Abstract
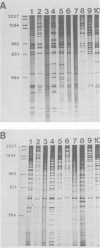
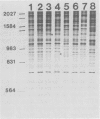
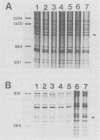
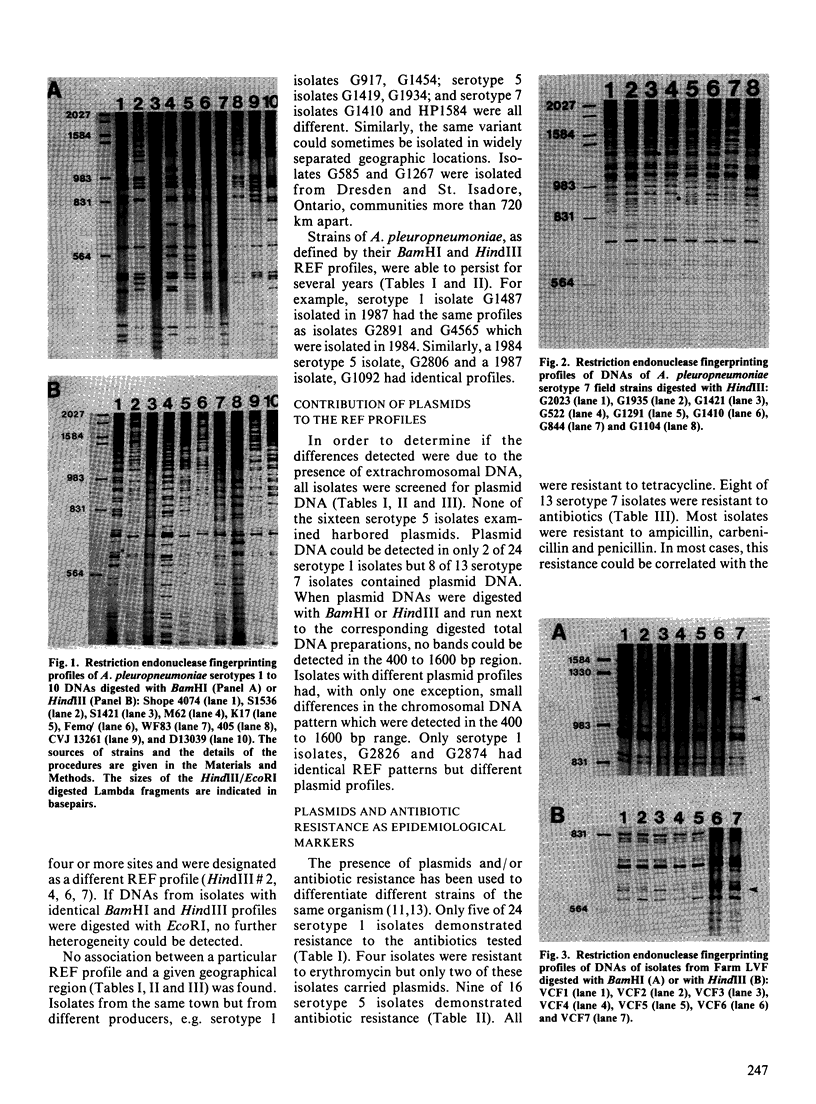
Isolates of Actinobacillus (Haemophilus) pleuropheumoniae were studied by restriction endonuclease fingerprinting (REF) analysis using the enzymes BamHI and HindIII. Restriction fragments were resolved by polyacrylamide gel electrophoresis and visualized by silver staining. Except for serotypes 1 and 9, reference strains of A. pleuropneumoniae serotypes 1 to 10 had clearly distinguishable REF profiles. Analysis of REF profiles of southern Ontario field isolates revealed limited heterogeneity amongst isolates of serotype 1 or serotype 5. The REF profiles of the serotype 7 isolates studied showed greater variation. Heterogeneity could not be correlated with the presence of plasmids nor with antibiotic resistance. Limited heterogeneity could also be detected amongst REF profiles of A. pleuropneumoniae isolates recovered from a closed herd suggesting that there is a small amount of genetic variation within clonal populations.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edelstein P. H., Nakahama C., Tobin J. O., Calarco K., Beer K. B., Joly J. R., Selander R. K. Paleoepidemiologic investigation of Legionnaires disease at Wadsworth Veterans Administration Hospital by using three typing methods for comparison of legionellae from clinical and environmental sources. J Clin Microbiol. 1986 Jun;23(6):1121–1126. doi: 10.1128/jcm.23.6.1121-1126.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen B. E., Sørensen B., Bjorvatn B., Falk E. S., Fosse E., Bryn K., Frøholm L. O., Gaustad P., Bøvre K. An outbreak of group B meningococcal disease: tracing the causative strain of Neisseria meningitidis by DNA fingerprinting. J Clin Microbiol. 1986 Apr;23(4):764–767. doi: 10.1128/jcm.23.4.764-767.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijper E. J., Oudbier J. H., Stuifbergen W. N., Jansz A., Zanen H. C. Application of whole-cell DNA restriction endonuclease profiles to the epidemiology of Clostridium difficile-induced diarrhea. J Clin Microbiol. 1987 Apr;25(4):751–753. doi: 10.1128/jcm.25.4.751-753.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenberg W., Rauws E. A., Widjojokusumo A., Tytgat G. N., Zanen H. C. Identification of Campylobacter pyloridis isolates by restriction endonuclease DNA analysis. J Clin Microbiol. 1986 Sep;24(3):414–417. doi: 10.1128/jcm.24.3.414-417.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnes J. I., Rosendal S. Analysis of major antigens of Haemophilus (Actinobacillus) pleuropneumoniae and related organisms. Infect Immun. 1987 Jul;55(7):1626–1634. doi: 10.1128/iai.55.7.1626-1634.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- McClenaghan M., Herring A. J., Aitken I. D. Comparison of Chlamydia psittaci isolates by DNA restriction endonuclease analysis. Infect Immun. 1984 Aug;45(2):384–389. doi: 10.1128/iai.45.2.384-389.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal K. R., Higgins R., Lariviere S. Quantitation of serotype-specific and cross-reacting group-specific antigens by coagglutination and immunodiffusion tests for differentiating Actinobacillus (Haemophilus) pleuropneumoniae strains belonging to cross-reacting serotypes 3, 6, and 8. J Clin Microbiol. 1988 May;26(5):985–989. doi: 10.1128/jcm.26.5.985-989.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal K. R., Higgins R., Larivière S. Identification and serotyping of Haemophilus pleuropneumoniae by coagglutination test. J Clin Microbiol. 1983 Dec;18(6):1351–1354. doi: 10.1128/jcm.18.6.1351-1354.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Rapp V. J., Selander R. K. Clonal diversity in Haemophilus pleuropneumoniae. Infect Immun. 1987 May;55(5):1207–1215. doi: 10.1128/iai.55.5.1207-1215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R. Haemophilus pleuropneumoniae (Actinobacillus pleuropneumoniae). Serotypes 8, 3 and 6. Serological response and cross immunity in pigs. Nord Vet Med. 1985 Jul-Aug;37(4):217–227. [PubMed] [Google Scholar]
- Nielsen R. Seroepidemiology of Actinobacillus pleuropneumoniae. Can Vet J. 1988 Jul;29(7):580–582. [PMC free article] [PubMed] [Google Scholar]
- Nielsen R. Serological characterization of Haemophilus pleuropneumoniae (Actinobacillus pleuropneumoniae) strains and proposal of a new serotype: serotype 9. Acta Vet Scand. 1985;26(4):501–512. doi: 10.1186/BF03546522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara M. J., Collins D. M., de Lisle G. W. Restriction endonuclease analysis of Brucella ovis and other Brucella species. Vet Microbiol. 1985 Aug;10(5):425–429. doi: 10.1016/0378-1135(85)90024-0. [DOI] [PubMed] [Google Scholar]
- Rapp V. J., Munson R. S., Jr, Ross R. F. Outer membrane protein profiles of Haemophilus pleuropneumoniae. Infect Immun. 1986 May;52(2):414–420. doi: 10.1128/iai.52.2.414-420.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp V. J., Ross R. F., Erickson B. Z. Serotyping of Haemophilus pleuropneumoniae by rapid slide agglutination and indirect fluorescent antibody tests in swine. Am J Vet Res. 1985 Jan;46(1):185–192. [PubMed] [Google Scholar]
- Rosendal S., Boyd D. A. Haemophilus pleuropneumoniae serotyping. J Clin Microbiol. 1982 Nov;16(5):840–843. doi: 10.1128/jcm.16.5.840-843.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983 Jun 15;182(12):1331–1337. [PubMed] [Google Scholar]
- Smart N. L., Miniats O. P., MacInnes J. I. Analysis of Haemophilus parasuis isolates from southern Ontario swine by restriction endonuclease fingerprinting. Can J Vet Res. 1988 Jul;52(3):319–324. [PMC free article] [PubMed] [Google Scholar]
- Tompkins L. S., Troup N. J., Woods T., Bibb W., McKinney R. M. Molecular epidemiology of Legionella species by restriction endonuclease and alloenzyme analysis. J Clin Microbiol. 1987 Oct;25(10):1875–1880. doi: 10.1128/jcm.25.10.1875-1880.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]