Abstract
In Drosophila, phototransduction is mediated by Gq-activation of phospholipase C and is a well studied model system for understanding the kinetics of signal initiation, propagation and termination controlled by G proteins. The proper intracellular targeting and spatial arrangement of most proteins involved in fly phototransduction require the multi-domain scaffolding protein InaD, composed almost entirely of five PDZ domains, which independently bind various proteins including NorpA, the relevant phospho lipase C-β isozyme. We have determined the crystal structure of the N-terminal PDZ domain of InaD bound to a peptide corresponding to the C-terminus of NorpA to 1.8 Å resolution. The structure highlights an intermolecular disulfide bond necessary for high affinity interaction as determined by both in vitro and in vivo studies. Since other proteins also possess similar, cysteine-containing consensus sequences for binding PDZ domains, this disulfide-mediated ‘dock-and-lock’ interaction of PDZ domains with their ligands may be a relatively ubiquitous mode of coordinating signaling pathways.
Keywords: disulfide bond/InaD/PDZ domain/phospholipase C-β/scaffolding protein
Introduction
G protein-regulated phospholipase C (PLC)-β isozymes play key roles in hormone, neurotransmitter and growth factor action, given their capacity to generate the second messengers inositol trisphosphate (IP3) and diacylglycerol (DAG). Much has been learned about G protein-coupled PLC-β signaling cascades by study of a model system, the Drosophila phototransduction pathway. In Drosophila photoreceptor cells, light activates the heptahelical G protein-coupled receptor (GPCR) rhodopsin, which in turn induces Gq to dissociate into the signaling-competent Gαq-GTP and Gβγ subunits. Activated Gαq-GTP induces no-receptor potential A (NorpA), a PLC-β4 homolog, to cleave phosphatidylinositol bisphosphate (PIP2) into IP3 and DAG, activating the eye-specific protein kinase C (eyePKC) and opening the transient receptor potential (TRP) and Trp-like (TRPL) cation channels. The basis of this transient response is still unknown, but could be due to depletion of PIP2 stores by NorpA (Hardie et al., 2001; Montell, 2001).
The scaffolding protein inactivation no after-potential D (InaD) is absolutely required for coordinating the molecular activities of phototransduction. In the absence of functional InaD, both localization of phototransduction proteins and depolarization of the retinal cells in response to photonic stimulation are severely impaired (Shieh et al., 1997; Tsunoda et al., 1997; Li and Montell, 2000). InaD is mainly composed of five post-synaptic density (PSD)-95/Discs-large/zonular occludens (ZO)-1 (PDZ) domains (Tsunoda et al., 1997). PDZ domains bind preferentially to the C-terminal 3–4 residues of protein targets, but some interact with internal sequences (van Huizen et al., 1998; Hillier et al., 1999). Based upon sequence similarity and ligand specificity, PDZ domains are divided into two classes. The prototypical class I PDZ domain recognition sequence is S/T-X-V-COO–, where X represents any residue, while the class II PDZ domains bind to the more general sequence Φ-X-Φ-COO–, where Φ is usually a large, hydrophobic residue (Daniels et al., 1998).
The PDZ domains of InaD bind to most of the proteins involved in Drosophila phototransduction. The N-terminal PDZ domain of InaD (PDZ1) binds to the unconventional myosin NinaC (Wes et al., 1999), which in turn binds actin. PDZ2, PDZ3 and PDZ4 bind to eyePKC, while PDZ3 and PDZ4 also bind TRP, TRPL, rhodopsin and each other (Fanning and Anderson, 1999). NorpA interacts with both PDZ1 and PDZ5, where PDZ1 binds to the extreme C-terminal residues of NorpA, F-C-A-COO–, and PDZ5 interacts with an undetermined internal sequence (Tsunoda et al., 1997; van Huizen et al., 1998). The ability of InaD to bind multiple partners allows the formation of a massive ‘signalplex’ (Montell, 1999), localizing the phototransduction proteins to the plasma membrane and tethering them to the actin cytoskeleton through NinaC.
PDZ domains are composed of a 5- to 6-stranded anti-parallel β-barrel and 2–3 α-helices (Hata et al., 1998). Several atomic resolution structures of PDZ domains bound to peptides or other proteins have been determined. The third PDZ domain of PSD-95 was crystallized and structurally characterized in the presence of a nine-residue peptide (Doyle et al., 1996). The structure of a type II PDZ domain, hCASK, was solved by crystallography and revealed that hCASK homodimerizes by binding to its own C-terminal tail (Daniels et al., 1998). Structures of the PDZ domain of neuronal nitric oxide synthase (nNOS) were determined by NMR spectroscopy in complex with a peptide (Tochio et al., 1999) and by crystallography bound to the PDZ domain of syntrophin (Hillier et al., 1999). The structure of the syntrophin PDZ domain was itself solved by NMR in the presence of a peptide ligand (Schultz et al., 1998). However, no structure of any of the PDZ domains of InaD has yet been reported.
To begin to understand the structural determinants necessary for the coordination of Drosophila phototransduction by InaD, we have solved the crystal structure of the first PDZ domain of InaD bound to a heptapeptide (Gly-Lys-Thr-Glu-Phe-Cys-Ala) corresponding to the C-terminus of NorpA. This structure reveals the formation of an intermolecular disulfide bond between PDZ1 and the penultimate residue of NorpA. Results from in vitro protein-binding studies and cell co-immunoprecipitations indicate that this disulfide bond is critical for high-affinity heterotetramer formation between InaD and NorpA, showing a need to revise standard models of Drosophila phototransduction. Furthermore, many consensus motifs indicative of PDZ interaction contain similar cysteines, suggesting that the ‘dock-and-lock’ interaction revealed by the PDZ1–NorpA structure may be a relatively ubiquitous interaction between PDZ domains and their target proteins.
Results
Purification and crystal structure analysis of the PDZ1–peptide complex
Recombinant PDZ1 of InaD (residues 11–107) with a cleavable His6 tag was expressed in BL21(DE3) Escherichia coli and purified from the soluble lysate. Crystals were grown of PDZ1 in complex with a mercury-substituted heptapeptide (Gly-Lys-Thr-Glu-Phe-Cys-Ala) corresponding to the C-terminal seven residues of NorpA. The last three residues in the peptide sequence conform to the previously described type II PDZ domain-binding motif Φ-X-Φ-COO–. PDZ1–heptapeptide crystals grew in the P1 space group with two molecules per asymmetric unit and diffracted to 1.7 Å resolution. A mercury multiwavelength anomalous dispersion data set was collected from a single crystal at 100 K.
Although heavy atom locations were found using several different methods, the phases obtained from mercury anomalous diffraction were not of sufficient quality for model building. As another approach, the third PDZ domain of PSD-95 (Doyle et al., 1996) was used as a molecular replacement model for data obtained at the mercury absorption peak wavelength (Table I). Using standard molecular replacement methods (see Materials and methods), the structure of the PDZ1–heptapeptide complex was determined (Table II).
Table I. Data processing statistics.
| Hg peak | |
|---|---|
| Wavelength (Å) | 1.0072 |
| Energy (eV) | 12 309.9 |
| Resolution (Å) | 20–1.76 (1.83–1.76) |
| Redundancy | 3.6 (2.9) |
| No. of unique reflections | 25 906 (2424) |
| I/σ | 17.5 (8.5) |
| Completeness (%) | 95.1 (86.7) |
| Linear R-factor | 0.058 (0.178) |
| Square R-factor | 0.059 (0.156) |
| χ2 | 1.81 (1.075) |
The values for the highest resolution shell are given in parentheses.
Table II. Final model statistics.
| Protein atoms | 1504 |
| Mercury atoms | 2 |
| Water atoms | 172 |
| Resolution range (Å) | 20.0–1.8 |
| R-factor (%) | 21.0 |
| Free R-factor (%) | 23.0 |
| R.m.s. bond length deviations (Å) | 0.007 |
| R.m.s. bond angle deviations (°) | 1.306 |
| Ramachandran plot | |
| most favored regions (%) | 92.3 |
| additionally allowed regions (%) | 6.5 |
| generously allowed regions (%) | 1.2 |
| disallowed regions (%) | 0.0 |
After several rounds of model building interspersed with automated positional and temperature factor refinement, the peptide density became clear in 2Fo – Fc and Fo – Fc maps calculated using model phases (note: the peptide was not included in the model, thus the peptide electron density is not affected by model bias). Hereafter, the residues of the peptide will be referred to with respect to the C-terminus: NH3+-Gly(–6)-Lys(–5)-Thr(–4)-Glu (–3)-Phe(–2)-Cys(–1)-Ala(0)-COO–. The final refined electron density and model for the PDZ1–heptapeptide complex are shown in Figure 1, centered on the binding site. Density for the C-terminal five residues of the peptides is unambiguous, indicating they are well ordered in the structure. Density for Gly(–6) and Lys(–5) is missing, indicating a high degree of mobility for these residues. Surprisingly, even though the peptide was incubated with a 5-fold molar excess of methylmercury chloride prior to crystallization, there is no electron density near Cys(–1) indicative of a covalently bound mercury. Instead, Cys(–1) is participating in an intermolecular disulfide bond to Cys31 of PDZ1. Furthermore, an unanticipated 9σ peak of positive electron density near Cys62 was modeled as the missing mercury and suggests displacement of the methylmercury from the peptide upon disulfide formation. The temperature factors of both mercury atoms in the asymmetric unit are high (∼90 Å2), indicating that the mercuries are either very mobile or poorly substituted, and may explain why the phases obtained from multiwavelength anomalous dispersion could not be used to solve the structure a priori.
Fig. 1. The NorpA C-terminal peptide forms an intermolecular disulfide bond to InaD PDZ1 in the crystalline state. The 2Fo – Fc electron density map is contoured at 1σ, and shows the NorpA C-terminal peptide (orange) bound to PDZ1 (yellow). The five ordered residues of the peptide are labeled with respect to the C-terminus. Crystallographic waters are depicted as red spheres. The inset shows a view of the continuous density for the disulfide bond in a 2Fo – Fc omit map calculated without the cysteines in the model.
General description of the structure
PDZ domains are composed of a 5- to 6-stranded anti-parallel β-barrel and 2–3 α-helices (Morais Cabral et al., 1996; Doyle, 1997). PDZ1 conforms to this general topology, with a β-barrel composed of six β-strands (B1–B6), one short α-helix (A1) and one long α-helix (A2) (Figure 2). The C-terminal portion of the NorpA peptide hydrogen bonds to strand B2 of PDZ1, extending the β-barrel similar to other PDZ–peptide structures (Doyle et al., 1996; Daniels et al., 1998; Schultz et al., 1998; Hillier et al., 1999; Tochio et al., 1999), while the disulfide bond between Cys31 of PDZ1 and Cys(–1) of the NorpA peptide is a unique interaction. Similar to other PDZ domains, the N- and C-termini of PDZ1 are nearly superimposed on the opposite side of the molecule from the peptide binding cleft, presumably to facilitate transfer of the PDZ module between proteins without loss of binding specificity (Doyle et al., 1996).
Fig. 2. Ribbon diagram showing the secondary and tertiary structure of PDZ1 complexed with the NorpA C-terminal peptide. The peptide is shown in red ball-and-stick format. Cys31, which participates in disulfide bonding to the peptide, and Cys62, which binds the crystallographic mercury ion, are labeled.
The structure profile of PDZ1 directed a multiple sequence alignment of select PDZ domains using ClustalW (Higgins and Sharp, 1988) followed by manual adjustment of the alignment (Figure 3). The carboxylate binding loop, spanning the region between strands B1 and B2, is easily discernible by the G-L-G-F motif in the case of class I PDZ domains such as Discs-large, PSD-95 PDZ2 and PDZ3, syntrophin and nNOS, or the more general X-Φ-G-Φ motif in the case of class II PDZ domains, of which hCASK and InaD PDZ1 are members. These conserved, flexible glycine linkers allow simultaneous binding of peptide C-termini while satisfying the overall fold of the β-barrel (Doyle et al., 1996).
Fig. 3. Sequence alignment of PDZ domains of known tertiary structure. Identical residues are shaded in red, conserved residues in black and similar residues in gray. Cys31 is shaded in gold. The secondary structural elements of InaD PDZ1 are indicated above the alignment, with colors corresponding to the structure in Figure 2. The numbers above the alignment indicate residue positions of InaD PDZ1.
As highlighted by the similarly oriented structures of InaD PDZ1 and PSD-95 PDZ3 bound to their cognate peptides (Figure 4), InaD PDZ1 shares many characteristics with other PDZ domains. For example, the footprint of the five ordered residues of the peptide in the binding site are nearly identical in the InaD PDZ1 and PSD-95 PDZ3 structures. Consequently, the hydrogen bonding patterns for the two complexes are essentially identical. Residues in strands B1, B2 and B3 and helix A2 make important contacts directly with the peptide or through ordered waters, and these contacts are conserved between InaD PDZ1 and PSD-95 PDZ3. Similarly, the carboxylate-binding loop of PDZ domains is in general highly electropositively charged, and this feature is maintained in PDZ1 (Doyle et al., 1996; Daniels et al., 1998; Schultz et al., 1998; Tochio et al., 1999) (Figure 5A). PDZ1 also contains the deep binding cleft for the peptide characteristic of other PDZ domains (Figure 5B).
Fig. 4. Comparison of the peptide binding sites of (A) InaD PDZ1 and (B) PSD-95 PDZ3 with their respective peptide ligands. The peptides are shown in red ball-and-stick format. The protein backbones are shown as coils, color-coded to match the structure in Figure 2. Ordered waters are represented as green spheres. Hydrogen bonds and ionic interactions of 3.3 Å or less are shown as dashed lines.

Fig. 5. Molecular surface representations of the PDZ1–peptide structure. (A) Electrostatic surface representation. Electropositive residues are shown in blue, while electronegative residues are shown in red. There is a large electropositive pocket composed of residues in the carboxylate-binding loop. (B) Surface curvature representation. Highly convex regions are shown in green, while highly concave regions are shown as increasingly darker gray. The C-terminus of the peptide resides in a deep declivity of PDZ1.
However, there are also important differences between the interaction of PDZ1 with the NorpA peptide and other PDZ–peptide interactions. For example, the NorpA peptide contains an abrupt turn at Phe(–2) while all other peptides bound to PDZ domains are in extended conformations (Doyle et al., 1996; Daniels et al., 1998; Schultz et al., 1998; Tochio et al., 1999). Furthermore, while PDZ1 possesses the characteristic hydrophobic cleft near the C-terminal-binding loop normally occupied by the side chain of the final residue within the peptide ligand, Ala(0) of the NorpA peptide is exposed to solvent. Burial of the C-terminal side chain is generally assumed to be a major factor in determining binding specificity (Songyang et al., 1997). For example, mutagenesis of the C-terminal Val of the Kv1.4 K+ channel to Ala abolishes binding of the channel to PSD-95 PDZ2 (Kim et al., 1995). Most importantly, Cys(–1) of the NorpA peptide is involved in a disulfide bond to Cys31 of PDZ1, a covalent interaction that has not been observed in any previous PDZ–peptide structures.
Physiological relevance of the intermolecular disulfide bond
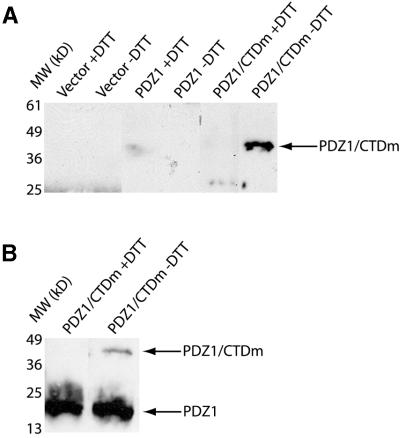
From the structure it is clear that the major interaction between the peptide and PDZ1 is an intermolecular disulfide bond. In order to determine the importance of the disulfide bond for PDZ1 and NorpA interaction, PDZ1 was incubated with the C-terminal domain of NorpA (CTDm), spanning residues 863–1095, at a 1:1 molar ratio, and analyzed by gel exclusion chromatography (Figure 6A). The complex species was separated from free CTDm and PDZ1, and the fractions analyzed by SDS–PAGE to resolve the proteins by molecular weight in non-reducing and reducing conditions. PDZ1 (10.9 kDa) and CTDm (27.6 kDa) form a stable complex (38.5 kDa) in denaturing conditions that is completely dissociable with addition of reductant (Figure 6B and C). An unexpected result of this experiment is that CTDm elutes from the gel exclusion column as a homodimer, and the PDZ1–CTDm complex as a heterotetramer (Figure 6A).
Fig. 6. PDZ1 binds to CTDm via a disulfide bond in vitro. (A) PDZ1 and CTDm elute as a heterotetramer by gel exclusion chromatography. The molecular weights were calculated from an equation obtained from standardization of the column. PDZ1 and CTDm co-migrate as a 38 kDa species during SDS–PAGE under non-reducing conditions (B) and as separate species upon reduction (C).
As evidenced by co-immunoprecipitation of PDZ1 and CTDm under conditions designed to prevent spurious disulfide bond formation, interaction of PDZ1 and CTDm is dependent on intermolecular disulfide bonding in a eukaryotic system (Figure 7). When hemagglutinin (HA) epitope-tagged PDZ1 is immunoprecipitated after co-expression with myc-CTDm, a 46 kDa complex corresponding to a HA-PDZ1–myc-CTDm dimer can be isolated by non-reducing SDS–PAGE. However, the complex is not observed upon co-immunoprecipitation in the presence of dithiothreitol (DTT), indicating the requirement of the disulfide bond for high-affinity complex formation, even in the reducing environment of a eukaryotic cell. These results highlight the importance of the disulfide bond for PDZ1–CTDm interaction in vivo (see Discussion).
Fig. 7. PDZ1 binds to CTDm via a disulfide bond in vivo. 293T cells expressing HA-PDZ1 and/or myc-CTDm were lysed in the presence of NEM, and PDZ1 was immunoprecipitated with anti-HA antibody in the absence and presence of 10 mM DTT. Immunoprecipitated proteins were separated on denaturing polyacrylamide gels in the absence of reducing agent, transferred to PVDF membranes, and blotted with (A) anti-myc-horseradish peroxidase (HRP) or (B) anti-HA-HRP antibodies. A band corresponding to the theoretical molecular weight of an HA-PDZ1–myc-CTDm heterodimer is present when the immuno precipitation was performed in the absence of reductant, but is not observed with the addition of 10 mM DTT during immunoprecipitation.
Discussion
The proper localization of signaling machinery is crucial for efficient cell signaling and regulation. In Drosophila, the proteins of the phototransduction pathway localize to the rhabdomeres, subcellular compartments that house the ∼108 molecules of rhodopsin found in each photoreceptor cell. TRP, eyePKC and NorpA require interaction with InaD for localization to and retention in the rhabdomeres (Chevesich et al., 1997; Tsunoda et al., 1997, 2001; Li and Montell, 2000). In inaD null mutants, TRP, eyePKC and NorpA are distributed randomly throughout the photoreceptor cells, leading to near-complete loss of signaling (Tsunoda et al., 1997). Since binding of NorpA to InaD is critical for phototransduction, we were interested in elucidating the structural determinants of NorpA–InaD interaction through the structure of InaD PDZ1 in complex with the C-terminus of NorpA. Discovering that PDZ1–NorpA interaction is mediated by an intermolecular disulfide bond led us to conduct several interesting experiments that have furthered our knowledge of PDZ–protein interactions in G protein-mediated signaling cascades.
As shown by gel exclusion chromatography, NorpA forms a stable homodimer through its C-terminal domain (Figure 6A), supporting the recent discovery that other PLC-βs, namely mammalian PLC-β1 and -β2 and turkey PLC-β, form stable dimers (A.U.Singer, G.L.Waldo, T.K.Harden and J.Sondek, in preparation). As is shown in the present work, a stable CTDm dimer can be covalently linked to two PDZ1 molecules through intermolecular disulfide bond formation. Evidence of dimerization of NorpA and other PLC-β isoforms, along with disulfide-mediated interaction of NorpA and InaD, necessitates a revision of the current model of Drosophila phototransduction (Figure 8). In this revised model, a NorpA homodimer can covalently bind two InaD molecules, each of which can homodimerize through PDZ3 and PDZ4 (Xu et al., 1998). This increases the number and strength of connections between phototransduction components by linking several rhodopsins, eyePKCs and TRP isozymes through NorpA dimers. As in the former model, PDZ1 can also bind to the myosin NinaC, linking the entire membrane-bound signalplex to the actin cytoskeleton (Figure 8). Binding of PDZ1 to NinaC, which has the C-terminal sequence VDI-COO–, requires at least the C-terminal 21 residues of NinaC (Wes et al., 1999), suggesting that PDZ1 may interact with NinaC in a different mode than it does with NorpA.
Fig. 8. Revised model of Drosophila phototransduction. Light activation of rhodopsin (Rh) is indicated by curved arrows. Rhodopsin is held in tight complex with Gαq and NorpA (PLC) both by dimerization of NorpA and interaction with the PDZ domains of InaD, which itself can dimerize. EyePKC and the TRP and TRPL cation channels are also bound by InaD. Finally, PDZ1 interacts with the myosin NinaC, tethering the entire signalplex to both the plasma membrane and the actin cytoskeleton.
The spatial localization and connectivity of relevant proteins would help explain why the Drosophila phototransduction pathway is one of the fastest G protein-coupled signaling cascades known, with activation and termination occurring within tens of milliseconds (Ranganathan et al., 1991). Another factor contributing to the rapid cycling of the Drosophila phototransduction cascade is that NorpA functions not only as an effector for Gq signaling, but also as a GTPase-activating protein (GAP) for GTP-bound Gαq (Cook et al., 2000; Montell, 2000). Drosophila norpA mutations suggest that the isosteric Cys1094Ser point mutation effectively abrogates all functional PDZ1 binding to NorpA, mimicking the effects of completely deleting the PDZ1 binding site (NorpA ΔCterm 25) (Table III). The combination of well characterized norpA mutants together with our structural and biochemical analyses strongly suggest that disulfide-bond formation between InaD and the penultimate residue of NorpA is critical for proper phototransduction in Drosophila. By holding NorpA in a tight, membrane-bound complex, a covalent association between InaD and NorpA aids in the rapid regulation of Gq by NorpA.
Table III. Effects of Drosophila norpA mutations.
| Reference | Mutation | Experiment | Result |
|---|---|---|---|
| Shieh et al. (1997) | NorpA C1094S | in vitro | NorpA binding to PDZ1 abolished |
| NorpA ΔCterm 25 | in vitro and in vivo | PDZ1 binding abolished; NorpA mislocalized; slow kinetics | |
| van Huizen et al. (1998) | NorpA C1094S | in vitro and in vivo | PDZ1 binding abolished; NorpA mislocalized; slow kinetics |
| Cook et al. (2000) | NorpA C1094S | in vivo | NorpA mislocalized; slow kinetics |
While intracellular disulfide-bond formation is not common, there is precedence for its occurrence. Some results have shown that PSD-95 homotetramerizes through intermolecular disulfide bond formation in vivo (Hsueh et al., 1997; Hsueh and Sheng, 1999). In addition, elongation factor (EF) Ts from Thermus thermophilus homodimerizes through the formation of intermolecular disulfide bonds, enabling EF Ts to catalyze nucleotide exchange on EF Tu (Blank et al., 1996; Jiang et al., 1996; Hwang et al., 1997; Nesper et al., 1998).
Intracellular disulfide bond formation may indicate that a protein or protein complex is regulated by the redox potential of the cell. For example, ultraviolet light promotes the disulfide bond-mediated dimerization and superactivation of Ret tyrosine kinase by increasing reactive oxygen species (ROS), which directly attack the intracellular domains of Ret (Kato et al., 2000). As another example, the molecular chaperone Hsp33 is activated during oxidative stress by the formation of two intramolecular disulfide bonds (Graumann et al., 2001). In Drosophila, redox regulation could be an overall assurance that the InaD–NorpA complex is maintained under conditions of high ambient light, where oxidizing conditions might prevail.
While the PDZ1–NorpA peptide structure is the first example of a PDZ domain forming a disulfide-bonded complex with its ligand, it is not expected to be the only example. A motif search of the Swiss-Prot database (http://motif.genome.ad.jp) indicates that there are many proteins with a putative PDZ binding sequence and a Cys in the (–1) position (Table IV). Of particular interest is the 5-hydroxytryptamine (5-HT)-2A serotonin receptor, with the C-terminal sequence S-C-V-COO–. The 5-HT-2C isoform has been shown to bind the human multiple PDZ domain protein MUPP1 through its tenth PDZ domain (Becamel et al., 2001). MUPP1 also binds 5-HT-2A and 5-HT-2B (Becamel et al., 2001), suggesting that the 5-HT-2A isoform could also interact with a PDZ domain protein. Also of interest is Frizzled 10, with the C-terminal sequence T-C-V-COO–. In canonical Wnt signaling, extracellular Wnt activates a heptahelical Frizzled receptor, which in turn activates Dishevelled. Activated Dishevelled affects many different pathways, including those involved in development, glycogen metabolism, transcription, cell adhesion and insulin signaling (Arias et al., 1999). While Frizzled 10 has not been shown to bind a PDZ domain, the PDZ domain of Dishevelled 1-like (DVL1-L PDZ) contains a sequence nearly identical to InaD PDZ1 in the peptide binding cleft (Figure 9), potentially linking two proteins involved in Wnt signaling by a covalent disulfide bond.
Table IV. Selected proteins with putative Cys-containing PDZ binding sequences.
| Organism | Protein | SwissProt accession No. | Function | C-terminus |
|---|---|---|---|---|
| Drosophila | NorpA | P13217 | phospholipase C | FCA |
| Wingless | P09615 | differentiation factor | TCL | |
| Knirps | P10734 | transcription repressor | VCV | |
| netrin A | Q24567 | extracellular matrix protein | TCA | |
| Human | ZFP36 | P17209 | Zn finger transcription factor | SCV |
| ZAP70 | P43403 | T-cell Tyr kinase | ACA | |
| Ulk-1 | O75385 | ubiquitious Ser/Thr kinase | ICA | |
| adenylosuccinase | P30566 | purine biosynthesis | LCL | |
| P53 induced protein 10 | O14682 | apoptosis | FCL | |
| NAG-2 | O14817 | TM4 superfamily/binds integrin | YCA | |
| c-Myc | P01106 | transcription factor | SCA | |
| insulin-like peptide 4 | Q14641 | insulin family signal peptide | LCT | |
| glutathione peroxidase | P07203 | Se-dependent ROS scavenger | SCA | |
| 5-HT-2A | P28223 | GPCR/serotonin receptor | SCV | |
| T-cadherin receptor | P55290 | GPI-anchored glycoprotein | ACL | |
| CD86 precursor | P42081 | CD28 ligand | TCF | |
| estradiol 17B hydrogenase | P56937 | steroid hormone biosynthesis | SCL | |
| EGR-3 | Q06889 | transcription factor | TCA | |
| galactokinase 1 | P51570 | galactose metabolism | LCL | |
| Frizzled 10 | Q9ULW2a | wingless receptor/putative GPCR | TCV | |
| Rat | hexokinase III | P27296 | multiple metabolic pathways | ACV |
| Olif. Rec. like prot I15 | P23274 | GPCR; odorant receptor | FCL | |
| Olif. Rec. like prot F3 | P23265 | GPCR; odorant receptor | FCY | |
| D3 phosphoglycerate dehydrogenase | O08651 | serine biosynthesis | FCF |
ZFP, zinc finger protein; ZAP, zeta-associated protein; UlK-1, UNC-51-like kinase-1; NAC, novel antigen; TM4, transmembrane helix 4. atrEMBL accession No.
Fig. 9. Alignment of PDZ domains containing Cys in the peptide-binding cleft. DVL1-L PDZ, InaD PDZ1 and Sitac18 PDZ1 sequences were aligned with those of all PDZ domains of known structure, but for clarity only the three sequences of interest are shown. Identical residues are depicted in red, conserved residues are shown in black and similar residues are shown in gray. Cysteines in the putative peptide binding pockets are shown in gold. The numbers below the alignment refer to residue positions in the InaD sequence.
Recently, it was discovered that the extreme C-terminal Cys of the tetraspanin L6 antigen is required for its interaction with SITAC18, which contains two PDZ domains (Borrell-Pages et al., 2000). Interestingly, Cys30 of SITAC18 PDZ1 aligns with Ile30 of InaD PDZ1 and DVL1-L PDZ, putting it in position, structurally, to interact with Cys(0) of the tetraspanin L6 antigen (Figure 9). These alignments indicate that intermolecular disulfide-bond formation to the extreme C-terminal residue of a ligand may be yet another mode of PDZ domain binding.
In conclusion, the three-dimensional structure of the PDZ1–NorpA C-terminus complex has revealed a novel mode of PDZ domain binding, mediated by the formation of an intermolecular disulfide bond. As determined by in vitro protein binding studies, eukaryotic cell co-immunoprecipitations and in vivo norpA mutations, the PDZ1–NorpA disulfide bond is critical for the ‘dock-and-lock’ interaction of InaD and NorpA. Sequence motif searches indicate that intermolecular disulfide bond formation between a PDZ domain and its ligand may not be restricted to the Drosophila phototransduction pathway, and that some PDZ domain interactions may be regulated by the redox state of the cell.
Materials and methods
Cloning and protein expression
PDZ1- and CTDm-specific primers were used to amplify residues 11–107 of InaD and 863–1095 of NorpA, respectively. NcoI cleavage sites were engineered into the sense primers, and XhoI cleavage sites into the antisense primers. The sequences of the PDZ1 primers used were 5′-ACCATGGCGGGTGAGCTCATTCACATGGTGA-3′ (sense) and 5′-ACTCGAGTTACTTGTCGAAGGTCTGAATCTCCAG-3′ (antisense). The sequences of the CTDm primers used were 5′-ACCATGGAACCACCACTAGTCTTTGAGC-3′ (sense) and 5′-GCTCGAGTTAGGCACAAAATTCCGTTTTTCC-3′ (antisense). The PDZ1 and CTDm coding sequences were obtained by PCR using the gene-specific primers and plasmid templates generously provided by Bih-hwa Shieh (Vanderbilt University). The cDNA was ligated into the pCR2.1/TOPO vector and transformed into TOP10F′-competent cells using the TOPO TA cloning system (Invitrogen). pCR2.1/PDZ1 and pCR2.1/CTDm were purified from overnight cultures of positive transformants. Both plasmids were restriction digested with NcoI and XhoI at 37°C for 3 h. The cleaved PDZ1 and CTDm inserts were separated from the vector by agarose gel electrophoresis followed by gel purification (Qiagen). The PDZ1 and CTDm genes were ligated into similarly cleaved and purified pPROEX HTa (Life Technologies), which encodes an N-terminal His tag that is specifically cleaved by Tobacco etch virus (TEV) protease.
Escherichia coli strain BL21(DE3) was transformed with pPRO/PDZ1 or pPRO/CTDm for protein overexpression. Overnight cultures were used to inoculate 4 l of super medium (12 g of tryptone and 24 g of yeast extract per liter), which was grown at 37°C with shaking to an optical density at 600 nm of ∼1. The temperature was reduced to 30°C and protein expression induced with addition of 1 mM isopropyl- β-d-thiogalactopyranoside for 6–7 h. The cultures were pelleted by centrifugation, resuspended in N1 (100 mM sodium phosphate, 150 mM sodium chloride, 10% glycerol, 10 mM imidazole) and lysed by French press. The cell lysate was centrifuged for 30 min at >13 000 g, and the supernatant passed over a 5 ml Ni2+ chelating column (Amersham Pharmacia) to bind the His-tagged protein. The column was washed with 8 column volumes of N1 and 8 column volumes of 5% N2 (N1 + 1M imidazole) and His-PDZ1 or His-CTDm eluted with 30% N2. DTT (5 mM) was added to the elutant, and the His tag specifically cleaved with TEV protease at 4°C overnight. The digested PDZ1 was subjected to gel exclusion chromatography by passage over a 100 ml Superdex 200 (S200) gel exclusion column (Amersham Pharmacia) to separate PDZ1 from the TEV protease and free His tag. The PDZ1 peak fractions were pooled and buffer exchanged into crystallization buffer (20 mM PIPES, 10 mM NaCl, 10% glycerol, 2 mM DTT) using an Amicon concentrator with a YM3 membrane, concentrated to 11 mg/ml (1 mM) and stored at –80°C. The digested CTDm was passed over a Ni2+ chelating column to bind the free tag and TEV protease. The flow-through, containing cleaved CTDm, was loaded onto a Source-S column (Amersham Pharmacia) and eluted with a NaCl gradient. Fractions containing CTDm were pooled and applied to an S200 gel exclusion column for final purification. The CTDm peak fractions were concentrated and buffer exchanged into crystallization buffer and stored at –80°C.
Crystallization and structure determination
The NorpA C-terminal heptapeptide was synthesized by the UNC-CH Protein Chemistry facility. The peptide was purified by HPLC and the peptide content quantitated by mass spectrometric analysis. The peptide was resuspended in crystallization buffer at a concentration of 10 mg/ml and mixed with PDZ1 at a 1.3:1 molar ratio for use in crystallization screens using sitting-drop vapor diffusion. Crystals grew in 24% PEG 4000, 100 mM Tris pH 8.5, 200 mM LiCl and 10% glycerol, and good crystals could be routinely obtained by microseeding. The crystals were characterized on a home X-ray source.
For derivative preparation, methylmercury chloride was solubilized in 50% acetonitrile and mixed with 10 mg of peptide at a 5:1 molar ratio at room temperature overnight. The solution was dried in a Savant SpeedVac and resuspended in water. The rehydrated peptide was centrifuged at 14 000 r.p.m. in a microcentrifuge and the supernatant, which contains the mercury-substituted peptide, was saved and the pellet discarded. Native PDZ1–mercurated peptide crystals were obtained by microseeding equilibrated drops with native crystal shards.
Mercury-substituted PDZ1–peptide complex crystals were transferred to a cryo-protective solution (24% PEG 4000, 15% glycerol, 40 mM LiCl, 80 mM Tris–HCl pH 8.5), flash-frozen in liquid nitrogen, and stored at 100 K. Data were collected from a single frozen crystal at beamline X4A at the National Synchrotron Light Source (NSLS) at Brookhaven National Labs. Fluorescence revealed the presence of mercury in the crystals. A 6-wavelength anomalous dispersion data set was collected around the mercury absorbance wavelength. The crystal diffracted to beyond 1.7 Å.
Data were integrated and scaled using the HKL program suite (Otwinowski, 1993). The crystals belong to the space group P1 (a = 42.17, b = 44.15, c = 44.46, α = 106.55, β = 100.61, γ = 118.25) with two molecules per unit cell. SOLVE (Terwilliger and Berendzen, 1999), ShelX (Sheldrick, 1986) and CNS (Brünger, 1992) were all used to locate the two mercury heavy atom sites, but resulting electron density maps using the mercury phase information were of poor quality. The structure of PSD-95 PDZ3 (Protein Data Bank accession No. 1BE9) was truncated and non-identical residues changed to alanine to provide a suitable molecular replacement model for PDZ1. Because of its superior data reduction statistics, the mercury peak wavelength was chosen as the native data for use in molecular replacement using the CCP4 program, AMoRe (Otwinowski, 1991). Rigid-body refinement, simulated annealing, energy minimization and B-factor refinement were carried out using CNS (Brünger, 1992) with bulk solvent correction. Model building was performed using O (Jones et al., 1991). The quality of the structure was analyzed using PROCHECK (Laskowski et al., 1993). The final model consists of two PDZ1 molecules (residues 12–105 of InaD), the C-terminal five residues of the heptapeptide, two mercury ions and 172 water molecules. The coordinates have been deposited in the Protein Data Bank (accession No. 1IHJ).
In vitro protein binding
Seven milligrams of purified CTDm and 2.75 mg of purified PDZ1 were incubated at 4°C overnight, and the species separated by molecular weight using a 100 ml Superdex 200 gel exclusion column (Amersham Pharmacia) in non-reducing conditions. Since PDZ1 does not absorb at 280 nm, protein content of the fractions was assayed at 600 nm using the BioRad Protein Assay and plotted versus elution volume. The column was standardized using broad-range molecular weight standards (Sigma Aldrich) and elution volume found to be linear from 29–700 kDa. Thus, the molecular weight of PDZ1 or CTDm species separated on the S200 column could be accurately calculated, and the stoichiometry of binding determined. Protein identity was confirmed by separating the species in the peak fractions by non-reducing and reducing SDS–PAGE using 16% polyacrylamide gels.
Cell culture
293T cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and 40 µg/ml gentamycin. Prior to transfection, 293T cells were seeded on 10 mm plates and cultured overnight to ∼70% confluence. Control cells were transfected with 2 µg of pcDNA3.1 (Invitrogen) encoding an N-terminal HA tag and 2 µg of pcDNA3.1 encoding an N-terminal Myc tag, and experimental cells were transfected with 1 µg of pcDNA3.1/HA-PDZ1 and/or 6 µg of pcDNA3.1/Myc-CTDm (a 1:2.5 molar ratio) using FuGENE 6 (Roche). After 48 h, cells were suspended in lysis buffer (20 mM Tris pH 7.5, 100 mM NaCl, 1% Triton X-100, 2.5 mM MgCl2, 1 mM EDTA and protease inhibitors) with 10 mM N-ethylmaleimide (NEM) added to block free sulfhydryl groups. After incubation at 4°C for 1 h, insoluble fractions of cell lysates were pelleted in a microcentrifuge at 10 000 r.p.m. for 10 min. The supernatant was pre-cleared with protein A/G–agarose beads (Roche) for 45 min at 4°C. The beads were pelleted at 10 000 r.p.m. for 15 s in a microcentrifuge, and the supernatant split into two aliquots. DTT (10 mM) was added to one aliquot and HA-specific 12CA5 antibody (Roche) was incubated with each sample for 60 min at 23°C with stirring. Protein A/G beads were added, and the samples incubated for 1 h at 23°C. The beads were pelleted, and the supernatant removed and discarded. The beads were washed three times with lysis buffer. The proteins bound to the beads were denatured in SDS–PAGE sample buffer lacking reductants for 5 min at 100°C. The immunoprecipitates were separated by SDS–PAGE and analyzed by immunoblotting.
Acknowledgments
Acknowledgements
We would like to thank M.Pham for technical assistance; A.Singer and J.Snyder for assistance with data collection; D.Worthylake and K.Rossman for assistance with structure solution; B.Blake, J.Hillmann and R.Kimple for assistance with cell culture; B.-H.Shieh for providing NorpA and InaD plasmids; C.Ogata and R.Abramowitz of beamline X4A, and the staff of NSLS for help with synchrotron data collection. M.E.K. is supported by a Molecular and Cellular Biophysics Training Grant, D.P.S. acknowledges support from the NIH, the EJLB Foundation, the Burroughs-Wellcome Fund and Inspire Pharmaceuticals, and J.S. acknowledges support from the NIH, the Pew Charitable Trusts and GlaxoSmithKline.
References
- Arias A.M., Brown,A.M. and Brennan,K. (1999) Wnt signalling: pathway or network? Curr. Opin. Genet. Dev., 9, 447–454. [DOI] [PubMed] [Google Scholar]
- Becamel C., Figge,A., Poliak,S., Dumuis,A., Peles,E., Bockaert,J., Lubbert,H. and Ullmer,C. (2001) Interaction of serotonin 5-hydroxytryptamine type 2C receptors with PDZ10 of the multi-PDZ domain protein MUPP1. J. Biol. Chem., 276, 12974–12982. [DOI] [PubMed] [Google Scholar]
- Blank J., Nock,S., Kreutzer,R. and Sprinzl,M. (1996) Elongation factor Ts from Thermus thermophilus—overproduction in Escherichia coli, quaternary structure and interaction with elongation factor Tu. Eur. J. Biochem., 236, 222–227. [DOI] [PubMed] [Google Scholar]
- Borrell-Pages M., Fernandez-Larrea,J., Borroto,A., Rojo,F., Baselga,J. and Arribas,J. (2000) The carboxy-terminal cysteine of the tetraspanin L6 antigen is required for its interaction with SITAC, a novel PDZ protein. Mol. Biol. Cell, 11, 4217–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brünger A.T. (1992) X-PLOR v. 3.1. A System for X-ray Crystallography and NMR. Yale University Press, New Haven, CT.
- Chevesich J., Kreuz,A.J. and Montell,C. (1997) Requirement for the PDZ domain protein, INAD, for localization of the TRP store-operated channel to a signaling complex. Neuron, 18, 95–105. [DOI] [PubMed] [Google Scholar]
- Cook B., Bar-Yaacov,M., Cohen,B.H., Goldstein,R.E., Paroush,Z., Selinger,Z. and Minke,B. (2000) Phospholipase C and termination of G-protein-mediated signalling in vivo. Nature Cell Biol., 5, 296–301. [DOI] [PubMed] [Google Scholar]
- Daniels D.L., Cohen,A.R., Anderson,J.M. and Brünger,A.T. (1998) Crystal structure of the hCASK PDZ domain reveals the structural basis of class II PDZ domain target recognition. Nature Struct. Biol., 5, 317–325. [DOI] [PubMed] [Google Scholar]
- Doyle D.A., Lee,A., Lewis,J., Kim,E., Sheng,M. and MacKinnon,R. (1996) Crystal structures of a complexed and peptide-free membrane protein-binding domain: molecular basis of peptide recognition by PDZ. Cell, 85, 1067–1076. [DOI] [PubMed] [Google Scholar]
- Doyle M.L. (1997) Characterization of binding interactions by isothermal titration calorimetry. Curr. Opin. Biotechnol., 8, 31–35. [DOI] [PubMed] [Google Scholar]
- Fanning A.S. and Anderson,J.M. (1999) Protein modules as organizers of membrane structure. Curr. Opin. Cell Biol., 11, 432–439. [DOI] [PubMed] [Google Scholar]
- Graumann J., Lilie,H., Tang,X., Tucker,K.A., Hoffmann,J.H., Vijayalakshmi,J., Saper,M., Bardwell,J.C. and Jakob,U. (2001) Activation of the redox-regulated molecular chaperone Hsp33— a two-step mechanism. Structure, 9, 377–387. [DOI] [PubMed] [Google Scholar]
- Hardie R.C., Raghu,P., Moore,S., Juusola,M., Baines,R.A. and Sweeney,S.T. (2001) Calcium influx via TRP channels is required to maintain PIP2 levels in Drosophila photoreceptors. Neuron, 30, 149–159. [DOI] [PubMed] [Google Scholar]
- Hata Y., Nakanishi,H. and Takai,Y. (1998) Synaptic PDZ domain-containing proteins. Neurosci. Res., 32, 1–7. [DOI] [PubMed] [Google Scholar]
- Higgins D.G. and Sharp,P.M. (1988) CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene, 73, 237–244. [DOI] [PubMed] [Google Scholar]
- Hillier B.J., Christopherson,K.S., Prehoda,K.E., Bredt,D.S. and Lim,W.A. (1999) Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS–syntrophin complex. Science, 284, 812–815. [PubMed] [Google Scholar]
- Hsueh Y.P. and Sheng,M. (1999) Requirement of N-terminal cysteines of PSD-95 for PSD-95 multimerization and ternary complex formation, but not for binding to potassium channel Kv1.4. J. Biol. Chem., 274, 532–536. [DOI] [PubMed] [Google Scholar]
- Hsueh Y.P., Kim,E. and Sheng,M. (1997) Disulfide-linked head-to-head multimerization in the mechanism of ion channel clustering by PSD-95. Neuron, 18, 803–814. [DOI] [PubMed] [Google Scholar]
- Hwang Y.W., Sanchez,A., Hwang,M.C. and Miller,D.L. (1997) The role of cysteinyl residues in the activity of bacterial elongation factor Ts, a guanosine nucleotide dissociation protein. Arch. Biochem. Biophys., 348, 157–162. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Nock,S., Nesper,M., Sprinzl,M. and Sigler,P.B. (1996) Structure and importance of the dimerization domain in elonga tion factor Ts from Thermus thermophilus. Biochemistry, 35, 10269–10278. [DOI] [PubMed] [Google Scholar]
- Jones T.A., Zou,J.Y., Cowan,S.W. and Kjelgard,M. (1991) Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A, 47, 110–119. [DOI] [PubMed] [Google Scholar]
- Kato M. et al. (2000) Ultraviolet light induces redox reaction-mediated dimerization and superactivation of oncogenic Ret tyrosine kinases. Mol. Biol. Cell, 11, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Niethammer,M., Rothschild,A., Jan,Y.N. and Sheng,M. (1995) Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature, 378, 85–88. [DOI] [PubMed] [Google Scholar]
- Laskowski R.A., MacArthur,M.W., Moss,D.S. and Thornton,J.M. (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr., 26, 283–291. [Google Scholar]
- Li H.S. and Montell,C. (2000) TRP and the PDZ protein, INAD, form the core complex required for retention of the signalplex in Drosophila photoreceptor cells. J. Cell Biol., 150, 1411–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. (1999) Visual transduction in Drosophila. Annu. Rev. Cell Dev. Biol., 15, 231–268. [DOI] [PubMed] [Google Scholar]
- Montell C. (2000) PLC fills a GAP in G-protein-coupled signalling. Nature Cell Biol., 2, E82–E83. [DOI] [PubMed] [Google Scholar]
- Montell C. (2001) An end in sight to a long TRP. Neuron, 30, 3–5. [DOI] [PubMed] [Google Scholar]
- Morais Cabral J.H., Petosa,C., Sutcliffe,M.J., Raza,S., Byron,O., Poy,F., Marfatia,S.M., Chishti,A.H. and Liddington,R.C. (1996) Crystal structure of a PDZ domain. Nature, 382, 649–652. [DOI] [PubMed] [Google Scholar]
- Nesper M., Nock,S., Sedlak,E., Antalik,M., Podhradsky,D. and Sprinzl,M. (1998) Dimers of Thermus thermophilus elongation factor Ts are required for its function as a nucleotide exchange factor of elongation factor Tu. Eur. J. Biochem., 255, 81–86. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z. (1991) Maximum likelihood refinement of heavy atom parameters. In Wolf,W., Evans,P.R. and Leslie,A.G.W. (eds), Isomorphous Replacement and Anomalous Scattering. SERC Daresbury Laboratory, Warrington, UK, pp. 80–86.
- Otwinowski Z. (1993) Oscillation data reduction program. In Sawyer,L., Isaacs,N. and Bailey,S. (eds.), Data Collection and Processing, SERC Daresbury Laboratory, Warrington, UK, pp. 56–62.
- Ranganathan R., Harris,G.L., Stevens,C.F. and Zuker,C.S. (1991) A Drosophila mutant defective in extracellular calcium-dependent photoreceptor deactivation and rapid desensitization. Nature, 354, 230–232. [DOI] [PubMed] [Google Scholar]
- Schultz J., Hoffmuller,U., Krause,G., Ashurst,J., Macias,M.J., Schmieder,P., Schneider-Mergener,J. and Oschkinat,H. (1998) Specific interactions between the syntrophin PDZ domain and voltage-gated sodium channels. Nature Struct. Biol., 5, 19–24. [DOI] [PubMed] [Google Scholar]
- Sheldrick G.M. (1993) The application of direct methods and Patterson interpretation to high-resolution native protein data. Acta Crystallogr. D, 49, 18–23. [DOI] [PubMed] [Google Scholar]
- Shieh B.H., Zhu,M.Y., Lee,J.K., Kelly,I.M. and Bahiraei,F. (1997) Association of INAD with NORPA is essential for controlled activation and deactivation of Drosophila phototransduction in vivo. Proc. Natl Acad. Sci. USA, 94, 12682–12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z. et al. (1997) Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science, 275, 73–77. [DOI] [PubMed] [Google Scholar]
- Terwilliger T.C. and Berendzen,J. (1999) Automated structure solution for MIR and MAD. Acta Crystallogr. D, 55, 849–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tochio H., Zhang,Q., Mandal,P., Li,M. and Zhang,M. (1999) Solution structure of the extended neuronal nitric oxide synthase PDZ domain complexed with an associated peptide. Nature Struct. Biol., 6, 417–421. [DOI] [PubMed] [Google Scholar]
- Tsunoda S., Sierralta,J., Sun,Y., Bodner,R., Suzuki,E., Becker,A., Socolich,M. and Zuker,C.S. (1997) A multivalent PDZ-domain protein assembles signalling complexes in a G-protein-coupled cascade. Nature, 388, 243–249. [DOI] [PubMed] [Google Scholar]
- Tsunoda S., Sun,Y., Suzuki,E. and Zuker,C. (2001) Independent anchoring and assembly mechanisms of INAD signaling complexes in Drosophila photoreceptors. J. Neurosci., 21, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Huizen R., Miller,K., Chen,D.M., Li,Y., Lai,Z.C., Raab,R.W., Stark,W.S., Shortridge,R.D. and Li,M. (1998) Two distantly positioned PDZ domains mediate multivalent INAD–phospholipase C interactions essential for G protein-coupled signaling. EMBO J., 17, 2285–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wes P.D., Xu,X.Z., Li,H.S., Chien,F., Doberstein,S.K. and Montell,C. (1999) Termination of phototransduction requires binding of the NINAC myosin III and the PDZ protein INAD. Nature Neurosci., 2, 447–453. [DOI] [PubMed] [Google Scholar]
- Xu X.Z., Choudhury,A., Li,X. and Montell,C. (1998) Coordination of an array of signaling proteins through homo- and heteromeric interactions between PDZ domains and target proteins. J. Cell Biol., 142, 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]