Abstract
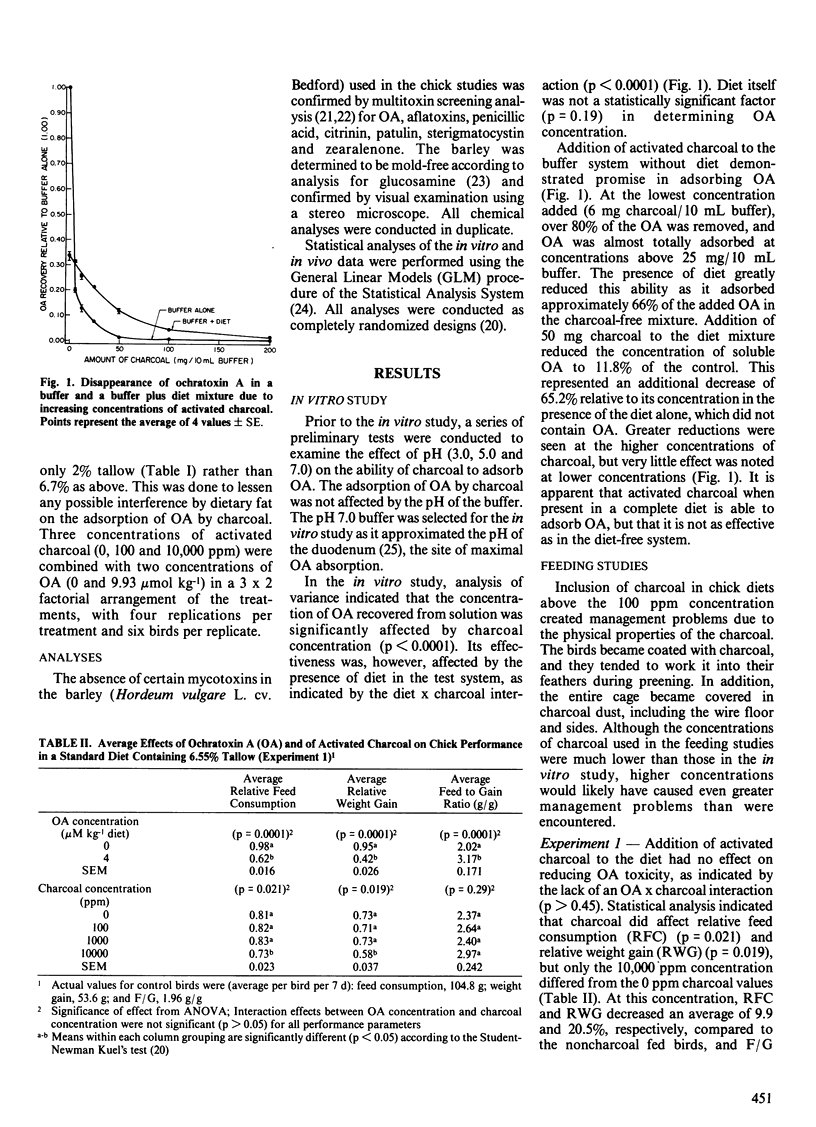
The ability of activated charcoal to adsorb ochratoxin A (OA) in vitro and to reduce the toxic effects of OA in vivo when added to the diet of growing Leghorn chicks was studied. Activated charcoal (50 mg) was able to adsorb 90% of the OA (150 micrograms) contained in 10 mL of citrate-phosphate buffer (pH 7.0). When 2 g of a complete chick diet were mixed with OA in buffer, it adsorbed 66% of the OA, while addition of 50 mg of charcoal to this mixture further reduced the concentration of OA to 11.8% of the control, an additional 65% compared to the diet alone. In the first of two feeding studies, charcoal addition of up to 10,000 parts per million (ppm) to diets (6.7% tallow) containing 9.93 mumol (4 ppm) OA kg-1 diet had no effect on OA toxicity. Feed consumption and weight gain, however, were reduced 10 and 20%, respectively, in chicks fed diets which contained 10,000 ppm of charcoal compared to those fed no charcoal. In the second study, reducing dietary tallow to 2% did not alter the effects of OA or charcoal on weight gain and feed to gain ratio, but birds fed OA with 10,000 ppm charcoal had an 8.5% increase in feed consumption. An additional management problem was associated with the propensity of charcoal to blacken the feed, the birds and their environment. Addition of charcoal to OA contaminated diets appeared to be an ineffective method for reducing the toxic effects of OA in growing chicks.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ademoyero A. A., Dalvi R. R. Efficacy of activated charcoal and other agents in the reduction of hepatotoxic effects of a single dose of aflatoxin B1 in chickens. Toxicol Lett. 1983 Apr;16(1-2):153–157. doi: 10.1016/0378-4274(83)90024-3. [DOI] [PubMed] [Google Scholar]
- Chelkowski J., Goliński P., Szebiotko K. Mycotoxins in cereal grain. Part II. The fate of ochratoxin A after processing of wheat and barley grain. Nahrung. 1981;25(5):423–426. [PubMed] [Google Scholar]
- Chełkowski J., Goliński P., Godlewska B., Radomyska W., Szebiotko K., Wiewiórowska M. Mycotoxins in cereal grain. Part IV. Inactivation of ochratoxin A and other mycotoxins during ammoniation. Nahrung. 1981;25(7):631–637. doi: 10.1002/food.19810250705. [DOI] [PubMed] [Google Scholar]
- Chełkowski J., Szebiotko K., Goliński P., Buchowski M., Godlewska B., Radomyska W., Wiewiórowska M. Mycotoxins in cereal grain. Part 5. Changes of cereal grain biological value after ammoniation and mycotoxins (ochratoxins) inactivation. Nahrung. 1982;26(1):1–7. doi: 10.1002/food.19820260102. [DOI] [PubMed] [Google Scholar]
- Dalvi R. R., Ademoyero A. A. Toxic effects of aflatoxin B1 in chickens given feed contaminated with Aspergillus flavus and reduction of the toxicity by activated charcoal and some chemical agents. Avian Dis. 1984 Jan-Mar;28(1):61–69. [PubMed] [Google Scholar]
- Dalvi R. R., McGowan C. Experimental induction of chronic aflatoxicosis in chickens by purified aflatoxin B1 and its reversal by activated charcoal, phenobarbital, and reduced glutathione. Poult Sci. 1984 Mar;63(3):485–491. doi: 10.3382/ps.0630485. [DOI] [PubMed] [Google Scholar]
- Davis N. D., Sansing G. A., Ellenburg T. V., Diener U. L. Medium-scale production and purification of ochratoxin A, a metabolite of Aspergillus ochraceus. Appl Microbiol. 1972 Feb;23(2):433–435. doi: 10.1128/am.23.2.433-435.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich A. A., Marquardt R. R., Bernatsky A. Quantitation of ochratoxin A: use of reverse phase thin-layer chromatography for sample cleanup followed by liquid chromatography or direct fluorescence measurement. J Assoc Off Anal Chem. 1988 Sep-Oct;71(5):949–953. [PubMed] [Google Scholar]
- Hatch R. C., Clark J. D., Jain A. V., Weiss R. Induced acute aflatoxicosis in goats: treatment with activated charcoal or dual combinations of oxytetracycline, stanozolol, and activated charcoal. Am J Vet Res. 1982 Apr;43(4):644–648. [PubMed] [Google Scholar]
- Josefsson B. G., Möller T. E. Heat stability of ochratoxin A in pig products. J Sci Food Agric. 1980 Dec;31(12):1313–1315. doi: 10.1002/jsfa.2740311215. [DOI] [PubMed] [Google Scholar]
- Josefsson B. G., Möller T. E. Screening method for the detection of aflatoxins, ochratoxin, patulin, sterigmatocystin, and zearalenone in cereals. J Assoc Off Anal Chem. 1977 Nov;60(6):1369–1371. [PubMed] [Google Scholar]
- Sands D. C., McIntyre J. L., Walton G. S. Use of activated charcoal for the removal of patulin from cider. Appl Environ Microbiol. 1976 Sep;32(3):388–391. doi: 10.1128/aem.32.3.388-391.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. M., Tabor W. H., Trucksess M. W. Screening method for the detection of aflatoxin, ochratoxin, zearalenone, penicillic acid, and citrinin. J Assoc Off Anal Chem. 1976 Jan;59(1):125–127. [PubMed] [Google Scholar]
- Yamazaki M., Maebayashi Y., Miyaki K. Production of ochratoxin A by Aspergillus ochraceus isolated in Japan from moldy rice. Appl Microbiol. 1970 Sep;20(3):452–454. doi: 10.1128/am.20.3.452-454.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


