Abstract
Mitochondrial membrane permeabilization (MMP) is a critical step of several apoptotic pathways. Some infectious intracellular pathogens can regulate (induce or inhibit) apoptosis of their host cells at the mitochondrial level, by targeting proteins to mitochondrial membranes that either induce or inhibit MMP. Pathogen-encoded mitochondrion-targeted proteins may or may not show amino acid sequence homology to Bcl-2-like proteins. Among the Bcl-2-unrelated, mitochondrion-targeted proteins, several interact with the voltage-dependent anion channel (VDAC) or with the adenine nucleotide translocator (ANT). While VDAC-targeted proteins show homology to VDAC/porin, ANT-targeted proteins possess relatively short cationic binding domains, which may facilitate insertion into the negatively charged inner mitochondrial membrane. It may be speculated that such proteins employ pre-existing host-intrinsic mechanisms of MMP control.
Keywords: adenine nucleotide translocator/Bcl-2/programmed cell death/voltage-dependent anion channel
Introduction
For millions of years, pathogenic infectious agents have adapted to the molecular and cellular biology of their hosts to acquire and defend an ecological niche. Apoptotic cell death can be considered as an innate cellular response to limit the propagation of certain viruses and obligate intracellular bacteria. Indeed, infectious microorganisms were found to encode proteins that block the death response. However, apoptosis may also promote viral dissemination within apoptotic bodies, and viruses may purposefully employ mechanisms to induce apoptosis. This could represent a coordinated strategy utilized by professional intracellular pathogens to invade, proliferate within and eventually exit the specific host cell. As a general rule, infectious intracellular pathogens tend to inhibit apoptosis of their host cells during an early stage of their life cycle, when replication of the microorganism is desired. At later stages, they may then induce apoptosis, either in their host cells, or via a variety of different strategies in immunologically relevant cells, with the specific aim to subvert the host’s immune and inflammatory response (Tschopp et al., 1998; Gao and Kwaik, 2000). Inhibition of apoptosis in host cells has also been viewed as a strategy to minimize immune responses (Restifo, 2000). To modulate apoptosis, viruses employ a whole arsenal of different proteins that specifically intercept a variety of pro-apoptotic signal-transducing pathways (for review see Tschopp et al., 1998; Gao and Kwaik, 2000). Recently, it has been discovered that mitochondria, the relics of endosymbiotic bacteria, play a central role in the control of apoptosis. This review summarizes the current state of knowledge concerning apoptosis regulatory, pathogen-encoded proteins targeted to mitochondria.
Mitochondrial proteins involved in apoptosis control
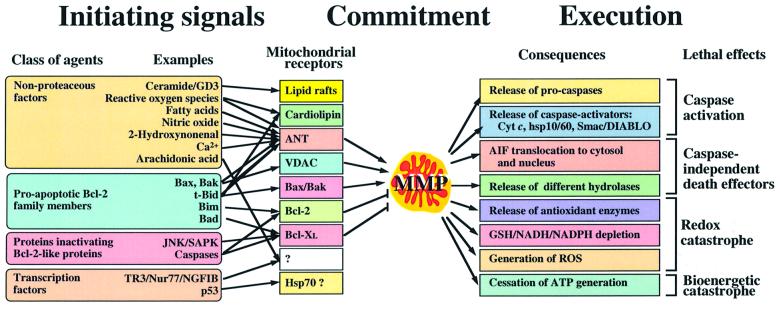
Mitochondria continuously collect information on various aspects of cellular metabolism and signal transduction cascades, process this information, then decide on the cell’s fate and participate in the execution of the death sentence (for review see Kroemer and Reed, 2000). In this regard, apoptosis can be conceived as a triphasic process: initiation, decision/commitment and degradation/execution (Figure 1). During the initiation phase, pro-apoptotic second messengers accumulate in the cell and will finally act on mitochondria to increase the permeability of their membranes. The nature of such permeabilizing agents depends on the death-inducing stimulus and thus, is rather heterogeneous. During the decision or commitment phase, mitochondrial membrane permeabilization (MMP) occurs, presumably via a limited set of mechanisms. MMP differentially affects the outer membrane (OMMP), which becomes protein permeable, and the inner membrane (IMMP), which continues to retain matrix proteins, yet may dissipate the mitochondrial transmembrane potential (Δψm). The temporary order of OMMP and IMMP, as well as their relative contribution to cell death, are a matter of debate. The degradation or execution phase is triggered by the activation of catabolic hydrolases, mainly caspases (apoptosis-specific cysteine proteases) and nucleases. Such hydrolases are activated secondary to the release of proteins that are normally strictly confined to the mitochondrial intermembrane space, in particular cytochrome c (which stimulates the cytosolic assembly of the apoptosome, the caspase activation complex) and apoptosis-inducing factor (AIF, which activates a DNase located in the nucleus). MMP is a near-universal hallmark of apoptosis. Therefore, the simple observation that pathogen-encoded proteins can induce or prevent MMP in intact cells, correlating with their apoptosis regulatory potential, is not an indication that such proteins actually act on mitochondria in a direct fashion (Ferri et al., 2000). Here, we will only discuss reports in which the effect of pathogen-encoded proteins on mitochondria has been documented in cell-free systems involving isolated mitochondria.
Fig. 1. Phases of the apoptotic process with respect to MMP. In the left part of the figure, pro-apoptotic pathways converging on mitochondria are shown. Different apoptogenic molecules act on a variety of tentatively identified mitochondrial receptors (arrows), which in turn regulate MMP. The degradation pathways triggered by MMP are depicted on the right. Apoptosis regulatory proteins encoded by pathogens can either target the signal-transducing pathways upstream of mitochondria or the mitochondrion itself. This has functional consequences. Thus, apoptosis inhibitors acting at the mitochondrial level are likely to have a broader spectrum of cytoprotective action than inhibitors acting on upstream signal. GSH, glutathione; ROS, reactive oxygen species.
The central role of mitochondria in the control of apoptosis is highlighted by the fact that numerous endogenous proteins translocate to, reside in, act on, or are released from mitochondria in different paradigms of cell death induction (Table I). For the purpose of the present discussion, it appears particularly important that anti-apoptotic members of the Bcl-2 family (e.g. Bcl-2, Bcl-XL) reside in mitochondrial membranes, where they locally inhibit MMP (Kroemer, 1997b). Pro-apoptotic members of the Bcl-2 family such as Bax and Bak can translocate from other cellular localizations to mitochondria, oligomerize within mitochondrial membranes and facilitate MMP. This translocation–oligomerization– permeabilization reaction is inhibited by anti-apoptotic members of the Bcl-2 family and is stimulated by pro-apoptotic BH3-only members of the Bcl-2 family (e.g. Bid). It is an ongoing conundrum whether Bax-like proteins induce MMP in an autonomous fashion, without the requirement of interaction with proteins that do not belong to the Bcl-2 family (Martinou and Green, 2001), or whether they interact with a multiprotein complex built up at the outer/inner membrane contact sites, the permeability transition pore complex (PTPC) (Zamzami and Kroemer, 2001). The backbone of the PTPC is constituted by two proteins, namely the voltage-dependent anion channel (VDAC, outer membrane) and the adenine nucleotide translocase (ANT, inner membrane). Both VDAC and ANT have been reported to physically and functionally interact with Bax and Bcl-2 (Zamzami and Kroemer, 2001).
Table I. Endogenous apoptosis regulatory proteins acting at the mitochondrial level.
| Class of proteins | Examples | Mode of action | References |
|---|---|---|---|
| Sessile membrane proteins in the PTPC | VDAC | regulates (enhances/increases) OMMP | Kroemer and Reed (2000) |
| ANT | enhances IMMP | ||
| Bcl-2, Bcl-XL | prevent IMMP/OMMP | ||
| Nip3 | enhances IMMP/OMMP | ||
| Proteins translocating to mitochondria | Bax, Bid, Bak, Bim | enhance OMMP and IMMP | Kroemer and Reed (2000) |
| p53 | enhances OMMP | Vogelstein et al. (2000) | |
| Nur77/TR1/NGFIB | enhances OMMP | Li et al. (2000) | |
| c-Abl | enhances OMMP | Kumar et al. (2001) | |
| p53-induced proteins | Bax | enhances OMMP and IMMP | Vogelstein et al. (2000) |
| NoxA, PUMA | BH3-only Bcl-2 family members | E.Oda et al. (2000) | |
| proline oxidase | locally generates ROS | Donald et al. (2001) | |
| p53AIP1 | induces IMMP | K.Oda et al. (2000) | |
| Proteins released from mitochondria | cytochrome c | activates apoptosome | De Laurenzi and Melino (2000) |
| Hsp10 | coactivates apoptosome | ||
| Pro-caspases | facilitate caspase activation | ||
| Smac/DIABLO | inhibits IAP | ||
| AIF | activates DNase | Susin et al. (2000) |
Bcl-2-like pathogen-encoded proteins
Several pathogenic viruses produce apoptosis inhibitory (and presumably MMP inhibitory) Bcl-2 analogs which preferentially localize to mitochondria (reviewed in Kroemer, 1997b): african swine fever virus (protein 5-HL/ A179L), herpes virus saimiri (HVS-Bcl-2), Kaposi sarcoma-associated herpes virus 8 (KSBcl-2), bovine herpes virus 4 (BHRF-1) and murine gamma herpes virus-68 (M11). Epstein–Barr virus encodes two structural and functional homologs of Bcl-2: BORFB2F and BALF1. Another strategy of apoptosis inhibition may be the induction of endogenous Bcl-2 (the case of Epstein–Barr virus). Among the viral Bcl-2 homologs, several have been described to inhibit MMP, much as Bcl-2 does (Derfuss et al., 1998). Whether this effect is obtained through interactions with other members of the Bcl-2 protein family, interactions with proteins not related to Bcl-2 or autonomous membrane effect is not yet known. Intriguingly, several viral Bcl-2-like proteins only possess two Bcl-2 homology (BH) regions, namely BH1 and BH2, yet lack BH3 and BH4. It has been suggested that the BH4 domain of Bcl-2 interacts with VDAC and prevents it from forming protein-permeable conduits (Shimizu et al., 2000b). Viral Bcl-2-like proteins that lack a BH4 domain thus may act in another fashion, perhaps by neutralizing pro-apoptotic members of the Bcl-2 family, as has been suggested for the the adenovirus protein E1B19K. E1B19K possesses homology to Bcl-2, yet predominantly localizes to nuclear lamina in non-apoptotic cells. Upon treatment with tumor necrosis factor (TNF) or transfection with tBID, E1B19K translocates to mitochondria, together with Bax, and prevents the Bax/t-Bid-mediated MMP, presumably by local effects on Bax (Perez and White, 2000).
VDAC-targeted proteins
The outer membrane of Gram-negative bacteria contains the porin proteins, which allow for the diffusion of small hydrophilic molecules (up to ∼600 Da) into the periplasmic space. Porins possess a trimeric β-pleated barrel structure, similar to VDAC. The porin B (PorB) proteins from Neisseria meningitidis and Neisseria gonorrhoeae (65% amino acid sequence identity) translocate from the outer bacterial membrane into host cell membranes and finally localize intracellularly in the mitochondrial compartment. Neisseria meningitidis PorB (PorBm) is anti-apoptotic and N.gonorrhoeae PorB (PorBg) is pro-apoptotic. PorBm co-immunoprecipitates with VDAC. It inhibits MMP and apoptosis triggered by the universal apoptosis inducer staurosporin (Massari et al., 2000). In contrast, PorBg causes MMP and apoptosis, which is inhibited by Bcl-2 overexpression (Muller et al., 2000). This MMP-inducing effect can be obtained by incubating intact cells or purified mitochondria with purified PorBg. The major structural difference between PorBg and PorBm resides in the loops separating the β-sheets, in particular loops L3 and L5, which in OmpF (an Escherichia coli porin) have been determined to be, in part, α-helical (Cowan et al., 1992). L3 is proposed to fold into the lumen of the β-pleated barrel, thereby regulating the pore size and perhaps determining voltage gating. Intriguingly, this loop is longer in PorBg than in PorBm, by four amino acids, exhibits a different distribution of charged residues, and lacks a proline residue that, in other porins, initiates the turn of the loop. L5, which faces the cytosolic site of VDAC, is also longer in PorBg than in PorBm, by 12 amino acids. These differences may allow PorBg to form relatively large non-specific pores in the outer mitochondrial membrane.
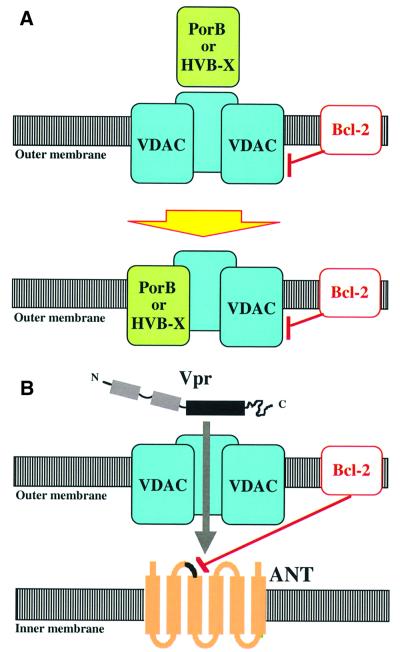
Hepatitis B virus X protein (HBV-X) colocalizes to mitochondria while interacting with one particular VDAC isoform, HVDAC3 (Rahmani et al., 2000). HBV-X is a potent apoptosis inducer, whose effects are neutralized by Bcl-2 (Schuster et al., 2000). VDAC, porins and HBV-X are homologous to each other. For instance, HBV-X and HVDAC3 share 31% amino acid similarity. Incorporation of VDAC into mitochondrial membranes is self-catalyzed in the sense that VDAC present in the membrane facilitates the insertion of further VDAC molecules (Xu and Colombini, 1996). It is tempting to speculate that a similar mechanism involving heterologous protein interactions facilitates the insertion of PorB and HBV-X into the VDAC-containing outer mitochondrial membrane (Figure 2A). It has been proposed that Bax and VDAC cooperatively form large protein-permeable conduits in the outer membrane of mitochondria, while Bcl-2 would reduce the conductance of VDAC channels (Shimizu et al., 2000a). As a possibility, PorB/VDAC and HBV-X/VDAC interactions thus could directly impinge on MMP.
Fig. 2. Hypothetical mechanisms of MMP induced by pathogen-encoded proteins targeting the PTPC compounds VDAC or ANT. (A) Effects of PorB from N.gonorrhoeae or HBV-X from hepatitis B virus. Upon interaction with VDAC, these proteins incorporate into the outer membrane, where they form heterotrimers (or perhaps higher-order oligomers) with VDAC. Bcl-2 would prevent the formation of large non-specific pores in the outer membrane by local effects on VDAC, PorB and/or HVB-X. (B) Model of the Vpr–ANT interactions. Vpr crosses the outer membrane through VDAC and then interacts with ANT, and in particular with the second loop of ANT facing the intermembrane space. Bcl-2 would inhibit the binding of Vpr to ANT. The physical interaction between ANT and Vpr would culminate in cooperative channel formation leading to a primary permeabilization of the inner mitochondrial membrane.
ANT-targeted proteins
The UL37 exon 1 of cytomegalovirus encodes the protein vMIA (viral mitochondrial inhibitor of apoptosis). vMIA localizes to mitochondria, where it binds to ANT (Goldmacher et al., 1999) but not VDAC (Vieira et al., 2001). vMIA inhibits apoptosis induced by a variety of inducers, including ligation of the death receptors Fas/Apo-1/CD95, tumor necrosis factor receptor I, TRAIL, the cytotoxic drugs staurosporine and maytansine, pro-oxidants, and infection with E1B19K-deficient adenovirus (Goldmacher et al., 1999; Vieira et al., 2001) (V.S.Goldmacher, unpublished). Mitochondria isolated from vMIA-transfected cells are more resistant than control mitochondria to the OMMP-inducing effect of truncated Bid (V.S.Goldmacher and A.Skalteskaya, unpublished). Deletion mutations revealed two regions of vMIA to be essential for its anti-apoptotic action, namely amino acids 5–34 and 118–147 (Hayajneh et al., 2001). The N-terminal domain is required for mitochondrial localization of vMIA (Hayajneh et al., 2001). The second domain possesses a net excess of positive charges (six in total; theoretical pI: 10.75), a feature that is shared by the ANT-binding domain of Vpr (see below). This domain does not possess obvious homologs in other species; however, it is strongly conserved among different cytomegalovirus isolates.
As does vMIA, viral protein R (Vpr), a small (96 aa) accessory protein of human immunodeficiency virus-1 (HIV-1), as well as its C-terminal moiety (Vpr52–96), can interact with ANT (Jacotot et al., 2001). In contrast to vMIA, however, Vpr does induce apoptosis. The Vpr–ANT interaction involves an α-helical dodecapeptide domain (aa 71–82) of Vpr (Schüler et al., 1999), characterized by the presence of three critical arginine residues (Jacotot et al., 2000). Disruption of this α-helix (by substitution of an l-Pro by d-Pro) or mutation of the arginine residues abolishes the action of this aa 71–82 peptide on purified mitochondria (Jacotot et al., 2001). Peptides derived from the apoptogenic domain (aa 106–154) of ANT (Jacotot et al., 2000), the same domain that interacts with Bcl-2 (Marzo et al., 1998), disrupt the Vpr–ANT interaction. Electrophysiological studies have revealed that Vpr and ANT cooperatively form non-specific ion channels when reconstituted into artificial membranes (Figure 2B). The physical and functional interaction between Vpr and ANT is inhibited by Bcl-2, while Bax increases the conductance and the opening probability of channels formed by Vpr and ANT (Brenner et al., 2000; Jacotot et al., 2000, 2001). Although data bank homology searches fail to identify obvious Vpr relatives, it appears that some pro-apoptotic viral proteins IIpossess α-helical, arginine-rich, mitochondriotoxic domains related to that of Vpr (Schüler et al., 1999). This applies to a mitochondrion-targeted, MMP-inducing decapeptide derived from the p13 (II) protein encoded by Human T-lymphotropic virus-1 (HTLV-1) (Ciminale et al., 1999).
Table II. Bacterial and viral proteins not related to Bcl-2 acting on mitochondria.
| Microorganism | Observation | References |
|---|---|---|
| Cytomegalovirus | vMIA (anti-apoptotic) interacts with ANT (not VDAC) and prevents MMP | Goldmacher et al. (1999); Colberg-Poley et al. (2000) |
| Helicobacter pylori | N-terminal cleavage product (p34) of vacuolating cytotoxin (Vac A) (pro-apoptotic) translocates to mitochondria and releases cytochrome c in a Bcl-2-inhibitable fashion | Galmiche et al. (2000) |
| Hepatitis B virus | HBV-X (pro-apoptotic) translocates to mitochondria, interacting with VDAC-3 | Rahmani et al. (2000) |
| HIV-1 | Vpr (pro-apoptotic) interacts with ANT and induces MMP, both in intact cells and in mitochondria in vitro; Tat (pro-apoptotic) translocates to mitochondria | Jacotot et al. (2000); Macho et al. (1999) |
| HTLV-1 | p13II protein (pro-apoptotic) interacts with mitochondria | Ciminale et al. (1999) |
| Myxoma virus | M11L (anti-apoptotic) translocates to mitochondria and prevents MMP | Everett et al. (2000) |
| Neisseria meningitidis | porin B (pro-apoptotic) translocates to mitochondria; porin B causes isolated mitochondria to release cytochrome c and to swell | Muller et al. (2000) |
| Neisseria gonorrhoeae | porin B (anti-apoptotic) translocates to mitochondria, where it interacts with VDAC; porin B prevents MMP | Massari et al. (2000) |
Mitochondrion-targeted proteins with an unknown mode of action
Myxoma virus, the causative agent of myxomatosis, encodes M11L, which is anti-apoptotic. Deletion of the M11L gene causes the virus to induce accelerated apoptosis in infected rabbit T lymphocytes (Macen et al., 1996) and monocytes (Everett et al., 2000). In vivo, M11L-negative strains have a greatly reduced virulence and induce more vigorous inflammatory reactions than pathogenic wild-type strains. The M11L protein targets mitochondria via a 25 amino acid C-terminal targeting sequence that is similar to a unique consensus sequence present in Bcl-2 family members (Everett et al., 2000). This kind of targeting signal, which includes a putative transmembrane domain, determines insertion of Bcl-2-like proteins in the outer membrane, facing the cytosol. Evidence for such a topology of M11L has been reported (Everett et al., 2000). M11L homologs are encoded by other poxviruses, namely rabbit fibroma virus (gp011L), swinepox virus (C10), and the virus responsible for Yaba-like disease (16L). All these proteins share the C-terminal mitochondrial localization motif, suggesting that M11L represents a prototype of a new class of mitochondrion-targeted apoptosis regulatory proteins.
Helicobacter pylori, the main cause of gastric ulcera, produces the vacuolating cytoxin VacA, thought to be essential for its pathogenic effect on gastric epithelial cells. p34 of VacA, which is pro-apoptotic, corresponds to the N-terminal fragment of VacA. p34 but not p58 (the C-terminal VacA fragment) translocates into mitochondria, both upon transfection of intact cells and upon addition to purified mitochondria. p34 of VacA causes OMMP in a Bcl-2-inhibitable fashion (Galmiche et al., 2000). Intriguingly, M11L and VacA share a domain of 122 amino acids homology with a similarity of 57%. However, the mechanism of mitochondrial targeting of VacA differs from that of M11L and involves both N- and C-terminal motifs (Galmiche et al., 2000). Moreover, differing from M11L, p34 localizes within the mitochondrial matrix or within the inner membrane after in vitro import.
Open questions and perspectives
The data summarized above establish that infectious pathogens can induce or inhibit apoptosis by producing proteins that target mitochondria. Given this precedent, it may be anticipated that further apoptosis modulatory proteins functioning in a similar fashion will be discovered. Some mitochondrion-targeted viral or bacterial proteins are certainly relevant to pathogenesis, as appears clear for M11L from myxoma virus and VacA from H.pylori. This hints at the possible clinical benefits of pharmacological targeting of mitochondria for the treatment of infectious diseases. Intriguingly, treatment of AIDS patients with HIV-1 protease inhibitors suppresses the increased apoptotic turnover of CD4-positive T cells before viral titers decrease, indicating that the anti-viral and biological activities of these agents can be uncoupled (Phenix et al., 2000). Indeed, such protease inhibitors inhibit apoptosis via a direct mitochondrial effect in vitro and reportedly abolish the MMP-inducing effect of Vpr on purified mitochondria (Phenix et al., 2001). Thus, HIV-1 protease inhibitors might achieve some of their therapeutic effects by apoptosis modulation at the mitochondrial level.
As stated in the introduction of this paper, viral proteins can prevent apoptosis by neutralizing specific pro-apoptotic signal transduction pathways. For example, viral proteins can neutralize pro-apoptotic cytokines (e.g. tumor necrosis factor, interferon-γ, interleukin-1), scavenge reactive oxygen species, inactivate the DNA damage sensor p53, target plasma membrane death receptors (such as CD95/Fas/Apo-1) to degradation, intercept death receptor-proximal signals (in particular CD95-triggered caspase-8 activation), or inhibit serine/threonine kinases (Tschopp et al., 1998; Gao and Kwaik, 2000). This kind of inhibition may be expected to be specific in the sense that it will prevent apoptosis only in response to one or few inducers. In contrast, inhibition of apoptosis at the mitochondrial level, where different pro-apoptotic signals converge (Figure 1), should have a comparatively broad spectrum of cytoprotective action. Intriguingly, some viruses encode several different anti-apoptotic proteins. For instance, cytomegalovirus produces both vMIA and vICA (encoded by the gene UL36), a viral inhibitor of apoptosis that binds and inactivates caspase-8 (Skalteskaya et al., 2001). CD95-triggered, caspase-8-dependent cell death is not inhibited by Bcl-2 or vMIA in so-called type I cells, in which massive caspase-8 activation results in direct proteolytic activation of caspase-3 (Scaffidi et al., 1998). However, in such cells, vICA does prevent CD95-triggered apoptosis (Skalteskaya et al., 2001). This illustrates how infectious microorganisms may subvert apoptotic signaling at several levels to enhance the probability of host cell survival.
Is there a general rule by which proteins encoded by viruses or bacteria act on mitochondria? It appears that all pathogen-encoded proteins discussed in this review lack a canonical N-terminal mitochondrial localization sequence, which would be proteolytically removed upon mitochondrial import. Thus, it may be speculated that such proteins employ the same membrane insertion pathways as do VDAC, ANT and Bcl-2-like proteins. VDAC is a prominent target of viral or bacterial proteins. PorB as well as HBV-X directly interact with VDAC (Massari et al., 2000; Muller et al., 2000; Schuster et al., 2000). Vpr, which targets ANT, crosses the outer mitochondrial membrane through VDAC, as indicated by inhibitor studies (Jacotot et al., 2001), and then acts on ANT. Thus, the fact that proteins interact with VDAC may reveal their mode of mitochondrial membrane insertion rather than their MMP-inducing mechanism. Several ANT-targeted proteins share amphipathic α-helical motifs with positive charges, which may facilitate protein–protein interactions or, alternatively, interactions with lipids of the inner mitochondrial membrane. The inner membrane contains a comparatively large amount of acidic lipids and is unique, compared with other cellular membranes, due to the absence of cholesterol and the presence of cardiolipin. This peculiar lipid composition (which resembles that of bacteria) may favor the insertion of mitochondriotoxic peptides [which induce MMP yet fail to permeabilize other membranes including the plasma membrane (del Rio et al., 2001)] and can determine the action of pro-apoptotic members of the Bcl-2 family (Lutter et al., 2000).
In evolutionary terms, it is possible that the mitochondrial (pristine?) level of apoptosis regulation has been established as a by-product of the integration into the host cell genome of genes coding for virus-encoded, mitochondrion-targeted proteins. Alternatively and in addition, the endosymbiotic incorporation of obligate aerobic, intracellular bacteria, the precursors of mitochondria, into the anaerobic eukaryotic host cell might have created the basic machinery of apoptosis (Kroemer, 1997a). In this context, some parallels may be drawn between cell death regulation in bacteria and in eukaryotes. Indeed, bacterial porins (the homologs of mitochondrial VDAC) may serve as receptor sites for the binding of phages and bacteriocins. For instance, colicins, the prototypic bacteriocins, bear a structural domain similar to the channel-forming domain of Bcl-2-like proteins. Certain colicins (colicins B and N) bind to E.coli OmpF, a protein from the porin family (Stroud et al., 1998). Nonetheless, in the bacterial system the cytotoxic effect of a colicin is not due to a direct effect on the porin-containing outer bacterial membrane, but rather involves translocation to, insertion into, and permeabilization of the inner membrane (Stroud et al., 1998). Given this evolutionary background, the hypothesis that host genome- or pathogen-encoded, mitochondrion-targeted proteins actually act on the inner mitochondrial membrane warrants addressing. Moreover, the tantalizing possibility that yet-to-be-discovered endogenous homologs or analogs of pathogen-encoded proteins participate in physiological cell death control needs to be explored.
Acknowledgments
Acknowledgements
We thank Drs Badley (Ottawa Hospital), V.S.Goldmacher (ImmunoGen, Inc., Cambridge, MA) and N.Zamzami (CNRS, Villejuif) for critical discussion and sharing unpublished data. This work has been supported by grants from ANRS (to G.K. and B.R.), as well as by grants from the Ligue Nationale contre le Cancer and the European Commission (QLG1-1999-00739 to G.K.). P.B. receives a fellowship from the European Commission.
References
- Brenner C. et al. (2000) Bcl-2 and Bax regulate the channel activity of the mitochondrial adenine nucleotide translocator. Oncogene, 19, 329–336. [DOI] [PubMed] [Google Scholar]
- Ciminale V., Zotti,L., D’Agostini,D.M., Ferro,T., Casareto,L., Franchini,G., Bernardi,P. and Chieco Bianchi,L. (1999) Mitochondrial targeting of the p13(II) protein coded by the x-II ORF of human T-cell leukemia/lymphotropic virus type I (HTLV-1). Oncogene, 18, 4505–4514. [DOI] [PubMed] [Google Scholar]
- Colberg-Poley A.M., Patel,M.B., Erezo,D.P. and Slater,J.E. (2000) Human cytomegalovirus UL37 immediate-early regulatory proteins traffic through the secretory apparatus and to mitochondria. J. Gen. Virol., 81, 1779–1789. [DOI] [PubMed] [Google Scholar]
- Cowan S.W., Schirmer,T., Rummel,G., Steier,M., Gosh,R., Pauptit,R.A., Jansonius,J.N. and Rosenbusch,J.P. (1992) Crystal structures explain functional properties of two E.coli porins. Nature, 358, 727–733. [DOI] [PubMed] [Google Scholar]
- De Laurenzi V. and Melino,G. (2000) Apoptosis. The little devil of death. Nature, 406, 135–136. [DOI] [PubMed] [Google Scholar]
- del Rio G., Castro-Obregon,C., Rao,R., Ellerby,M. and Bredesen,D.E. (2001) APAP, a sequence-pattern recognition approach identifies substance P as a potential apoptogenic peptide. FEBS Lett., 494, 213–219. [DOI] [PubMed] [Google Scholar]
- Derfuss T., Fickenscher,H., Kraft,M.S., Henning,G., Fleckenstein,B. and Meinl,E. (1998) Antiapoptotic activity of the herpesvirus saimiri-encoded Bcl-2 homolog: stabilization of mitochondria and inhibition of caspase-3-like activity. J. Virol., 72, 5897–5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald S.P., Sun,X.Y., Hu,C.A., Yu,J., Mei,J.M., Valle,D. and Phang,J.M. (2001) Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res., 61, 1810–1815. [PubMed] [Google Scholar]
- Everett H., Barry,M., Lee,S.F., Sun,X., Graham,K., Stone,J., Bleackley,R.C. and McFadden,G. (2000) M11L: a novel mitochondria-localized protein of myxoma virus that blocks apoptosis of infected leukocytes. J. Exp. Med., 191, 1487–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri K.F. et al. (2000) Apoptosis control in syncytia induced by the HIV-1-envelope glycoprotein complex. Role of mitochondria and caspases. J. Exp. Med., 192, 1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmiche A. et al. (2000) The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J., 19, 6361–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L.-Y. and Kwaik,Y.A. (2000) The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends Microbiol., 8, 306–313. [DOI] [PubMed] [Google Scholar]
- Goldmacher V.S. et al. (1999) A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl Acad. Sci. USA, 96, 12536–12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayajneh W.A., Colberg-Oley,A.M., Skaleskaya,A., Bartle,L.M., Lesperance,M.M., Contopoulos-Ionnidis,D.G., Kedersha,N.L. and Goldmacher,V.S. (2001) The sequence and antiapoptotic functional domains of the human cytomegaovirus UL37 exon 1 immediate early protein are conserved in multiple primary strains. Virology, 279, 233–240. [DOI] [PubMed] [Google Scholar]
- Jacotot E. et al. (2000) The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J. Exp. Med., 191, 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacotot E. et al. (2001) Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 Vpr and Bcl-2. J. Exp. Med., 193, 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G. (1997a) Mitochondrial implication in apoptosis. Towards an endosymbiotic hypothesis of apoptosis evolution. Cell Death Differ., 4, 443–456. [DOI] [PubMed] [Google Scholar]
- Kroemer G. (1997b) The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nature Med., 3, 614–620. [DOI] [PubMed] [Google Scholar]
- Kroemer G. and Reed,J.C. (2000) Mitochondrial control of cell death. Nature Med., 6, 513–519. [DOI] [PubMed] [Google Scholar]
- Kumar S., Bharti,A., Mishra,N.C., Raina,D., Kharbanda,S., Saxena,S. and Kufe,D. (2001) Targeting of the c-Abl tyrosine kinase to mitochondria in the necrotic cell death response to oxidative stress. J. Biol. Chem., 276, 17281–17285. [DOI] [PubMed] [Google Scholar]
- Li H. et al. (2000) Cytochrome c release and apoptosis induced by mitochondrial targeting of orphan receptor TR3-nur77-NGFI-B. Science, 289, 1159–1164. [DOI] [PubMed] [Google Scholar]
- Lutter M., Fang,M., Luo,X., Nishijima,M., Xie,X.S. and Wang,X. (2000) Cardiolipin provides specificity for targeting tBid to mitochondria. Nature Cell Biol., 2, 754–756. [DOI] [PubMed] [Google Scholar]
- Macen J.L., Graham,K.A., Lee,S.F., Schreiber,M., Boshkov,L.K. and McFadden,G. (1996) Expression of the myxoma virus tumor necrosis factor receptor homologue and M11L genes is required to prevent virus-induced apoptosis in infected rabbit T lymphocytes. Virology, 218, 232–237. [DOI] [PubMed] [Google Scholar]
- Macho A., Calzado,M.A., Jimenez-Reina,L., Ceballos,E., Leon,J. and Munoz,E. (1999) Susceptibility of HIV-1-TAT transfected cells to undergo apoptosis. Biochemical mechanisms. Oncogene, 18, 7543–7551. [DOI] [PubMed] [Google Scholar]
- Martinou J.-C. and Green,D.R. (2001) Breaking the mitochondrial barrier. Nature Rev. Mol. Cell. Biol., 2, 63–67. [DOI] [PubMed] [Google Scholar]
- Marzo I. et al. (1998) Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science, 281, 2027–2031. [DOI] [PubMed] [Google Scholar]
- Massari P., Ho,Y. and Wetzler,L.M. (2000) Neisseria meningitidis porin PorB interacts with mitochondria and protects cells from apoptosis. Proc. Natl Acad. Sci. USA, 97, 9070–9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A., Gunther,D., Brinkmann,V., Hurwitz,R., Meyer,T.F. and Rudel,T. (2000) Targeting of the pro-apoptotic VDAC-like porin (PorB) of Neisseria gonorrhoeae to mitochondria of infected cells. EMBO J., 19, 5332–5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda E., Ohki,R., Murasawa,H., Nemoto,J., Shibue,T., Yamashita,T., Tokino,T., Taniguchi,T. and Tanaka,N. (2000) Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science, 288, 1053–1058. [DOI] [PubMed] [Google Scholar]
- Oda K. et al. (2000) p53AIP1, a potential mediator of p53-dependent apoptosis and its regulation by Ser-46-phosphorylated p53. Cell, 102, 849–862. [DOI] [PubMed] [Google Scholar]
- Perez D. and White,E. (2000) TNF-α signals apoptosis through a bid-dependent conformational change in Bax that is inhibited by E1B 19K. Mol. Cell, 6, 53–63. [PubMed] [Google Scholar]
- Phenix B.N. et al. (2000) Decreased HIV-associated T cell apoptosis by HIV protease inhibitors. AIDS Res. Hum. Retroviruses, 16, 559–566. [DOI] [PubMed] [Google Scholar]
- Phenix B.N., Lum,J.J., Nie,Z., Sanchez-Dardon,J. and Badley,A.D. (2001) HIV protease inhibitors possess antiapoptotic effects that are mediated at the level of the mitochondrial transmembrane potential loss. Blood, in press. [DOI] [PubMed] [Google Scholar]
- Rahmani Z., Huh,K.W., Lasher,R. and Siddiqui,A. (2000) Hepatitis B virus X protein colocalizes to mitochondria with a human voltage-dependent anion channel, HVDAC3 and alters its transmembrane potential. J. Virol., 74, 2840–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restifo N.P. (2000) Building better vaccines: how apoptotic cell death can induce inflammation and activate innate and adaptative immunity. Curr. Opin. Immunol., 12, 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi C., Fulda,S., Srinivasan,A., Friesen,C., Li,F., Tomaselli,K.J., Debatin,K.-M., Krammer,P.H. and Peter,M.E. (1998) Two CD95 (APO-1/Fas) signaling pathways. EMBO J., 17, 1675–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüler W., Wecker,K., de Rocquigny,H., Baudat,Y., Sire,J. and Roques,B.P. (1999) NMR structure of the (52–96) C-terminal domain of the HIV-1 regulatory protein Vpr: molecular insights into its biological functions. J. Mol. Biol., 285, 2105–2117. [DOI] [PubMed] [Google Scholar]
- Schuster R., Gerlich,W.H. and Schaefer,S. (2000) Induction of apoptosis by the transactivating domains of the hepatitis B virus X gene leads to suppression of oncogenic transformation of primary rat embryo fibroblasts. Oncogene, 19, 1173–1180. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Ide,T., Yanagida,T. and Tsujimoti,Y. (2000a) Electro physiological study of a novel large pore formed by Bax and the voltage-dependent anion channel that is permeable to cytochrome c. J. Biol. Chem., 275, 12321–12325. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Konishi,A., Kodama,T. and Tsujimoto,Y. (2000b) BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc. Natl Acad. Sci. USA, 97, 3100–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalteskaya A., Bartle,L.M., Chittenden,T., McCormick,A.L., Mocarski,E.S. and Goldmacher,V.S. (2001) A cytomegolavirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl Acad. Sci. USA, 38, 7823–7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud R.M., Reiling,K., Wiener,M. and Freymann,D. (1998) Ion-channel-forming colicins. Curr. Opin. Struct. Biol., 8, 525–533. [DOI] [PubMed] [Google Scholar]
- Susin S.A. et al. (2000) Two distinct pathways leading to nuclear apoptosis. J. Exp. Med., 192, 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J., Thome,M., Hofmann,K. and Meinl,E. (1998) The fight of viruses against apoptosis. Curr. Opin. Genet. Dev., 8, 82–87. [DOI] [PubMed] [Google Scholar]
- Vieira J.L.A. et al. (2001) The adenine nucleotide translocator: a target of nitric oxide, peroxynitrite and 4-hydroxynonenal. Oncogene, in press. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Lane,D. and Levine,A.J. (2000) Surfing the p53 network. Nature, 408, 307–310. [DOI] [PubMed] [Google Scholar]
- Xu X. and Colombini,M. (1996) Self-catalyzed insertion of proteins into phospholipid membranes. J. Biol. Chem., 271, 23675–23682. [DOI] [PubMed] [Google Scholar]
- Zamzami N. and Kroemer,G. (2001) Mitochondria in apoptosis. How Pandora’s box opens. Nature Rev. Mol. Cell. Biol., 2, 67–71. [DOI] [PubMed] [Google Scholar]