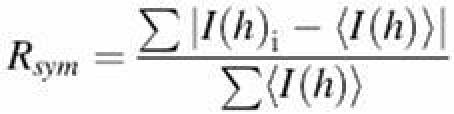Abstract
Insulin-like growth factors (IGFs) are key regulators of cell proliferation, differentiation and transformation, and are thus pivotal in cancer, especially breast, prostate and colon neoplasms. They are also important in many neurological and bone disorders. Their potent mitogenic and anti-apoptotic actions depend primarily on their availability to bind to the cell surface IGF-I receptor. In circulation and interstitial fluids, IGFs are largely unavailable as they are tightly associated with IGF-binding proteins (IGFBPs) and are released after IGFBP proteolysis. Here we report the 2.1 Å crystal structure of the complex of IGF-I bound to the N-terminal IGF-binding domain of IGFBP-5 (mini-IGFBP-5), a prototype interaction for all N-terminal domains of the IGFBP family. The principal interactions in the complex comprise interlaced hydrophobic side chains that protrude from both IGF-I and the IGFBP-5 fragment and a surrounding network of polar interactions. A solvent-exposed hydrophobic patch is located on the IGF-I pole opposite to the mini-IGFBP-5 binding region and marks the IGF-I receptor binding site.
Keywords: cancer/complex/IGFBP-5/insulin-like growth factor/structure
Introduction
Insulin-like growth factors (IGFs) are members of the insulin superfamily of hormones, growth factors and neuropeptides whose biological actions are achieved through binding to cell surface receptors. IGF actions are regulated by IGF-binding proteins (IGFBPs), which act as transporters of IGFs, protect them from degradation, limit their binding to receptors and maintain a ‘reservoir’ of biologically inactive IGF (Jones and Clemmons, 1995; Hwa et al., 1999; Martin and Baxter, 1999; Khandwala et al., 2000). The IGF and growth hormone (GH) axis plays a large part in regulating fetal and childhood somatic growth, and several decades of basic and clinical research have demonstrated that it is also critical in maintaining neoplastic growth (Khandwala et al., 2000). High circulating IGF-I concentrations may also be an important determinant of cancer incidence (Hankinson et al., 1998; Holly, 1998; Wolk, 2000). Virtually every level of the IGF system mediating response on the tumour tissues (IGFs, IGFBPs, IGF receptors) can be targeted for therapeutic approaches (Fanayan et al., 2000; Imai et al., 2000; Khandwala et al., 2000). It should also be mentioned here that IGFBP-3 has IGF-independent anti-proliferative and pro-apoptotic effects (Wetterau et al., 1999; Butt et al., 2000).
IGF-I and IGF-II are 67% identical single polypeptide chains of 70 and 67 amino acids, respectively, sharing with insulin ∼40% sequence identity and presumed structural homology. The first 29 residues of IGFs are homologous to the B-chain of insulin (B region, 1–29), followed by 12 residues that are analogous to the C-peptide of proinsulin (C region, 30–41), and a 21-residue region that is homologous to the A-chain of insulin (A region, 42–62). The C-terminal octapeptide (D region, 63–70) has no counterpart in insulins and proinsulins (Figure 1A) (Baxter et al., 1992; Murray-Rust et al., 1992). The IGFs are the only members of the insulin superfamily in which the C region is not removed proteolytically after translation. The three-dimensional structure of insulin has been studied intensively since the first crystal structure determination in the 1960s (Adams et al., 1969). There are now structures of insulins in several oligomeric states, for insulins crystallized in different solvent conditions, and for many variants from different species and chemical modifications. This is in stark contrast to IGFs (and proinsulins), for which no high-definition structure has been available prior to this report. Instead, the tertiary structure of IGF-I has been modelled after porcine insulin (Blundell et al., 1978). Two-dimensional nuclear magnetic resonance (NMR) studies of IGF-I have confirmed that the solution structure is consistent with the model (Cooke et al., 1991; Sato et al., 1993). However, NMR studies of IGF-I have yielded structures only of low resolution, probably because IGF-I is soluble at the concentrations required for NMR only at pH values <3 (Cooke et al., 1991; Sato et al., 1993). More recently, better defined structures have been obtained for IGF-II (Terasawa et al., 1994; Torres et al., 1995) and for a Glu3 to Arg variant of IGF-I (long-[Arg3]IGF-I) that additionally possesses a 13-amino acid extension at the N-terminus (Laajoki et al., 2000). It is worth mentioning here that the affinity of this IGF analogue for IGFBPs is decreased by several orders of magnitude compared with IGF-I.
Fig. 1. Sequence and structure alignment (A) of IGFs and single-chain insulin (SCI). Residues that make contacts with mini-IGFBP-5 within 4 Å are highlighted in magenta; residues responsible for binding to IGF-1R are in red and residues in green showed no electron density. (B) Mini-IGFBP-5 with the corresponding N-terminal domains of IGFBP-3, IGFBP-rP1 and IGFBP-rP2; consensus amino acid residues are shown above the sequences; conserved residues are indicated by blue letters. Residues that interact with IGF-I (within 4 Å) are highlighted in magenta. The mini-IGFBP-5 construct had additional Gly and Ser residues from the cloning vector at the N-terminus; residues in green showed no electron density.
IGFBPs are proteins of 216–289 residues, with mature IGFBP-5 consisting of 252 residues (Wetterau et al., 1999). All IGFBPs share a common domain organization. The highest conservation is found in the N- (residues 1 to ∼100) and C- (from residue 170) terminal cysteine rich regions. Twelve conserved cysteines are found in the N-terminal domain (exception is IGFBP-6 with only 10 conserved cysteines) and six in the C-terminal domain. The central, weakly conserved part (L-domain) contains most of the cleavage sites for specific proteases (Chernausek et al., 1995). Several different fragments of IGFBPs have been described and biochemically characterized so far (Mazerbourg et al., 1999). Mutagenesis studies suggest that the high affinity IGF-binding site is located in the N-terminal domain (Chernausek et al., 1995; Wetterau et al., 1999) and that at least IGFBP-3 and IGFBP-2 contain two binding determinants, one in the N- and one in the C-terminal domain (Wetterau et al., 1999). Recently, a group of IGFBP-related proteins (IGFBP-rPs) that bind IGFs with lower affinity have been described (Hwa et al., 1999). IGFBPs and IGFBP-rPs share the highly conserved and cysteine-rich N-terminus, which appears to be crucial for several biological actions, including their binding to IGFs and high affinity binding to insulin (Hwa et al., 1999). N-terminal fragments of IGFBP-3, generated, for example, by plasma digestion, also bind insulin and physiologically are thus likely to be relevant for insulin action. Beyond the N-terminal domain, there is a lack of sequence similarity between the IGFBPs and IGFBP-rPs.
We have recently described proteolytic studies of human IGFBP-5 and the cloning and expressing of the domain of IGFBP-5 between residues 40 and 92 (mini-IGFBP-5; Figure 1B); this domain binds IGF-I and IGF-II with KD values of 37 and 6 nM, respectively (Kalus et al., 1998). We have also determined the solution structure of uncomplexed mini-IGFBP-5 by NMR (Kalus et al., 1998). Here we describe the X-ray structure of the complex of mini-IGFBP-5 with IGF-I (Figures 2 and 3).
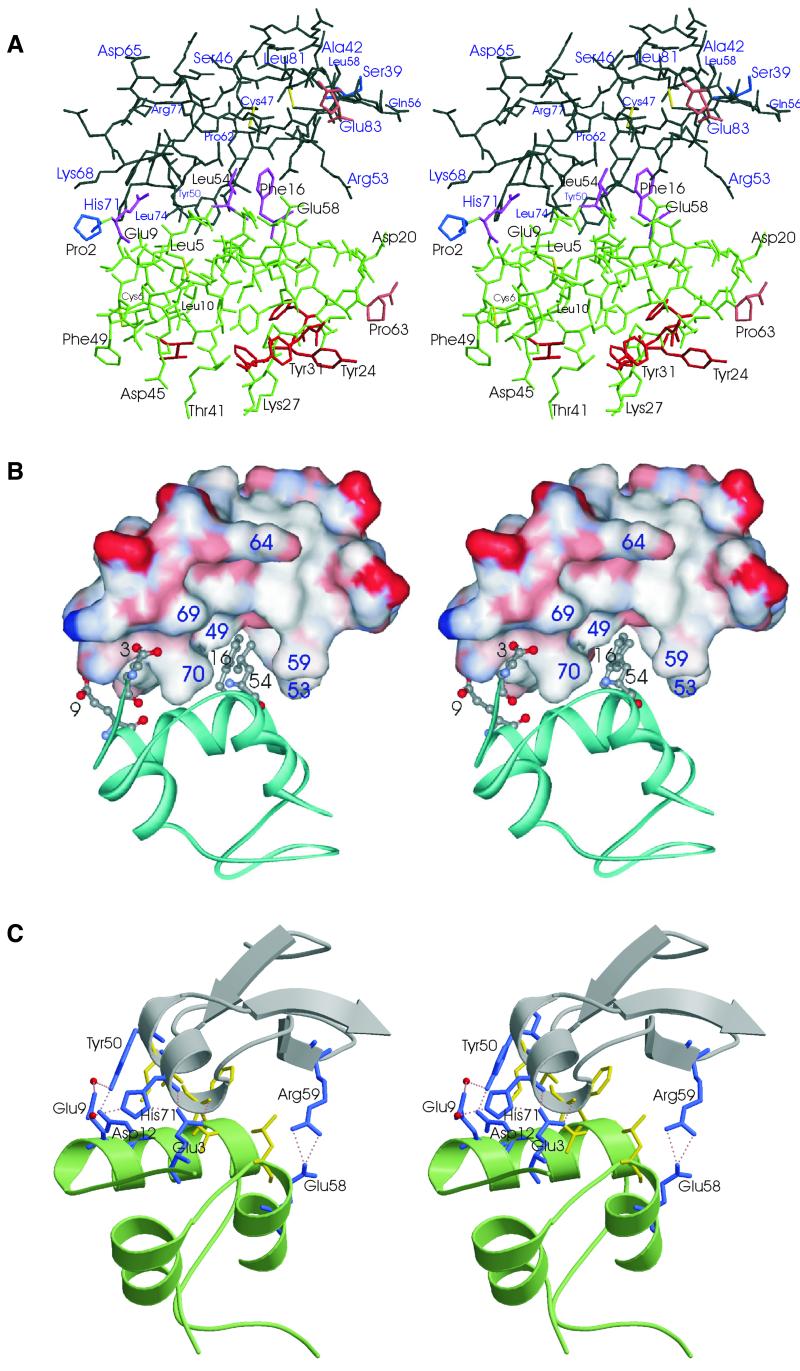
Fig. 2. The overall structure of the IGF-I (green)–mini-IGFBP-5 (black) complex. (A) A heavy atom plot. Residues shown in magenta constitute the primary binding sites for interaction with mini-IGFBP-5. Residues in red are determinants for binding to IGF-1R. The first N- and last C-terminal residues are shown in brown and blue, respectively. (B) Interface of the IGF-I–mini-IGFBP-5 complex interactions. Mini-IGFBP-5 is shown as a surface plot (residues in red, negatively charged; blue, positive; white, neutral), IGF is shown in blue. Side chains of the primary binding residues of IGF for mini-IGFBP-5 are shown. (C) Ribbon plot of IGF (green)–mini-IGFBP-5 (grey) with interface residues that form hydrogen bonds highlighted (blue). The interface hydrophobic residues are shown in yellow.
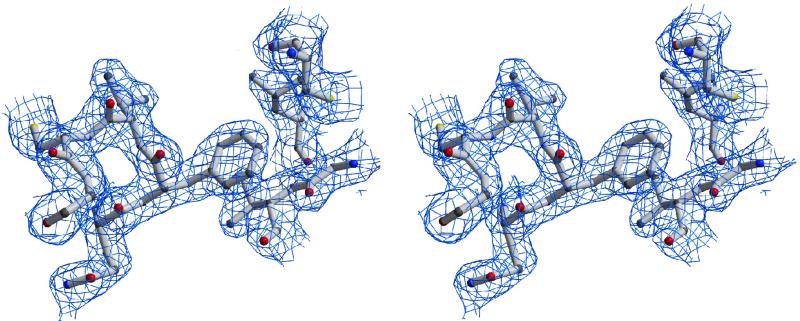
Fig. 3. Stereo figure of the 2Fo – Fc electron density map contoured at 1.0σ over the mean at the mini-IGFBP-5–IGF-I interface. The IGF-I peptide segment is at the left, including the interfacial Phe16, seen here packed against two segments of mini-IGFBP-5, between Val49 (below) and Cys60 (above).
Results and discussion
The IGF-I–mini-IGFBP-5 complex
Formation of the IGF-I–mini-IGFBP-5 complex buries a binding surface totalling ∼550 Å2. Of the 11 IGFBP-5 residues within 4 Å of IGF, six are hydrophobic, three of which are surface-exposed leucines and valines and are of primary importance for hydrophobic interaction to IGFs (Figures 1, 2 and 3A). On the IGF side, four of the 11 residues within 4 Å of mini-IBFBP-5 are hydrophobic (Figures 1 and 2).
The principal IGF-I–mini-IGFBP-5 interaction is a hydrophobic sandwich that consists of interlaced protruding side chains of IGF-I and solvent-exposed hydrophobic side chains of the mini-IGFBP-5 (Figure 2A). The side chains of IGF-I Phe16, Leu54 and also Glu3 are inserted deep into a cleft on the mini-IGFBP-5 (Figure 2A and B). This cleft is formed by side chains of Arg53 and Arg59 on the solvent-exposed side of the molecule and by Val49, Leu70 and Leu74 on the opposite inner side, with a base formed by residues Cys60 and Leu61. Phe16 makes direct contacts with the backbone and side chain of Val49, and with Cys60 of mini-IGFBP-5 (Figure 3). The hydrophobic cluster is closed on the solvent side by side chains of Glu3 and Glu9 of IGF-I, and His71 and Tyr50 of mini-IGFBP-5. These residues form a network of hydrogen bonds; in addition, Arg59 of mini-IGFBP-5 makes hydrogen bonds with Glu58 (Figure 2C).
Arg53 and Arg59 of mini-IGFBP-5 isolate the hydrophobic sandwich from the solvent close to the C-terminus. In the full-length IGFBP-5, the segment corresponding to the C-terminus of mini-IGFBP-5 is followed by nine hydrophilic residues and then by at least 30 residues of mixed types. Thus, we can postulate that the conformations seen in the structure of the complex near the C-terminus of mini-IGFBP-5 are likely to be preserved in the complex of IGF-I with the full-length IGFBP-5 (Figure 2A). The mini-IGFBP-5 domain begins at residue 40 of full length IGFBP-5. Our previous NMR study of binding of the N-terminal domain of IGFBP-5 (residues 1–102) showed unequivocally that this 39-residue segment did not interact with IGFs and that the first 39 residues of IGFBP-5 have no influence on the structure of the following mini-IGFBP-5 domain (Kalus et al., 1998).
Mutagenesis studies for IGFs indicated that IGF residues Glu3, Thr4, Gln15 and Phe16 of IGF-I and Glu6, Phe48, Arg49 and Ser50 in IGF-II are important for binding to IGFBPs (Baxter et al., 1992; Luthi et al., 1992; Bach et al., 1993; Jansson et al., 1997). Baxter et al. (1992) suggested that IGF-I Glu3, Thr4, Gln15 and Phe16 are crucial for interaction with IGFBP-3, whereas residues Phe49, Arg50 and Ser51 are of secondary importance. It was also suggested that Phe26 of IGF-II plays a role in changing the local structures of IGFs but does not bind directly to IGFBPs (Terasawa et al., 1994). Not all of these residues make direct contacts (within 4 Å) with mini-IGFBP-5; of the residues identified by mutagenesis, Gln15 neighbours the important Phe16, the IGF-I residues Phe49, Arg50 and Ser51 (equivalent to IGF-II 48, 49 and 50) are within three residues from the interface, and Phe23 (IGF-II Phe26) is far from the complex contact.
Our previous NMR study showed that the hydrophobic residues Val49, Leu70 and Leu73 of IGFBP-5 are crucial for binding to IGFs, which is fully in agreement with the current structure. Since these residues are highly conserved among all IGFBPs we expect that these hydrophobic interactions dominate the IGF-binding properties of all IGFBPs and also IGFBP-rPs. For IGFBP-rPs, it possible to produce a model of the structure of the N-terminal domains bound to IGFs using the structure of mini-IGFBP-5 as a template (data not shown; c.f. Figure 1B). In IGFBP-rP1, the crucial Leu70 of IGFBPs is replaced by Lys72. In the model of the complex, βs and γs of Lys72 make hydrophobic contacts to IGF residues sideways, similarly to Leu70 of IGFBPs. The terminal NH2s of Lys72 can insert deep into the pocket of IGF-I.
2.1 Å resolution atomic structure of IGF-I
The general fold of the free IGF-I found in the best NMR structure, that of long-[Arg3]IGF-I (Laajoki et al., 2000), is preserved in the complex, but the average root mean square deviation (r.m.s.d.) between the NMR and the X-ray structures for well defined parts of the NMR structures (residues 3–25 and 41–63) is high, with 3.7 ± 1.6 Å for α-carbons. Regrettably, the coordinates of the best quality NMR structure of IGFs, that of IGF-II, are not available (Terasawa et al., 1994). For these structures, the ensemble of the structures seems to be highly defined for most of the residues. However, large variabilities in the structures were seen for residues 1–6, the C-terminal residues 62–67, and most importantly, for the C chain residues 31–40, which form a peripheral loop. This is interesting because most of the C chain and the C-terminus in our IGF-I structure (e.g. residues 32–40 and 64–70) showed no electron density (Figures 1A and 2B). The NMR and the present X-ray data therefore indicate an increased motional flexibility in these regions of the IGF molecules. The N-terminal residues of IGF-I are well defined in the X-ray structure of the complex and unstructured in the NMR structures. The N-terminus of IGFs includes several key residues responsible for the interaction with IGFBPs and therefore the conformations of these residues most probably become fixed only upon complex formation.
The model structure constructed by Blundell et al. (1978) is closest to the present X-ray structure. R.m.s.d. values for residues between 3–25 and 41–63 are 1.07 Å for α-carbons and 2.2 Å for heavy atoms. The side chain conformation of Phe16, the residue responsible for primary interactions with IGFBP-5, is similar in both structures; however, the conformations of Glu3 and Leu54 differ, although the χ1 rotamers are similar in both structures.
Comparison between complexed and free mini-IGFBP-5
The fold of the uncomplexed mini-IGFBP-5 determined by NMR (Kalus et al., 1998) is preserved in the complex. A solvent-exposed loop between Pro62 and Pro69 was the least precisely defined segment of the structure, and five C-terminal residues of mini-IGFBP-5 were unstructured. 15N relaxation measurements indicated that the backbone of the variable loop 62–69 does not exhibit any fast picosecond time scale motions; instead, the loop residues in the free mini-IGFBP-5 show contributions from slower exchange processes with millisecond range (data not shown). IGF complex formation, however, rigidifies this loop. In the crystal structure, the loop adopts one of the many conformations that were possible for the free mini-IGFBP-5.
Implications for IGF binding to its receptor (IGF-1R)
The IGF-I receptor (IGF-1R) is a transmembrane heterotetrameric protein complex that has ∼60% sequence homology to the insulin receptor (IR). IGF-1R also binds IGF-II and insulin with 2- to 15- and 1000-fold lower affinity, respectively (Khandwala et al., 2000). There is also an IGF-II-specific receptor: the IGF-II/mannose 6-phosphate receptor, a monomeric receptor that binds IGF-II with a 500- to 1000-fold increased affinity over IGF-I but does not bind insulin. Most of the actions of IGF-II are, however, believed to be mediated via the IGF type 1 receptor (Khandwala et al., 2000). Since the ligands IGF-I, IGF-II and insulin share a common architecture and cross-react with IGF-1R and IR, it is thought that they bind to these receptors in a structurally equivalent fashion (Torres et al., 1995; Gill et al., 1996).
Extensive site-directed mutagenesis studies of mapping binding sites of IGFs and insulin for IGF-1R and IR showed that the major determinants of binding are located in the N-terminal region of the A-chain and the C-terminal strand of the B-chain (Murray-Rust et al., 1992). In IGF-I, the three aromatic residues Phe23, Tyr24 and Phe25 are known to be crucial for receptor binding (Cascieri et al., 1988), and also the A-chain Val44 is important for binding (Figure 2). Bayne et al. (1990) have demonstrated that IGF-1R recognizes in addition Tyr31 and Tyr60; in fact, all three tyrosines (24, 31 and 60) are protected from iodination when bound to IGF-1R, indicating that these residues are part of or are near to the binding site. The C region of IGF-I seems to be important in maintaining high affinity binding to the type 1 IGF receptor, since the replacement of the C region of IGF-I with a four-glycine span such as in [1–27,Gly4,38–62]hIGF-I results in a 30-fold loss of affinity for IGF-1R. More recently it was shown that binding to the IGF receptor is lost in a ‘mini’ deletion construct of IGF-I in which Pro28 and Gly42 are peptide linked. Removal of the D region has little effect on binding to IGF-1R.
Figures 1A and 2A show the location of the residues involved in the IGF-1R binding in our IGF-I structure. In most cases these residues correspond to those mapped on the structures of IGFs derived previously from NMR studies (Cooke et al., 1991; Sato et al., 1993; Laajoki et al., 2000). A general trend established from comparing IGFs binding to IGF-1R, IGF-2R, IR and IGFBPs was that the residues that bind to the type 1 receptor appear to overlap those that bind to the insulin receptor, whereas those that bind to type 2 receptor overlap those that interact with IGF-binding proteins.
The most notable feature evident from Figure 2 is that the binding site for IGF-1R consists of a fully solvent-exposed hydrophobic patch that is located on the opposite side of IGF to that for the binding to mini-IGFBP-5. This is in contrast to insulin, where the binding site for IR is partially occluded by the C-terminus of the B-chain, and it is now uniformly accepted that the C-terminus moves away from the surface of the insulin monomer on receptor binding and makes the highly conserved side chains of Ile2 and Val3 accessible for binding (Hua et al., 1991).
The current structure also supports an attractive explanation of the results of our studies on inhibition of IGF binding to the IGF-1R by IGFBP-5 and mini-IGFBP-5, and on the influence of IGFBP-5–IGF complex formation on IGF-mediated stimulation of IGF-1R autophosphorylation (Kalus et al., 1998). Whereas a complete inhibition of IGF-1R–IGF binding was observed as soon as IGFBP-5 was in excess to IGF, a 103-fold excess of mini-IGFBP-5 was needed to block IGF-II binding to its receptor. Obviously, the C-terminal domain of IGFBP-5 is essential for effective inhibition of receptor binding of IGF-I. In addition, incomplete inhibition of receptor binding was observed for mini-IGFBP-5 even at the highest concentrations used. From our structure, it appears that IGF-I can still freely bind to the receptor even when complexed to the truncated IGFBP-5 fragment. The lower inhibitory potency of mini-IGFBP-5, compared with the full-length IGFBP-5, would also be decreased by its 10-fold reduction in binding to IGF-I.
Implication for therapeutic modulation of the GH/IGF system for stroke and tumorigenesis
The present structure enables in silico screens for small IGFBP ligand inhibitors with the potential to release ‘free’ bioactive IGF-I. Displacement of IGF from their binding proteins in brain tissue, for example, should have therapeutic benefits for stroke and other neurodegenerative diseases. It has recently been demonstrated that a high-affinity IGFBP ligand inhibitor, [Leu24,59,60, Ala31] hIGF-I, which binds to IGFBPs but not to IGF-1R, elicits neuroprotective effects comparable to those produced by the administration of exogenous IGF. In a rat model of focal ischaemia, administration of this analogue after ischaemic insult to the rat brain had potent neuroprotective action comparable to IGF-I (Loddick et al., 1998).
The association of insulin-like growth factors with neoplasia indicates that modulation of the IGFBP environment might be a successful strategy in cancer therapy. Such modulation might be accomplished, for example, through exogenous administration of recombinant protein effective fragments. Additionally, tumour cell IGFBP production, inhibition or degradation may be controlled by agents such as tamoxifen and ICI 182,780, which modify tumour IGFBP production (Khandwala et al., 2000). The consequent alteration in IGFBP-3 levels appears in certain instances to inhibit IGF-I-stimulated cell proliferation (Khandwala et al., 2000). There is also recent evidence that IGFBP-3 may be a p53-independent effector of apoptosis in breast cancer cells via its modulation of the Bax:Bcl-2 protein ratio (Wetterau et al., 1999; Butt et al., 2000).
Based on the knowledge of the mini-IGFBP-5 structure (Kalus et al., 1998), mutants have been produced with modulated IGF action and altered cleavage susceptibility for IGFBP-5 protease (Imai et al., 2000). Such mutants may identify roles for IGFBPs that require IGF-I binding and distinguish them from those that are IGF independent (Imai et al., 2000). In conclusion, the structure of the IGF-I–mini-IGFBP-5 complex will advance the development of IGFs with reduced binding affinity for IGFBPs and consequently enhanced activity, and of IGFBPs with higher affinity for IGFs and consequent inhibition of IGF signalling. Furthermore, it should contribute to the search for small IGFBP ligand inhibitors that release IGFs from the inactive complex with IGFBPs.
Materials and methods
Crystallization, data collection and derivatization
Mini-IGFBP-5 was produced as described recently (Kalus et al., 1998) and IGF-I was obtained from OvoPepi, Australia. Crystallization was successful with 10% Jeffamine M-600, 0.1 M sodium citrate, 0.01 M ferric chloride pH 5.6. Within 11 days, crystals appeared at 4°C, growing to a final size of ∼0.3 × 0.2 × 0.2 mm3. They belong to the cubic space group P213 and have unit cell dimensions a, b, c = 74.385 Å, with one complex molecule per asymmetric unit. Soaking in precipitation buffer with heavy atom compounds yielded a derivative K2PtCl4 (2.7 mM, 3 days), which was interpretable. All diffraction data were collected using a 300 mm MAR Research (Hamburg, Germany) image plate detector mounted on a Rigaku (Tokyo, Japan) RU300 rotating anode X-ray generator with graphite monochromatized CuKα radiation. All image plate data were processed with MOSFLM (Leslie, 1991) and the CCP4 program suite (CCP4, 1994).
Phase calculation, model building and refinement
The structure of the IGF–BP5 complex was solved by the single isomorphous replacement (SIR) method using the heavy atom derivative described above. Derivative data were analysed with the native data set, first using isomorphous difference Patterson maps and employing the Patterson vector superposition methods implemented in SHELX-97 (Sheldrick, 1991). The two heavy site locations were confirmed by difference Fourier methods with appropriate initial single site SIR phases using CCP4 programs. The refinement of heavy atom parameters and calculation of SIR phases were done with SHARP (La Fortelle and de Bricogne, 1997). The final parameters are given in Table I. The initial SIR phases were improved with SOLOMON (Abrahams and Leslie, 1996) using a solvent fraction of 45%, resulting in a 2.1 Å electron density map that was of such high quality as to enable automated structure building with ARP (Lamzin and Wilson, 1993). All further model building was carried out with the program O (Jones et al., 1991). Refinement was performed by conjugate gradient and simulated annealing protocols as implemented in CNS 1.0 (Brünger et al., 1998). All protocols included refinement of individual isotropic B-factors and using the amplitude-based maximum likelihood target function. The R-factor dropped to 21.8% (Rfree = 26.2%, resolution range 16.2–2.1 Å) for the final model including 47 water molecules. The water model was calculated using ARP and verified by visual inspection. The final refinement statistics are shown in Table I. Coordinates have been deposited in the Protein Data Bank (accession code 1H59).
Table I. Statistics from the crystallographic analysis.
| Native | K2PtCl4 | |
|---|---|---|
| Resolution (Å) | 16.2–2.1 | 18.6–2.5 |
| Measurements | 45 345 | 32 833 |
| Unique measurements | 8035 | 4925 |
| % complete (last shell/Å) | 99.3 (96.9/2.23–2.11) | 99.9 (95.4/2.64–2.5) |
| Rsym (%) (last shell) | 8.2 (44.8) | 8.8 (49.5) |
| RCullis-iso | – | 0.77 |
| Piso | – | 1.48 |
| Resolution for phase calculation (Å) | – | 18.6–2.5 |
| Mean FOM |
– |
0.4 |
| Refinement statistics |
|
|
| Resolution range (Å) | 16.2–2.1 | |
| Reflections in working set | 7522 | |
| Reflections in test set | 501 | |
| Rcryst (%) | 21.8 | |
| Rfree (%) | 26.2 | |
| Protein atoms (non-H) | 765 | |
| Solvent atoms (non-H) | 47 | |
| Average B-factor (Å2) | 38.1 | |
| R.m.s. ΔB (2 Å cutoff) | 3.4 | |
| Deviations from ideality (r.m.s.) | ||
| bond lengths (Å) | 0.013 | |
| bond angles (°) | 1.7 |
Acknowledgments
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 469). W.Ż. (on a leave of absence from the Department of Chemistry, Jagiellonian University, PL-30-060 Krakow, Poland) was a recipient of a postdoctoral fellowship from the Alexander von Humboldt Foundation.
References
- Abrahams J.P. and Leslie,A.G.W. (1996) Methods used in the structure determination of bovine mitochondrial F1 ATPase. Acta Crystallogr. D, 52, 30–42. [DOI] [PubMed] [Google Scholar]
- Adams M.J., Blundell,T.L., Dodson,E.J., Vijazan,M., Baker,E.N., Hodgkin,D.C., Rimm,B. and Sheat,S. (1969) Structure of rhombohedral 2 zinc insulin crystals. Nature, 224, 491–495. [Google Scholar]
- Bach L.A., Hsieh,S., Sakano,K., Fujiwara,H., Perdue,J.F. and Rechler,M.M. (1993) Binding of mutants of human insulin-like growth factor II to insulin-like growth factor binding proteins 1–6. J. Biol. Chem., 268, 9246–9254. [PubMed] [Google Scholar]
- Baxter R.C., Bayne,M.L. and Cascieri,M.A. (1992) Structural determinants for binary and ternary complex formation between insulin-like growth factor-I (IGF-I) and IGF binding protein-3. J. Biol. Chem., 267, 60–65. [PubMed] [Google Scholar]
- Bayne M.L., Applebaum,J., Chicchi,G.G., Miller,R.E. and Cascieri,M.A. (1990) The roles of tyrosine 24, 31, and 60 in the high affinity binding of insulin-like growth factor-I and the type 1 insulin-like growth factor receptor. J. Biol. Chem., 265, 15648–15652. [PubMed] [Google Scholar]
- Blundell T.L., Berdarkar,S., Rinderknecht,E. and Humblel,R.E. (1978) Insulin-like growth factor: a model for tertiary structure accounting for immunoreactivity and receptor binding. Proc. Natl Acad. Sci. USA, 75, 180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brünger A.T. et al. (1998) Crystallography and NMR system—a new software suite for macromolecular structure determination. Acta Crystallogr. D, 54, 905–921. [DOI] [PubMed] [Google Scholar]
- Butt A.J., Firth,S.M., King,M.A. and Baxter,R.C. (2000) Insulin-like growth factor-binding protein-3 modulates expression of Bax and Bcl-2 and potentiates p53-independent radiation-induced apoptosis in human breast cancer cells. J. Biol. Chem., 275, 39174–39181. [DOI] [PubMed] [Google Scholar]
- Cascieri M.A., Chicchi,G.C., Applebaum,J., Hazes,N.S., Green,B.C. and Bayne,M.L. (1988) Mutants of human insulin-like growth factor I with reduced affinity for the type I insulin-like growth factor receptor. Biochemistry, 27, 3229–3233. [DOI] [PubMed] [Google Scholar]
- Chernausek S.D., Smith,C.E., Duffin,K.L., Busby,W.H., Wright,G. and Clemmons,D.R. (1995) Proteolytic cleavage of insulin-like growth factor binding protein 4 (IGFBP-4). Localization of cleavage site to non-homologous region of native IGFBP-4. J. Biol. Chem., 270, 11377–11382. [DOI] [PubMed] [Google Scholar]
- Collaborative Computational Project, Number 4 (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D, 50, 760–763. [DOI] [PubMed] [Google Scholar]
- Cooke R.M., Harvey,T.S. and Campbell,I.D. (1991) Solution structure of human insulin-like growth factor 1: a nuclear magnetic resonance and restrained molecular dynamics study. Biochemistry, 30, 5484–5491. [DOI] [PubMed] [Google Scholar]
- Fanayan S., Firth,S.M., Butt,A.J. and Baxter,R.C. (2000) Growth inhibition by insulin-like growth factor binding protein-3 in T47D breast cancer cells requires transforming growth factor-β (TGF-β) and type II TGF-β receptor. J. Biol. Chem., 275, 39146–39151. [DOI] [PubMed] [Google Scholar]
- Gill R. et al. (1996) Engineering the C-region of human insulin-like growth factor-1. Implications for receptor binding. Protein Eng., 9, 1011–1019. [DOI] [PubMed] [Google Scholar]
- Hankinson S.E., Willet,W.C., Colditz,G.A., Hunter,D.J., Michaud,D.S., Deroo,B., Rosner,B., Speizer,F.E. and Pollak,M. (1998) Circulating concentrations of insulin-like growth factor I and risk of breast cancer. Lancet, 351, 1393–1396. [DOI] [PubMed] [Google Scholar]
- Holly J. (1998) Insulin-like growth factor-I and new opportunities for cancer prevention. Lancet, 351, 1373–1375. [DOI] [PubMed] [Google Scholar]
- Hua Q.X., Shoelson,S.E., Kochoyan,M. and Weiss,M.A. (1991) Receptor binding redefined by a structural switch in a mutant human insulin. Nature, 354, 238–241. [DOI] [PubMed] [Google Scholar]
- Hwa V. et al. (1999) The IGF binding protein superfamily. In Rosenfeld,R.G. and Roberts,C.T. (eds), The IGF System. Molecular Biology, Physiology, and Clinical Applications. Humana Press, Totowa, NJ, pp. 315–327.
- Imai Y., Moralez,A., Andag,U., Clarke,J.B., Busby,W.H. and Clemmons,D.R. (2000) Substitutions for hydrophobic amino acids in the N-terminal domains of IGFBP-3 and -5 markedly reduce IGF-I binding and alter their biologic actions. J. Biol. Chem., 275, 18188–18194. [DOI] [PubMed] [Google Scholar]
- Jansson M., Uhlen,M. and Nilsson,B. (1997) Structural changes in insulin-like growth factor IGF I mutant proteins affecting binding kinetic rates to IGF binding protein 1 and IGF-I receptor. Biochemistry, 36, 4108–4117. [DOI] [PubMed] [Google Scholar]
- Jones J.L. and Clemmons,D.R. (1995) Insulin-like growth factors and their binding proteins: biological actions. Endocr. Rev., 16, 3–34. [DOI] [PubMed] [Google Scholar]
- Jones T.A., Zou,J.Y., Cowan,S.W. and Kjeldgaard,M. (1991) Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A, 47, 110–119. [DOI] [PubMed] [Google Scholar]
- Kalus W. et al. (1998) Structure of the IGF binding domain of the insulin-like growth factor-binding protein-5 (IGFBP-5): implications for IGF and IGF-I receptor interactions. EMBO J., 17, 6558–6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandwala H.M., McCutcheon,I.E., Flyvbjerg,A. and Friend,K.E. (2000) The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr. Rev., 21, 215–244. [DOI] [PubMed] [Google Scholar]
- Laajoki L.G., Francis,G.L., Wallace,J.C., Carver,J.A. and Keniry,M.A. (2000) Solution structure and backbone dynamics of long-[Arg3]insulin-like growth factor-I. J. Biol. Chem., 275, 10009–10015. [DOI] [PubMed] [Google Scholar]
- La Fortelle E. and de Bricogne,G. (1997) Maximum-likelihood heavy-atom parameter refinement for multiple isomorphous replacement and multiwavelength anomalous diffraction methods. Methods Enzymol., 276, 472–494. [DOI] [PubMed] [Google Scholar]
- Lamzin V.S. and Wilson,K.S. (1993) Automated refinement of protein models. Acta Crystallogr. D, 49, 129–147. [DOI] [PubMed] [Google Scholar]
- Leslie A.G.W. (1991) Molecular data processing. In Moras,D., Podjarny,A.D. and Thierry,J.C. (eds), Crystallographic Computing 5. Oxford University Press, Oxford, UK, pp. 50–61.
- Loddick S.A. et al. (1998) Displacement of insulin-like growth factors from their binding proteins as a potential treatment for stroke. Proc. Natl Acad. Sci. USA, 95, 1894–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi C., Roth,B.V. and Humbel,R.E. (1992) Mutants of human insulin-like growth factor II (IGF II). Expression and characterization of truncated IGF II and of two naturally occurring variants. Eur. J. Biochem., 205, 483–490. [DOI] [PubMed] [Google Scholar]
- Martin J.L. and Baxter,R.C. (1999) IGF binding proteins as modulators of IGF actions. In Rosenfeld,R.G. and Roberts,C.T. (eds), The IGF System. Molecular Biology, Physiology, and Clinical Applications. Humana Press, Totowa, NJ, pp. 227–255.
- Mazerbourg S., Zapf,J., Bar,R.S., Brigstock,D.R. and Monget,P. (1999) Insulin-like growth factor binding protein-4 proteolytic degradation in ovine preovulatory follicles: studies of underlying mechanisms. Endocrinology, 140, 4175–4184. [DOI] [PubMed] [Google Scholar]
- Murray-Rust J., McLeod,A.N., Blundell,T.L. and Wood,S.P. (1992) Structure and evolution of insulins: implications for receptor binding. BioEssays, 14, 325–331. [DOI] [PubMed] [Google Scholar]
- Sato A., Nishimura,S., Ohkuba,T., Kyogoku,Y., Koyama,S., Kobayashi,M., Ysuda,T. and Kobayashi,Y. (1993) Three-dimensional structure of human insulin-like growth factor-1 (IGF-1) determined by 1H NMR and distance geometry. Int. J. Pept. Protein Res., 41, 433–440. [DOI] [PubMed] [Google Scholar]
- Sheldrick G. (1991) Tutorial on automated Patterson interpretation to find heavy atoms. In Moras,D., Podjarny,A.D. and Thierry,J.C. (eds), Crystallographic Computing 5. Oxford University Press, Oxford, UK, pp. 145–157.
- Terasawa H., Kohda,D., Hatanaka,H., Nagata,K., Higashihashi,N., Fujiwara,H., Sakano,K. and Inagaki,F. (1994) Solution structure of human insulin-like growth factor II; recognition sites for receptors and binding proteins. EMBO J., 13, 5590–5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres A.M., Forbes,B.E., Aplin,S.E., Wallace,J.C., Francis,G.L. and Norton,R.S. (1995) Solution structure of human insulin-like growth factor II. Relationship to receptor and binding protein interactions. J. Mol. Biol., 248, 385–401. [DOI] [PubMed] [Google Scholar]
- Wetterau L.A., Moore,M.G., Lee,K.W., Shim,M.L. and Cohen,P. (1999) Novel aspects of the insulin-like growth factor binding proteins. Mol. Gen. Metab., 68, 161–181. [DOI] [PubMed] [Google Scholar]
- Wolk A. (2000) Can measurements of IGF-1 and IGFBP-3 improve the sensitivity of prostate-cancer screening. Lancet, 356, 1902–1903. [DOI] [PubMed] [Google Scholar]