Abstract
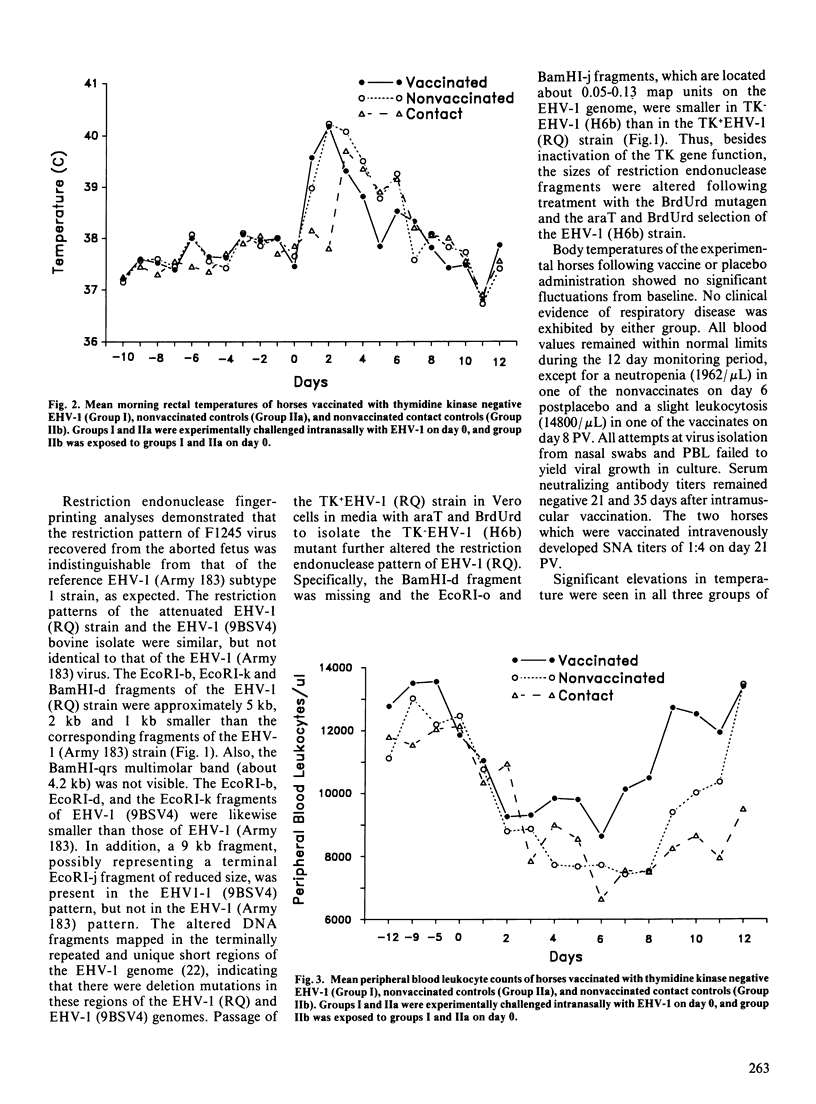
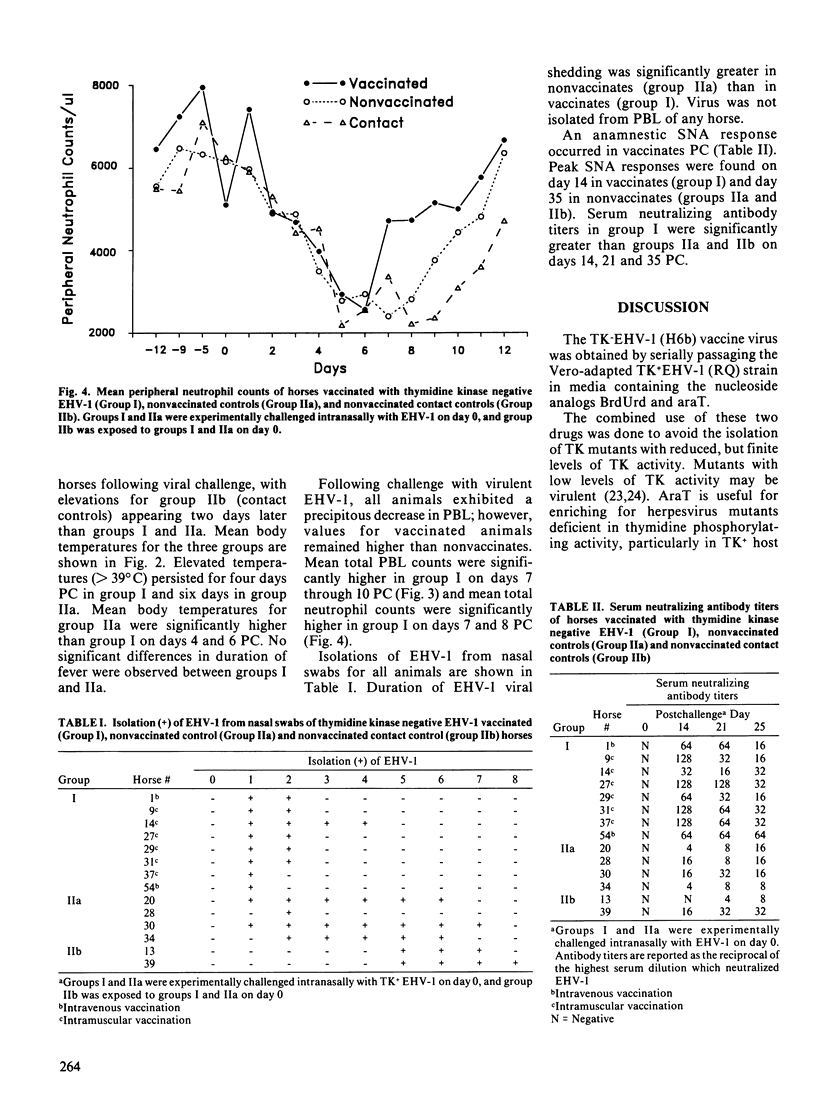
A drug induced equine herpesvirus-1 (EHV-1) mutant lacking thymidine kinase inducing activity was developed and evaluated as a vaccine. The safety and effectiveness of the vaccine to protect against experimentally induced EHV-1 respiratory disease were evaluated in weanling horses free of EHV-1 neutralizing antibody. The vaccine was safe when administered either intramuscularly or intravenously, and EHV-1 was not shed intranasally during the 12 days following administration. Intranasal challenge with virulent EHV-1 was used to evaluate vaccine efficacy. Following challenge, there was a significantly (p less than 0.05) greater increase in peak body temperatures and duration of nasal virus shedding in the nonvaccinates, and a significant (p less than 0.05) increase in serum neutralizing antibody titers in the vaccinates.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. P., Bryans J. T. Molecular epizootiology, pathogenesis, and prophylaxis of equine herpesvirus-1 infections. Prog Vet Microbiol Immunol. 1986;2:78–144. [PubMed] [Google Scholar]
- Bryans J. T. On immunity to disease caused by equine herpesvirus 1. J Am Vet Med Assoc. 1969 Jul 15;155(2):294–300. [PubMed] [Google Scholar]
- Chowdhury S. I., Kubin G., Ludwig H. Equine herpesvirus type 1 (EHV-1) induced abortions and paralysis in a Lipizzaner stud: a contribution to the classification of equine herpesviruses. Arch Virol. 1986;90(3-4):273–288. doi: 10.1007/BF01317376. [DOI] [PubMed] [Google Scholar]
- Crandell R. A., Drysdale S., Stein T. L. A comparative study of bovine herpesvirus 1247 and equine herpesvirus 1 in ponies. Can J Comp Med. 1979 Jan;43(1):94–97. [PMC free article] [PubMed] [Google Scholar]
- Crandell R. A., Ichimura H., Kit S. Isolation and comparative restriction endonuclease DNA fingerprinting of equine herpesvirus-1 from cattle. Am J Vet Res. 1988 Nov;49(11):1807–1813. [PubMed] [Google Scholar]
- DOLL E. R., BRYANS J. T. Epizootiology of equine viral rhinopneumonitis. J Am Vet Med Assoc. 1963 Jan 1;142:31–37. [PubMed] [Google Scholar]
- DOLL E. R., RICHARDS M. G., WALLACE M. E. Cultivation of the equine influenza virus in suckling Syrian hamsters. Its similarity to the equine abortion virus. Cornell Vet. 1954 Jan;44(1):133–138. [PubMed] [Google Scholar]
- DOLL E. R., WALLACE E., RICHARDS M. G. Thermal, hematological, and serological responses of weanling horses following inoculation with equine abortion virus: its similarity to equine influenza. Cornell Vet. 1954 Apr;44(2):181–190. [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. MUTANT STRAINS OF HERPES SIMPLEX DEFICIENT IN THYMIDINE KINASE-INDUCING ACTIVITY. Virology. 1964 Apr;22:493–502. doi: 10.1016/0042-6822(64)90070-4. [DOI] [PubMed] [Google Scholar]
- Engels M., Giuliani C., Wild P., Beck T. M., Loepfe E., Wyler R. The genome of bovine herpesvirus 1 (BHV-1) strains exhibiting a neuropathogenic potential compared to known BHV-1 strains by restriction site mapping and cross-hybridization. Virus Res. 1986 Oct;6(1):57–73. doi: 10.1016/0168-1702(86)90057-2. [DOI] [PubMed] [Google Scholar]
- Gordon Y., Gilden D. H., Shtram Y., Asher Y., Tabor E., Wellish M., Devlin M., Snipper D., Hadar J., Becker Y. A low thymidine kinase-producing mutant of herpes simplex virus type 1 causes latent trigeminal ganglia infections in mice. Arch Virol. 1983;76(1):39–49. doi: 10.1007/BF01315702. [DOI] [PubMed] [Google Scholar]
- Kit S., Ichimura H., De Clercq E. Phosphorylation of nucleoside analogs by equine herpesvirus type 1 pyrimidine deoxyribonucleoside kinase. Antiviral Res. 1987 Jan;7(1):53–67. doi: 10.1016/0166-3542(87)90039-8. [DOI] [PubMed] [Google Scholar]
- Kit S., Kit M., McConnell S. Intramuscular and intravaginal vaccination of pregnant cows with thymidine kinase-negative, temperature-resistant infectious bovine rhinotracheitis virus (bovine herpes virus 1). Vaccine. 1986 Mar;4(1):55–61. doi: 10.1016/0264-410x(86)90098-8. [DOI] [PubMed] [Google Scholar]
- Kit S., Kit M., Pirtle E. C. Attenuated properties of thymidine kinase-negative deletion mutant of pseudorabies virus. Am J Vet Res. 1985 Jun;46(6):1359–1367. [PubMed] [Google Scholar]
- Kit S., Qavi H. Thymidine kinase (TK) induction after infection of TK-deficient rabbit cell mutants with bovine herpesvirus type 1 (BHV-1): isolation of TK- BHV-1 mutants. Virology. 1983 Oct 30;130(2):381–389. doi: 10.1016/0042-6822(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Klein R. J. The pathogenesis of acute, latent and recurrent herpes simplex virus infections. Arch Virol. 1982;72(3):143–168. doi: 10.1007/BF01348961. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Lisle J. J., Darby G. Restoration of wild-type pathogenicity to an attenuated DNA polymerase mutant of herpes simplex virus type 1. J Gen Virol. 1986 Nov;67(Pt 11):2501–2506. doi: 10.1099/0022-1317-67-11-2501. [DOI] [PubMed] [Google Scholar]
- Lomniczi B., Blankenship M. L., Ben-Porat T. Deletions in the genomes of pseudorabies virus vaccine strains and existence of four isomers of the genomes. J Virol. 1984 Mar;49(3):970–979. doi: 10.1128/jvi.49.3.970-979.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka H., Hazen M., Kit M., Qavi H., Kit S. Cloning of the marmoset herpesvirus thymidine kinase gene and analyses of the boundaries of the coding region. Virology. 1981 Aug;113(1):196–213. doi: 10.1016/0042-6822(81)90148-3. [DOI] [PubMed] [Google Scholar]
- Purdy C. W., Ford S. J., Grant W. F. Equine rhinopneumonitis virus (herpesvirus type 1): attenuation in stable monkey cell line. Am J Vet Res. 1977 Aug;38(8):1211–1215. [PubMed] [Google Scholar]
- Studdert M. J., Simpson T., Roizman B. Differentiation of respiratory and abortigenic isolates of equine herpesvirus 1 by restriction endonucleases. Science. 1981 Oct 30;214(4520):562–564. doi: 10.1126/science.6270790. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Ressel S., Dunstan M. E. Herpes simplex virus thymidine kinase expression in trigeminal ganglion infection: correlation of enzyme activity with ganglion virus titer and evidence of in vivo complementation. Virology. 1981 Jul 15;112(1):328–341. doi: 10.1016/0042-6822(81)90638-3. [DOI] [PubMed] [Google Scholar]
- Whalley J. M., Robertson G. R., Davison A. J. Analysis of the genome of equine herpesvirus type 1: arrangement of cleavage sites for restriction endonucleases EcoRI, BglII and BamHI. J Gen Virol. 1981 Dec;57(Pt 2):307–323. doi: 10.1099/0022-1317-57-2-307. [DOI] [PubMed] [Google Scholar]



