Abstract
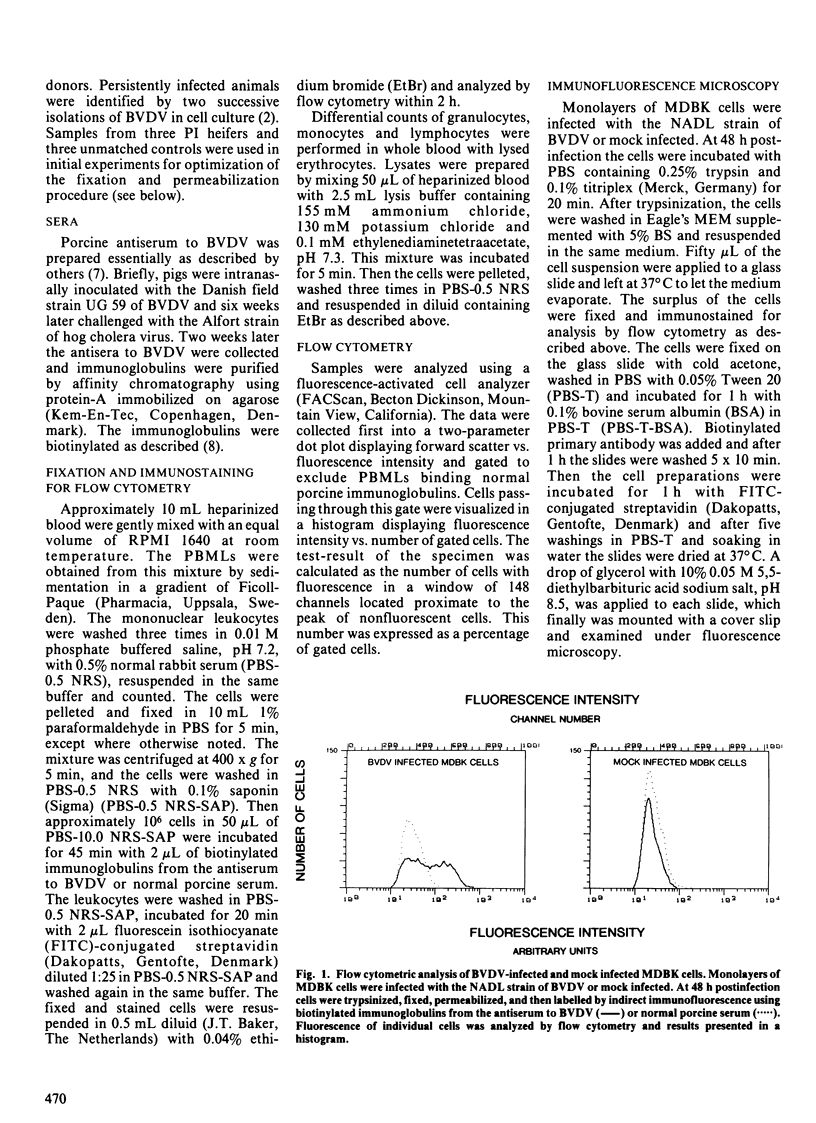
Flow cytometry was investigated for detection of bovine viral diarrhea virus (BVDV) in peripheral blood mononuclear leukocytes of persistently infected cattle. The mononuclear leukocytes were purified by sedimentation in a gradient of Ficoll-Paque, fixed, permeabilized, and then labelled by indirect immunofluorescence using biotinylated immunoglobulins from a porcine antiserum to BVDV. Flow cytometric analysis of blood samples obtained from persistently infected cattle revealed virus in 3.0-21.0% (mean +/- SD, 11.2% +/- 6.4%) of the mononuclear leukocytes. Fluorescent cells were not observed in controls. Flow cytometric detection of BVDV in blood cells of persistently infected bovines is a rapid and objective technique which does not require cell culture facilities.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aurelian L., Gupta P. K., Frost J. K., Rosenshein N. B., Smith C. C., Tyrer H. W., Mantione J. M., Albright C. D. Fluorescence-activated separation of cervical abnormal cells using herpesvirus antigenic markers. Anal Quant Cytol. 1979 Jul-Aug;1(2):89–102. [PubMed] [Google Scholar]
- Bezek D. M., Baker J. C., Kaneene J. B. Immunofluorescence of bovine virus diarrhea viral antigen in white blood cells from experimentally infected immunocompetent calves. Can J Vet Res. 1988 Apr;52(2):288–290. [PMC free article] [PubMed] [Google Scholar]
- Bielefeldt Ohmann H., Rønsholt L., Bloch B. Demonstration of bovine viral diarrhoea virus in peripheral blood mononuclear cells of persistently infected, clinically normal cattle. J Gen Virol. 1987 Jul;68(Pt 7):1971–1982. doi: 10.1099/0022-1317-68-7-1971. [DOI] [PubMed] [Google Scholar]
- Bolin S. R., McClurkin A. W., Coria M. F. Effects of bovine viral diarrhea virus on the percentages and absolute numbers of circulating B and T lymphocytes in cattle. Am J Vet Res. 1985 Apr;46(4):884–886. [PubMed] [Google Scholar]
- Bolin S. R., Sacks J. M., Crowder S. V. Frequency of association of noncytopathic bovine viral diarrhea virus with mononuclear leukocytes from persistently infected cattle. Am J Vet Res. 1987 Oct;48(10):1441–1445. [PubMed] [Google Scholar]
- Brock K. V., Brian D. A., Rouse B. T., Potgieter L. N. Molecular cloning of complementary DNA from a pneumopathic strain of bovine viral diarrhea virus and its diagnostic application. Can J Vet Res. 1988 Oct;52(4):451–457. [PMC free article] [PubMed] [Google Scholar]
- Cassidy L. F., Lyles D. S., Abramson J. S. Synthesis of viral proteins in polymorphonuclear leukocytes infected with influenza A virus. J Clin Microbiol. 1988 Jul;26(7):1267–1270. doi: 10.1128/jcm.26.7.1267-1270.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffell S. J., Harkness J. W. Bovine virus diarrhoea-mucosal disease infection in cattle. Vet Rec. 1985 Sep 7;117(10):240–245. doi: 10.1136/vr.117.10.240. [DOI] [PubMed] [Google Scholar]
- Elmendorf S., McSharry J., Laffin J., Fogleman D., Lehman J. M. Detection of an early cytomegalovirus antigen with two-color quantitative flow cytometry. Cytometry. 1988 May;9(3):254–260. doi: 10.1002/cyto.990090311. [DOI] [PubMed] [Google Scholar]
- Gershey E. L. SV40-infected muntjac cells: cell cycle kinetics, cell ploidy and T antigen concentration. Cytometry. 1980 Jul;1(1):49–56. doi: 10.1002/cyto.990010111. [DOI] [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Horan M., Horan P. K., Williams C. A. Quantitative measurement of SB 40 T-antigen production. Exp Cell Res. 1975 Mar 15;91(2):247–252. doi: 10.1016/0014-4827(75)90101-9. [DOI] [PubMed] [Google Scholar]
- Hyera J. M., Dahle J., Liess B., Moennig V., Frey H. R. Gewinnung hochtitriger Antiseren gegen BVD-Virus aus Schweinen und ihre Verwendung für direkte Immunfluoreszenz- und Immunperoxidase Techniken. Dtsch Tierarztl Wochenschr. 1987 Nov-Dec;94(10):576–580. [PubMed] [Google Scholar]
- Jacobberger J. W., Fogleman D., Lehman J. M. Analysis of intracellular antigens by flow cytometry. Cytometry. 1986 Jul;7(4):356–364. doi: 10.1002/cyto.990070410. [DOI] [PubMed] [Google Scholar]


