Abstract
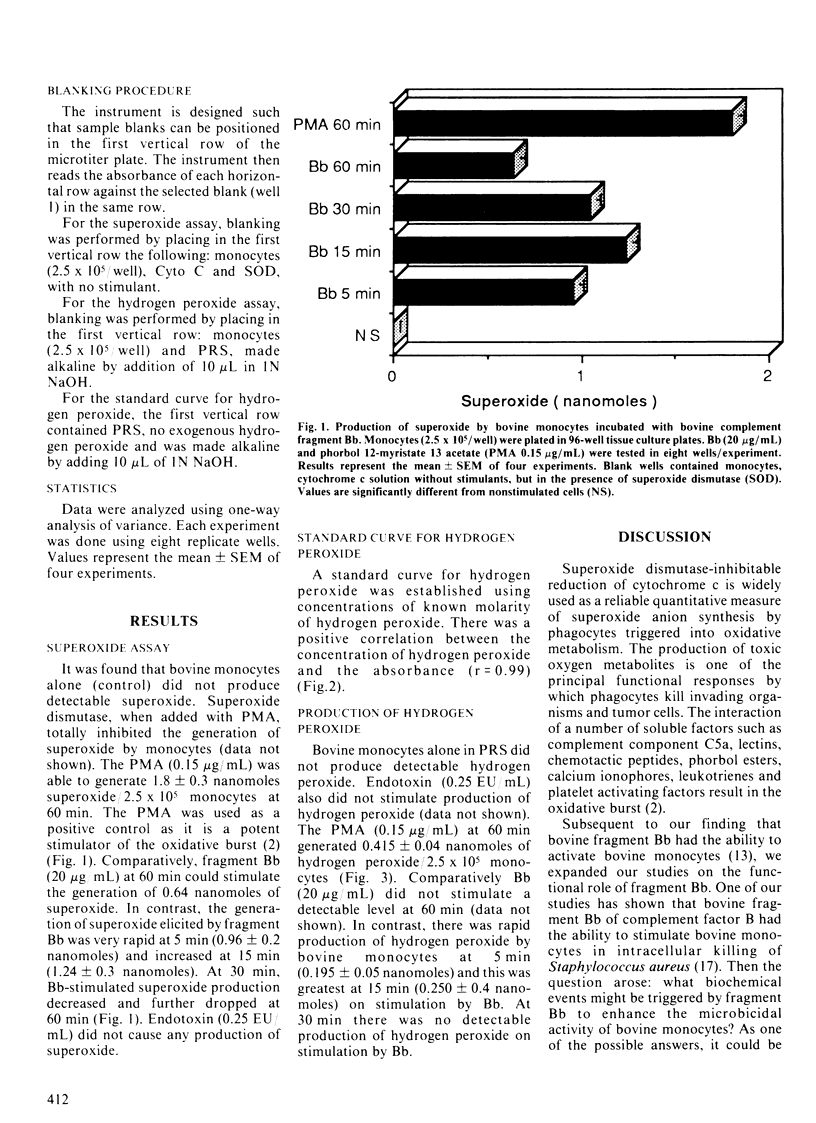
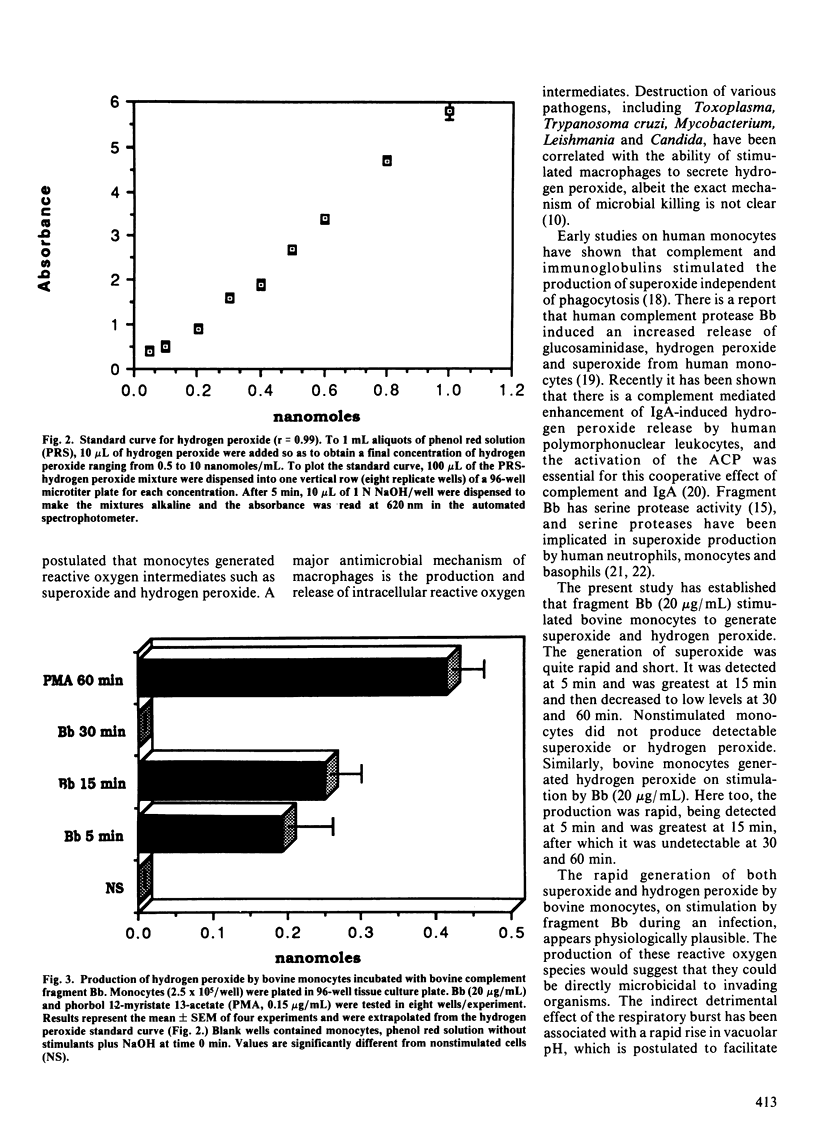
In a previous study, we reported that fragment Bb of bovine complement factor B activated bovine monocytes, as demonstrated by the uptake of 3H-deoxyglucose. In the present study, the effects of Bb on the production of superoxide anion and hydrogen peroxide by bovine monocytes was investigated. The production of superoxide was measured by the superoxide dismutase inhibitable reduction of cytochrome c. The change in absorbance was determined by a spectrophotometer at a wavelength of 550 nm. Hydrogen peroxide production was measured by the horse-radish peroxidase-dependent oxidation of phenol red. The resulting color change was measured by a spectrophotometer at a wavelength of 620 nm. Fragment Bb (20 micrograms/mL) induced the generation of 0.96 +/- 0.2 (mean +/- SEM) nanomoles of superoxide/2.5 x 10(5) monocytes at 5 min. The production of superoxide peaked at 15 min (1.24 +/- 0.3 nanomoles). The production of hydrogen peroxide was also rapid: 0.195 +/- 0.05 nanomoles/2.5 x 10(5) monocytes at 5 min with a peak at 15 min (0.250 +/- 0.04 nanomoles). These observations show that fragment Bb, which has serine protease activity, induces bovine monocytes to generate reactive oxygen intermediates such as superoxide and hydrogen peroxide.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adinolfi M., Lehner T. C9 and factor B as acute phase proteins and their diagnostic and prognostic value in disease. Exp Clin Immunogenet. 1988;5(2-3):123–132. [PubMed] [Google Scholar]
- Aneshansley D. J., Eisner T., Widom J. M., Widom B. Biochemistry at 100{degrees}C: Explosive Secretory Discharge of Bombardier Beetles (Brachinus). Science. 1969 Jul 4;165(3888):61–63. doi: 10.1126/science.165.3888.61. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Bianco C., Götze O., Cohn Z. A. Regulation of macrophage migration by products of the complement system. Proc Natl Acad Sci U S A. 1979 Feb;76(2):888–891. doi: 10.1073/pnas.76.2.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeldt Ohmann H., Babiuk L. A. In vitro generation of hydrogen peroxide and of superoxide anion by bovine polymorphonuclear neutrophilic granulocytes, blood monocytes, and alveolar macrophages. Inflammation. 1984 Sep;8(3):251–275. doi: 10.1007/BF00916415. [DOI] [PubMed] [Google Scholar]
- Cohen M. S., Britigan B. E., Hassett D. J., Rosen G. M. Phagocytes, O2 reduction, and hydroxyl radical. Rev Infect Dis. 1988 Nov-Dec;10(6):1088–1096. doi: 10.1093/clinids/10.6.1088. [DOI] [PubMed] [Google Scholar]
- Dyer R. M., Erney S., Spencer P., Benson C. E. Oxidative metabolism of the bovine alveolar macrophage. Am J Vet Res. 1989 Apr;50(4):448–454. [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter A., Hiemstra P. S., Leijh P. C., van der Sluys M. E., van den Barselaar M. T., Van Es L. A., Daha M. R. Complement-mediated enhancement of IgA-induced H2O2 release by human polymorphonuclear leucocytes. Immunology. 1989 May;67(1):120–125. [PMC free article] [PubMed] [Google Scholar]
- Gschwend P. M., Macfarlane J. K., Newman K. A. Volatile halogenated organic compounds released to seawater from temperate marine macroalgae. Science. 1985 Mar 1;227(4690):1033–1035. doi: 10.1126/science.227.4690.1033. [DOI] [PubMed] [Google Scholar]
- Götze O., Bianco C., Cohn Z. A. The induction of macrophage spreading by factor B of the properdin system. J Exp Med. 1979 Feb 1;149(2):372–386. doi: 10.1084/jem.149.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa S., Takaku F. Role of serine proteases in superoxide production by human neutrophils, monocytes and basophils. Adv Exp Med Biol. 1982;141:441–451. doi: 10.1007/978-1-4684-8088-7_42. [DOI] [PubMed] [Google Scholar]
- Kitagawa S., Takaku F., Sakamoto S. Evidence that proteases are involved in superoxide production by human polymorphonuclear leukocytes and monocytes. J Clin Invest. 1980 Jan;65(1):74–81. doi: 10.1172/JCI109662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicus R. G., Götze O., Müller-Eberhard H. J. The serine protease nature of the C3 and C5 convertases of the classical and alternative complement pathways. Scand J Immunol. 1976;5(9):1049–1055. doi: 10.1111/j.1365-3083.1976.tb03056.x. [DOI] [PubMed] [Google Scholar]
- Miyagawa N., Okamoto Y., Miyagawa S. Effect of C-reactive protein on peritoneal macrophages. I. Human C-reactive protein inhibits migration of guinea pig peritoneal macrophages. Microbiol Immunol. 1988;32(7):709–719. doi: 10.1111/j.1348-0421.1988.tb01432.x. [DOI] [PubMed] [Google Scholar]
- Miyagawa N., Okamoto Y., Nakano H. Effect of C-reactive protein on peritoneal macrophages. II. Human C-reactive protein activates peritoneal macrophages of guinea pigs to release superoxide anion in vitro. Microbiol Immunol. 1988;32(7):721–731. doi: 10.1111/j.1348-0421.1988.tb01433.x. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. III. Enhanced oxidative metabolism as an expression of macrophage activation. J Exp Med. 1980 Dec 1;152(6):1596–1609. doi: 10.1084/jem.152.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Mechanisms of macrophage antimicrobial activity. Trans R Soc Trop Med Hyg. 1983;77(5):620–630. doi: 10.1016/0035-9203(83)90190-6. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Cohn Z. A. The macrophage as an effector cell. N Engl J Med. 1980 Sep 11;303(11):622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Goldberger G., Dinarello C. A., Mizel S. B., Colten H. R. Regulation of class III major histocompatibility complex gene products by interleukin-1. Science. 1986 May 16;232(4752):850–852. doi: 10.1126/science.3010455. [DOI] [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Saeki T., Nagasawa S. Expression of the binding molecules for factor B and its fragment, Bb, of human complement on human monocytes after in vitro cultivation. Immunol Lett. 1989 Jan 31;20(2):97–101. doi: 10.1016/0165-2478(89)90092-8. [DOI] [PubMed] [Google Scholar]
- Sethi M. S., Tabel H. Fragment Bb of bovine complement factor B: stimulatory effect on the microbicidal activity of bovine monocytes. Can J Vet Res. 1990 Oct;54(4):405–409. [PMC free article] [PubMed] [Google Scholar]
- Sethi M. S., Tabel H., Misra V. Activation of bovine monocytes and neutrophils by the Bb fragment of complement factor B: demonstration by the uptake of 3H-deoxyglucose. Can J Vet Res. 1990 Jan;54(1):106–112. [PMC free article] [PubMed] [Google Scholar]
- Sundsmo J. S., Götze O. Human monocyte spreading induced by factor B of the alternative pathway of complement activation. Cell Immunol. 1980 Jun;52(1):1–17. doi: 10.1016/0008-8749(80)90395-0. [DOI] [PubMed] [Google Scholar]


