Abstract
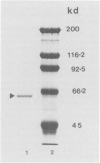
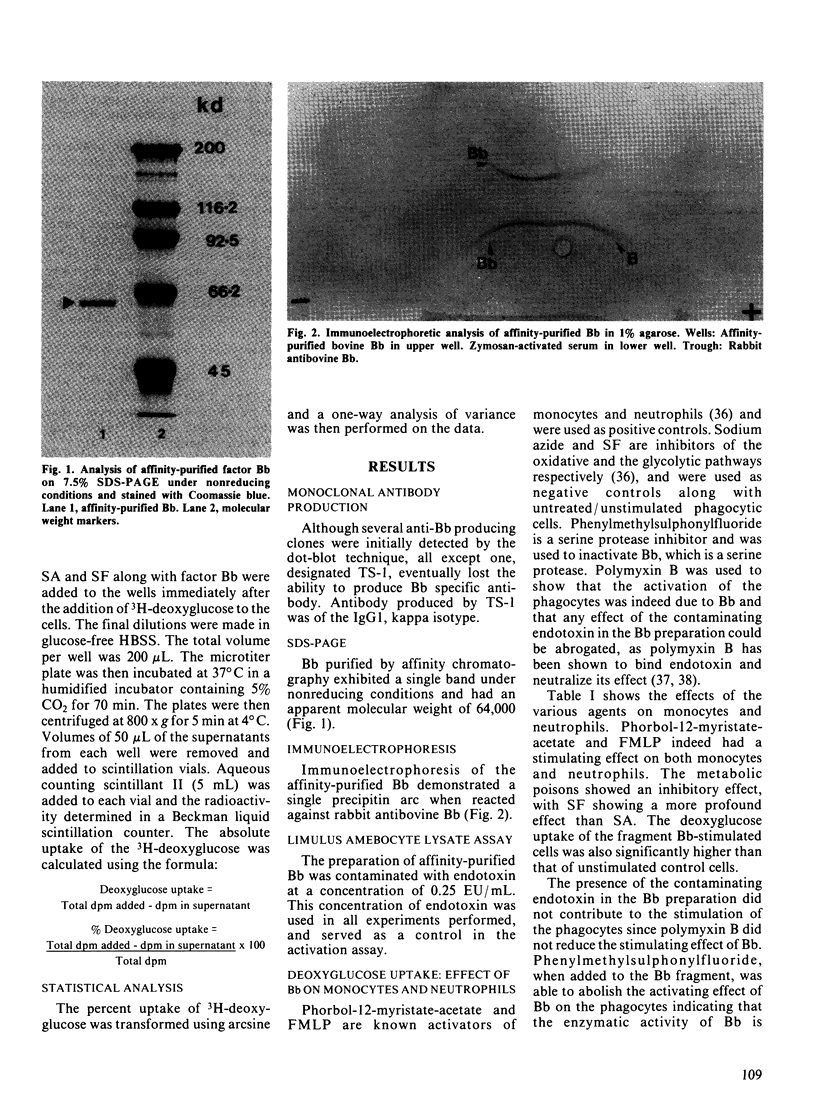
The Bb fragment is the enzymatically active split product of bovine complement factor B. The Bb fragment was obtained after zymosan treatment of fresh bovine serum and fractionation of the treated serum, first over diethylaminoethyl-Sephacel and then over an affinity column made up of monoclonal antibody to bovine Bb, coupled to cyanogen-bromide-activated Sepharose. Purified Bb has a molecular weight of 64,000, as determined by sodium dodecyl sulphate-polyacrylamide gel electrophoresis. The ability of purified Bb to activate phagocytes was assessed. The activation assay was based on the principle that the primary source of energy for the phagocytes is obtained from glucose. 3H-deoxyglucose, a nonmetabolizable analogue of glucose, was used to obtain the quantitative measurement of the activation process. The activation by Bb was shown by the uptake of the labelled deoxyglucose in the phagocytic cells and was comparable to the activation caused by phorbol myristate acetate and N-formyl-L-methionyl-L-leucyl-L-phenylalanine, run in parallel. These data showed that fragment Bb activates bovine monocytes and neutrophils and also suggested that, when generated after complement activation, Bb may stimulate monocytes and neutrophils for enhanced phagocytosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Gilman N., Karnovsky M. L. Respiration and glucose oxidation in human and guinea pig leukocytes: comparative studies. J Clin Invest. 1970 Apr;49(4):692–700. doi: 10.1172/JCI106281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. L., Sigman M., Oliver J. M. Superoxide production induced in rabbit polymorphonuclear leukocytes by synthetic chemotactic peptides and A23187. Am J Pathol. 1979 Apr;95(1):81–97. [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Eden A., Cohn Z. A. The induction of macrophage spreading: role of coagulation factors and the complement system. J Exp Med. 1976 Dec 1;144(6):1531–1544. doi: 10.1084/jem.144.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Götze O., Cohn Z. A. Regulation of macrophage migration by products of the complement system. Proc Natl Acad Sci U S A. 1979 Feb;76(2):888–891. doi: 10.1073/pnas.76.2.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Mukkada A. J. Augmentation of glucose transport in macrophages after particle ingestion. Infect Immun. 1974 Dec;10(6):1391–1396. doi: 10.1128/iai.10.6.1391-1396.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Straus D., Baughn R. E., Imhoff J. Enhancement of carrier-mediated transport after immunologic activation of peritoneal macrophages. J Immunol. 1977 May;118(5):1827–1835. [PubMed] [Google Scholar]
- Borregaard N., Herlin T. Energy metabolism of human neutrophils during phagocytosis. J Clin Invest. 1982 Sep;70(3):550–557. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M. C., Campbell R. D., Bentley D. R., Porter R. R. A molecular map of the human major histocompatibility complex class III region linking complement genes C4, C2 and factor B. Nature. 1984 Jan 19;307(5948):237–241. doi: 10.1038/307237a0. [DOI] [PubMed] [Google Scholar]
- Cohen M. S., Britigan B. E., Hassett D. J., Rosen G. M. Phagocytes, O2 reduction, and hydroxyl radical. Rev Infect Dis. 1988 Nov-Dec;10(6):1088–1096. doi: 10.1093/clinids/10.6.1088. [DOI] [PubMed] [Google Scholar]
- Curman B., Sandberg-Trägårdh L., Peterson P. A. Chemical characterization of human factor B of the alternate pathway of complement activation. Biochemistry. 1977 Nov 29;16(24):5368–5375. doi: 10.1021/bi00643a031. [DOI] [PubMed] [Google Scholar]
- Duff G. W., Atkins E. The inhibitory effect of polymyxin B on endotoxin-induced endogenous pyrogen production. J Immunol Methods. 1982 Aug 13;52(3):333–340. doi: 10.1016/0022-1759(82)90005-9. [DOI] [PubMed] [Google Scholar]
- Fishelson Z., Müller-Eberhard H. J. C3 convertase of human complement: enhanced formation and stability of the enzyme generated with nickel instead of magnesium. J Immunol. 1982 Dec;129(6):2603–2607. [PubMed] [Google Scholar]
- Fishelson Z., Müller-Eberhard H. J. Residual hemolytic and proteolytic activity expressed by Bb after decay-dissociation of C3b,Bb. J Immunol. 1984 Mar;132(3):1425–1429. [PubMed] [Google Scholar]
- Fishelson Z., Pangburn M. K., Müller-Eberhard H. J. C3 convertase of the alternative complement pathway. Demonstration of an active, stable C3b, Bb (Ni) complex. J Biol Chem. 1983 Jun 25;258(12):7411–7415. [PubMed] [Google Scholar]
- Goddeeris B. M., Baldwin C. L., ole-MoiYoi O., Morrison W. I. Improved methods for purification and depletion of monocytes from bovine peripheral blood mononuclear cells. Functional evaluation of monocytes in responses to lectins. J Immunol Methods. 1986 May 22;89(2):165–173. doi: 10.1016/0022-1759(86)90354-6. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götze O., Bianco C., Cohn Z. A. The induction of macrophage spreading by factor B of the properdin system. J Exp Med. 1979 Feb 1;149(2):372–386. doi: 10.1084/jem.149.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The C3-activator system: an alternate pathway of complement activation. J Exp Med. 1971 Sep 1;134(3 Pt 2):90s–108s. [PubMed] [Google Scholar]
- Hamuro J., Hadding U., Bitter-Suermann D. Fragments Ba and Bb derived from guinea pig factor B of the properdin system: purification, characterization, and biologic activities. J Immunol. 1978 Feb;120(2):438–444. [PubMed] [Google Scholar]
- Issekutz A. C. Removal of gram-negative endotoxin from solutions by affinity chromatography. J Immunol Methods. 1983 Jul 29;61(3):275–281. doi: 10.1016/0022-1759(83)90221-1. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr Defects of neutrophil function. N Engl J Med. 1982 Aug 12;307(7):434–436. doi: 10.1056/NEJM198208123070709. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E. Elaboration of toxic oxygen by-products by neutrophils in a model of immune complex disease. J Clin Invest. 1976 Apr;57(4):836–841. doi: 10.1172/JCI108359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., Daha M. R., van Furth R. Stimulation of the intracellular killing of Staphylococcus aureus by monocytes: regulation by immunoglobulin G and complement components C3/C3b and B/Bb. J Immunol. 1982 Jul;129(1):332–337. [PubMed] [Google Scholar]
- McCormack J. G., Moore A. L., Johnson J. E., 3rd, Philp J. R. Amplified lymphokine production phenomenon confirmed by a macrophage 2-D-[3H]deoxyglucose transport assay. Infect Immun. 1981 May;32(2):707–715. doi: 10.1128/iai.32.2.707-715.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicus R. G., Götze O., Müller-Eberhard H. J. The serine protease nature of the C3 and C5 convertases of the classical and alternative complement pathways. Scand J Immunol. 1976;5(9):1049–1055. doi: 10.1111/j.1365-3083.1976.tb03056.x. [DOI] [PubMed] [Google Scholar]
- Minta J. O. Synthesis of complement factor B by the human monocyte U-937 cell line. Augmentation by immunostimulatory agents. J Immunol. 1988 Sep 1;141(5):1636–1641. [PubMed] [Google Scholar]
- Mole J. E., Anderson J. K., Davison E. A., Woods D. E. Complete primary structure for the zymogen of human complement factor B. J Biol Chem. 1984 Mar 25;259(6):3407–3412. [PubMed] [Google Scholar]
- Mukkada A. J., Bonventre P. F. Membrane transport by mouse and guinea pig macrophages: characteristics of the glucose transport system. J Reticuloendothel Soc. 1975 Jan;17(1):20–29. [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Götze O. C3 proactivator convertase and its mode of action. J Exp Med. 1972 Apr 1;135(4):1003–1008. doi: 10.1084/jem.135.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Cohn Z. A. The macrophage as an effector cell. N Engl J Med. 1980 Sep 11;303(11):622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang A. S., Aston W. P. The alternative complement pathway in bovine serum: the isolation of a serum protein with factor B activity. Immunochemistry. 1978 Aug;15(8):529–534. doi: 10.1016/0161-5890(78)90004-4. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. The alternative pathway of complement. Springer Semin Immunopathol. 1984;7(2-3):163–192. doi: 10.1007/BF01893019. [DOI] [PubMed] [Google Scholar]
- Sandborg R. R., Smolen J. E. Early biochemical events in leukocyte activation. Lab Invest. 1988 Sep;59(3):300–320. [PubMed] [Google Scholar]
- Seow W. K., Smith S. E., McCormack J. G., Thong Y. H. Uptake of 3H-deoxyglucose as a microassay of human neutrophil and monocyte activation. J Immunol Methods. 1987 Apr 2;98(1):113–118. doi: 10.1016/0022-1759(87)90443-1. [DOI] [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I. Chemotactic factor-induced generation of superoxide radicals by human neutrophils: evidence for the role of sodium. J Immunol. 1979 Nov;123(5):2428–2435. [PubMed] [Google Scholar]
- Sumimoto H., Takeshige K., Minakami S. Superoxide production of human polymorphonuclear leukocytes stimulated by leukotriene B4. Biochim Biophys Acta. 1984 Apr 16;803(4):271–277. doi: 10.1016/0167-4889(84)90117-4. [DOI] [PubMed] [Google Scholar]
- Sundsmo J. S., Chin J. R., Papin R. A., Fair D. S., Werb Z. Factor B, the complement alternative pathway serine proteinase, is a major constitutive protein synthesized and secreted by resident and elicited mouse macrophages. J Exp Med. 1985 Feb 1;161(2):306–322. doi: 10.1084/jem.161.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundsmo J. S., Götze O. Human monocyte spreading induced by factor B of the alternative pathway of complement activation. Cell Immunol. 1980 Jun;52(1):1–17. doi: 10.1016/0008-8749(80)90395-0. [DOI] [PubMed] [Google Scholar]
- Sundsmo J. S., Götze O. Human monocyte spreading induced by factor Bb of the alternative pathway of complement activation. A possible role for C5 in monocyte spreading. J Exp Med. 1981 Sep 1;154(3):763–777. doi: 10.1084/jem.154.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundsmo J. S. Leukocyte complement: a possible role for C5 in lymphocyte stimulation. J Immunol. 1983 Aug;131(2):886–891. [PubMed] [Google Scholar]
- Sundsmo J. S., Wood L. M. Activated factor B (Bb) of the alternative pathway of complement activation cleaves and activates plasminogen. J Immunol. 1981 Sep;127(3):877–880. [PubMed] [Google Scholar]
- Tabel H. Activation of the alternative pathway of bovine complement by Trypanosoma congolense. Parasite Immunol. 1982 Sep;4(5):329–335. doi: 10.1111/j.1365-3024.1982.tb00444.x. [DOI] [PubMed] [Google Scholar]
- Tabel H. Alternative pathway fof bovine complement Immunochemical studies on factor B-like serum protein and its conversion product B gamma 2. Can J Comp Med. 1981 Jul;45(3):291–298. [PMC free article] [PubMed] [Google Scholar]
- Webster R. O., Hong S. R., Johnston R. B., Jr, Henson P. M. Biologial effects of the human complement fragments C5a and C5ades Arg on neutrophil function. Immunopharmacology. 1980 Jun;2(3):201–219. doi: 10.1016/0162-3109(80)90050-8. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Smolen J. E., Korchak H. M. Release of inflammatory mediators from stimulated neutrophils. N Engl J Med. 1980 Jul 3;303(1):27–34. doi: 10.1056/NEJM198007033030109. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]