Abstract
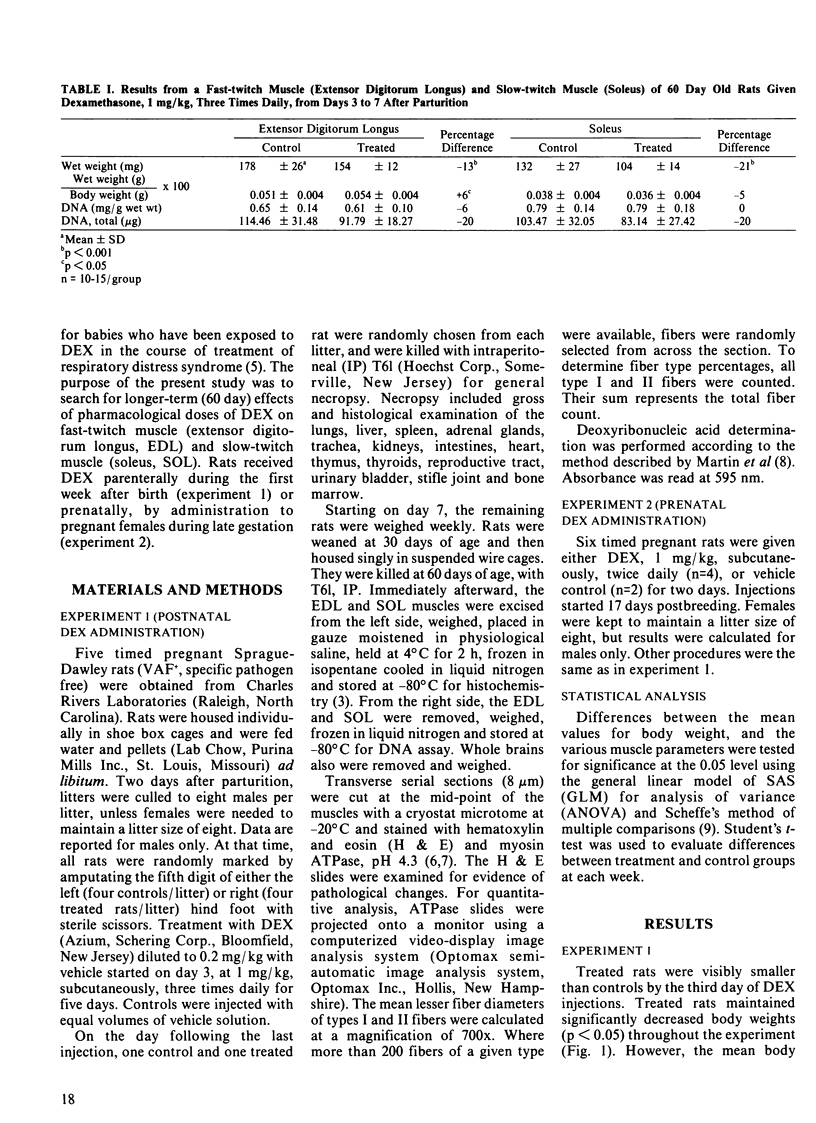
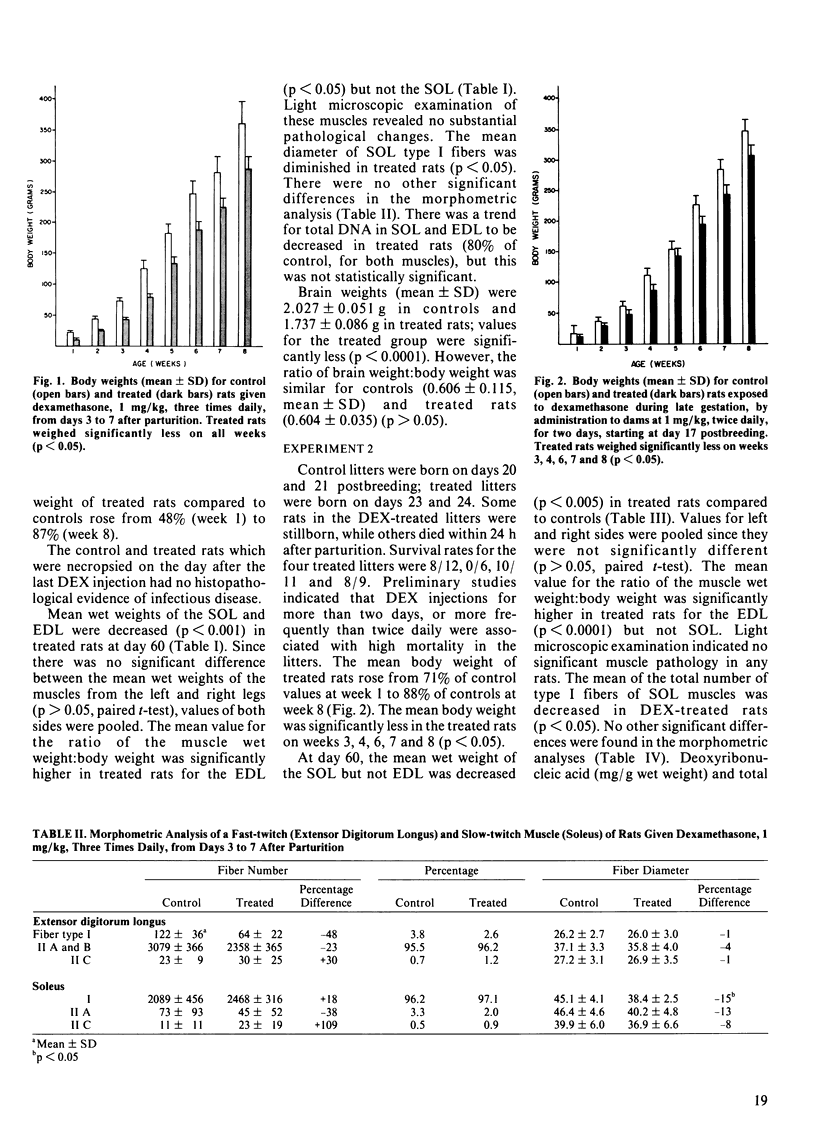
Five litters of suckling rats were given either dexamethasone (DEX), 1 mg/kg, subcutaneously, three times daily (n = 4/litter) or vehicle control (n = 4/litter) from day 3 through day 7 after birth. Rats were weighed weekly and were weaned on day 30. On day 60, rats were killed and the soleus (SOL) and extensor digitorum longus (EDL) were removed for the following analyses: 1) wet weight, 2) light microscopic examination of hematoxylin and eosin stained transverse sections, 3) quantitative morphometric analysis of myosin ATPase stained transverse sections (fiber numbers, fiber type percentages and mean fiber diameters), and 4) DNA (total and mg/g wet weight). The following parameters were significantly reduced in treated rats: 1) body weight, 2) wet weight of SOL and EDL, and 3) mean diameter of SOL type I fibers. There was a trend for total DNA of SOL and EDL to be decreased in treated rats but this was not statistically significant. In a second experiment, pregnant rats (n = 4) were given DEX, 1 mg/kg, subcutaneously, twice daily, on days 17 and 18 of gestation. Two rats served as vehicle controls. The prenatally DEX-exposed rats weighed significantly less on weeks 3, 4, 6, 7 and 8. There were significant reductions in the following parameters for treated rats: 1) SOL wet weight, and 2) total number of SOL type I fibers. There was a trend for SOL DNA to be reduced but this was not statistically significant.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. E., Merkel R. A., Young R. B. Cellular aspects of muscle growth: myogenic cell proliferation. J Anim Sci. 1979 Jul;49(1):115–127. doi: 10.2527/jas1979.491115x. [DOI] [PubMed] [Google Scholar]
- Bedi K. S., Birzgalis A. R., Mahon M., Smart J. L., Wareham A. C. Early life undernutrition in rats. 1. Quantitative histology of skeletal muscles from underfed young and refed adult animals. Br J Nutr. 1982 May;47(3):417–431. doi: 10.1079/bjn19820053. [DOI] [PubMed] [Google Scholar]
- Beermann D. H. Effects of maternal dietary restriction during gestation and lactation, muscle, sex and age on various indices of skeletal muscle growth in the rat. J Anim Sci. 1983 Aug;57(2):328–337. doi: 10.2527/jas1983.572328x. [DOI] [PubMed] [Google Scholar]
- Braund K. G., Hoff E. J., Richardson E. Y. Histochemical identification of fiber types in canine skeletal muscle. Am J Vet Res. 1978 Apr;39(4):561–565. [PubMed] [Google Scholar]
- Braunstein P. W., Jr, DeGirolami U. Experimental corticosteroid myopathy. Acta Neuropathol. 1981;55(3):167–172. doi: 10.1007/BF00691314. [DOI] [PubMed] [Google Scholar]
- Effect of antenatal dexamethasone administration on the prevention of respiratory distress syndrome. Am J Obstet Gynecol. 1981 Oct 1;141(3):276–287. [PubMed] [Google Scholar]
- Furcht L. T., Wendelschafer-Crabb G., Woodbridge P. A. Cell surface changes accompanying myoblast differentiation. J Supramol Struct. 1977;7(3-4):307–322. doi: 10.1002/jss.400070305. [DOI] [PubMed] [Google Scholar]
- Glore S. R., Layman D. K. Cellular development of skeletal muscle during early periods of nutritional restriction and subsequent rehabilitation. Pediatr Res. 1983 Jul;17(7):602–605. doi: 10.1203/00006450-198307000-00017. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Timson B. F., Moore R. L., Riedy M. Muscular enlargement and number of fibers in skeletal muscles of rats. J Appl Physiol Respir Environ Exerc Physiol. 1981 May;50(5):936–943. doi: 10.1152/jappl.1981.50.5.936. [DOI] [PubMed] [Google Scholar]
- Haltia M., Berlin O., Schucht H., Sourander P. Postnatal differentiation and growth of skeletal muscle fibres in normal and undernourished rats. A histochemical and morphometric study. J Neurol Sci. 1978 Mar;36(1):25–39. doi: 10.1016/0022-510x(78)90159-4. [DOI] [PubMed] [Google Scholar]
- Jirmanová I., Soukup T., Zelená J. The pathomorphology of developing skeletal muscles of rabbits treated with glucocorticoids. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;38(3):323–335. doi: 10.1007/BF02892828. [DOI] [PubMed] [Google Scholar]
- Kelly F. J., Goldspink D. F. The differing responses of four muscle types to dexamethasone treatment in the rat. Biochem J. 1982 Oct 15;208(1):147–151. doi: 10.1042/bj2080147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone I., Johnson M. A., Mastaglia F. L. Effects of dexamethasone on fibre subtypes in rat muscle. Neuropathol Appl Neurobiol. 1981 Sep-Oct;7(5):381–398. doi: 10.1111/j.1365-2990.1981.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Martin R., White J., Herbein J., Ezekwe M. O. Muscle and adipose cell development in mice selected for postweaning growth rate. Growth. 1979 Sep;43(3):167–173. [PubMed] [Google Scholar]
- Morikawa Y., Kasubuchi Y., Mino M., Yoshioka H., Kusunoki T., Yamano T., Shimada M. [Effects of dexamethasone on cortical dendritic growth in mice (author's transl)]. No To Shinkei. 1978 Mar;30(3):327–336. [PubMed] [Google Scholar]
- Odedra B. R., Bates P. C., Millward D. J. Time course of the effect of catabolic doses of corticosterone on protein turnover in rat skeletal muscle and liver. Biochem J. 1983 Aug 15;214(2):617–627. doi: 10.1042/bj2140617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen A. H. The distribution and relative sized of fibre types in the extensor digitorum longus and soleus muscles of the adult rat. J Anat. 1977 Apr;123(Pt 2):467–486. [PMC free article] [PubMed] [Google Scholar]
- Rannels S. R., Jefferson L. S. Effects of glucocorticoids on muscle protein turnover in perfused rat hemicorpus. Am J Physiol. 1980 Jun;238(6):E564–E572. doi: 10.1152/ajpendo.1980.238.6.E564. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Dana R., Krichevsky A., Bilezikian J. P., Schonberg M. Inhibition of beta-adrenergic responsiveness in muscle cell cultures by dexamethasone. Endocrinology. 1981 Dec;109(6):2110–2116. doi: 10.1210/endo-109-6-2110. [DOI] [PubMed] [Google Scholar]
- Steiss J. E. Effect of high- and low-dose dexamethasone on regeneration of minced skeletal muscle autografts in rats. Exp Neurol. 1986 Aug;93(2):300–310. doi: 10.1016/0014-4886(86)90191-3. [DOI] [PubMed] [Google Scholar]
- Stickland N. C. The arrangement of muscle fibers and tendons in two muscles used for growth studies. J Anat. 1983 Jan;136(Pt 1):175–179. [PMC free article] [PubMed] [Google Scholar]
- Timson B. F. The effect of varying postnatal growth rate on skeletal muscle fiber number in the mouse. Growth. 1982 Spring;46(1):36–45. [PubMed] [Google Scholar]


