Abstract
The chloroplast-based photosynthetic apparatus of plants and algae associates various redox cofactors and pigments with ∼70 polypeptides to form five major transmembrane protein complexes. Among these are two photosystems that have distinct light absorption properties but work in series to produce reducing equivalents aimed at the fixation of atmospheric carbon. A short term chromatic adaptation known as ‘State transitions’ was discovered thirty years ago that allows photosynthetic organisms to adapt to changes in light quality and intensity which would otherwise compromise the efficiency of photosynthetic energy conversion. A two-decade research effort has finally unraveled the major aspects of the molecular mechanism responsible for State transitions, and their physiological significance has been revisited. This review describes how a—still elusive—regulatory kinase senses the physiological state of the photosynthetic cell and triggers an extensive supramolecular reorganization of the photosynthetic membranes. The resulting picture of the photosynthetic apparatus is that of a highly flexible energy convertor that adapts to the ever-changing intracellular demand for ATP and/or reducing power.
Keywords: cyclic electron flow/cytochrome b6f complexes/LHCII phosphorylation/photosynthesis/State transitions
Introduction
Oxygenic photosynthesis relies on the function of two distinct types of—membrane-embedded—photochemical reaction centers, termed RCI and RCII, which operate in series. In order to increase the probability that incident photons will trigger charge separation in the reaction centers, photosynthetic eukaryotes have developed a specific set of transmembrane proteins that bind pigments, termed antenna proteins or light-harvesting proteins, which collect light excitation and channel it to the reaction centers. However, the absorption spectra of these antenna proteins differ between photosystem I (PSI) and photosystem II (PSII), the former being enriched in chlorophyll a holochromes absorbing in the far red region whereas the latter are enriched in chlorophyll b, whose maximum absorption stands at shorter wavelengths in the red region, ∼650 nm. Thus, a spectral unbalance of the exciting light may result in an unbalanced excitation of the two photosystems. Since the two photosystems work in series, the overall efficiency of the resulting electron flow should decrease. This is not observed in vivo because of a short term adaptation process called ‘State transitions’.
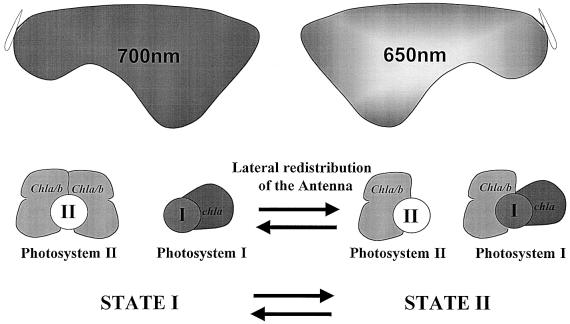
State transitions were discovered ∼30 years ago in unicellular photosynthetic organisms (Bonaventura and Myers, 1969; Murata, 1969). They were described as ‘a control mechanism of excitation transfer between the two photosystems’ (Murata, 1970). In a typical experiment (Figure 1), light II, i.e. 650 nm, which excites mainly PSII, induces an increased sensitization of PSI within a few minutes, whereas a subsequent transfer to light I, i.e. far red light, which excites mainly PSI, induces an increased sensitization of PSII. It was later found that chla/b-containing organisms display an extreme lateral heterogeneity in the distribution of the two photosystems (Andersson and Anderson, 1980; Vallon et al., 1986). They are located along the thylakoid membranes within the chloroplast compartment, with PSI being present in stroma lamellae, which are thylakoid domains with no membrane-to-membrane contacts, while PSII is enriched in grana stacks in which several thylakoids are in membrane-to-membrane contacts along several hundreds of nanometers. Thus, the mechanism underlying State transitions became a challenging issue: what were the means by which light excitation could be redirected between two protein complexes located >500 nm apart?
Fig. 1. The early view of State transitions: two photosystems, Photosystem I (PSI) and Photosystem II (PSII), cooperate in gathering light energy aimed at a photosynthesis-dependent carbon fixation. Light harvesting is improved by the association of distinct and specific chlorophyll beds to PSI and PSII. The PSI antenna is enriched in far-red light absorbing holochromes of Chla whereas the PSII antenna is enriched in Chlb whose absorption peak is at 650 nm in the red region. When exposed to light preferentially absorbed by PSI (far red light), plants or algae display an antenna distribution that favors PSII (State I). In contrast, when exposed to light preferentially absorbed by PSII (650 nm light), plants or algae display an antenna distribution that favors PSI (State II).
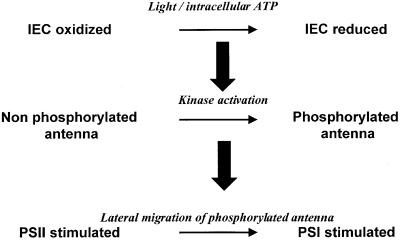
It took ∼10 years before the molecular basis for this regulation process was understood by Bennett and coworkers (for reviews see Bennett, 1991; Allen, 1992a). In a series of incisive papers (Bennett, 1977, 1979a, b, 1980; Bennett et al., 1980; Allen et al., 1981) they concluded to a regulation scheme that is still the framework of our present research: when the intersystem electron carriers are reduced—a situation that prevails if PSII is overexcited relative to PSI—an activated kinase phosphorylates the major chlb-containing antenna proteins, LHCII, leading to their lateral redistribution in favor of PSI (Figure 2). Oxidation of the intersystem electron carriers leads to the opposite effect: kinase deactivation allows LHCII dephosphorylation by a phosphatase and re-association of these mobile antenna proteins with PSII.
Fig. 2. Schematic representation of the main steps in a transition to State II: upon illumination of plants or algae with a light preferentially absorbed by PSII, or upon intracellular ATP depletion, the intersystem electron carriers (IEC) of the photosynthesis electron transfer chain switch to a more reduced state. A regulatory kinase becomes activated in these reducing conditions and phosphorylates the chla/b-containing peripheral antenna, termed LHCII. Phospho-LHCII moves away from PSII and is associated with PSI, thereby changing the relative absorption cross-sections of the two photosystems.
This purported regulation raises a number of questions that have been in part resolved only recently. What is the nature of the LHCII-kinase? What is the signal transduction chain that starts with a redox change in the photosynthetic electron transport chain and ends up with new phosphate groups added to the antenna proteins? Which structural parameters govern the movement and differential affinities of (phospho)-LHCII for PSII and PSI membrane domains? What physiological changes accompany this supramolecular reorganization of the thylakoid membranes?
Hunting for the kinase
As soon as John Bennett had identified a membrane-bound kinase activity responsible for the phosphorylation of chla/b-binding proteins from the LHCII family (Bennett, 1977, 1979b), several laboratories launched a biochemical effort for the purification of this LHCII-kinase. From the various reports describing kinase activities and putative kinase-polypeptides (Lin et al., 1982; Sokolenko et al., 1995; Snyders and Kohorn, 1999; Weber et al., 2001) one can conclude that at least four distinct kinases are associated with the thylakoid membranes with apparent molecular weights of ∼64, 56, 55 and 33 kDa. Although there is, as yet, no definite biochemical identification of the LHCII-kinase itself, significant progress was made with the elegant approach of Snyders and Kohorn (1999), aimed at fetching proteins that interact with the phosphorylatable N-terminus of LHCII. They identified three related kinases, called TAK1–3 (for thylakoid-associated kinase), which lacked a cleavable chloroplast-targeting sequence but were imported into the chloroplast, immunodetected in thylakoid membrane fractions and able to phosphorylate LHCII in vitro. In a recent study with TAK1 antisense Arabidopsis plants that showed decreased levels of expression of the kinase, S.Snyders and B.D.Kohorn (manuscript submitted) observed fluorescence modifications and changes in the LHCII-phosphorylation pattern that suggest a contribution of TAK1 to State transitions. As an alternative route, a unicellular alga well suited for genetic studies, Chlamydomonas reinhardtii, was used for the isolation of mutants defective in State transitions. Two laboratories have successfully used a simple screening procedure using video-imaging systems that are based on the changes in fluorescence yields at room temperature that accompany State transitions, from a high yield in State I to a low yield in State II (Fleischmann et al., 1999; Kruse et al., 1999). Those colonies that do not show such a fluorescence change are bona fide candidates for being altered in the kinase activity that develops during this adaptation process. At present several tagged mutants—i.e. where the interrupted gene can be identified through the flanking regions of the construct used for insertional mutagenesis—have been isolated and the gene controlling the mutant phenotype is close to being sequenced (J.D.Rochaix, personal communication).
The cytochrome b6f, a key partner in kinase activation
Among the seminal contributions of J.Bennett, J.Allen and their coworkers was the suggestion that a change in the redox state of the plastoquinone pool—a set of small lipophilic molecules that funnel redox equivalents between PSII and PSI—delivered the triggering signal for State transitions (Allen et al., 1981); a reduced PQ pool led to State II whereas an oxidized pool led to State I. Since State transitions were accompanied by changes in LHCII phosphorylation, it strongly suggested that the LHCII-kinase might be itself—or be associated with—a plastoquinol-binding protein. Two protein complexes display quinone/quinol-binding sites in the membrane-embedded photosynthetic electron transport chain: PSII and the cytochrome b6f complex. Chlamydomonas mutants lacking either of the two protein complexes were used to identify the sensor of the redox state of the PQ pool that controls State transitions: the cytochrome b6f complex (Lemaire et al., 1986; Wollman and Lemaire, 1988).
In the absence of cytochrome b6f complex, algae remained in State I even in conditions that fully reduced the plastoquinone pool. Accordingly they lacked the ability to activate the LHCII-kinase. However, a basal LHCII-kinase activity and a PSII-kinase activity were still observed in these mutants, independently of the redox state of the PQ pool, an observation consistent with the multiple kinases detected in the thylakoid membranes (see above). That the cytochrome b6f complex controlled the activation of the LHCII-kinase was subsequently confirmed with various mutants from higher plants (Gal et al., 1987; Bennett et al., 1988; Coughlan, 1988). The cytochrome b6f complex contains two plastoquinone-binding sites termed Qo, close to the lumenal surface of the thylakoid membranes, and Qi close to their stromal surface where the phosphorylatable residues of LHCII protrude. Although Qi was the most likely candidate for LHCII-kinase activation, Qo was unambiguously identified by two groups as the triggering site for kinase activation. Using an in vitro approach based on a jump to acidic pHs, Vener and coworkers (Vener et al., 1995, 1997) produced enough quinol to occupy the Qo site of the cytochrome b6f complex in isolated thylakoids and demonstrated that the LHCII-kinase remained activated upon restoration of neutral pHs as long as a quinol remained bound to the cytochrome b6f complex. Using an in vivo approach based on site-directed mutagenesis of the Qo site that led to the loss of plastoquinol binding, Zito et al. (1999) demonstrated that the resulting mutant of Chlamydomonas remained locked in State I and had lost its ability to activate the LHCII-kinase.
These studies raised a new and fundamental question: how would the information that binding of a quinol had occurred on a protein site close to the lumenal face of the membrane be transduced to the kinase whose active site is stromally exposed, i.e. on the opposite side of the thylakoid membranes? To understand this aspect of kinase activation, one needs first to go into some details of the three-dimensional (3D) structure of cytochrome b6f complexes.
The impressive progress in the crystallization of membrane proteins has allowed the recent resolution of the 3D structure of the mitochondrial cytochrome bc complex from various sources (Iwata et al., 1998; Kim et al., 1998; Zhang et al., 1998; Hunte et al., 2001). This protein is highly homologous to the chloroplast cytochrome b6f complex despite the presence of additional subunits in the former type of complexes and widely different sequences between the constitutive c-type cytochromes. The protein complex crystallizes as a dimer, which is consistent with the biochemical behavior of purified cytochrome bc and cytochrome b6f complexes. The 3D structures show how one of the major subunits, the Rieske protein, whose major part is hydrophilic and stands in the soluble phase (i.e. the lumen space in the case of the chloroplast Rieske), is bound to the membrane: it displays a single transmembrane helix located at its N-terminal end. This 37-residue transmembrane helix is longer than average, and is tilted with respect to the membrane plane by an angle of 68° (Zhang et al., 1998). The N-terminal part of the helix interacts with one monomer of the dimer but the soluble domain of the Rieske, which contains the 2Fe2S cluster, interacts with the other monomer of the same dimer. This unexpected topology provides the structural basis for the earlier biochemical observation that the Rieske contributes to the stability of the dimeric form of the protein complex (Breyton et al., 1997). Still, the most surprising conclusion from these structural studies was that, depending on the presence or absence of stigmatellin—a Qo site inhibitor— the crystal structure of cytochrome bc1 showed two contrasted positions for the Rieske iron–sulfur protein (Zhang et al., 1998). With an empty Qo site, the Rieske protein was found in a distal position from the membrane, with the 2Fe2S cluster close enough to the c-type cytochrome for electron transfer to occur but too distant from the Qo pocket and the b-type heme for any alternative electron transfer route. With stigmatellin present, the Rieske protein was found proximal to the membrane, i.e. suitable for a redox interaction with a quinol bound to the Qo pocket but too distant from the c-type cytochrome for electron transfer. Our present knowledge of the mechanism of electron transfer at the Qo site, known as the Q cycle (Mitchell, 1975; Crofts et al., 1983), requires that, in situ, the Rieske oscillates between the two positions identified by crystallization. Physical evidence for such a movement has recently been documented for the Rieske in cytochrome bc (Brugna et al., 2000; Darrouzet et al., 2000).
The idea that the activation of the kinase would result from a switch of the Rieske from one position to the other was proposed by us (Zito et al., 1999) and others (Vener et al., 1998). Based on a purported transmembrane segment of the hypothetical LHCII-kinase that would develop protein interactions with the Rieske on the lumen side of the membranes, Vener and colleagues (Vener et al., 1998) first suggested that the distal position of the Rieske was required for kinase activation. In contrast, following the demonstration that acidic pHs favor the proximal position of the Rieske (Brugna et al., 1999), we argued that the experiments of Vener and colleagues (Vener et al., 1995, 1997), which resorted to an acidic pH jump to place the kinase in an active state, supported the proximal position of the Rieske as the kinase-activating position. Still, independently of the identification of which position of the Rieske would be competent in kinase activation, the transduction of this positional information from the lumen side to the stromal side of the membranes where the kinase operates remained unresolved. The missing link was uncovered by the structural study of Breyton (2000), who analyzed two-dimensional crystals of the cytochrome b6f complex in the presence or absence of stigmatellin. She confirmed that the Rieske from cytochrome b6f complexes showed the same flexibility as in cytochrome bc1 complexes. But the novelty of this study lay in the demonstration that the movement of the Rieske from one position to the other was accompanied by a significant reorganization of the transmembrane moiety of the cytochrome b6f complex, particularly at the interface between the two monomers. Thus, a lumen-located movement of the polar domain of the Rieske could be sensed on the stromal side of the protein through a conformational change of the whole cytochrome b6f complex.
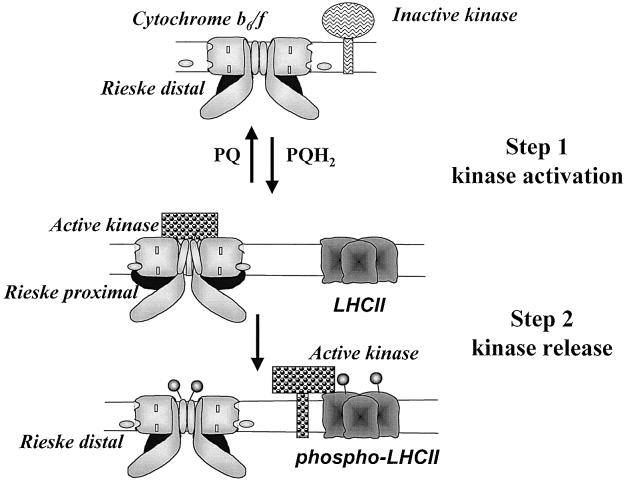
Using distinct inhibitors of the Qo site, we subsequently demonstrated that neither the distal nor the proximal position of the Rieske per se was able to activate the kinase and induce a transition to State II (Finazzi et al., 2001). However, we observed that suV, a phosphorylatable subunit of cytochrome b6f that we had recently identified (Hamel et al., 2000), became phosphorylated in the presence of stigmatellin, which stabilizes the proximal position of the Rieske. Thus, we proposed a two-step model for the activation of the LHCII-kinase, which requires the movement of the Rieske protein (Figure 3) (Finazzi et al., 2001). The putative LHCII-kinase would be activated through its interaction with the cytochrome b6f complex when the Rieske protein is placed in the proximal position to the membrane. This conformation would be promoted by the occupancy of the site by a plastoquinol molecule, consistent with the observation that a reduced PQ pool is required for the activation of the kinase. Stigmatellin would mimic this effect that can be probed by the immediate phosphorylation of the cytochrome b6f-associated polypeptide suV. The release of the activated kinase from the cytochrome b6f complex, required for its interaction with the LHCII substrates, would occur upon relaxation of the Rieske to its distal position, a movement elicited by the turnover of PQH2 at its binding site, but not by stigmatellin, which locks the complex in the proximal position for the Rieske protein. As discussed by Gal et al. (1997), given the low abundance of the kinase in comparison with the concentration of cytochrome b6f complexes and LHCII in the thylakoid membranes, a successful transition to State II requires that the kinase, before it relaxes to its inactive state, visits several LHCII substrates and also several cytochrome b6f activators if the activated state is short-lived. Thus, the dynamic model of kinase activation presented above also fulfills the stoichiometric constraints that apply to the partners of the phosphorylation process.
Fig. 3. A hypothetical model for the cytochrome b6f-mediated activation of the LHCII-kinase: cytochrome b6f complexes stand as dimers in the thylakoid membranes with most of the Rieske protein (in black) exposed to the lumen, in either a distal or proximal position with respect to the thylakoid membranes. The reversible binding of reduced plastoquinones ( , PQH2) drives the Rieske protein from one position to the other. A transmembrane reorganization of the cytochrome b6f complexes takes place when the Rieske switches from a distal to a proximal position. New interactions then develop with the LHCII-kinase, whose catalytic site, facing the stroma, becomes activated. The active kinase phosphorylates a subunit of the cytochrome b6f complex. It is released in the membrane space when the Rieske protein moves back to its distal position because of the binding turnover of PQH2 at the Qo site. Upon its release, the active kinase can interact with LHCII and promote its phosphorylation (
, PQH2) drives the Rieske protein from one position to the other. A transmembrane reorganization of the cytochrome b6f complexes takes place when the Rieske switches from a distal to a proximal position. New interactions then develop with the LHCII-kinase, whose catalytic site, facing the stroma, becomes activated. The active kinase phosphorylates a subunit of the cytochrome b6f complex. It is released in the membrane space when the Rieske protein moves back to its distal position because of the binding turnover of PQH2 at the Qo site. Upon its release, the active kinase can interact with LHCII and promote its phosphorylation ( ).
).
Revisiting the physiology of State transitions
As mentioned in the Introduction, State transitions were first identified as a short-term adaptation process that allows plants and microalgae to cope with unbalanced light excitation of the two photosystems. In this view, the lateral migration of a fraction of the LHCII antenna proteins between the grana domains, enriched in PSII, and the stroma lamellae domains, enriched in PSI, served the function of modulating the size and quality of the light-harvesting antenna in order that the two types of photosystem remain equally excited. The movement of LHCII was documented both by biochemical fractionation of thylakoid membrane domains (Kyle et al., 1983; Larsson et al., 1983) and by immunocytochemistry (Vallon et al., 1991). The detachment of phospho-LHCII from PSII was ascribed to an electrostatic repulsion due to the increased negative charges at the stromal surface of each thylakoid in a granum as well as at the stromal tip of the PSII–LHCII complex (Barber, 1986), and to conformational changes of the N-terminus part of LHCII (Allen, 1992b; Stys et al., 1995). In that respect, the recent report that LHCII undergoes a light-induced conformational change that contributes to its increased phosphorylation by an active kinase (Zer et al., 1999) further supports the idea that LHC has a lower affinity for PSII in State II conditions. Several subunits of the PSII core complex are reversibly phosphorylated (Delepelaire and Wollman, 1985; Millner et al., 1986). They may contribute to the reversible formation of the PSII–LHCII supercomplexes since (i) a Chlamydomonas mutant fully devoid of LHCII proteins shows no PSII core phosphorylation (de Vitry and Wollman, 1988) and (ii) a tobacco mutant, lacking the PsbZ subunit of the PSII cores, shows an increased rate of PSII core phosphorylation relative to that of LHCII together with a loss of PSII–LHCII supercomplexes upon membrane fractionation (Swiatek et al., 2001). However, the phosphorylation of LHCII and its ability to migrate away from the stacked membrane domains in State II conditions do not require the presence of PSII cores; PSII mutants from Chlamydomonas undergo a regular transition to State II in reducing conditions; LHCII phosphorylation increases (Wollman and Delepelaire, 1984) and becomes functionally associated with PSI (Delepelaire and Wollman, 1985; Delosme et al., 1996). The Förster theory of excitation transfer accounts for interactions between neighboring chlorophyll-binding proteins without a need for an actual specific binding. Thus, the migration of phospho-LHCII out of PSII domains was long considered as a suitable condition for energy transfer between phospho-LHCII and PSI in the stroma lamellae regions. However, a Chlamydomonas mutant lacking PSI centers still showed increased LHCII-phosphorylation in State II conditions, but the whole antenna complement remained functionally connected to PSII (Delosme et al., 1996). The explanation for this unexpected behavior came with the recent work of Lunde et al. (2000), who analyzed an antisense mutant of Arabidopsis that lacked the PsaH subunit of PSI but still accumulated close to wild-type amounts of the rest of the PSI cores; this mutant still showed increased phosphorylation of LHCII in State II conditions but the antenna proteins failed to associate with PSI and remained connected with PSII. Thus, the association of phospho-LHCII with PSI and PSII should be regarded as a competitive process, in which a PsaH-dependent high-affinity binding site for phospho-LHCII on PSI displaces the antenna complex from its binding site on PSII that has affinity for both the phosphorylated and dephosphorylated forms of LHCII.
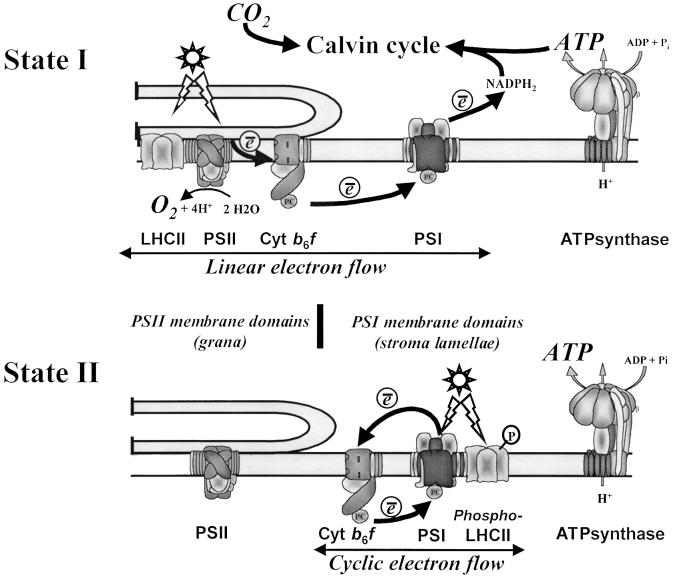
As mentioned above, cytochrome b6f complexes are part of the activation process for the LHCII-kinase. They are present both in the grana stacks and the stroma lamellae regions (Allred and Staehelin, 1985; Olive et al., 1986). Although the granal fraction of cytochrome b6f complexes is higher that that in the stroma lamellae in all conditions, the latter increase significantly upon State transitions (Vallon et al., 1991). That this lateral displacement of cytochrome b6f requires ATP suggests that its phosphorylated subunit, suV (Hamel et al., 2000), may govern its diffusion out of the grana through a similar mechanism to that for LHCII. What could be the rationale for the recruitment of more cytochrome b6f in the PSI domains? In unicellular algae, State II conditions can be driven in darkness by depleting the intracellular content in ATP (Bulte et al., 1990). We had noted that the co-localization of increased fractions of antenna proteins and cytochrome b6f together with PSI was particularly appropriate for an efficient cyclic electron flow, which differs from linear electron flow in that no CO2 is fixed, thereby leaving photosynthetic ATP available for other metabolic purposes (Bulte et al., 1990). This led us to suggest that the supramolecular organization of thylakoid membranes in State II would be aimed at restoring higher intracellular ATP levels. This proposal recently received experimental support with the very elegant spectroscopic study of Finazzi et al. (1999). These authors showed that the re-reduction of cytochrome f in Chlamydomonas became insensitive to the PSII inhibitor 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) in State II. Thus, no linear electron flow occurred in these conditions. In addition, a Chlamydomonas mutant incapable of kinase activation and locked in State I (Fleischmann et al., 1999) kept its ability to perform linear electron flow in the reducing conditions used to place a wild-type control in State II (G.Finazzi, personal communication). Conversely, we concluded that there was no cyclic electron flow around PSI in State I, as judged from the absence of a light-induced pH gradient in ATP-synthase mutants defective in PSII activity when placed in State I (Majeran et al., 2001). Thus, State transitions in the unicellular alga Chlamy domonas are primarily aimed at switching the photosynthetic apparatus from the oxygenic type—two photosystems working in series, evolving oxygen and fixing CO2— to the bacterial type—an ATP generator resulting from cyclic electron flow around one photosystem (Figure 4).
Fig. 4. The present view of State transitions: in State I, the supramolecular organization of the photosynthetic apparatus is adapted to the fixation of CO2 in the Calvin cycle. A linear electron flow from PSII (which extracts electrons from water leading to O2 evolution) to PSI generates reducing power (NADPH) and ATP, both of which are required for the biosynthesis of carbohydrates. In State II, an extensive supramolecular reorganization converts the photosynthesis apparatus in an ATP generator. A fraction of the major antenna proteins (LHCII) and cytochrome b6f complexes undergo a lateral redistribution from the PSII membrane domains to the PSI membrane domains, which switches on a cyclic electron flow around PSI exclusively aimed at ATP synthesis. The flexibility in the functional organization of the photosynthetic apparatus is established for work with the unicellular eukaryote Chlamydomonas reinhardtii. The extent to which it also applies to higher plant photosynthesis remains to be assessed.
What is next?
The kinase-mediated redox control of the supramolecular architecture of the photosynthetic apparatus offers a new view of photosynthesis as a flexible energy conversion system in a plant cell. It behaves as a carbon fixation device in State I and an ATP generator in State II. State transitions appear as a—thylakoid-borne—sensor mechanism in which the light environmental effects and the metabolic state of the chloroplast merge, primarily determined by the redox poise and ATP levels. Thylakoid membranes are also the site of chloroplast gene expression, since a major fraction of their protein complement is inserted co-translationally. Chloroplast protein biogenesis is heavily ATP consuming and has been suggested to be partly redox controlled (Allen, 1993). Thus, the ATP charge and the redox poise in the chloroplast contribute to the dynamics of the thylakoid membranes, both in terms of supramolecular architecture and de novo protein insertion. The interplay between these two levels and the signal transduction processes within the chloroplast and between the chloroplast and the nucleocytosol (Goldschmidt- Clermont, 1998) certainly depends on the multiple kinase activities associated with thylakoid membranes, the molecular dissection of which is presently underway as a result of the lengthy hunt for the LHCII-kinase responsible for State transitions.
Acknowledgments
Acknowledgements
Many thanks to Giovanni Finazzi, Fabrice Rappaport and Francesca Zito for stimulating discussions and critical reading of this manuscript. The work from the author’s laboratory was supported by the CNRS (UPR1261).
References
- Allen J.F. (1992a) Protein phosphorylation in regulation of photosynthesis. Biochim. Biophys. Acta, 1098, 275–335. [DOI] [PubMed] [Google Scholar]
- Allen J.F. (1992b) How does protein phosphorylation regulate photosynthesis. Trends Biochem. Sci., 17, 12–17. [DOI] [PubMed] [Google Scholar]
- Allen J.F. (1993) Control of gene expression by redox potential and the requirement for chloroplast and mitochondrial genomes. J. Theor. Biol., 165, 609–631. [DOI] [PubMed] [Google Scholar]
- Allen J.F., Bennett,J., Steinback,K.E. and Arntzen,C.J. (1981) Chloroplast protein phosphorylation couples plastochinone redox state to distribution of excitation energy between photosystems. Nature, 291, 25–29. [Google Scholar]
- Allred D.R. and Staehelin,L.A. (1985) Intra-thylakoid distribution of cytochrome b6/f. Plant Physiol., 78, 199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson B. and Anderson,J.M. (1980) Lateral heterogeneity in the distribution of chlorophyll–protein complexes of the thylakoid membranes of spinach chloroplasts. Biochim. Biophys. Acta, 593, 427–440. [DOI] [PubMed] [Google Scholar]
- Barber J. (1986) Surface electrical charges and protein phosphorylation. In Staehelin,L.A. and Arntzen,C.J. (eds), Encyclopedia of Plant Physiology; Photosynthesis III. Springer-Verlag, Berlin, Germany, pp. 653–664.
- Bennett J. (1977) Phosphorylation of chloroplast membrane proteins. Nature, 269, 344–346. [Google Scholar]
- Bennett J. (1979a) Chloroplast phosphoproteins. Eur. J. Biochem., 99, 133–137. [DOI] [PubMed] [Google Scholar]
- Bennett J. (1979b) The protein kinase of thylakoid membranes is light-dependent. FEBS Lett., 103, 342–344. [DOI] [PubMed] [Google Scholar]
- Bennett J. (1980) Chloroplast phosphoproteins. Evidence for a thylakoid-bound phosphoprotein phosphatase. Eur. J. Biochem., 104, 85–89. [DOI] [PubMed] [Google Scholar]
- Bennett J. (1991) Protein phosphorylation in green plant chloroplasts. Annu. Rev. Plant Physiol., 42, 281–311. [Google Scholar]
- Bennett J., Steinback,K.E. and Arntzen,C.J. (1980) Chloroplast phosphoproteins: regulation of excitation energy transfer by phosphorylation of thylakoid membrane polypeptides. Proc. Natl Acad. Sci. USA, 77, 5253–5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J., Shaw,E.K. and Michel,H. (1988) Cytochrome b6/f complex is required for phosphorylation of light-harvesting chlorophyll a/b complex in chloroplast photosynthetic membranes. Eur. J. Biochem., 171, 95–100. [DOI] [PubMed] [Google Scholar]
- Bonaventura C. and Myers,J. (1969) Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim. Biophys. Acta, 189, 366–383. [DOI] [PubMed] [Google Scholar]
- Breyton C. (2000) Conformational changes in the cytochrome b6f complex induced by inhibitor binding. J. Biol. Chem., 275, 13195–13201. [DOI] [PubMed] [Google Scholar]
- Breyton C., Tribet,C., Olive,J., Dubacq,J.P. and Popot,J.L. (1997) Dimer to monomer conversion of the cytochrome b6f complex. Causes and consequences. J. Biol. Chem., 272, 21892–21900. [DOI] [PubMed] [Google Scholar]
- Brugna M., Nitschke,W., Asso,M., Guigliarelli,B., Lemesle-Meunier,D. and Schmidt,C. (1999) Redox components of cytochrome bc-type enzymes in acidophilic prokaryotes. II. The Rieske protein of phylogenetically distant acidophilic organisms. J. Biol. Chem., 274, 16766–16772. [DOI] [PubMed] [Google Scholar]
- Brugna M., Rodgers,S., Schricker,A., Montoya,G., Kazmeier,M., Nitschke,W. and Sinning,I. (2000) A spectroscopic method for observing the domain movement of the Rieske iron–sulfur protein. Proc. Natl Acad. Sci. USA, 97, 2069–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulte L., Gans,P., Rebeille,F. and Wollman,F.-A. (1990) ATP control on state transitions in vivo in Chlamydomonas reinhardtii. Biochim. Biophys. Acta, 1020, 72–80. [Google Scholar]
- Coughlan S.J. (1988) Chloroplast thylakoid protein phosphorylation is influenced by mutations in the cytochrome b6f complex. Biochim. Biophys. Acta, 933, 413–422. [Google Scholar]
- Crofts A.R., Meinhardt,S.W., Jones,K.R. and Snozzi,M. (1983) The role of the quinone pool in the cyclic electron-transfer chain of Rhodopseudomonas sphaeroides: a modified Q-cycle mechanism. Biochim. Biophys. Acta, 723, 202–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrouzet E., Valkova-Valchanova,M., Moser,C.C., Dutton,P.L. and Daldal,F. (2000) Uncovering the [2Fe2S] domain movement in cytochrome bc1 and its implications for energy conversion. Proc. Natl Acad. Sci. USA, 97, 4567–4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delepelaire P. and Wollman,F.-A. (1985) Correlations between fluorescence and phosphorylation changes in thylakoid membranes of Chlamydomonas reinhardtii in vivo: a kinetic analysis. Biochim. Biophys. Acta, 809, 277–283. [Google Scholar]
- Delosme R., Olive,J. and Wollman,F.A. (1996) Changes in light energy distribution upon state transitions: an in vivo photoacoustic study of the wild type and photosynthesis mutants from Chlamydomonas reinhardtii. Biochim. Biophys. Acta, 1273, 150–158. [Google Scholar]
- de Vitry C. and Wollman,F.-A. (1988) Changes in phosphorylation of thylakoid membrane proteins in light-harvesting complex mutants from Chlamydomonas reinhardtii. Biochim. Biophys. Acta, 933, 444–449. [Google Scholar]
- Finazzi G., Furia,A., Barbagallo,R.P. and Forti,G. (1999) State transitions, cyclic and linear electron transport and photophos phorylation in Chlamydomonas reinhardtii. Biochim. Biophys. Acta, 1413, 117–129. [DOI] [PubMed] [Google Scholar]
- Finazzi G., Zito,F., Barbagallo,R.P. and Wollman,F.A. (2001) Contrasted effects of inhibitors of cytochrome b6f complex on state transitions in Chlamydomonas reinhardtii: the role of Qo site occupancy in LHCII kinase activation. J. Biol. Chem., 276, 9770–9774. [DOI] [PubMed] [Google Scholar]
- Fleischmann M.M., Ravanel,S., Delosme,R., Olive,J., Zito,F., Wollman,F.-A. and Rochaix,J.D. (1999) Isolation and characteriz ation of photoautotrophic mutants of Chlamydomonas reinhardtii deficient in state transition. J. Biol. Chem., 274, 30987–30994. [DOI] [PubMed] [Google Scholar]
- Gal A., Shahak,Y., Schuster,G. and Ohad,I. (1987) Specific loss of LHCII phosphorylation in Lemna mutant 1073 lacking the cytochrome b6f complex. FEBS Lett., 221, 205–210. [Google Scholar]
- Gal A., Zer,H. and Ohad,I. (1997) Redox-controlled thylakoid protein phosphorylation. News and views. Physiol. Plant, 100, 869–885. [Google Scholar]
- Goldschmidt-Clermont M. (1998) Coordination of nuclear and chloroplast gene expression in plant cells. Int. Rev. Cytol., 177, 115–180. [DOI] [PubMed] [Google Scholar]
- Hamel P., Olive,J., Pierre,Y., Wollman,F.A. and de Vitry,C. (2000) A new subunit of cytochrome b6f complex undergoes reversible phosphorylation upon state transition. J. Biol. Chem., 275, 17072–17079. [DOI] [PubMed] [Google Scholar]
- Hunte C., Koepke,J., Lange,C., Rossmanith,T. and Michel,H. (2001) Structure at 2.3 Å resolution of the cytochrome bc1 complex from the yeast Saccharomyces cerevisiae co-crystallized with an antibody Fv fragment. Struct. Fold. Des., 8, 669–684. [DOI] [PubMed] [Google Scholar]
- Iwata S., Lee,J.W., Okada,K., Lee,J.K., Iwata,M., Rasmussen,B., Link,T.A., Ramaswamy,S. and Jap,B.K. (1998) Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science, 281, 64–71. [DOI] [PubMed] [Google Scholar]
- Kim H., Xia,D., Yu,C.A., Xia,J.Z., Kachurin,A.M., Zhang,L., Yu,L. and Deisenhofer,J. (1998) Inhibitor binding changes domain mobility in the iron–sulfur protein of the mitochondrial bc1 complex from bovine heart. Proc. Natl Acad. Sci. USA, 95, 8026–8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse O., Nixon,P.J., Schmid,G.H. and Mullineaux,C.W. (1999) Isolation of state transition mutants of Chlamydomonas reinhardtii by fluorescence video imaging. Photosynth. Res., 61, 43–51. [Google Scholar]
- Kyle D.J., Staehelin,L.A. and Arntzen,C.J. (1983) Lateral mobility of the light-harvesting complex in chloroplast membranes controls excitation energy distribution in higher plants. Arch. Biochem. Biophys., 222, 527–541. [DOI] [PubMed] [Google Scholar]
- Larsson U.K., Jergil,B. and Andersson,B. (1983) Changes in the lateral distribution of the light-harvesting chlorophyll-a/b–protein complex induced by its phosphorylation. Eur. J. Biochem., 136, 25–29. [DOI] [PubMed] [Google Scholar]
- Lemaire C., Girard-Bascou,J. and Wollman,F.-A. (1986) Character ization of the b6/f complex subunits and studies on the LHC-kinase in Chlamydomonas reinhardtii using mutants strains altered in the b6/f. In Biggins,J. (ed.), Progress in Photosynthesis Research. Nijhoff, Dordrecht, The Netherlands, pp. 655–658.
- Lin Z.-F., Lucero,H.A. and Racker,E. (1982) Protein kinases from spinach chloroplasts. I Purification and identification of two distinct protein kinases. J. Biol. Chem., 257, 12153–12156. [PubMed] [Google Scholar]
- Lunde C., Jensen,P.E., Haldrup,A., Knoetzel,J. and Scheller,H.V. (2000) The PSI-H subunit of photosystem I is essential for state transitions in plant photosynthesis. Nature, 408, 613–615. [DOI] [PubMed] [Google Scholar]
- Majeran W., Olive,J., Drapier,D., Vallon,O. and Wollman,F.A. (2001) The light sensitivity of ATP synthase mutants of Chlamydomonas reinhardtii. Plant Physiol., 126, 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millner P., Marder,J.B., Gounaris,K. and Barber,J. (1986) Localization and identification of phosphoproteins within the photosystem II core of higher plant thylakoid membranes. Biochim. Biophys. Acta, 852, 30–37. [Google Scholar]
- Mitchell P. (1975) Protonmotive redox mechanisms of the cytochrome bc1 complex in the respiratory chain: protonmotive ubiquinone cycle. FEBS Lett., 56, 1–6. [DOI] [PubMed] [Google Scholar]
- Murata N. (1969) Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim. Biophys. Acta, 172, 242–251. [DOI] [PubMed] [Google Scholar]
- Murata N. (1970) Control of excitation energy transfer in photosynthesis. IV. Kinetics of chlorophyll a fluorescence in Porphyra yezoensis. Biochim. Biophys. Acta, 205, 379–389. [DOI] [PubMed] [Google Scholar]
- Olive J., Vallon,O., Wollman,F.-A., Recouvreur,M. and Bennoun,P. (1986) Studies on the cytochrome b6f complex. II Localization of the complex in the thylakoid membranes from spinach and Chlamydomonas reinhardtii by immunocytochemistry and freeze-fracture analysis of b6f mutants. Biochim. Biophys. Acta, 851, 239–248. [Google Scholar]
- Snyders S. and Kohorn,B.D. (1999) TAKs, thylakoid membrane protein kinases associated with energy transduction. J. Biol. Chem., 274, 9137–9140. [DOI] [PubMed] [Google Scholar]
- Sokolenko A., Fulgosi,H., Gal,A., Altschmied,L., Ohad,I. and Herrmann,R.G. (1995) The 64 kDa polypeptide of spinach may not be the LHCII kinase, but a lumen-located polyphenol oxidase. FEBS Lett., 362, 235–238. [DOI] [PubMed] [Google Scholar]
- Stys D., Drakenberg,T., Spangfort,M.D., Forsen,S. and Allen,J.F. (1995) Structure and magnesium binding of peptide fragments of LHCII in its phosphorylated and unphosphorylated forms studied by multinuclear NMR. In Mathis,P. (ed.), Photosynthesis: From Light to Biosphere, I. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp. 127–130.
- Swiatek M. et al. (2001) The chloroplast gene ycf9 encodes a photosystem II core subunit, PsbZ, that participates in photosystem II supramolecular architecture. Plant Cell, 13, 1347–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon O., Wollman,F.-A. and Olive,J. (1986) Lateral distribution of the main protein complexes of the photosynthetic appartus in Chlamydomonas reinhardttii and in spinach: an immunocyto chemical study using intact thylakoid membranes and PS II enriched membrane preparation. Photobiochem. Photobiophys., 12, 203–220. [Google Scholar]
- Vallon O., Bulté‘L., Dainese,P., Olive,J., Bassi,R. and Wollman,F.A. (1991) Lateral redistribution of cytochrome b6/f complexes along thylakoid membranes upon state transitions. Proc. Natl Acad. Sci. USA, 88, 8262–8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vener A.V., Van Kan,P.J., Gal,A., Andersson,B. and Ohad,I. (1995) Activation/deactivation cycle of redox-controlled thylakoid protein phosphorylation. Role of plastoquinol bound to the reduced cytochrome bf complex. J. Biol. Chem., 270, 25225–25232. [DOI] [PubMed] [Google Scholar]
- Vener A.V., Van Kan,P.J., Rich,P.R., Ohad,I. and Andersson,B. (1997) Plastoquinol at the quinol oxidation site of reduced cytochrome bf mediates signal trasduction between light and protein phosphorylation: thylakoid protein kinase deactivation by a single turnover flash. Proc. Natl Acad. Sci. USA, 94, 1585–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vener A.V., Ohad,I. and Andersson,B. (1998) Protein phosphorylation and redox sensing in chloroplast thylakoids. Curr. Opin. Plant Biol., 1, 217–223. [DOI] [PubMed] [Google Scholar]
- Weber P., Sokolenko,A., Eshaghi, Fulgosi,H., Vener,A.V., Andersson,B., Ohad,I. and Herrmann,R. (2001) Element of signal transduction involved in thylakoid membrane dynamics. In Sopory,S.K., Oelmüller,L. and Maeshwari,S.C. (eds), Signal Transduction in Plants: Current Advances. Kluwer, Dordrecht, The Netherlands, pp. 222-237
- Wollman F.A. and Delepelaire,P. (1984) Correlation between changes in light energy distribution and changes in thylakoid membrane polypeptide phosphorylation in Chlamydomonas reinhardtii. J. Cell Biol., 98, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman F.-A. and Lemaire,C. (1988) Studies on kinase-controlled state transitions in photosystem II and b6f mutants from Chlamydomonas reinhardtii which lack quinone-binding proteins. Biochim. Biophys. Acta, 85, 85–94. [Google Scholar]
- Zer H., Vink,M., Keren,N., Dilly-Hartwig,H.G., Paulsen,H., Herrmann,R.G., Andersson,B. and Ohad,I.I. (1999) Regulation of thylakoid protein phosphorylation at the substrate level: reversible light-induced conformational changes expose the phosphorylation site of the light-harvesting complex II. Proc. Natl Acad. Sci. USA, 96, 8277–8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Huang,L., Shulmeister,V.M., Chi,Y.I., Kim,K.K., Hung,L.W., Crofts,A.R., Berry,E.A. and Kim,S.H. (1998) Electron transfer by domain movement in cytochrome bc1. Nature, 392, 677–684. [DOI] [PubMed] [Google Scholar]
- Zito F., Finazzi,G., Delosme,R., Nitschke,P., Picot,D. and Wollman,F.-A. (1999) The Qo site of cytochrome b6f complexes controls the activation of the LHCII kinase. EMBO J., 18, 2961–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]