Introduction
The High Mobility Group Box (HMGB) chromosomal proteins have been known and studied for a long time, but we have only recently started to understand their biological functions. They now have a clear reputation for being important architectural factors: they facilitate the assembly of site-specific DNA binding proteins to their cognate binding sites within chromatin. Beyond this intranuclear function, they also have an extracellular function, which will be the prime focus of this short review.
The HMGB family: structure, expression and nuclear function
The HMGB family comprises the three proteins HMGB1 (previously HMG1), HMGB2 (previously HMG2) and HMGB3 (previously HMG4 or HMG2b) (Bustin, 2001). The structure of these three proteins is highly conserved (>80% amino acid identity), and their biochemical properties are so far indistinguishable. HMGBs are composed of three different domains. The two homologous DNA binding domains, HMG boxes A and B, are each ∼75 amino acids in length. The C-terminal domain is highly negatively charged, consisting of a continuous stretch of glutamate or aspartate residues, and is longest in HMGB1 and shortest in HMGB3 (reviewed in Bustin, 1999; Bianchi and Beltrame, 2000). HMGB1 is ubiquitous and only 10 times less abundant than core histones, at ∼106 molecules per typical mammalian cell. Expression of the other two family members is more restricted: HMGB3 is only expressed to a significant amount during embryogenesis (Vaccari et al., 1998); HMGB2 is widely expressed during embryonic development, but restricted mainly to lymphoid organs and testis in the adult mouse (Ronfani et al., 2001).
The localization of these proteins in most cells is nuclear. In their nuclear identity, HMGB1 and HMGB2 bind to the minor groove of DNA, causing a local distortion of the double helix. They have little or no sequence preference, and they are recruited to the site of action by specific DNA binding proteins. HMGB1 has been shown to interact with, and increase the apparent binding affinity of, several transcription factors (including the Hox and Pou proteins, and the steroid hormone receptors p53 and TBP), some viral proteins, and the RAG1 protein, which performs V(D)J recombination (reviewed in Bianchi and Beltrame, 2000). The functional importance of HMGB1 as regulator of transcription has been confirmed by the phenotype of the Hmgb1 knockout mouse, which dies shortly after birth due to hypoglycemia and shows a defect in the transcriptional enhancement of the glucocorticoid receptor (Calogero et al., 1999).
Secretion of HMGB1 and inflammation
In surprising constrast to its intranuclear role, HMGB1 can also be secreted by certain cells, and plays important roles in inflammation and tumour metastasis (Wang et al., 1999a; Taguchi et al., 2000).
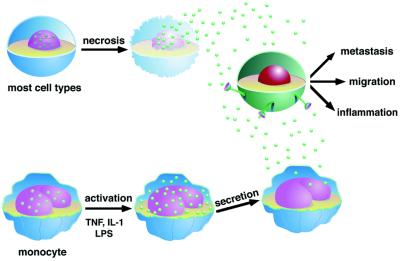
Wang et al. (1999a) identified HMGB1 as a late mediator of endotoxin lethality in mice. They showed that monocytes/macrophages stimulated by lipopolysaccharide (LPS), tumour necrosis factor (TNF) or interleukin-1 (IL-1) secreted HMGB1 as a delayed response. In mice, administration of anti-HMGB1 antibodies attenuated LPS-induced endotoxemia; conversely, injection of HMGB1 caused toxic shock. Moreover, septic patients showed increased serum levels of HMGB1, which correlated with the severity of the infection. Subsequently, HMGB1 was also shown to cause acute lung inflammation when administered intratracheally (Abraham et al., 2000). Antibodies against HMGB1 decreased lung edema and neutrophile migration, whereas they did not reduce the levels of the other proinflammatory cytokines TNF-α, IL-1β or macrophage inflammatory protein-2 (MIP2). Pituicytes, which provide an important link between the immune and the neuroendocrine systems, release HMGB1 in response to specific stimuli like TNF-α and IL-1, suggesting that HMGB1 also participates in the regulation of neuroendocrine and immune responses to inflammatory processes (Wang et al., 1999b). However, most cells (including lymphocytes, adrenal cells or kidney cells) are not able to secrete HMGB1 (Wang et al., 1999a).
It is not clear so far how HMGB1 is secreted by monocytes and the other competent cell types: it possesses no signal peptide that would direct it to the endoplasmic reticulum (ER) (Figure 1). This lack of a secretion leader peptide is a feature shared with a small number of other secreted proteins, like the cytokine IL-1β (Andrei et al., 1999). Studies in murine erythroleukemia (MEL) cells have shown that HMGB1 export does not involve the ER and the Golgi complex, but is promoted by intracellular Ca2+ increase and possibly by activation of a Ca-dependent protein kinase C isoform (Passalacqua et al., 1997). In any event, some sort of nucleus–cytoplasm shuttling must also be invoked if the protein has to leave the nucleus altogether.
Fig. 1. Extracellular HMGB1 mediates migration of cells, metastasis and inflammatory responses. Most types of cells (upper) passively release nuclear HMGB1 (green spheres) into their surroundings after they have died. Alternatively, HMGB1 can be actively secreted from monocytes (and a limited number of other cells, lower part of the drawing) upon stimulation with LPS, TNF-α or IL-1. Secretion does not involve passage through the endoplasmic reticulum and Golgi apparatus. Extracellular HMGB1 provokes in other cells (green) that bear a receptor for HMGB1 an appropriate response for that cell type, like cell migration or inflammation.
Once released, HMGB1 is able to activate several other cells involved in the immune response or inflammatory reactions, and can act as a cytokine itself (Andersson et al., 2000). HMGB1 stimulates monocytes to secrete a specific subset of proinflammatory cytokines, including TNF-α and IL-1. In comparison with the well known inflammatory stimulus LPS, HMGB1 causes a delayed and biphasic release of TNF-α. It appears, therefore, that HMGB1 is not only secreted by macrophages in response to proinflammatory stimuli, but also itself provokes a delayed response; thereby, it prolongs and sustains inflammation. This response is cell type specific: no cytokine was released from lymphocytes stimulated with HMGB1.
Local, controlled export of HMGB1 enhances specific differentiation processes
Before the discovery of the role of HMGB1 in inflammation, it had already been observed that several cells release the protein in their surroundings as part of their differentiation process. MEL cells, stimulated with a chemical inducer, release HMGB1 (also called DEF for differentiation enhancing factor) in the medium; extracellular HMGB1 in turn is required for the differentiation of these cells (Sparatore et al., 1996; Passalacqua et al., 1997). Likewise, neurons secrete HMGB1 (also called amphoterin) and retain it associated to their plasma membranes at the leading edge of migration, which in turn promotes the outgrowth of neurites (Fages et al., 2000 and references therein).
Alternatively, HMGB1 can also be set free by groups of cells that support the differentiation of other spatially associated cell types. Stimulated astrocytes release HMGB1, which then induces LAN5 neuroblastoma cells to differentiate (Passalacqua et al., 1998). Human promyelocytic HL60 cells, although not secreting HMBG1 themselves, show an accelerated differentiation when exposed to extracellular HMGB1 (Sparatore et al., 1996).
In all these cases, the release of HMGB1 into the extracellular space is well controlled and local, in the sense that HMGB1 is either secreted in an autocrine manner, or in a paracrine manner by closely associated cells. Remarkably, this spatially restricted secretion of HMGB1 does not seem to trigger an inflammatory response.
Cell migration and metastasis
HMGB1 can also promote cell migration. Cells migrate in response to extracellular stimuli, channelled through specific signal transduction pathways, which ultimately elicit cytoskeletal remodelling. We showed that vascular smooth muscle cells (Degryse et al., 2001) and fibroblasts (B.Degryse and M.E.Bianchi, unpublished) respond to extracellular HMGB1 in cytokinesis and chemoattraction assays. Anti-HMGB1 antibodies inhibit neurite outgrowth and migration of glioma cells (Fages et al., 2000). Perhaps most strikingly, Taguchi et al. (2000) showed that HMGB1 is also involved in tumour invasion and metastasis: admin istration of anti-HMGB1 antibodies suppressed metastasis formation by Lewis lung tumour cells implanted under the skin of recipient mice.
In vivo, cell migration depends to a large extent on the capability of a cell to invade the surrounding tissue, for which the activation of extracellular proteases is required. HMGB1 appears to play a role here as well: it binds to several components of the plasminogen activation system and enhances the activation of t-PA (Parkkinen and Rauvala, 1991). Moreover, Taguchi et al. (2000) showed HMGB1-elicited activation of metalloproteases MMP-2 and MMP-9, which are downstream targets of the plasmin activation cascade. While it enables the degradation of the extracellular matrix, the activation of proteases also leads to degradation of HMGB1 and might serve as a feedback mechanism.
Signalling mechanisms
The signalling mechanisms by which HMGB1 activates cells to respond are incompletely understood (Figure 2). HMGB1 is rather ‘sticky’, and binds to many different molecules on the cell surface: heparin, proteoglycans, but also sulfoglycolipids and phospholipids (Bianchi, 1988; Degryse et al., 2001; Rouhiainen et al., 2001). This could be a mechanism to restrict the diffusion of extracellular HMGB1, and thereby keep the effect of this potentially dangerous molecule local. A molecule that binds HMGB1 in a specific and saturable way is syndecan-1, which is a cell surface proteoglycan containing heparan sulfate and chondoitinsulfate (Salmivirta et al., 1992). HMGB1 can also be taken up by the cell in an as yet unidentified manner, and promote the co-uptake of DNA. This property was therefore used to transfect cells (Mistry et al., 1997). It is unclear, however, whether binding to cell surface glycans and cellular uptake are causally related.
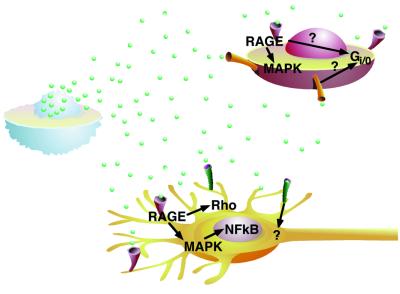
Fig. 2. Known signalling mechanisms of extracellular HMGB1. Extracellular HMGB1 (green spheres) stimulates smooth muscle cells (red) to migrate. This response is mediated through RAGE, a Gi/o protein and the MAP kinase pathway, but the exact connection between these participants in the signal transduction cascade is not known. Neurite outgrowth in neurons (yellow) is activated by HMGB1 binding to RAGE, and the signal is channelled through the Rho family of small GTPases. Signalling of HMGB1 through RAGE also activates the Ras/MAP kinase pathway, leading ultimately to activation of the transcription factor NF-κB and its nuclear translocation. Other receptors (green) might also mediate responses to extracellular HMGB1.
However, HMGB1 definitely has one high-affinity receptor: RAGE (receptor for advanced glycation endproducts) (Hori et al., 1995). RAGE belongs to the immunoglobulin superfamily and binds a variety of ligands: the glycated proteins that are present in the serum of diabetic patients (advanced glycation endproducts, AGEs), but also calgranulin, a proinflammatory peptide that derives from the proteolytic processing of the cytoplasmic protein S100, and amyloid β-peptide in Alzheimer patients (reviewed by Schmidt et al., 2000). It is expressed on a wide set of cells, including endothelial cells, smooth muscle cells, mononuclear phagocytes and neurons, and has been implicated in several pathological processes, such as diabetes, amyloidoses and atherosclerosis. HMGB1 binding on the cell surface itself induces the transcriptional upregulation of RAGE (Li et al., 1998).
The knowledge of the signal transduction pathways activated by HMGB1 binding to RAGE is still fragmentary. Extension of neurites requires the small GTPases Cdc42 and Rac, but not the ras-MAP kinase pathway (Huttunen et al., 1999). However, in neural cells, HMGB1 binding to RAGE also activates the Ras-MAP kinase pathway and leads ultimately to the activation of NF-κB, the transcription factor classically linked to inflammatory processes (Huttunen et al., 1999). During tumour invasion the MAP kinases p38MAPK, JNK and p42/p44 MAPK have been shown to be activated by RAGE-HMGB1 (Taguchi et al., 2000). HMGB1-mediated migration of smooth muscle cells also results in activation of the MAP kinase pathway and translocation of phosphorylated ERK 1 and 2 into the nucleus, but also involves a Gi/o protein (Degryse et al., 2001). How and whether these pathways are linked remains an open question; moreover, different pathways may be activated in different cell types. One potentially interesting observation is that cells which respond to extracellular HMGB1 appear to contain very little HMGB1 themselves, and almost none in the nucleus. In support of this observation, we have shown that Hmgb1–/– embryonic fibroblasts respond better to extracellular HMGB1 in cell migration assays than their wild-type counterparts (B.Degryse and M.E.Bianchi, unpublished data).
A unifying hypothesis: spilled chromatin means cell death
Several years ago it was shown that HMGB1 binds tightly to nucleosomes reconstituted from purified DNA and histones (Nightingale et al., 1996; Ura et al., 1996), in a way similar to histone H1. However, differently from histone H1, HMGB1 is not stably associated with the chromatin of cells: it does not appear to be a component of condensed mitotic chromosomes, and is rapidly lost to the surrounding medium when cells are permeabilized with non-ionic detergents (Falciola et al., 1997; Spada et al., 1998). Recently, we showed that HMGB1 is also released in the medium as a soluble molecule by necrotic cells, whereas histones are retained associated to the DNA in the cell remnants (Figure 1; Degryse et al., 2001).
HMGB1 released by mechanically damaged or necrotic cells is indeed capable of binding RAGE and elicit migratory responses in smooth muscle cells (Degryse et al., 2001). We proposed that, following damage, endothelial cells could release the abundant nuclear HMGB1 they contain both in the bloodstream (possibly causing inflammation of nearby endothelia) and towards the intimal layer of the vessel, causing migration and proliferation of smooth muscle cells. This would be consistent with a repair process being elicited by damage. Significantly, intimal thickening also occurs as a process associated with inflammation. Monocyte-derived ‘foam cells’ infiltrate the intima in early steps of atherogenesis: it would be illuminating to know whether they then secrete HMGB1 as a mediator of local inflammation. Necrotic cells accumulate in later stages of atherosclerosis, and they are also expected to release their HMGB1, supporting a continuing process of inflammation and vascular smooth muscle cell stimulation. We note too that the receptor mediating the signalling of the damage, RAGE, also binds glycated proteins, and thereby causes the same response (vessel wall thickening and accelerated atherosclerosis) in diabetic patients (Schmidt et al., 1999).
Vessels are certainly not the only theatres of the HMGB1 signalling action. For example, we speculate that cancer metastasis (Taguchi et al., 2000) could be caused by the release of HMGB1 from necrotic cells in the interior of large tumour masses, and its impingement on RAGE receptors of cancer cells at the periphery of the same masses.
The proinflammatory action of HMGB1 also suggests that dead cells initiate inflammation through the local release of their nuclear content. The interiors of nuclei can only be spilled around when cells have disintegrated, through trauma or lysis. DNA and histones are not solubilized in necrotic cells, at least in early stages, and this leaves HMGB1 as the only candidate for a sufficiently abundant and diffusible component. The use of a chromatin protein as a diffusible signal for cell death might have evolved as a simple mechanism of natural immunity, and could have been elaborated upon by professional inflammatory cells, which have evolved to release HMGB1 without dying.
A ‘chromatin spillage’ signal for cell death is an exceedingly simple and versatile notion, and could be used in a number of different pathological contexts. For example, stem cells could use this cue to start to divide and replace dead tissue. Several issues remain outstanding: for example, why is there no inflammation when HMGB1 is released locally by differentiating cells? Is the binding to glycans involved in inflammation, or in its dampening? At present, Hmgb1 null mutant mice cannot be used to test the chromatin spillage hypothesis, since they are too sick; however, conditional mutants where HMGB1 can be ablated in endothelia or neurons should help clarify the issue considerably. It would also be interesting to know whether only cells equipped with RAGE are able to read out the signal, or whether HMGB1 can activate other receptors and thus function as a general message of cell death.
Acknowledgments
Acknowledgements
The authors are supported by grants from Associazione Italiana Ricerca sul Cancro, CNR Programma Finalizzato Biotecnologie, Ministero dell’Universitá e della Ricerca Scientifica, and the TMR Program of the European Union.
References
- Abraham E., Arcaroli,J., Carmody,A., Wang,H. and Tracey,K.J. (2000) HMG-1 as a mediator of acute lung inflammation. J. Immunol., 165, 2950–2954. [DOI] [PubMed] [Google Scholar]
- Andersson U. et al. (2000) High Mobility Group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med., 192, 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrei C., Dazzi,C., Lotti,L., Torrisi,M.R., Chimini,G. and Rubartelli,A. (1999) The secretory route of the leaderless protein interleukin 1β involves exocytosis of endolysosome-related vesicles. Mol. Biol. Cell, 10, 1463–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M.E. (1988) Interaction of a protein from rat liver nuclei with cruciform DNA. EMBO J., 7, 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M.E. and Beltrame,M. (2000) Upwardly mobile proteins. The role of HMG proteins in chromatin structure, gene expression and neoplasia. EMBO Rep., 1, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M. (1999) Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol. Cell. Biol., 19, 5237–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M. (2001) Revised nomenclature for high mobility group (HMG) chromosomal proteins. Trends Biochem. Sci., 26, 152–153. [DOI] [PubMed] [Google Scholar]
- Calogero S., Grassi,F., Aguzzi,A., Voigtländer,T., Ferrier,P., Ferrari,S. and Bianchi,M.E. (1999) The lack of chromosomal protein HMG1 does not disrupt cell growth, but causes lethal hypoglycaemia in newborn mice. Nature Genet., 22, 276–280. [DOI] [PubMed] [Google Scholar]
- Degryse B., Bonaldi,T., Scaffidi,P., Müller,S., Resnati,M., Sanvito,F., Arrigoni,G. and Bianchi,M.E. (2001) The High Mobility Group Boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J. Cell Biol., 152, 1197–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fages C., Nolo,R., Huttunen,H.J., Eskelinen,E. and Rauvala,H. (2000) Regulation of cell migration by amphoterin. J. Cell Sci., 113, 611–620. [DOI] [PubMed] [Google Scholar]
- Falciola L., Spada,F., Calogero,S., Längst,G., Voit,R., Grummt,I. and Bianchi,M.E. (1997) High mobility group 1 (HMG1) protein is not stably associated with the chromosomes of somatic cells. J. Cell Biol., 137, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori O. et al. (1995) The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. J. Biol. Chem., 270, 25752–25761. [DOI] [PubMed] [Google Scholar]
- Huttunen H., Fages,C. and Rauvala,H. (1999) Receptor for advanced glycation end products (RAGE)-mediated neurite outgrowth and activation of NF-κB require the cytoplasmic domain of the receptor but different downstream signaling pathways. J. Biol. Chem., 274, 19919–19924. [DOI] [PubMed] [Google Scholar]
- Li J., Qu,X. and Schmidt,A.M. (1998) Sp1-binding elements in the promoter of RAGE are essential for amphoterin-mediated gene expression in cultured neuroblastoma cells. J. Biol. Chem., 273, 30870–30878. [DOI] [PubMed] [Google Scholar]
- Mistry A. et al. (1997) Recombinant HMG1 protein produced in Pichia pastoris: a non-viral gene delivery agent. Biotechniques, 22, 718–729. [DOI] [PubMed] [Google Scholar]
- Nightingale K., Dimitrov,S., Reeves,R. and Wolffe,A.P. (1996) Evidence for a shared structural role for HMG1 and linker histones B4 and H1 in organizing chromatin. EMBO J., 15, 548–561. [PMC free article] [PubMed] [Google Scholar]
- Parkkinen J. and Rauvala,H. (1991) Interactions of plasminogen and tissue plasminogen activator (t-PA) with amphoterin. Enhancement of t-PA-catalyzed plasminogen activation by amphoterin. J. Biol. Chem., 266, 16730–16735. [PubMed] [Google Scholar]
- Passalacqua M., Zicca,A., Sparatore,B., Patrone,M., Melloni,E. and Pontremoli,S. (1997) Secretion and binding of HMG1 protein to the external surface of the membrane are required for murine erythroleukemia cell differentiation. FEBS Lett., 400, 275–279. [DOI] [PubMed] [Google Scholar]
- Passalacqua M., Patrone,M., Picotti,G.B., Del Rio,M., Sparatore,B., Melloni,E. and Pontremoli,S. (1998) Stimulated astrocytes release high-mobility group 1 protein, an inducer of LAN-5 neuroblastoma cell differentiation. Neuroscience, 82, 1021–1028. [DOI] [PubMed] [Google Scholar]
- Ronfani R., Ferraguti,M., Croci,L., Ovitt,C.E., Schöler,H.R., Consalez,G.G. and Bianchi,M.E. (2001) Reduced fertility and spermatogenesis defects in mice lacking chromosomal protein Hmgb2. Development, 128, 1265–1273. [DOI] [PubMed] [Google Scholar]
- Rouhiainen A., Imai,S., Rauvala,H. and Parkkinen,J. (2001) Occurrence of amphoterin (HMG1) as an endogenous protein of human platelets that is exported to the cell surface upon platelet activation. Thromb. Haemost., 84, 1087–1094. [PubMed] [Google Scholar]
- Salmivirta M., Rauvala,H., Elenius,K. and Jalkanen,M. (1992) Neurite growth-promoting protein (amphoterin, p30) binds syndecan. Exp. Cell Res., 200, 444–451. [DOI] [PubMed] [Google Scholar]
- Schmidt A.M., Yan,S.D., Wautier,J.L. and Stern,D. (1999) Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ. Res., 84, 489–497. [DOI] [PubMed] [Google Scholar]
- Schmidt A.M., Yan,S.D., Yan,S.F. and Stern,D.M. (2000) The biology of the receptor for advanced glycation end products and its ligands. Biochim. Biophys. Acta, 1498, 99–111. [DOI] [PubMed] [Google Scholar]
- Spada F., Brunet,A., Mercier,Y., Renard,J.-P., Bianchi,M.E. and Thompson,E.M. (1998) High mobility group 1 (HMG1) protein in mouse preimplantation embryos. Mech. Dev., 76, 57–66. [DOI] [PubMed] [Google Scholar]
- Sparatore B., Passalacqua,M., Patrone,M., Melloni,E. and Pontremoli,S. (1996) Extracellular high-mobility group 1 protein is essential for murine erythroleukaemia cell differentiation. Biochem. J., 320, 253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A. et al. (2000) Blockage of RAGE-amphoterin signalling suppresses tumour growth and metastasis. Nature, 405, 354–360. [DOI] [PubMed] [Google Scholar]
- Ura K., Nightingale,K. and Wolffe,A.P. (1996) Differential association of HMG1 and linker histones B4 and H1 with dinucleosomal DNA: structural transitions and transcriptional repression. EMBO J., 15, 4959–4969. [PMC free article] [PubMed] [Google Scholar]
- Vaccari T., Beltrame,M., Ferrari,S. and Bianchi,M.E. (1998) Hmg4, a new member of the Hmg1/2 gene family. Genomics, 49, 247–252. [DOI] [PubMed] [Google Scholar]
- Wang H. et al. (1999a) HMG-1 as a late mediator of endotoxin lethality in mice. Science, 285, 248–251. [DOI] [PubMed] [Google Scholar]
- Wang H., Vishnubhakat,J.M., Bloom,O., Zhang,M., Ombrellino,M., Sama,A. and Tracey,K.J. (1999b) Proinflammatory cytokines (tumor necrosis factor and interleukin 1) stimulate release of high mobility group protein-1 by pituicytes. Surgery, 126, 389–392. [PubMed] [Google Scholar]