Abstract
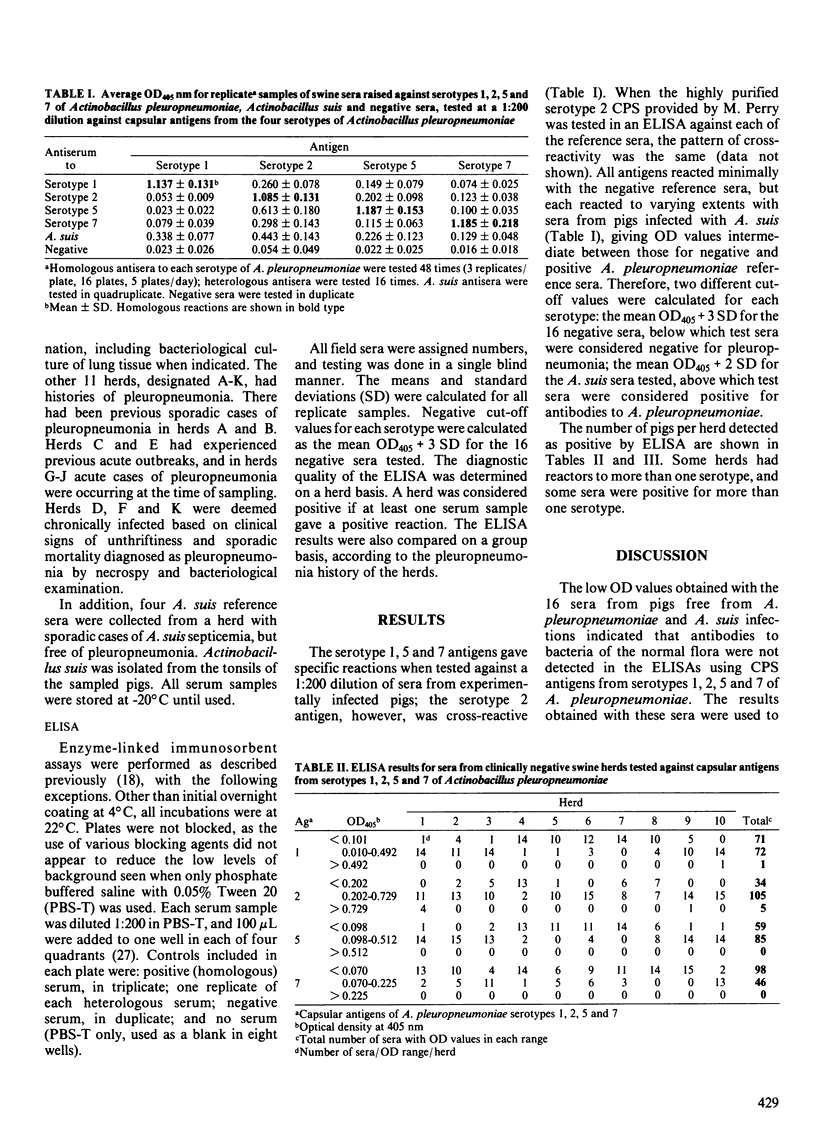
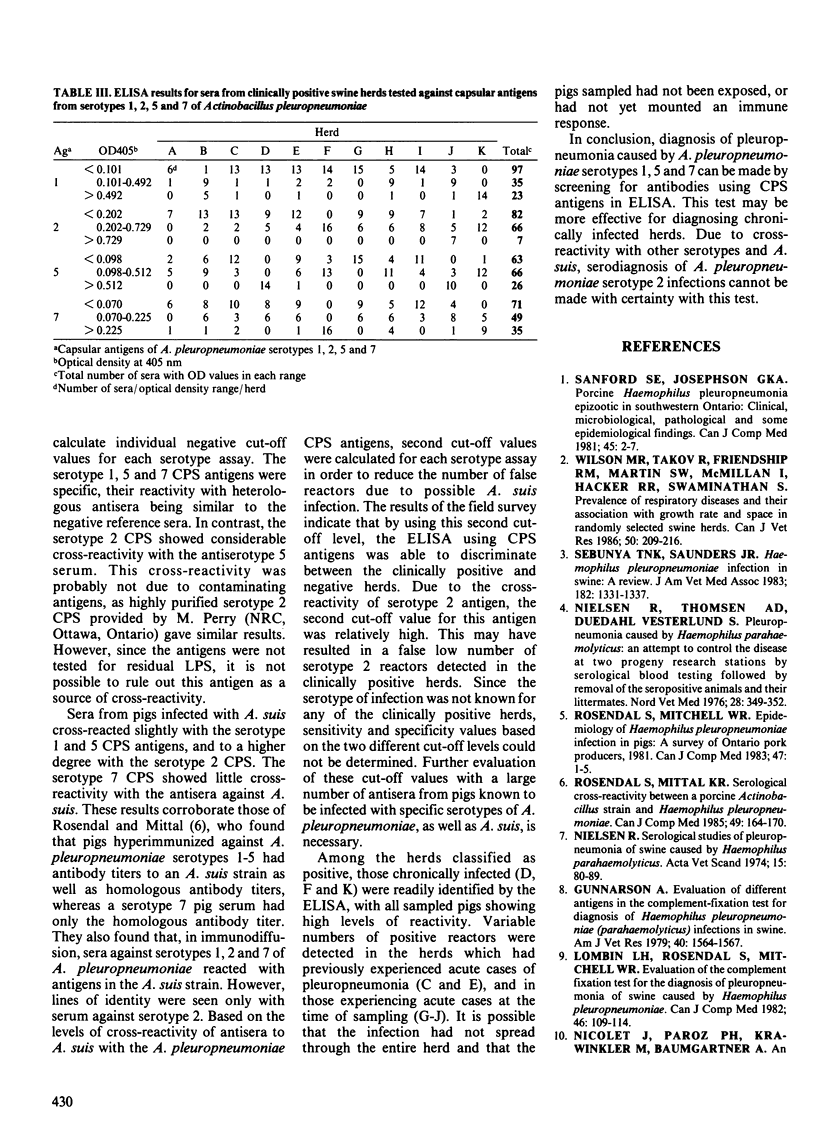
Capsular polysaccharide antigens of serotypes 1, 2, 5 and 7 of Actinobacillus pleuropneumoniae were used in enzyme-linked immunosorbent assays (ELISAs) to test sera from experimentally infected and field pigs. Specific reactions were found in sera of experimental pigs with antigens of serotypes 1, 5 and 7 whereas the serotype 2 antigen was cross-reactive. A 1:200 serum dilution was used for testing of 300 sera from 21 swine herds in southern Ontario. Cases of pleuropneumonia had occurred in 11 of these herds, but not in the others. The negative cut-off value was the mean optical density at 405 nm (OD405) + three standard deviations (SD) for 16 negative reference sera. Sera from four pigs naturally infected with Actinobacillus suis were tested and found to react to varying degrees with each of the antigens. Therefore a second cut-off value was determined as the mean OD405 + 2 SD for the A. suis sera. Sera which, in the ELISA produced OD readings above the latter cut-off were considered positive for antibodies to A. pleuropneumoniae; those which were lower than the former cut-off were considered negative. Readings between the two cut-off values may have been due to low positive titers or cross-reactivity, possibly with A. suis, and could not be used to predict pleuropneumonia. Of the pleuropneumonia-free herds, none had positive reactors to serotypes 5 or 7, whereas one and two herds had positive reactors to serotypes 1 and 2, respectively. Of the pleuropneumonia positive herds, six had positive reactors to serotype 1, one to serotype 2, four to serotype 5, and eight to serotype 7.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman E., Brisson J. R., Perry M. B. Structural studies of the capsular polysaccharide from Haemophilus pleuropneumoniae serotype 1. Biochem Cell Biol. 1986 Aug;64(8):707–716. doi: 10.1139/o86-097. [DOI] [PubMed] [Google Scholar]
- Altman E., Brisson J. R., Perry M. B. Structural studies of the capsular polysaccharide from Haemophilus pleuropneumoniae serotype 2. Biochem Cell Biol. 1987 May;65(5):414–422. doi: 10.1139/o87-053. [DOI] [PubMed] [Google Scholar]
- Altman E., Brisson J. R., Perry M. B. Structure of the capsular polysaccharide of Haemophilus pleuropneumoniae serotype 5. Eur J Biochem. 1987 Dec 30;170(1-2):185–192. doi: 10.1111/j.1432-1033.1987.tb13685.x. [DOI] [PubMed] [Google Scholar]
- Bossé J. T., Johnson R. P., Rosendal S. Capsular polysaccharide antigens for detection of serotype-specific antibodies to Actinobacillus pleuropneumoniae. Can J Vet Res. 1990 Jun;54(3):320–325. [PMC free article] [PubMed] [Google Scholar]
- Gunnarsson A. Evaluation of different antigens in the complement-fixation test for diagnosis of Haemophilus pleuropneumoniae (parahaemolyticus) infections in swine. Am J Vet Res. 1979 Nov;40(11):1564–1567. [PubMed] [Google Scholar]
- Gunnarsson A. Serologic studies on porcine strains of Haemophilus parahaemolyticus (pleuropneumoniae): extraction of type-specific antigens. Am J Vet Res. 1979 Apr;40(4):469–472. [PubMed] [Google Scholar]
- Inzana T. J., Mathison B. Serotype specificity and immunogenicity of the capsular polymer of Haemophilus pleuropneumoniae serotype 5. Infect Immun. 1987 Jul;55(7):1580–1587. doi: 10.1128/iai.55.7.1580-1587.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J. Purification and partial characterization of the capsular polymer of Haemophilus pleuropneumoniae serotype 5. Infect Immun. 1987 Jul;55(7):1573–1579. doi: 10.1128/iai.55.7.1573-1579.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombin L. H., Rosendal S., Mitchell W. R. Evaluation of the complement fixation test for the diagnosis of pleuropneumonia of swine caused by Haemophilus pleuropneumoniae. Can J Comp Med. 1982 Apr;46(2):109–114. [PMC free article] [PubMed] [Google Scholar]
- Miniats O. P., Spinato M. T., Sanford S. E. Actinobacillus suis septicemia in mature swine: two outbreaks resembling erysipelas. Can Vet J. 1989 Dec;30(12):943–947. [PMC free article] [PubMed] [Google Scholar]
- Mittal K. R., Higgins R., Larivière S., Leblanc D. A 2-mercaptoethanol tube agglutination test for diagnosis of Haemophilus pleuropneumoniae infection in pigs. Am J Vet Res. 1984 Apr;45(4):715–719. [PubMed] [Google Scholar]
- Nicolet J., Paroz P., Krawinkler M., Baumgartner A. An enzyme-linked immunosorbent assay, using an EDTA-extracted antigen for the serology of Haemophilus pleuropneumoniae. Am J Vet Res. 1981 Dec;42(12):2139–2142. [PubMed] [Google Scholar]
- Nielsen R. Serological and immunological studies of pleuropneumonia of swine caused by Haemophilus parahaemolyticus. Acta Vet Scand. 1974;15(1):80–89. doi: 10.1186/BF03547495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R., Thomsen A. D., Vesterlund S. D. Pleuropneumonia caused by Haemophilus parahaemolyticus. An attempt to control the disease at two progeny testing stations by serological blood testing followed by removal of the seropositive animals and their litter mates. Nord Vet Med. 1976 Jul-Aug;28(7-8):349–352. [PubMed] [Google Scholar]
- Rosendal S., Miniats O. P., Sinclair P. Protective efficacy of capsule extracts of Haemophilus pleuropneumoniae in pigs and mice. Vet Microbiol. 1986 Sep;12(3):229–240. doi: 10.1016/0378-1135(86)90052-0. [DOI] [PubMed] [Google Scholar]
- Rosendal S., Mitchell W. R. Epidemiology of Haemophilus pleuropneumoniae infection in pigs: a survey of Ontario Pork Producers, 1981. Can J Comp Med. 1983 Jan;47(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Mittal K. R. Serological cross-reactivity between a porcine Actinobacillus strain and Haemophilus pleuropneumoniae. Can J Comp Med. 1985 Apr;49(2):164–170. [PMC free article] [PubMed] [Google Scholar]
- Sanford S. E., Josephson G. K. Porcine Haemophilus pleuropneumonia epizootic in southwestern Ontario: clinical, microbiological, pathological and some epidemiological findings. Can J Comp Med. 1981 Jan;45(1):2–7. [PMC free article] [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983 Jun 15;182(12):1331–1337. [PubMed] [Google Scholar]
- Willson P. J., Falk G., Klashinsky S. Detection of Actinobacillus pleuropneumoniae Infection in Pigs. Can Vet J. 1987 Mar;28(3):111–116. [PMC free article] [PubMed] [Google Scholar]
- Wilson M. R., Takov R., Friendship R. M., Martin S. W., McMillan I., Hacker R. R., Swaminathan S. Prevalence of respiratory diseases and their association with growth rate and space in randomly selected swine herds. Can J Vet Res. 1986 Apr;50(2):209–216. [PMC free article] [PubMed] [Google Scholar]
- Wright P. Enzyme immunoassay: observations on aspects of quality control. Vet Immunol Immunopathol. 1987 Dec;17(1-4):441–452. doi: 10.1016/0165-2427(87)90160-7. [DOI] [PubMed] [Google Scholar]


