Abstract
Myosin VI is involved in membrane traffic and dynamics and is the only myosin known to move towards the minus end of actin filaments. Splice variants of myosin VI with a large insert in the tail domain were specifically expressed in polarized cells containing microvilli. In these polarized cells, endogenous myosin VI containing the large insert was concentrated at the apical domain co-localizing with clathrin- coated pits/vesicles. Using full-length myosin VI and deletion mutants tagged with green fluorescent protein (GFP) we have shown that myosin VI associates and co-localizes with clathrin-coated pits/vesicles by its C-terminal tail. Myosin VI, precipitated from whole cytosol, was present in a protein complex containing adaptor protein (AP)-2 and clathrin, and enriched in purified clathrin-coated vesicles. Over-expression of the tail domain of myosin VI containing the large insert in fibroblasts reduced transferrin uptake in transiently and stably transfected cells by >50%. Myosin VI is the first motor protein to be identified associated with clathrin-coated pits/vesicles and shown to modulate clathrin-mediated endocytosis.
Keywords: actin/clathrin-coated vesicles/endocytosis/myosin
Introduction
The movement of vesicles between different compartments within mammalian cells is partly dependent on interactions with the cytoskeleton. In general it is believed that long distance transport involves motor proteins such as kinesin and dynein moving vesicles along microtubules, whereas movement over short distances may require interactions with actin filaments. There is a wealth of evidence that the actin cytoskeleton is important in post-Golgi membrane traffic both in the secretory and endocytic pathways (Kübler and Riezman, 1993; Goodson et al., 1997; Kroschewski et al., 1999; Qualmann et al., 2000). Thus, the disruption of actin filaments inhibits receptor-mediated endocytosis (Lamaze et al., 1997), having a particularly dramatic effect at the apical surface of polarized epithelial cells (Gottlieb et al., 1993; Jackman et al., 1994; Shurety et al., 1996). More recent evidence suggests that clathrin-coated pits are intimately associated with the actin cytoskeleton directly underlying the plasma membrane bilayer (Gaidarov et al., 1999) and that endocytic vesicles may use actin polymerization to move into the cytosol after pinching off from the plasma membrane (Merrifield et al., 1999). Furthermore, endocytic uptake via clathrin-coated pits is inhibited in cells overexpressing an activated form of Rac, which is a major player in a growth factor stimulated signalling pathway shown to regulate the actin cytoskeleton (Lamaze et al., 1996). However, relatively little is known about the actin-associated proteins required for these actin-dependent membrane traffic events, especially the myosins, which are likely to be the molecular motors involved. Myosins are motor proteins that move along actin filaments and generate force for membrane protrusion and retraction or potentially carry membrane vesicles (Mermall et al., 1998; Sellers, 2000). There is evidence that members of three out of the 18 classes so far identified (Berg et al., 2001), namely myosins I, V and VI, are involved in membrane traffic (Hasson and Mooseker, 1995; DePina and Langford, 1999).
In this paper we focus on myosin VI. In mammalian cells myosin VI is concentrated in the terminal web of the apical brush border of polarized epithelial cells (Heintzelman et al., 1994) and in sensory hair cells of the inner ear where it is localized in the cuticular plate close to the stereocilia rootlets and in the pericuticular necklace (Hasson et al., 1997). Mutations in the myosin VI gene lead to the recessive deafness disorder and morphological abnormalities of the intestinal brush border observed in Snell’s waltzer mice (Avraham et al., 1995). The absence of myosin VI leads to fusion of stereocilia during development of the sensory hair cells in the first weeks after birth (Self et al., 1999). Interestingly, myosin VI is an actin-based motor protein with a very unusual property as it is the only myosin known to move towards the minus end of actin filaments (Wells et al., 1999). Thus, its movement overturns the dogma that all myosin motors move in the same direction along actin filaments, i.e. towards the plus end.
Myosin VI is expressed as a number of different splice variants, as first described in Drosophila (Kellerman and Miller, 1992). In the striped bass (Morone saxatilis), myosin VI isoforms containing either a large 13 or 17 amino acid (aa) insert or a small 9 aa insert in the C-terminal tail have also been identified (Breckler et al., 2000). Looking at human and other mammalian tissues we found a tissue specific expression of splice variants of myosin VI containing a large, a small or no insert in their tails. The splice variant containing the large insert was specifically expressed in polarized cells with microvilli at their apical surface. In these polarized cells the endogenous myosin VI co-localized with clathrin-coated vesicles. To extend these studies to probe the targeting of myosin VI to specific intracellular compartments and to explore the importance of the large insert in the tail domain on the localization of myosin VI we have, in the present study, expressed full-length and domain fragments of myosin VI tagged with green fluorescent protein (GFP) in normal rat kidney (NRK) cells. Full-length myosin VI tagged with GFP containing the large tail insert localizes to the Golgi complex and to membrane ruffles at the leading edge but in addition gives a distinct punctate staining pattern close to the plasma membrane. Double labelling experiments showed that GFP-tagged myosin VI co-localizes with adaptor protein (AP)-2, the adaptor complex for clathrin-coated pits/vesicles at the plasma membrane, and that the very C-terminal globular domain of the tail of myosin VI is essential for targeting to these AP-2-containing structures. The large insert in the tail enhances binding to clathrin-coated pits/vesicles, but by itself is not sufficient. The localization of myosin VI containing the large insert to clathrin-coated pits/vesicles was confirmed at the electron microscope (EM) level. Using pull-down and co-immunoprecipitation assays we have further shown that myosin VI is present in a protein complex containing AP-2 and clathrin. By measuring transferrin uptake we were able to demonstrate that the C-terminal tail of myosin VI containing the large insert could act as a dominant-negative inhibitor of clathrin-mediated endocytosis.
Results
Differential expression of myosin VI isoforms in polarized or non-polarized cells
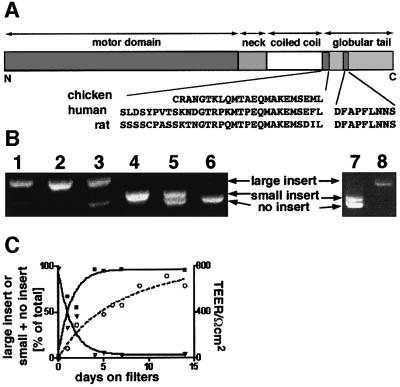
Using rat and human tissues we cloned and sequenced three different splice variants containing inserts in a similar position in the tail to those previously described in the fish. The large insert, which occurs at the C-terminal end of the coiled-coil region, is highly conserved between human, rat and chicken, and is 31 aa long in human and rat but only 23 aa long in chicken. In fish there are two myosin VI isoforms with large inserts of 13 or 17 aa (Figure 1A; Buss et al., 1998; Breckler et al., 2000). The small insert, which occurs in the C-terminal globular tail (GT) region, is completely conserved between rat and human and shares a significant homology with the insert in the fish (DHAPPVKKA). No recognizable sequence motifs have been identified so far in these inserts. Using a PCR-based strategy we observed that myosin VI with the large insert was predominantly expressed in rat tissues containing a high proportion of polarized epithelial cells with microvilli on their apical domain such as in liver, kidney and small intestine, whereas the isoforms with the small or no insert were expressed in tissues such as brain, testis and lung, which contain mostly cells lacking apical microvilli (Figure 1B). To investigate further the expression and localization of different myosin VI splice variants we used Caco-2 cells. These cells are derived from human small intestine and polarize by developing highly differentiated apical microvilli (similar in appearance to brush border cells in the small intestine) when grown on filter supports (Pinto et al., 1983). Caco-2 cells grown on plastic are much less polarized and have less well developed microvilli. In unpolarized Caco-2 cells, myosin VI with the small insert or no insert was expressed, whereas when the cells were grown on membrane filters to encourage maximum polarization, expression of the isoform with the large insert was observed (Figure 1B). After 5 days growth on filters, when the transepithelial electrical resistance (TEER) had reached half its maximum value, the smaller isoforms of myosin VI were hardly detectable (Figure 1C).
Fig. 1. Expression of myosin VI tail isoforms in different tissues and polarized and unpolarized cells. (A) Schematic representation of myosin VI showing the position and sequence of the tail inserts. (B) The myosin VI tail isoform with the large insert is expressed in rat tissues containing many polarized cells having microvilli (liver, lane 1; kidney, lane 2; and small intestine, lane 3) and in polarized Caco-2 cells (lane 8), whereas unpolarized Caco-2 cells (lane 7) and rat tissues containing few polarized cells with microvilli on their apical surface (brain, lane 4; testis, lane 5; and lung, lane 6) contain myosin VI with either the small or no insert in the tail domain. mRNA prepared from rat tissues and Caco-2 cells was used in RT–PCR reactions with primers flanking the region of the tail containing the two inserts (∼300 bp). (C) Expression levels of myosin VI tail isoforms (large insert, closed squares; small or no insert, closed triangles) during polarization of Caco-2 cells plotted as percent of total isoforms. Polarization was monitored by measuring the TEER (open circle). Caco-2 cells were grown on filters and harvested for RT–PCR reactions at different times after seeding.
Localization of myosin VI in polarized Caco-2 cells
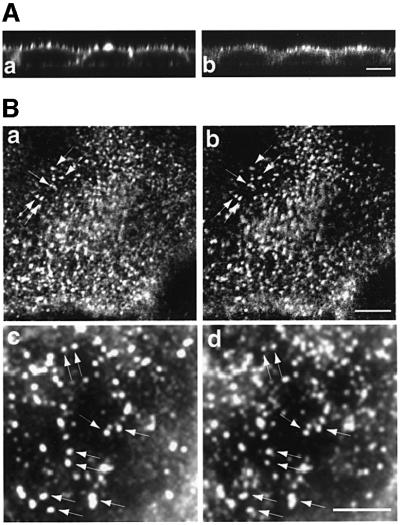
Although in polarized Caco-2 cells the endogenous myosin VI containing the large insert is enriched in the terminal web just underneath the microvilli where it shows a punctate staining pattern (Figure 2A), it is also present at the basolateral domain. To establish the identity of the apical punctate structures, double labelling experiments for the endogenous proteins with antibodies to the tail of myosin VI (Figure 2B, a and c) and to the subunit of the adaptor complex AP-2 (b and d) were carried out by immunofluorescence. Vesicular structures positive for myosin VI show very good co-localization with structures containing AP-2 or clathrin, suggesting that endogenous myosin VI in Caco-2 cells is present in clathrin-coated vesicles (Figure 2B).
Fig. 2. Endogenous myosin VI is localized to clathrin-coated vesicles in the apical domain of polarized Caco-2 cells. (A) Caco-2 cells, grown on filters for 14 days, were (a) labelled for immunofluorescence with rhodamine-phalloidin to localize actin or (b) with antibody to myosin VI. Confocal vertical X–Z optical sections are shown. (B) Myosin VI co-localizes with clathrin-coated pits/vesicles at the apical domain of Caco-2 cells. Immunofluorescence (a) and (c) with antibodies to whole myosin VI tail or (b) and (d) with antibodies to AP-2. The polyclonal antibody to the whole tail of myosin VI has been described previously (Buss et al., 1998). A section through the apical domain above the nucleus is shown. (c) and (d) show enlarged images of co-localization of myosin VI (c) and AP-2 (d). Arrows identify a few of the spots showing co-localization of myosin VI and AP-2. (A) Bar: 10 µm; (B) bar: 3 µm.
Localization of myosin VI tagged with GFP in unpolarized cells
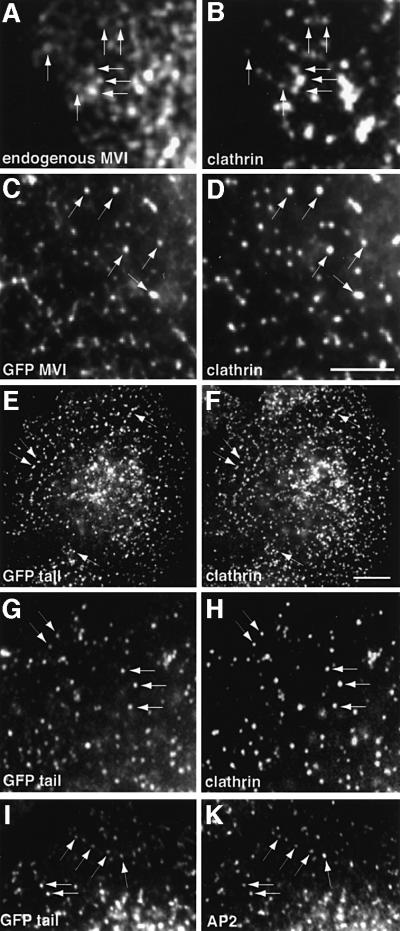
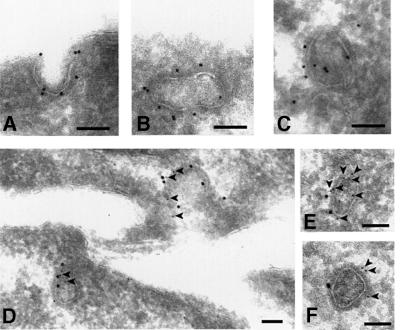
In unpolarized fibroblasts, like NRK cells, the myosin VI isoform without the large insert is expressed. In these cells the endogenous myosin VI co-localizes to a much lesser extent with clathrin-coated pits/vesicles at the plasma membrane (Figure 4A and B). To study localization further, GFP-tagged constructs of myosin VI and its various domains were prepared (Figure 3). When we overexpressed, in unpolarized NRK cells, a GFP-tagged myosin VI (Figure 4C) and the whole tail domain (Figure 4E, G and I), both containing the large insert, we observed that these expressed proteins were targeted to clathrin-coated pits/vesicles and showed almost complete co-localization with AP-2 and clathrin at the plasma membrane (Figure 4C–K). It should be noted that myosin VI cloned from chicken epithelial brush border cells (Buss et al., 1998) that was used in these expression studies contains the large insert in the tail domain and, therefore, is equivalent to the endogenous myosin VI found in Caco-2 cells. Localization in clathrin-coated pits/vesicles was confirmed at the EM level (Figure 5) using anti-GFP antibodies on stable cell lines overexpressing the GFP-tagged tail domain of myosin VI containing the large insert. This GFP-tagged protein was present in clathrin-coated pits at the plasma membrane (Figure 5A) and also on 100–150 nm coated vesicles that appear in the cytosol after apparent internalization (Figure 5B and C). Double labelling experiments show that clathrin and GFP-tagged myosin VI tail containing the large insert are indeed in close proximity on the same coated pit or vesicle (Figure 5D–F).
Fig. 4. Co-localization of myosin VI with AP-2 and clathrin in NRK cells. (A) An untransfected cell stained for endogenous myosin VI with an antibody to the whole tail. Endogenous myosin VI not containing the large insert (A) shows partial co-localization with clathrin (B) in NRK cells. NRK cells transiently expressing whole myosin VI from chicken brush border containing the large insert (C) or only the tail containing the large insert, both tagged with GFP (E, G and I), were stained with antibodies to AP-2 (K) or clathrin (D, F and H). The GFP–myosin VI and the tail show co-localization with clathrin and AP-2 at the plasma membrane. Bar: 20 µm.
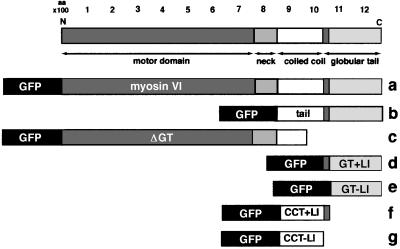
Fig. 3. Schematic representation of whole myosin VI and different myosin VI deletion mutants tagged with GFP. (a) Whole myosin VI; (b) whole tail (tail); (c) myosin VI without the ΔGT; (d) GT with the large insert (GT + LI); (e) GT without the large insert (GT – LI); (f) CCT with the large insert (CCT + LI) or (g) CCT without the large insert (CCT – LI). All constructs carried a GFP tag at their N-termini for expression in NRK cells.
Fig. 5. Localization of myosin VI in clathrin-coated pits/vesicles. For immuno-EM, stable NRK cells lines expressing GFP–GT were labelled (A, B and C) with antibodies to GFP (Molecular Probes, Leiden) followed by protein-A 15 nm gold as described by Buss et al. (1998). (D, E and F) The cryosections were double labelled for GFP–GT (5 nm gold, arrow heads) and for clathrin (15 nm gold). Bar: 100 nm.
Targeting of GFP-tagged myosin VI deletion mutants
The roles of different domains and in particular the importance of the large insert in the tail on the localization of myosin VI were investigated by transiently expressing full-length and several deletion mutants of GFP-tagged chicken brush border myosin VI in NRK cells. The deletion mutants consist of whole chicken brush border myosin VI without the GT (Figure 3c, ΔGT), the whole tail (b, tail), the globular domain with (d, GT + LI) or without the large insert (e, GT – LI) and the coiled-coil tail domain with (f, CCT + LI) or without the large insert (g, CCT – LI) all tagged with GFP at their N-termini (Figure 3). GFP–myosin VI (Figure 6B) was observed to be concentrated at the Golgi complex (small arrows), in ruffles at the leading edge (large arrows) and in a punctate staining pattern at the plasma membrane (arrow heads), which was identified as clathrin-coated pits/vesicles (see Figure 4). In these NRK cells, endogenous myosin VI (Figure 6A; Buss et al., 1998) was found by immunofluorescence in a very similar localization: in membrane ruffles, at the Golgi complex and in a punctate pattern, which partially co-localizes with clathrin-coated vesicles (see Figure 4A and B). When NRK cells were stimulated with platelet-derived growth factor (PDGF) to induce ruffling, GFP–myosin VI was incorporated into the newly formed ruffles, indicating that it was fully functional (data not shown). The punctate staining pattern at the plasma membrane was only observed in cells expressing low levels of GFP–myosin VI where there was only a very small pool of free cytosolic GFP-tagged protein. Targeting to these punctate structures and to the Golgi complex required the C-terminal GT domain, as only deletion mutants containing this domain showed a punctate staining pattern (Figure 6B, C, E and F), whereas constructs without this domain were mainly cytosolic (Figure 6D, G and H). The large insert enhanced localization of the globular domain to clathrin-coated vesicles when expression of the GT with or without the large insert was compared (see Figure 6E and F). On the other hand, expression of the large insert fused to the coiled-coil domain does not target this domain to clathrin-coated pits/vesicles, indicating that the large insert by itself does not contain all the targeting information (Figure 6G and H). It only enhances localization to clathrin-coated pits/vesicles in conjunction with the GT (Figure 6E and F). Interest ingly, the localization patterns of the GFP–myosin VI mutants also demonstrated that for the myosin VI to be incorporated into ruffles at the leading edge, a functional motor domain is necessary (Figure 6B and D).
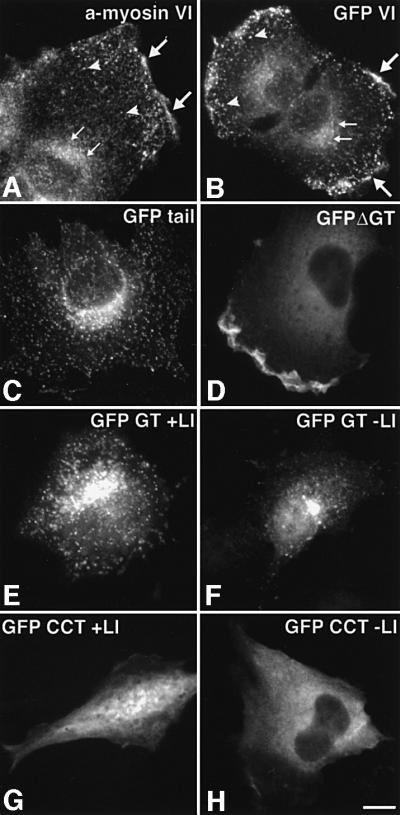
Fig. 6. Localization of GFP–myosin VI and GFP–myosin VI deletion mutants in NRK cells. (A) An untransfected cell stained for endo genous myosin VI with an antibody to the whole tail (a-myosin VI). Myosin VI is enriched in the perinuclear area (small arrows), in ruffles at the edge of the cell (large arrows) and in a vesicular staining pattern in the thin leading lamella (arrowheads). (B–H) NRK cells transiently expressing GFP-tagged constructs: (B) whole myosin VI tagged with GFP [symbols as in (A)]; (C) GFP–tail; (D) GFP–myosin VI without the GT (GFP ΔGT); (E) the GT containing the large insert (GFP GT + LI); (F) the GT without the large insert (GFP GT – LI); (G) the coiled-coil domain of the tail tagged with GFP containing the large insert (GFP CCT + LI); and (H) the coiled-coil domain without the large insert (GFP CCT – LI). The GT together with the large insert are important for targeting to vesicular structures [see (B), (C), (E) and (F) compared with (D), (G) and (H)]. Only expression constructs including the motor domain were found in ruffles [compare (B) and (D) with (C), (E) and (F)]. Bar: 15 µm.
In vitro binding of myosin VI to components of clathrin-coated pits/vesicles
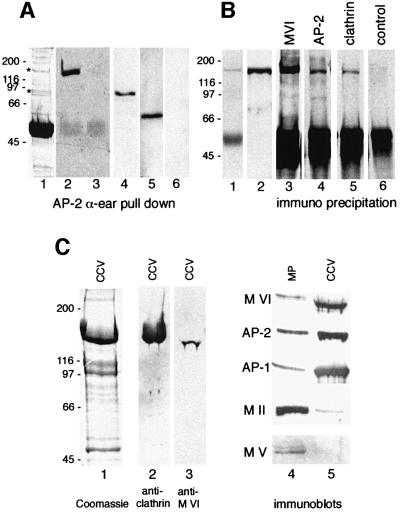
The interaction of myosin VI with components of clathrin-coated pits/vesicles was investigated using in vitro pull-down experiments with the ear domain of the α-subunit of the AP-2 adaptor. When this ear domain expressed as a glutathione S-transferase (GST) fusion protein was incubated with cytosol prepared from A431 cells, a substantial amount of endogenous myosin VI was bound, but neither GST alone (Figure 7A, lanes 2 and 3) nor a GST–γ ear (from AP-1) construct bound any myosin VI (not shown). In addition, GST–α ear was able to pull-down either the GFP-tagged chicken myosin VI whole tail or just the GT, but not GFP alone, from cytosol prepared from NRK cells overexpressing either one of these three constructs (Figure 7A, lanes 4–6). Similar pull-down experiments have been used previously to show interactions between the AP-2 α-ear and amphiphysin, Eps15, epsin, auxillin and AP180 (Owen et al., 1999). Bands corresponding in molecular weight to these proteins were observed in the Coomassie-stained gel of the AP-2 α-ear pull-down. The interaction of myosin VI with AP-2 was confirmed in native co-immunoprecipitation experiments using cytosol from A431 cells, where myosin VI was detected in the immunoprecipitates prepared with antibodies to AP-2 and also with antibodies to clathrin (Figure 7B). Myosin VI was also found to be enriched like AP-1 and AP-2 in purified rat liver clathrin-coated vesicles (Figure 7C, lane 5) when compared with a 100 000 g microsomal pellet prepared from rat liver homogenate (Figure 7C, lane 4). In contrast, non-muscle myosin II and myosin V were hardly detectable in these purified clathrin-coated vesicle preparations (Figure 7C, lane 5). The amount of myosin VI relative to clathrin was estimated using immunoblotting with purified proteins as standards (data not shown). Assuming that there are ∼200 molecules of clathrin per 100 nm clathrin-coated vesicle, we calculate that one clathrin-coated vesicle contains on average two myosin VI motor proteins.
Fig. 7. Myosin VI interacts in vitro with AP-2 and clathrin. (A) The pull-down experiments shown demonstrated the binding of myosin VI (lane 2), GFP–tail (lane 4) and GFP–GT (lane 5) to the ear of the α-subunit of AP-2. Cytosol for these experiments was prepared from A431 (lanes 1–3) or NRK cells (lanes 4–6). The latter were stably transfected with GFP–tail (lane 4), GFP–GT (lane 5) or GFP (lane 6). Protein binding to the α-subunit of AP-2 was analysed by SDS–PAGE (lane 1) or by immunoblotting with anti-myosin VI serum (lanes 2 and 3) or with an antibody to GFP (Molecular Probes, Leiden, The Netherlands) (lanes 4–6). Lanes 3 and 6 show a blank control, in which instead of GST–α ear only GST was used. In lane 1 a Coomassie stained gel of a GST–α ear pull-down from A431 cytosol is shown. The bands seen in lane 1 in addition to the GST–α ear (∼50 kDa) are consistent with the expected size of EPS 15, AP 180, Ampiphysin 1, Ampiphysin 2 and Epsin (as marked by the asterisk). ( B) Co-immunoprecipitation of myosin VI with AP-2 and clathrin. AP-2 (lane 4), clathrin (lane 5) and myosin VI (lane 3) as a control were immunoprecipitated under native conditions from cytosol of A431 cells and analysed by western blotting using anti-myosin VI serum. Some myosin VI can be immunoprecipitated with the AP-2 complex (lane 4) and with clathrin (lane 5) but not with pre-immune serum used as a control (lane 6). Lane 1 shows a Coomassie-stained gel of an immunoprecipitate with anti-myosin VI antiserum. Lane 2 is the input lane showing 1/25 of the total cytosol used for one immuno precipitation as blotted with antibodies to myosin VI. (C) Immunoblot of purified clathrin-coated vesicles. Purified clathrin-coated vesicle proteins were separated by SDS–PAGE and stained with Coomassie Blue (lane 1) or blotted onto nitrocellulose and reacted with antibodies to clathrin (lane 2) or myosin VI (lane 3). Myosin VI was observed in purified clathrin-coated vesicles (lane 5). Blotting the same amounts of protein of a 100 000 g microsomal pellet (MP) (lane 4) from rat liver and purified clathrin-coated vesicles (CCV) (lane 5) showed that there was an enrichment of myosin VI in clathrin-coated vesicles similar to that observed for AP-1 and AP-2. Myosin II (MII) and myosin V (MV) were present as expected in the microsomal pellet and were barely detectable in the clathrin-coated vesicles.
Overexpression of the myosin VI tail reduces endocytosis
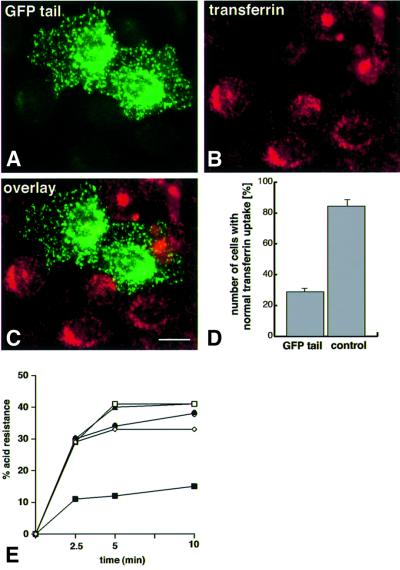
As the tail domain of myosin VI is able to bind to clathrin-coated pits/vesicles (Figures 4 and 6), but is non-functional without the motor domain, we predicted that it might act as a dominant-negative inhibitor of clathrin-mediated endocytosis. We therefore assessed transferrin uptake and transport into the perinuclear recycling compartment in transient as well as in stably transfected NRK cells overexpressing the GFP–myosin VI tail (Figure 8). The whole tail construct containing the large insert was used as this showed the greatest efficiency of localization to clathrin-coated pits/vesicles (see above). Quantitation of transferrin uptake by transiently transfected cells using immunofluorescence microscopy showed that in 70% of the cells expressing the GFP–myosin VI tail, transferrin uptake and transport to the perinuclear region was reduced by >50% compared with non-transfected cells (Figure 8A–D). This effect was not apparent in cells transfected with the coiled-coil domain of the tail tagged with GFP (data not shown), which does not bind to clathrin-coated pits/vesicles (see Figure 6H). As incomplete inhibition of transferrin endocytosis was achieved in transiently transfected cells, we generated several stable cell lines expressing the whole tail, myosin VI, the GT without the large insert or only GFP as a control. In stably transfected cells expressing the GFP–myosin VI tail with the large insert under the control of an inducible promotor, transferrin uptake was measured at 31°C to visualize the initial kinetics of transferrin endocytosis. In these experiments both the initial rate of transferrin uptake and steady state uptake were reduced by ∼70% when compared with untransfected cells or with cells expressing either whole myosin VI or the GT without the large insert or GFP alone (Figure 8E). Treatment of the cells with azide completely blocked transferrin uptake, showing that it was energy-dependent as expected. Data from a single stable, transfected cell line expressing the GFP–tail construct are shown in Figure 8E, although two other stable cell lines tested showed the same effect. The lack of complete inhibition may be a reflection of either differing levels of expression of the GFP–tail construct amongst the stably transfected cells or the low levels of expression, which are roughly equivalent to the amount of endogenous myosin in the cells, or the availability of other minor uptake routes or redundancy within the pathway (another myosin or some other protein could partially compensate for the inhibited myosin VI) or a combination of these. It was also observed that stable, transfected NRK cells expressing GFP–myosin VI tail constructs missing the large insert showed little or no significant reduction in transferrin uptake (see Figure 8E).
Fig. 8. Over-expression of the whole myosin VI tail causes a marked reduction in clathrin-mediated transferrin uptake. (A–C) Immuno fluorescence of transferrin uptake [red, (B)] in transiently transfected NRK cells expressing GFP–tail (see Figure 1) [green, (A)]; (C) overlay. The effect of the tail in transiently transfected cells on transferrin uptake is shown in the bar graph (D). This quantitation is based on cells showing a reduction in transferrin uptake by >50% compared with untransfected control cells in four different experiments ± SD. In each experiment, at least 100 transfected and 100 non-transfected cells as a control were counted. To measure quantitatively transferrin uptake, stable NRK cell lines expressing myosin VI GFP–tail or GFP alone were used (see Figure 3). The effect of the tail on transferrin uptake is shown by the graph (E); open squares show untransfected cells; open diamonds show cells stably transfected with GFP alone; filled circles show cells stably transfected with myosin VI; filled triangles show cells stably transfected with the GT without the large insert and filled squares show a representative stable transfected cell line overexpressing GFP–myosin VI tail containing the large insert. Data show a representative experiment. Bar: 25 µm.
Discussion
The expression of GFP-tagged myosin VI constructs in cells has allowed us to demonstrate a striking co-local ization of the C-terminal tail of chicken intestinal brush border myosin VI with both AP-2 and clathrin-coated pits/vesicles at the light microscope and EM levels. Using GFP-tagged deletion constructs we were able to show that distinct regions of the myosin VI molecule were required for different sites of localization, the GT being sufficient and necessary for localization to AP-2 positive, clathrin-coated pits/vesicles and the Golgi complex, whereas the motor domain was required for localization to membrane ruffles. The myosin VI cDNA cloned from chicken intestinal brush border cells (Buss et al., 1998), which was used in non-polarized NRK cells for over-expressing GFP-tagged myosin VI and deletion mutants, was the isoform with the large tail insert. Screening a range of different rat tissues indicated that the myosin VI isoform with the large tail insert is predominantly expressed in tissues containing many polarized cells with apical microvilli, whereas the isoforms with the small or no insert in their tails are expressed in tissues containing few or no polarized cells. Interestingly, in Caco-2 cells undergoing polarization during cell culture, the expression of myosin VI isoforms switched from the smaller to the large insert. Immunolocalization of endogenous myosin VI in polarized Caco-2 cells, expressing only the isoform with the large insert, showed very good co-localization with clathrin-coated pits/vesicles concentrated at the apical domain. In unpolarized NRK cells the endogenous myosin VI contains no, or only the small insert (data not shown). In these non-polarized fibroblastic cells endogenous myosin VI is also found in punctate structures (Buss et al., 1998 and Figure 6A), which are difficult to define against the background of a large cytosolic pool and only partially co-localize with clathrin-coated pits/vesicles (Figure 4A and B). These results taken together imply that the large insert may be involved in targeting myosin VI to apical clathrin-coated pits/vesicles in polarized cells. This would suggest that myosin VI is particularly important for clathrin-mediated endocytosis from the apical domain of polarized cells. We also found that the myosin VI tail domain containing the large insert acts as a dominant-negative inhibitor of endocytosis when expressed in non-polarized cells, but a construct without this insert, which localizes less well to clathrin-coated pits/vesicles, had no inhibitory effect. Thus, the functional importance of myosin VI in the early steps of endocytosis may be much greater in cells expressing the myosin VI variant with the large insert. Interestingly, in Snell’s waltzer mice, which have no functional myosin VI (Avraham et al., 1995), the major phenotypic features are associated with highly polarized cells, e.g. in these mutant mice there are morphological abnormalities in the stereocilia of the sensory hair cells of the inner ear which cause deafness and in the microvilli of the intestinal brush border cells (Self et al., 1999).
The challenge now is to discover the function(s) of myosin VI. Does the domain organization of myosin VI provide any clues? Myosin VI is a dimeric molecule with two motor domains each containing a neck or lever arm region with a single IQ or light chain binding site and a 53 aa insert (with a high content of hydrophobic and basic amino acids) in the converter region in the motor domain, just before the neck (also called the reverse gear), which is absent in all other myosins. This insert is believed to allow myosin VI to move towards the minus end of actin filaments (in the opposite direction to all other myosins) and thus has a major impact on the cellular function of this myosin (reviewed in Rodriguez and Cheney, 2000; Cramer, 2000). So what role(s) could myosin VI have in clathrin-mediated endocytosis? Our pull-down experiments show that the tail domain of myosin VI can form a protein complex that includes the ear domain of the clathrin adaptor AP-2. Interestingly, the α-ear has been shown to bind or recruit a number of other proteins such as EPS15, epsin, amphiphysin, auxillin and AP 180, which are now known to be involved in modulating endocytosis (Marsh and McMahon, 1999). Myosin VI is the first motor protein to be added to this list. Thus, it could be involved together with dynamic actin filaments and the cytoskeleton in one or more of the following steps: plasma membrane deformation and invagination, coated pit formation and sequestering, detachment of the newly formed vesicle and its movement away from the plasma membrane into the cell. However, actin has not been implicated so far in coated pit formation but is required for later events such as coated pit constriction and/or coated vesicle detachment (Lamaze et al., 1997). In theory, myosin VI could be involved in deforming and pulling in the plasma membrane, thus supporting the work of dynamin as it acts as a ‘poppase’ or ‘pinchase’ and pulling the newly forming coated vesicle into the cell. Another possibility is that myosin VI could transport the clathrin-coated vesicle away from the plasma membrane through the cortical actin network until the clathrin coat comes off. All of these processes require a myosin motor moving towards the minus end of actin filaments away from the plasma membrane, as the plus or barbed ends of actin filaments are oriented towards the plasma membrane. As myosin VI is the only motor so far identified that moves along the actin filament towards the minus end (Wells et al., 1999), it is an ideal candidate for an endocytic motor protein carrying vesicles away from the plasma membrane and into the cell. Another interesting possibility is that myosin VI may link endocytosis to components of signalling pathways. Lamaze et al. (1996) showed that activated Rac, which activates ruffling on the cell surface via actin, can inhibit receptor-mediated endocytosis. We have previously shown that myosin VI is phosphorylated and probably activated in the motor domain by a p21 (Rac)-activated kinase (Buss et al., 1998). Therefore, the reported link between Rac activation and endocytosis could be via myosin VI localized on AP-2 and clathrin-coated pits/vesicles at the plasma membrane.
In summary our data imply a role for myosin VI in clathrin-mediated endocytosis, which is most apparent at the apical microvilli-enriched surface of polarized cells. Current work is focusing on establishing which step(s) in the early endocytic pathway involves myosin VI participation and to which proteins in this pathway the myosin VI directly binds.
Materials and methods
Antibodies, proteins and subcellular fractions
The polyclonal antibody to the whole tail of myosin VI has been described previously (Buss et al., 1998). The antibodies to subunits of the adaptor complexes AP-1 and AP-2 as well as clathrin were a kind gift of Dr M.S.Robinson (Cambridge Institute for Medical Research, Cambridge, UK). Clathrin-coated vesicles were purified as described elsewhere (Manfredi and Bazari, 1987). Microsomal membranes were purified from rat liver, which was homogenized in 10 mM HEPES pH 7.4, 0.25 M sucrose and protease inhibitor cocktail (complete™, Boehringer, Germany). Cell debris was pelleted at 2500 g for 30 min and microsomal membranes pelleted from the supernatant at 100 000 g for 1 h.
Construction of GFP–myosin VI
The full-length (4 kb) chicken brush border myosin VI cDNA (DDBJ/EMBL/GenBank accession No. AJ278608) (Buss et al., 1998) or different restriction fragments covering different predicted domains of the molecule were cloned into the mammalian expression vector pEGFP (Clontech, Basingstoke, UK) so that they were expressed at the C-terminus of EGFP (see Figure 1). Stable cell lines expressing GFP–myosin VI whole tail (T), the GT or only GFP were established using ΔpMEP as the expression vector (Girotti and Banting, 1996). The constructs encoded were as follows: the CCT from amino acids 742 to 1030, the GT from amino acids 1036 to 1273, the whole tail (T or tail) from amino acids 846 to 1277 and myosin VI without the GT (ΔGT) from amino acids 1 to 988.
Transfection and immunofluorescence
NRK cells were obtained from the European Collection of Animal Cell Cultures and grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum and 2 mM l-glutamine. For transient transfection experiments, cells were grown on coverslips to ∼70% confluence and transfected according to the manufacturer’s instructions with 2 µg pEGFP–myosin VI or deletion mutants using FuGENE™ (Roche Diagnostics, UK). Sixteen to eighteen hours after transfection, the cells were fixed with 4% formaldehyde in phosphate-buffered saline (PBS) for 15 min. After permeabilization with 0.2% Triton X-100 or methanol at –20°C for 5 min, the coverslips were incubated with the first antibodies at room temperature for 1 h before incubating with the second antibody, which was either a goat anti-mouse IgG or a goat anti-rabbit IgG coupled to Texas red (Molecular Probes). After washing, the cells were mounted in Mowiol (Hoechst AG, Frankfurt, Germany) and analysed and photographed using a Zeiss Axiophot microscope equipped with a CCD camera. For selection of stable cell lines, NRK cells were transfected with myosin VI constructs cloned into ΔpMEP as described above. Selection was performed by addition of 200 µg/ml of hygromycin B (Roche Diagnostics, UK) to the culture medium. Expression of GFP-tagged protein was induced by addition of 5 µM CdCl2. In stable cell lines the concentration of expressed proteins was measured by immunoblotting as approximately equal to that of endogenous myosin VI. Caco-2 cells were cultured on clear filter supports (Becton Dickinson, France) as previously described (Jackman et al., 1994). For immunofluorescence on polarized cells, the cells were cultured for 14 days after seeding. At the end of this time the monolayer showed a TEER of ∼400 Ωcm2.
Transferrin uptake assay
To monitor transferrin uptake as a measure for clathrin-mediated endocytosis, NRK cells were transiently transfected with GFP–myosin VI tail. Twenty-four hours after transfection the cells were serum starved for 4 h and then incubated for 20 min in serum-free DMEM containing 25 µg/ml human transferrin coupled to biotin (Sigma, Poole, UK). After fixing and permeabilization (see above) the cells were incubated with Alexa™-conjugated streptavidin (Molecular Probes) to detect internalized transferrin. For quantitation of transferrin uptake cells were imaged in a Zeiss axiophot microscope. In each experiment at least 100 transfected cells were assessed for the transferrin taken up and compared with neighbouring untransfected cells. Cells that had taken up <50% of transferrin as compared with non-transfected cells were counted as showing ‘reduced’ transferrin uptake.
To measure quantitatively transferrin uptake, stable cell lines expressing GFP–myosin VI tail or GFP alone were compared with non-expressing cells. Cells grown in six-well plates were serum starved 1–2 h before binding of human biotinylated transferrin (Sigma, Poole, UK) to the cell surface receptor for 1 h on ice. Cells were washed twice with PBS/bovine serum albumin (BSA) (5 mg/ml) before transferrin was taken up at 31°C for various times. Transferrin, still bound to the cell surface, was stripped by washing twice on ice with 10 mM HCl, 150 mM NaCl. Afterwards, cells were washed with PBS and removed from the culture dish. Cells were then lysed in PBS containing 1% Triton X-100, the lysate clarified by centrifugation and diluted if necessary with PBS/BSA for quantification using an ELISA. The ELISA plates were coated with antibody to transferrin (1:1000) (The Binding Site Ltd, Birmingham, UK) in 50 mM NaHCO3 pH 9.6 overnight at 4°C. The plate was washed twice with PBS and blocked for 1 h at 37°C with 10 mM Tris pH 7.4, 50 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, 0.2% BSA. The diluted cell lysate was bound in PBS/BSA at 4°C overnight. The plate was washed again three times with PBS and incubated for 5 min at room temperature with blocking buffer. Afterwards, the ELISA plate was incubated with streptavidin HRP (Amersham Pharmacia, UK) (1:1000) at room temperature for 1 h, washed three times with PBS and incubated with 0.4 mg/ml of o-phenylenediamine in 0.1 M Na phosphate pH 5 containing 0.01% H2O2 at room temperature in the dark. The reaction was stopped by the addition of 50 µl of 2 M H2SO4 and the absorbance quantified at 492 nm using an ELISA plate reader.
Immunoprecipitation and GST pull-down
To prepare cytosol from A431 cells and stable expressing NRK cell lines, the cells were scraped into extraction buffer containing 25 mM Tris–HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.5% Triton X-100 and protease inhibitor cocktail (complete™). After sonication the cytosol was cleared by centrifugation at 50 000 g for 30 min. The cytosol was used for immunoprecipitation as described previously (Buss et al., 1998). For pull-down experiments, the cytosol was diluted to 1 mg/ml of protein with extraction buffer and then incubated for 90 min at 4°C with 20 µg of GST and 20 µl of 50% suspension of glutathione Sepharose. This pre-absorbed cytosol was then incubated for 90 min with 20 µg of the GST ear domain of the α-subunit of AP-2 (Owen et al., 1999) in the presence of glutathione Sepharose. After several washes proteins binding to the beads were analysed by SDS–PAGE and immunoblotting.
PCR analysis
To assess expression of myosin VI isoforms in various tissues by PCR, mRNA was prepared from rat tissues and Caco-2 cells, grown unpolarized on plastic dishes or polarized on filter support units, using the PURESCRIPT® RNA isolation kit (Gentra, MN). This RNA was used in RT–PCR reactions with primers flanking the region of the tail containing the two inserts. Expression levels of the isoform with the large insert versus the isoform with the small or no insert during Caco-2 polarization was quantified by scanning agarose gels of PCR products. Polarization of Caco-2 cells was monitored by measuring the TEER.
Acknowledgments
Acknowledgements
We thank Dr David Owen (MRC Laboratory of Molecular Biology, Cambridge) for the GST–α ear protein, Abigail Stewart for help with the EM work, Neil Cook for advice on the transferrin assay, Dr Paul Pryor for help with computing and Drs Margaret S.Robinson, Jenny Hirst and Gudrun Ihrke for much helpful discussion and critical reading of the manuscript. We thank the Medical Research Council for supporting this work.
References
- Avraham K.B., Hasson,T., Steel,K.P., Kingsley,D.M., Russell,L.B., Mooseker,M.S., Copeland,N.G. and Jenkins,N.A. (1995) The mouse Snell’s waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nature Genet., 11, 369–375. [DOI] [PubMed] [Google Scholar]
- Berg J.S., Powell,B.C. and Cheney,R.E. (2001) A millennial myosin census. Mol. Biol. Cell, 12, 780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckler J., Au,K., Cheng,J., Hasson,T. and Burnside,B. (2000) Novel myosin VI isoform is abundantly expressed in retina. Exp. Eye Res., 70, 121–134. [DOI] [PubMed] [Google Scholar]
- Buss F., Kendrick-Jones,J., Lionne,C., Knight,A.E., Cote,G.P. and Luzio,J.P. (1998) The localization of myosin VI at the Golgi complex and leading edge of fibroblasts and its phosphorylation and recruitment into membrane ruffles of A431 cells after growth factor stimulation. J. Cell Biol., 143, 1535–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer L.P. (2000) Myosin VI: roles for a minus end-directed actin motor in cells. J. Cell Biol., 150, F121–F126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePina A.S. and Langford,G.M. (1999) Vesicle transport: the role of actin filaments and myosin motors. Microsc. Res. Tech., 47, 93–106. [DOI] [PubMed] [Google Scholar]
- Gaidarov I., Santini,F., Warren,R.A. and Keen,J.H. (1999) Spatial control of coated-pit dynamics in living cells. Nature Cell Biol., 1, 1–7. [DOI] [PubMed] [Google Scholar]
- Girotti M. and Banting,G. (1996) TGN38-green fluorescent protein hybrid proteins expressed in stably transfected eukaryotic cells provide a tool for the real-time, in vivo study of membrane traffic pathways and suggest a possible role for ratTGN38. J. Cell Sci., 109, 2915–2926. [DOI] [PubMed] [Google Scholar]
- Goodson H.V., Valetti,C. and Kreis,T.E. (1997) Motors and membrane traffic. Curr. Opin. Cell Biol., 9, 18–28. [DOI] [PubMed] [Google Scholar]
- Gottlieb T.A., Ivanov,I.E., Adesnik,M. and Sabatini,D.D. (1993) Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarised epithelial cells. J. Cell Biol., 120, 695–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson T. and Mooseker,M.S. (1995) Molecular motors, membrane movements and physiology: emerging roles for myosins. Curr. Opin. Cell Biol., 7, 587–594. [DOI] [PubMed] [Google Scholar]
- Hasson T., Gillespie,P.G., Garcia,J.A., MacDonald,R.B., Zhao,Y., Yee,A.G., Mooseker,M.S. and Corey,D.P. (1997) Unconventional myosins in inner-ear sensory epithelia. J. Cell Biol., 137, 1287–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzelman M.B., Hasson,T. and Mooseker,M.S. (1994) Multiple unconventional myosin domains of the intestinal brush border cytoskeleton. J. Cell Sci., 107, 3535–3543. [DOI] [PubMed] [Google Scholar]
- Jackman M.R., Shurety,W., Ellis,J.A. and Luzio,J.P. (1994) Inhibition of apical but not basolateral endocytosis of ricin and folate in Caco-2 cells by cytochalasin D. J. Cell Sci., 107, 2547–2556. [DOI] [PubMed] [Google Scholar]
- Kellerman K.A. and Miller,K.G. (1992) An unconventional myosin heavy chain gene from Drosophila melanogaster. J. Cell Biol., 119, 823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroschewski R., Hall,A. and Mellman,I. (1999) Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nature Cell. Biol., 1, 8–13. [DOI] [PubMed] [Google Scholar]
- Kübler E. and Riezman,H. (1993) Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO J., 12, 2855–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamaze C., Chuang,T.-H., Terlecky,L.J., Bokoch,G.M. and Schmid,S.L. (1996) Regulation of receptor-mediated endocytosis by Rho and Rac. Nature, 382, 177–179. [DOI] [PubMed] [Google Scholar]
- Lamaze C., Fujimoto,L.M., Yin,H.L. and Schmid,S.L. (1997) The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J. Biol. Chem., 272, 20332–20335. [DOI] [PubMed] [Google Scholar]
- Manfredi J.J. and Bazari,W.L. (1987) Purification and characterisation of two distinct complexes of assembly polypeptides from calf brain coated vesicles that differ in their polypeptide composition and kinase activities. J. Biol. Chem., 262, 12182–12188. [PubMed] [Google Scholar]
- Marsh M. and McMahon,H.T. (1999) The structural ear of endocytosis. Science, 285, 215–220. [DOI] [PubMed] [Google Scholar]
- Mermall V., Post,P.L. and Mooseker,M.S. (1998) Unconventional myosins in cell movement, membrane traffic and signal transduc tion. Science, 279, 527–533. [DOI] [PubMed] [Google Scholar]
- Merrifield C.J., Moss,S.E., Ballestrem,C., Imhof,B.A., Giese,G., Wunderlich,I. and Almers,W. (1999) Endocytic vesicles move at the tips of actin tails in cultured mast cells. Nature Cell Biol., 1, 72–74. [DOI] [PubMed] [Google Scholar]
- Owen D.J., Vallis,Y., Noble,M.E., Hunter,J.B., Dafforn,T.R., Evans,P.R. and McMahon,H.T. (1999) A structural explanation for the binding of multiple ligands by the α-adaptin appendage domain. Cell, 97, 805–815. [DOI] [PubMed] [Google Scholar]
- Pinto M. et al. (1983) Enterocyte-like differentiation and polarization of a human colon carcinoma cell line Caco-2 in culture. Biol. Cell, 47, 323–330. [Google Scholar]
- Qualmann B., Kessels,M.M. and Kelly,R.B. (2000) Molecular links between endocytosis and the actin cytoskeleton. J. Cell Biol., 150, F111–F116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez O.C. and Cheney,R.E. (2000) A new direction for myosin. Trends Cell Biol., 10, 307–311. [DOI] [PubMed] [Google Scholar]
- Self T., Sobe,T., Copeland,N.G., Jenkins,N.A., Avraham,K.B. and Steel,K.P. (1999) Role of myosin VI in the differentiation of cochlear hair cells. Dev. Biol., 214, 331–341. [DOI] [PubMed] [Google Scholar]
- Sellers J.R. (2000) Myosins: a diverse superfamily. Biochim. Biophys. Acta, 1496, 3–22. [DOI] [PubMed] [Google Scholar]
- Shurety W., Bright,N.A. and Luzio,J.P. (1996) The effects of cyto chalasin D and phorbol myristate acetate on the apical endocytosis of ricin in polarized Caco-2 cells. J. Cell Sci., 109, 2927–2935. [DOI] [PubMed] [Google Scholar]
- Wells A.L., Lin,A.W., Chen,L.Q., Safer,D., Cain,S.M., Hasson,T., Carragher,B.O., Milligan,R.A. and Sweeney,H.L. (1999) Myosin VI is an actin-based motor that moves backwards. Nature, 401, 505–508. [DOI] [PubMed] [Google Scholar]