sulaeman H., et al. Estimated U.S. infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020–May 2021. JAMA. 2021;326(14):1400–1409. doi: 10.1001/jama.2021.15161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buitrago-Garcia D., Salanti G., Low N. Studies of prevalence: how a basic epidemiology concept has gained recognition in the COVID-19 pandemic. BMJ Open. 2022;12(10) doi: 10.1136/bmjopen-2022-061497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbasi J. The flawed science of antibody testing for SARS-CoV-2 immunity. JAMA. 2021;326(18):1781–1782. doi: 10.1001/jama.2021.18919. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Interim Guidelines for COVID-19 Antibody Testing.https://archive.cdc.gov/#/details?q=https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing/antibody-tests-guidelines.html&start=0&rows=10&url=https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing/antibody-tests-guidelines.html. | CDC Archive; Updated December 16, 2022. Accessed January 30, 2023.
- 9.Antibody (serology) testing for COVID-19: information for patients and consumers. U.S. Food and Drug Administration.https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/antibody-serology-testing-covid-19-information-patients-and-consumers. Updated April 24, 2025. Accessed May 11, 2022.
- 10.Shuren J., Stenzel T. The FDA’s experience with Covid-19 antibody tests. N Engl J Med. 2021;384(7):592–594. doi: 10.1056/NEJMp2033687. [DOI] [PubMed] [Google Scholar]
- 11.Colgrove R., Bruno-Murtha L.A., Chastain C.A., et al. Tale of the titers: serologic testing for SARS-CoV-2-Yes, no, and maybe, with clinical examples from the IDSA diagnostics committee. Open Forum Infect Dis. 2023;10(1):ofac674. doi: 10.1093/ofid/ofac674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.West R., Kobokovich A., Connell N., Gronvall GK. COVID-19 antibody tests: a valuable public health tool with limited relevance to individuals. Trends Microbiol. 2021;29(3):214–223. doi: 10.1016/j.tim.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West R.M., Kobokovich A., Connell N., Gronvall GK. Antibody (serology) tests for COVID-19: a case study. mSphere. 2021;6(3) doi: 10.1128/mSphere.00201-21. -21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedlund J. Risky business: safety regulations, risks compensation, and individual behavior. Inj Prev. 2000;6(2):82–90. doi: 10.1136/ip.6.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peltzman S. The effects of automobile safety regulation. J Pol Econ. 1975;83(4):677–725. doi: 10.1086/260352. [DOI] [Google Scholar]
- 16.Becker MH. The health belief model and sick role behavior. Health Educ Monogr. 1974;2(4):409–419. doi: 10.1177/109019817400200407. [DOI] [Google Scholar]
- 17.Limbu Y.B., Gautam R.K., Pham L. The health belief model applied to COVID-19 vaccine hesitancy: a systematic review. Vaccines (Basel) 2022;10(6):973. doi: 10.3390/vaccines10060973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badr H., Zhang X., Oluyomi A., et al. Overcoming COVID-19 vaccine hesitancy: insights from an online population-based survey in the United States. Vaccines (Basel) 2021;9(10):1100. doi: 10.3390/vaccines9101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbott Laboratories Diagnostics. Division. SARS-CoV-2 IgG. 2020.https://www.corelaboratory.abbott/int/en/offerings/segments/infectious-disease/sars-cov-2.html. Updated May 26, 2020. Accessed January 30, 2023.
- 20.Bryan A., Pepper G., Wener M.H., et al. Performance characteristics of the Abbott Architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58(8) doi: 10.1128/JCM.00941-20. -20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner A., Garner-Spitzer E., Schötta A.M., et al. SARS-CoV-2-mRNA booster vaccination reverses non-responsiveness and early antibody waning in immunocompromised patients - A Phase Four study comparing immune responses in patients with solid cancers, multiple myeloma and inflammatory bowel disease. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.889138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrière J., Carles M., Audigier-Valette C., et al. Third dose of anti-SARS-CoV-2 vaccine for patients with cancer: should humoral responses be monitored? A position article. Eur J Cancer. 2022;162:182–193. doi: 10.1016/j.ejca.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frings C., Foerster A., Moeller B., Pastötter B., Pfister R. The relation between learning and stimulus-response binding. Psychol Rev. 2024;131(5):1290–1296. doi: 10.1037/rev0000449. [DOI] [PubMed] [Google Scholar]
- 24.Klein N.P., Lewis N., Goddard K., et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326(14):1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta H.B., Li S., An H., Goodwin J.S., Alexander G.C., Segal JB. Development and validation of the summary Elixhauser comorbidity score for use with ICD-10-CM-coded data among older adults. Ann Intern Med. 2022;175(10):1423–1430. doi: 10.7326/M21-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elixhauser A., Steiner C., Harris D.R., Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Duly K., Farraye F.A., Bhat S. COVID-19 vaccine use in immunocompromised patients: a commentary on evidence and recommendations. Am J Health Syst Pharm. 2022;79(2):63–71. doi: 10.1093/ajhp/zxab344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen C., Risk M., Schiopu E., et al. Efficacy of COVID-19 vaccines in patients taking immunosuppressants. Ann Rheum Dis. 2022;81(6):875–880. doi: 10.1136/annrheumdis-2021-222045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yek C. Risk factors for severe COVID-19 outcomes among persons aged≥ 18 years who completed a primary COVID-19 vaccination series - 465 Health Care Facilities, United States, December 2020-October 2021. MMWR Morb Mortal Wkly Rep. 2022;71(1):19–25. doi: 10.15585/mmwr.mm7101a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersen K.M., Bates B.A., Rashidi E.S., et al. Long-term use of immunosuppressive medicines and in-hospital COVID-19 outcomes: a retrospective cohort study using data from the National COVID Cohort Collaborative. Lancet Rheumatol. 2022;4(1):e33–e41. doi: 10.1016/S2665-9913(21)00325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Houston D.J., Richardson LE. Risk compensation or risk reduction? Seatbelts, state laws, and traffic fatalities. Soc Sci Q. 2007;88(4):913–936. doi: 10.1111/j.1540-6237.2007.00510.x. [DOI] [Google Scholar]
- 32.Dinh-Zarr T.B., Sleet D.A., Shults R.A., et al. Reviews of evidence regarding interventions to increase the use of safety belts. Am J Prev Med. 2001;21(4):48–65. doi: 10.1016/s0749-3797(01)00378-6. (suppl) [DOI] [PubMed] [Google Scholar]
- 33.Esmaeilikia M., Radun I., Grzebieta R., Olivier J. Bicycle helmets and risky behaviour: a systematic review. Transp Res F. 2019;60:299–310. doi: 10.1016/j.trf.2018.10.026. [DOI] [Google Scholar]
- 34.Rojas Castro D., Delabre R.M., Molina J.M. Give PrEP a chance: moving on from the “risk compensation” concept. J Int AIDS Soc. 2019;22(suppl 6) doi: 10.1002/jia2.25351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Schreeb S., Pedersen S.K., Christensen H., et al. Questioning risk compensation: pre-exposure prophylaxis (PrEP) and sexually transmitted infections among men who have sex with men, capital region of Denmark, 2019 to 2022. Euro Surveill. 2024;29(13) doi: 10.2807/1560-7917.ES.2024.29.13.2300451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ortblad K.F., Stalter R.M., Bukusi E.A., et al. No evidence of sexual risk compensation following PrEP initiation among heterosexual HIV serodiscordant couples in Kenya and Uganda. AIDS Behav. 2020;24(5):1365–1375. doi: 10.1007/s10461-019-02720-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glanz J.M., Mueller S.R., Narwaney K.J., et al. Effectiveness of direct patient outreach with a narrative naloxone and overdose prevention video to patients prescribed long-term opioid therapy in the USA: the naloxone Navigator randomised clinical trial. BMJ Public Health. 2024;2(1) doi: 10.1136/bmjph-2023-000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leidner A.J., Chesson H.W., Talih M. HPV vaccine status and sexual behavior among young sexually-active women in the U.S.: evidence from the National Health and Nutrition Examination Survey, 2007–2014. Health Econ Policy Law. 2020;15(4):477–495. doi: 10.1017/S1744133119000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shannon H.S., Szatmari P. Seat-belt legislation and risk homeostasis: further analysis of the British data. Accid Anal Prev. 1994;26(6):803–805. doi: 10.1016/0001-4575(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 40.Evansm L., Wasielewski P., Von Buseck C.R. Compulsory seat belt usage and driver risk-taking behavior. Hum Factors. 1982;24(1):41–48. doi: 10.1177/001872088202400105. [DOI] [Google Scholar]
- 41.Yan Y., Bayham J., Richter A., Fenichel EP. Risk compensation and face mask mandates during the COVID-19 pandemic. Sci Rep. 2021;11(1):3174. doi: 10.1038/s41598-021-82574-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ludema C., Rosenberg M.S., Macy J.T., et al. Does receiving a SARS-CoV-2 antibody test result change COVID-19 protective behaviors? Testing risk compensation in undergraduate students with a randomized controlled trial. PLoS One. 2022;17(12) doi: 10.1371/journal.pone.0279347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown R.C.H., Savulescu J., Williams B., Wilkinson D. Passport to freedom? Immunity passports for COVID-19. J Med Ethics. 2020;46(10):652–659. doi: 10.1136/medethics-2020-106365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zampetakis L.A., Melas C. The health belief model predicts vaccination intentions against COVID-19: a survey experiment approach. Appl Psychol Health Well Being. 2021;13(2):469–484. doi: 10.1111/aphw.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hidayana I., Amir S., Pelupessy D.C., Rahvenia Z. Using a health belief model to assess COVID-19 vaccine intention and hesitancy in Jakarta, Indonesia. PLoS Glob Public Health. 2022;2(10) doi: 10.1371/journal.pgph.0000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith P.J., Humiston S.G., Marcuse E.K., et al. Parental delay or refusal of vaccine doses, childhood vaccination coverage at 24 months of age, and the Health Belief Model. Public Health Rep. 2011;126(2):135–146. doi: 10.1177/00333549111260S215. (suppl 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Center for Disease Control and Prevention. ACIP Vaccine Recommendations and Schedules. | ACIP | CDC.https://www.cdc.gov/acip/vaccine-recommendations/?CDC_AAref_Val=https://www.cdc.gov/vaccines/acip/recommendations.html. Updated June 28, 2024. Accessed August 2, 2024.
- 48.Kriss J.L., Hung M.C., Srivastav A., et al. COVID-19 vaccination coverage, by race and ethnicity - national immunization survey adult COVID module, United States, December 2020–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71(23):757–763. doi: 10.15585/mmwr.mm7123a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sukumaran L., McCarthy N.L., Li R., et al. Demographic characteristics of members of the Vaccine Safety Datalink (VSD): a comparison with the United States population. Vaccine. 2015;33(36):4446–4450. doi: 10.1016/j.vaccine.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis A.C., Voelkel J.L., Remmers C.L., Adams J.L., McGlynn EA. Comparing Kaiser Permanente members to the general population: implications for generalizability of research. Perm J. 2023;27(2):87–98. doi: 10.7812/TPP/22.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krebs N.M., D’Souza G., Bordner C., et al. COVID-19 vaccination uptake and hesitancy among current tobacco users. Tob Use Insights. 2021;14 doi: 10.1177/1179173X211068027. 1179173X211068027. [DOI] [PMC free article] [PubMed] [Google Scholar]