Abstract
The non-α-helical C terminus of Xenopus lamin B3 (LB3T) inhibits the polymerization of lamin B3 in vitro and prevents the assembly of nuclei in Xenopus egg interphase extracts. To more precisely define the functions of LB3T in nuclear assembly, we have expressed subdomains of LB3T and determined their effects on nuclear assembly in Xenopus extracts. The results demonstrate that the Ig-fold motif (LB3T-Ig) is sufficient to inhibit lamin polymerization in vitro. Addition of the LB3T-Ig to egg extracts before the introduction of chromatin prevents chromatin decondensation and the assembly of the lamina, membranes, and pore complexes comprising the nuclear envelope. When added to assembled nuclei, LB3T-Ig prevents the further incorporation of lamin B3 into the endogenous lamina and blocks nuclear growth. The introduction of a point mutation in LB3T-Ig (R454W; LB3T-IgRW), known to cause Emery–Dreifuss muscular dystrophy when present in lamin A, does not inhibit lamin polymerization, chromatin decondensation, or nuclear assembly and growth. These results shed light on the specific alterations in lamin functions attributable to a known muscular dystrophy mutation and provide an experimental framework for revealing the effects of other mutations causing a wide range of laminopathies.
Keywords: laminopathies, intermediate filaments, nuclear envelope
Most vertebrate cells express both A- and B-type nuclear lamins (1, 2). Lamins are type V intermediate filament proteins consisting of a short N-terminal “head,” an α-helical central rod, and C-terminal “tail” domains (3, 4). The crystal structures of three small regions within these domains are known. These structures include coils 1A and 2B of the central rod (5) and the Ig-like fold (Ig-fold) present in the tail (6, 7). The α-helical rod domain is required for the formation of coiled-coil lamin dimers, which are the building blocks of higher order lamin structures. The function(s) of the Ig-fold in the nuclear lamins is unknown. In other systems, however, this motif is involved in protein–protein, protein–DNA, and protein–phospholipid interactions (6).
Within nuclei, lamins are found throughout the nucleoplasm and are concentrated in the lamina, which forms an interface between the inner nuclear membrane and chromatin (8, 9). During the cell cycle, the organization of lamins in interphase nuclei is not static. For example, during S-phase, lamins associate with replication factors such as proliferating cell nuclear antigen and replication factor C (10–12), whereas in mitosis, lamins are phosphorylated by the mitotic kinase cdk1 (13), causing their disassembly during nuclear envelope breakdown (14–16). Lamins are subsequently dephosphorylated as they repolymerize around chromatin during nuclear assembly in daughter cells (17, 18). The process of lamin polymerization is initiated in the anaphase–telophase transition during which B-type lamins begin to assemble on chromosomes and continues into early G1 (9).
Insights into lamin functions have been obtained from studies of the cell-free assembly of nuclei in Xenopus egg interphase extracts (19). For example, addition of the non-α-helical C-terminal domain of Xenopus lamin B3 (LB3T) inhibits lamin polymerization, chromatin decondensation, and nuclear membrane and pore assembly (20). Other lamin functions have been implied by the remarkable array of diseases (termed laminopathies) attributed to mutations in the human lamin A gene, LMNA (21–23). To date, >230 LMNA mutations have been reported, producing mainly single amino acid substitutions throughout the protein (see the Leiden Muscular Dystrophy pages at www.dmd.nl/lmna_seqvar.html). These diseases display a wide range of phenotypes, including muscle wasting, limb contracture, dilated cardiomyopathy, nerve-conduction defects, abnormal fat distribution, diabetes, and accelerated aging in children with progeria (22, 23). The laminopathies are mostly heterozygous and autosomal dominant. In rare instances, mutations result in deletions of numerous amino acids. For example, the common form of Hutchinson–Gilford progeria syndrome is caused by a conservative point mutation that generates a splicing abnormality producing a 50-aa deletion near the C terminus of lamin A (LA) (24, 25). In contrast, no lamin B mutations have been reported, suggesting that mutations in lamin B lead to early embryonic lethality.
Cells from laminopathy patients exhibit a number of phenotypic changes. For example, their nuclei are frequently abnormally shaped. This shape change is accompanied by alterations in the distribution of nuclear pores, lamin B, nuclear membrane components, and heterochromatin (26–29). Many of these changes are replicated when human cells are transfected with LMNA cDNAs carrying known mutations (27–31). These morphological alterations are not surprising because it has been shown that the normal assembly and organization of lamins play a major role in determining nuclear shape and structural organization (22, 32). Little is known, however, about the molecular basis of the alterations in lamin assembly states and functions in the numerous laminopathies (33, 34). In light of this lack of knowledge, we have initiated studies to determine the effects of specific mutations on lamin functions in nuclear assembly and organization by using interphase extracts prepared from Xenopus eggs. We have concentrated our efforts on determining the role of the highly conserved C-terminal Ig-fold. This subdomain of ≈100 amino acids contains the sites of >24% of the mutations reported in all of the laminopathies to date (see the Leiden Muscular Dystrophy pages at www.dmd.nl/ and search for LMNA).
Materials and Methods
Nuclear Assembly in Xenopus Egg Interphase Extracts. Interphase egg extracts and demembranated sperm chromatin were prepared as described in refs. 11, 20, and 35. To assemble nuclei, 2 μl of sperm-head chromatin (≈1,500 sperm heads per μl) was combined with 5 μl of nucleoplasmin (36) for 10 min at 22°C. The chromatin was added to interphase extracts containing an ATP-regenerating system (35, 37). In some experiments, the entire C-terminal domain or a subdomain(s) of the C terminus of Xenopus lamin B3 in protein buffer (PB) (300 mM NaCl/20 mM Tris, pH 9.0/1 mM DTT) were added to a final concentration of 10 μM. The addition of these truncated proteins resulted in <10% dilution of the interphase extract. For controls, lamin B3 was added at a final concentration of 10 μM in PB or an equivalent volume of PB.
Bacterial Expression and Purification of Wild-Type and Mutant Nuclear Lamins. Xenopus LMNB3 cDNA was cloned into pCMV-2 containing an N-terminal FLAG epitope (FLAG-LB3; Sigma) and expressed and purified as described in refs. 11 and 20. The non-α-helical LB3T (amino acid residues 383–583) was made from a full-length LMNB3 clone by PCR. The product was inserted into the pGEX4T vector by using EcoRI and NotI. Smaller subfragments derived from LMNB3 were cloned into the pPEP-T vector (38) by using PCR (Fig. 1). The clones were inserted into this vector by using the SalI and EcoRI sites. The proteins expressed by using this vector contained an N-terminal His6 tag followed by 6 kDa of laminin rod (38). The lamin fragments were expressed in NovaBlue DE3 bacteria co-transfected with pLys-S (Novagen) and induced with 1 mM isopropyl β-d-thiogalactoside for 2–4 h. The protein fragments were solubilized from inclusion bodies in column buffer (6 M urea/10 mM Tris·HCl, pH 8.0/10 mM DTT/2 mM EDTA), purified on a Mono Q column by FPLC (Amersham Pharmacia), and eluted in column buffer containing 0–1.0 M NaCl (11). Column fractions containing the lamin fragments were identified by SDS/PAGE and either dialyzed into PB or further purified by using nitrilotriacetic acid-agarose (Qiagen) and then dialyzed into PB. The laminin rod could be removed by thrombin cleavage (38). However, there was no difference in the experimental results described in this paper whether the rod was present or absent. The experiments described in our study were carried out with fragments containing the rod. Purified fragments were stored at -80°C.
Fig. 1.
The Ig-fold subdomain is sufficient to inhibit nuclear assembly. The structure of lamin B3 is shown with red boxes for the coil-coiled central rod domain and blue arrows indicating the β-strands comprising the Ig-fold. Also shown is an expanded view of the C-terminal tail domain with some amino acid positions indicated. The numbers on the bars of the graph refer to the amino acid residues of lamin B3 fragments added to interphase Xenopus extracts. The nuclear area was determined from the midsection of nuclei by using Zeiss lsm software (n = 100), as described in Materials and Methods. The gray bars indicate the subdomains that inhibited nuclear assembly.
Protein modeling was carried out by using deepview v.3.7 (http://swissmodel.expasy.org/spdbv) provided by the Swiss Institute of Bioinformatics (Basel).
Site-Directed Mutagenesis of LB3T Subfragments. LB3T and LB3T-Ig (Fig. 1) were mutagenized by using the Stratagene QuikChange mutagenesis kit to convert Arg-454 to Trp. GIBCO/BRL synthesized the complimentary oligonucleotide primers to the lamin B3 sequence, which contained a single point mutation shown in bold (5′-GGGAACTATGTCTGGCTGTTGAACACA and 5′-TGTGTTCAACAGCCAGACATAGTTCCC). After mutagenesis, the mutant sequence was confirmed by DNA sequencing. These constructs were termed LB3TRW and LB3T-IgRW.
Nuclear Growth and Transport Assays. To determine the effects of lamin fragments on assembled nuclei, we used a modification of the procedure developed by Coverley et al. (39). Specifically, nuclei were assembled in 100 μl of interphase extract for 45 min and then diluted with 500 μl of nuclear wash buffer (NWB) (250 mM sucrose/50 mM KCl/2.5 mM MgCl2/12.5 mM Hepes, pH 7.4/1 mM DTT), containing 0.1% Nonidet P-40 and the lamin fragment or PB as a control, for 10 min at 22°C. Under these conditions, the lamin fragment LB3T or LB3T-IgRW was loaded into the permeabilized nuclei. The “loaded” nuclei were diluted with an additional 500 μl of NWB and then pelleted for 10 min at 1,500 × g in a swinging bucket rotor. The supernatants were carefully removed, and the nuclei were suspended in 30 μl of normal interphase extract to reseal their envelopes, incubated for 60 min (22°C), and subsequently prepared for immunoblotting or immunofluorescence. In some experiments, lamin incorporation into permeabilized nuclei was assayed by suspending nuclei in fresh, detergent-free extract containing FLAG-LB3 at a concentration of 60 μM. Nuclear growth after the Nonidet P-40-permeablization procedure was assayed by microscopy.
Nuclear transport was assayed by the addition of GST-nuclear localization signal (NLS)-GFP to Nonidet P-40-treated nuclei 60 min after resuspension in interphase extract. The vector encoding this transport substrate was prepared by cutting pGEX4T-1 (Amersham Pharmacia) with NotI and ligating in a double-stranded oligonucleotide encoding a HindIII site. The EGFP-coding region and multiple cloning site were cut from pEGFP (Clontech) with HindIII and NotI and then ligated to the pGEX4TH cut with the same enzymes. The resulting vector, pGEX4TH-EGFP, was digested with XhoI and SphI, and the double-stranded oligonucleotide, with XhoI and SphI overhangs encoding the simian virus 40 NLS, was inserted. The resulting vector was verified by DNA sequencing. The transport substrate protein was expressed in BL21(DE3) cells by induction with 1 mM isopropyl β-d-thiogalactoside. The fusion protein was purified by glutathione-Sepharose affinity chromatography, dialyzed into 20 mM Hepes (pH 7.3), 110 mM potassium acetate, and 2 mM MgCl2, and stored at -80°C. The integrity of the nuclear membrane in resealed nuclei was also assessed by exclusion of TRITC-labeled 70-kDa dextran (Molecular Probes) (11).
Immunofluorescence. Nuclei were suspended in NWB, fixed with either 2% ethylene glycol bis(succinimidylsuccinate) (Pierce) or 2.0% formaldehyde for 10 min, and spun onto poly(l-lysine)-coated coverslips through 20% sucrose in NWB, as described in ref. 11. The coverslips were washed with PBS containing 0.1% Nonidet P-40, washed again with PBS, and overlayed with primary Abs diluted 1:200 (11). These Abs included mAbs directed against Xenopus lamin B3 [L6-5D5 provided by Reimer Stick (Institute for Cell Biology, University of Bremen, Bremen, Germany); ref. 40], His6 tags (Qiagen), nucleoporins (mAb 414; Covance, Princeton), and a pAb directed against the FLAG tag (Zymed Laboratories). Secondary Abs used were species-specific FITC-labeled, lissamine rhodamine-labeled, and Alexa Fluor 568-labeled IgGs (Jackson ImmunoResearch and Molecular Probes). Nuclear membranes were stained with DiOC6 (2.5 mg/ml) (41), and DNA was stained with 1 μM TOTO-3 iodide (Molecular Probes). Nuclei were examined with either an Axioplan or an LSM 510 confocal microscope (Zeiss) (20). Midsection areas of nuclei were determined with the LSM 510 “measure area” function (20). The midsections of LB3T-Ig-blocked nuclei were identified by examination through focus-stacked images. In each experiment, the number of nuclei measured was between 50 and 100.
Gel Electrophoresis and Immunoblotting. Permeabilized, resealed nuclei, which had been loaded with C-terminal fragments of lamin B3 were returned to interphase extracts for an additional 60 min. Aliquots (30 μl) of these preparations were centrifuged through 20% sucrose in NWB for 5 min at 3,000 × g. The pellets were suspended in Laemmli sample buffer (42), and the proteins were separated by SDS/PAGE and transferred to nitrocellulose. Primary Abs were used at either 1:1,000 or 1:5,000 dilution, and secondary Abs conjugated to horseradish peroxidase were used at a dilution of 1:5,000 (Molecular Probes) and detected by enhanced chemiluminescence (Amersham Pharmacia) on Hyperfilm (Amersham Pharmacia).
In Vitro Lamin Polymerization Assays. Lamin polymerization was assayed as described in ref. 43. Specifically, bacterially expressed and purified lamin B3, LB3T-Ig, or LB3T-IgRW was dissolved in 400 μl to a concentration of 600 nM in lamin assembly buffer (LAB) (100 mM NaCl/1 mM DTT/25 mM Mes, pH 6.6) and maintained at 22°C for 30 min. In addition, lamin B3 was mixed and incubated with LB3T-Ig or LB3T-IgRW at a molar ratio of 1:3 and 1:5, respectively, under identical conditions. These preparations were centrifuged at 20,000 × g for 20 min in a TLS55 rotor (Beckman). The pellets were washed three times with 1 ml of LAB, and the supernatants and pellets were analyzed by SDS/PAGE. Assembled polymers were also assayed by negative staining with 1% uranyl acetate and viewed with a JEOL 1200EX, as described in ref. 44.
Results
LB3T-Ig Inhibits Nuclear Assembly. By using the Xenopus egg interphase extract, it is possible to examine the steps in nuclear assembly and growth after the addition of sperm chromatin (19). In this system, a nuclear envelope completely assembles around decondensing chromatin in ≈40 min, and the nuclei continue to grow in size for up to 4 h (19, 35, 45, 46). We have shown (20) that nuclear envelope assembly was inhibited when LB3T (residues 383–583) was added to extracts before the introduction of chromatin (Fig. 1). To determine the importance of the Ig-fold and other subdomains in this inhibitory process, fragments of LB3T (Fig. 1) were added to extracts before the introduction of chromatin. Nuclear assembly and growth were assayed by immunofluorescence 90 min after adding chromatin. The addition of LB3T-Ig inhibited chromatin decondensation, nuclear assembly, and growth in a fashion indistinguishable from the inhibitory effects of LB3T (Figs. 1 and 2 A–C). The size of these chromatin masses was ≈20% of nuclei assembled in normal extracts (Fig. 1; compare Fig. 2 A and M). A few small, discontinuous patches of membranes, lamins, and nucleoporins were associated with the chromatin (Fig. 2 B and C and data not shown). Another fragment containing an intact Ig-fold, LB3T-2 (Fig. 1; residues 433–583), also inhibited the assembly of nuclei in a fashion indistinguishable from LB3T-Ig (see Figs. 1 and 2 A–C and data not shown).
Fig. 2.
The Ig-fold inhibits nuclear membrane and lamina assembly and chromatin decondensation. Sperm chromatin was incubated in extracts containing LB3T-Ig (A–C), LB3T-58A (D–F), LB3T-58B (G–I), LB3T-52 (J–L), lamin B3 (M–O), and LB3-IgRW (P–R) for 90 min (see Materials and Methods). The resulting preparations were stained with the DNA dye TOTO (A, D, G, J, M, and P), the lipophilic dye DiOC6 (B, E, H, K, N, and Q), and a mAb directed against lamin B3 (C, F, I, L, O, and R). Chromatin in extracts containing LB3T-Ig (10 μM) remained highly condensed (A), surrounded by small patches of lamins and membranes (B and C). However, chromatin incubated in extracts containing LB3T-58A, LB3T-58B, LB3T-52, lamin B3, or LB3T-IgRW (all 10 μM) were decondensed (D, G, J, M, and P) and surrounded by rims of lamin and membrane fluorescence (E, F, H, I, K, L, N, O, Q, and R). All images were captured from confocal sections taken through the midregions of nuclei. (Scale bar, 10 μm.)
On the basis of these results, we designed additional LB3T fragments to test whether an intact Ig-fold was required to inhibit nuclear assembly. To this end, we prepared the fragments LB3T-58A (the N-terminal portion of the Ig-fold; residues 433–490), LB3T-58B (the C-terminal region of the Ig-fold; residues 483–540), LB3T-52 (the midregion of the Ig-fold; residues 458–509), and LB3T-3 (the Ig-fold C-terminal region to the C terminus of lamin B3; residues 483–583). None of these fragments blocked nuclear assembly, suggesting that an intact Ig-fold is required (Figs. 1 and 2 D–L and data not shown). In addition, the C-terminal 50-aa LB3T-4 (residues 533–583) had no effect on nuclear assembly or growth (Fig. 1). A combination of LB3T-58A and LB3T-58B (10 μM each) also had no effect on nuclear assembly, demonstrating that an intact Ig-fold is required to inhibit nuclear assembly (data not shown).
A Point Mutation Causing Muscular Dystrophy Blocks the Inhibitory Effects of LB3T. We determined the effects of a point mutation, R454W, in LB3T-Ig (LB3T-IgRW) on nuclear assembly. This site was chosen because it is one of the most highly conserved sites within lamin Ig-folds across invertebrate and vertebrate species [from hydra to human (47)] and corresponds to the human LA R453W mutant, which causes EDMD (48). This mutation removes a salt bridge between two β-strands, thereby destabilizing the structure of the Ig-fold (7). When LB3TRW and LB3T-IgRW were added to interphase extracts in a final concentration of 10 μM, nuclear lamins, membranes, and pores assembled around decondensed chromatin and the nuclei seemed normal at 90 min after the addition of chromatin (Fig. 2 P–R and data not shown). These results demonstrate the importance of the structural integrity of the Ig-fold in lamin and nuclear assembly.
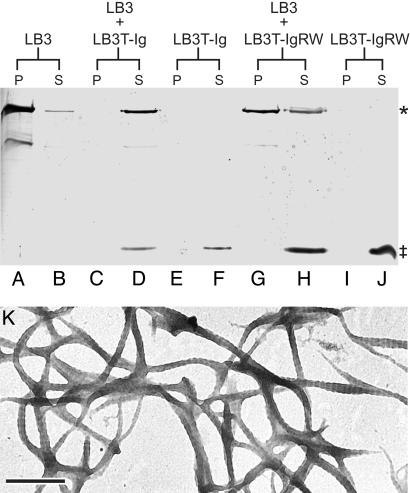
LB3T-Ig Blocks Lamin Polymerization in Vitro. To determine whether the effects of LB3T-Ig on nuclear assembly were related to the inhibition of lamin assembly, we carried out in vitro polymerization assays. In controls, lamin B3 polymerized into typical paracrystalline arrays (Fig. 3K), which were pelleted after centrifugation at 20,000 × g for 20 min (Fig. 3 A and B). The addition of a 3-fold molar excess of LB3T-Ig to lamin B3 in LAB rendered the lamin B3 nonpelletable, and no paracrystalline arrays could be detected by negative staining (Fig. 3 C and D and data not shown). In contrast, the addition of a 5-fold molar excess of LB3T-IgRW had only a slight effect on lamin B3 polymerization (Fig. 3 G and H) and paracrystalline arrays assembled (data not shown). Under identical assembly conditions, LB3T-Ig or LB3T-IgRW alone remained soluble after centrifugation (Fig. 3 E, F, I, and J).
Fig. 3.
The Ig-fold inhibits lamin polymerization in vitro. Purified lamin B3 (A and B), LB3T-Ig (E and F), or LB3T-IgRW (I and J) were diluted to a final concentration of 0.6 μM in LAB at 22°C. After 30 min, each of these preparations were centrifuged at 20,000 × g for 20 min. The supernatants and pellets were analyzed by SDS/PAGE. Under these conditions, ≈95% of the lamin B3 was detected in the pellet (A and B), whereas LB3T-Ig (E and F) and LB3T-IgRW (I and J) remained in the supernatant. When lamin B3 was mixed with LB3T-Ig (1:3 molar ratio; C and D), however, the majority of lamin B3 remained in the supernatant (C and D). In the presence of LB3T-IgRW (1:5 molar ratio), the majority of lamin B3 was found in the pellet fraction (G and H). Purified lamin B3 at a concentration of 0.6 μMin LAB was negatively stained with 1% uranyl acetate (K). The spacing of the paracrystal repeats is ≈24 nm. *, lamin B3; ‡, Ig-fold protein. (Scale bar, 250 nm.)
LB3T-Ig Inhibits the Growth of Assembled Nuclei. The finding that LB3T-Ig inhibited both lamin polymerization and nuclear assembly provided an opportunity to determine whether nuclear growth required the continuous assembly of lamins. Because the LB3T-Ig domain does not contain an NLS, we incorporated LB3T-Ig into assembled nuclei by transiently permeabilizing their nuclear membranes with Nonidet P-40 (see Materials and Methods). Nuclei were assembled for 45 min, loaded with 10 μM LB3T-Ig in the presence of 0.1% Nonidet P-40 in NWB for 10 min, washed with detergent-free NWB, and suspended in fresh interphase extract for 60 min. Identical experiments were carried out by using PB alone or PB containing LB3T or LB3T-IgRW. To make certain that nuclei had resealed at the end of this procedure, 70-kDa TRITC-labeled dextran beads were added to the extracts. The dextran did not enter nuclei showing that their nuclear envelopes had resealed. Furthermore, GFP-coupled simian virus 40 NLS added to these preparations accumulated normally within nuclei, demonstrating that their transport properties were normal (Materials and Methods and data not shown).
To determine whether nuclei containing LB3T, LB3T-Ig, and LB3T-IgRW were capable of continued growth, nuclei were transferred to fresh extract for an additional 60 min after resealing. After processing for immunofluorescence, their cross-sectional areas were measured and compared with controls (Fig. 4). The midplane area in control nuclei was 100 ± 15.0 μm2 immediately after resealing, and 60 min later, the nuclei had grown to 194 ± 27.6 μm2. The LB3T fragment significantly inhibited nuclear growth because the average increase in cross-sectional area was only 12% (112 ± 27.1 μm2), whereas LB3T-Ig resulted in an increase of only 2% (102 ± 26.5 μm2). In contrast, LB3T-IgRW-loaded nuclei showed an increase of 82% (182 ± 33.2 μm2), which was similar to controls. We also determined whether LB3T, LB3T-Ig, and LB3T-IgRW were retained in Nonidet P-40-treated nuclei using an antibody directed against their His-tags. The fluorescence patterns for each of these fragments were indistinguishable because each fragment was concentrated in the lamina and distributed in lesser amounts throughout the nucleoplasm (Fig. 5A and data not shown). Control nuclei treated with either PB or lamin fragments without a His-tag were not fluorescent (data not shown).
Fig. 4.
Nuclei were assembled in Xenopus interphase extract for 45 min. The resulting nuclei were treated with 0.1% Nonidet P-40 in NWB containing lamin B3, His-LB3T-Ig, or His-LB3T-IgRW for 10 min (see Materials and Methods). The nuclei were then washed in NWB, centrifuged, suspended in fresh interphase extract to reseal their membranes, and grown for an additional 60 min (see Materials and Methods). Under these conditions, the average increase in nuclear area was >90% relative to their size at 45 min after nuclear assembly was initiated. Nuclei exposed to LB3T increased in size by only ≈12%, whereas nuclei treated with LB3T-Ig grew ≈2%. In contrast, the LB3T-IgRW-treated nuclei grew ≈82% (n = 50).
Fig. 5.
Nuclei were assembled for 45 min, permeabilized with 0.1% Nonidet P-40 in the presence of either His-LB3T-Ig or His-LB3T-IgRW, and transferred to fresh interphase extract for 60 min (see Materials and Methods). Nuclei loaded with His-LB3T-Ig and stained with a mAb directed against His indicate that LB3T-Ig is incorporated into the nuclear lamina and nucleoplasm (A). In some cases, permeabilized nuclei loaded with either LB3T-Ig or LB3T-IgRW were resuspended in fresh interphase extract containing FLAG-LB3 and grown for an additional 60 min. Immunoblot analyses of these nuclei showed that LB3T-Ig significantly reduced the incorporation of FLAG-LB3 when compared with nuclei loaded with LB3T-RW (B). Preparations of these nuclei were also fixed and stained with TOTO (C and F), a mAb directed against lamin B3 (D and G), and a pAb directed against the FLAG epitope (E and H). Lamin B3 was present in nuclei treated with LB3T-Ig (D), but these nuclei did not incorporate FLAG-LB3 (E) into the lamina, whereas nuclei treated with LB3T-IgRW incorporated FLAG-LB3 (H) into the lamina. (Scale bars, 10 μm.)
Because LB3T-Ig prevented nuclear assembly and lamin polymerization, we asked whether continued nuclear growth also required new lamin assembly. Nuclei containing LB3T-Ig were washed, suspended in fresh extract containing FLAG-LB3 for 60 min, and then prepared either for immunofluorescence or immunoblotting. These nuclei did not incorporate FLAG-LB3 (Fig. 5 C–E) either into the lamina or nucleoplasm. Nuclei exposed to LB3T-IgRW, however, did incorporate lamin B3 both in the lamina region and throughout the nucleoplasm (Fig. 5 F–H). Immunoblotting analyses of nuclei treated in this fashion revealed dramatically less FLAG-LB3 in the LB3T-Ig-treated preparations compared with LB3T-IgRW-treated nuclei (Fig. 5 B and C). These results confirm the importance of both the structural integrity of the Ig-fold subdomain and lamin polymerization in nuclear growth.
Discussion
Nuclear lamins play important roles in nuclear assembly, organization, and shape (49, 50). Therefore, it is not surprising that cells from patients with laminopathies contain misshapen nuclei frequently accompanied by changes in the organization of nuclear membranes, pore complexes, and chromatin (29). To gain insight into the causes of these nuclear abnormalities, we focused our attention on the role of the highly conserved Ig-fold subdomain in lamin and nuclear assembly. Mutations in the Ig-fold of LA cause EDMD (11 sites), familial partial lipodystrophy (8 sites), dilated cardiomyopathy (4 sites), limb girdle muscular dystrophy (3 sites), mandibuloacral dysplasia (1 site), and Hutchinson–Gilford progeria syndrome (3 sites). In general, Ig-fold motifs are involved in protein–protein interactions (6). Therefore, we sought to determine whether the lamin B3 Ig-fold plays a role in lamin–lamin interactions during nuclear assembly in Xenopus egg extracts and whether a point mutation known to cause EDMD (LB3T-IgRW; ref. 48) modifies the properties of the Ig-fold. Note that the sequence and predicted structure of the human LA and Xenopus lamin B3 Ig-folds are highly similar, with a predicted root-mean-square deviation for the backbone atoms of 0.13 Å (http://swissmodel.expasy.org/spdbv).
When LB3T-Ig was added to interphase extracts before the addition of chromatin, both lamin and nuclear assembly were blocked. These results suggested that the Ig-fold inhibited the formation of the higher order structures formed during lamin polymerization. In support of this hypothesis, LB3T-Ig prevented the polymerization of lamin B3 into pelletable paracrystalline arrays. In contrast, LB3T-IgRW did not significantly inhibit lamin B3 polymerization in vitro and had no effect on nuclear assembly when added to interphase extracts. These results demonstrate that the Ig-fold inhibits the lamin–lamin interactions required for lamin polymerization and the formation of nuclei. The data also indicate that the Ig-fold can bind to lamins both within the nuclear lamina and nucleoplasm, thereby preventing the further incorporation of lamin B3 into the nuclear lamin network. This phenomenon is supported by the findings that FLAG-LB3 does not become integrated into the endogenous lamin network after the binding of the Ig-fold, and that under these conditions, there is a cessation of nuclear growth. These experimental findings suggest that the continuous integration of lamin subunits into lamin networks is necessary for nuclear growth. LB3T-IgRW also associates with the lamin network in assembled nuclei. LB3T-IgRW interacts weakly with lamin B3 in vitro, however, resulting only in slight effects on both the incorporation of lamin B3 into the lamina and nuclear growth. In light of these data, it is apparent that the Ig-fold plays an important and essential role in the lamin–lamin interactions that are required for lamin polymerization, as well as nuclear assembly and growth. Furthermore, the R454W mutation in LB3T-Ig removes a salt bridge, which destabilizes the Ig-fold (7), indicating that the structure of the Ig-fold is important for lamin assembly and function. The Ig-fold motif is known to be involved in protein–protein, protein–nucleic acid, and protein–phospholipid interactions. Therefore, this lamin subdomain possibly is involved in interactions between lamins, the inner nuclear membrane, integral membrane proteins such as emerin, lamin-associated proteins, or chromatin (22).
It has been shown that the structural organization of nuclear lamins is required for essential cellular functions. For example, when lamin organization is disrupted with a dominant-negative mutant consisting of N-terminally deleted LA, both DNA replication and transcription are inhibited (10, 51). Under these disruption conditions, the lamins are reorganized from their normal locations within the nucleoplasm and the lamina into large foci distributed throughout the nucleus. Interestingly, factors known to be involved in DNA replication, such as proliferating cell nuclear antigen and replication factor C, and Pol II transcription factors, such as TATA box-binding protein, are localized in the lamin foci (11, 51). In addition, the spliceosome distribution in these lamin-disrupted nuclei is significantly altered (51). These findings and the results of our present study suggest that lamins probably provide an infrastructure required for the assembly and growth of the nucleus, as well as the assembly and function of the molecular machines involved in transcription and DNA replication.
The single amino acid substitution in the Ig-fold R454W (LB3T-IgRW) negates the inhibitory effect of this subdomain on lamin polymerization and nuclear assembly. This effect suggests that the structural integrity of the Ig-fold is critically important for the formation of the higher order structures of lamins required for the assembly, organization, and normal functioning of nuclei. The conserved R453W mutation (R454W in Xenopus) is known to cause EDMD. As demonstrated by the in vitro polymerization and nuclear assembly assays, when this mutation is introduced into LB3T-Ig (LB3T-IgRW), it seems to bind to polymerized lamin B3 both in the lamina and the nucleoplasm; yet, this mutation does not block either the further incorporation of lamins or nuclear growth (Figs. 3 G and H and 5A). These results suggest that LB3T-IgRW binds to lamins with a lower affinity than the wild-type Ig-fold and may ultimately lead to an understanding of the clinical phenotypes presented in patients with EDMD. All known EDMD patients with the R453W mutation are heterozygous; however, the amount of mutant protein expressed relative to wild-type LA in these patients remains unknown. Furthermore, the time of onset of EDMD is variable, presenting sometime during the first decade of life (52). Although the mechanisms regulating the time of onset are not understood, the early indications of muscle wasting could be related to the accumulation of mutant protein to a level necessary to act in a dominant-negative fashion. In support of this theory, it has been shown that cells from patients with another laminopathy, Hutchinson–Gilford progeria syndrome, accumulate mutant lamin as cells age in culture (29). Although the LA R453W muscular dystrophy mutation results in the loss of a salt-bridge between β-strands in the Ig-fold, there is very little change in its overall structure (swiss pdb viewer v.3.7, www.expasy.org). Therefore, this subtle change in the stability of the Ig-fold (7) could possibly affect the structure and function of normal lamin polymers in an age-related, concentration-dependent manner.
Acknowledgments
This work was supported by National Cancer Institute Grant CA31760 and Muscular Dystrophy Association Grant MDA3604. R.I.L.-S. was supported by National Institute of General Medical Sciences Grant 5F31 GM20083-03. D.K.S. was supported by National Institutes of Health Grant T32 CA80621.
Author contributions: D.K.S., R.I.L.-S., and R.D.G. designed research; D.K.S. and R.I.L.-S. performed research; S.A.A., H.H., R.D.M., and T.P.S. contributed new reagents/analytic tools; D.K.S., R.I.L.-S., and R.D.G. analyzed data; and D.K.S., R.I.L.-S., and R.D.G. wrote the paper.
Abbreviations: LB3T, C-terminal domain of Xenopus lamin B3; LA, lamin A; PB, protein buffer; FLAG-LB3, FLAG-tagged lamin B3; LB3T-Ig, lamin B3 Ig-fold; LB3T-IgRW, lamin B3 with point mutation R454W; NWB, nuclear wash buffer; LAB, lamin assembly buffer; EDMD, Emery–Dreifuss muscular dystrophy; NLS, nuclear localization signal.
References
- 1.Riemer, D., Wang, J., Zimek, A., Swalla, B. J. & Weber, K. (2000) Gene 255, 317-325. [DOI] [PubMed] [Google Scholar]
- 2.Karabinos, A., Zimek, A. & Weber, K. (2004) Gene 326, 123-129. [DOI] [PubMed] [Google Scholar]
- 3.Gruenbaum, Y., Margalit, A., Goldman, R. D., Shumaker, D. K. & Wilson, K. L. (2005) Nat. Rev. Mol. Cell Biol. 6, 21-31. [DOI] [PubMed] [Google Scholar]
- 4.Hesse, M., Magin, T. M. & Weber, K. (2001) J. Cell Sci. 114, 2569-2575. [DOI] [PubMed] [Google Scholar]
- 5.Strelkov, S. V., Schumacher, J., Burkhard, P., Aebi, U. & Herrmann, H. (2004) J. Mol. Biol. 343, 1067-1080. [DOI] [PubMed] [Google Scholar]
- 6.Dhe-Paganon, S., Werner, E. D., Chi, Y. I. & Shoelson, S. E. (2002) J. Biol. Chem. 277, 17381-17384. [DOI] [PubMed] [Google Scholar]
- 7.Krimm, I., Ostlund, C., Gilquin, B., Couprie, J., Hossenlopp, P., Mornon, J. P., Bonne, G., Courvalin, J. C., Worman, H. J. & Zinn-Justin, S. (2002) Structure (Cambridge, MA) 10, 811-823. [DOI] [PubMed] [Google Scholar]
- 8.Fawcett, D. W. (1966) Am. J. Anat. 119, 129-145. [DOI] [PubMed] [Google Scholar]
- 9.Moir, R. D., Yoon, M., Khuon, S. & Goldman, R. D. (2000) J. Cell Biol. 151, 1155-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moir, R. D., Spann, T. P., Herrmann, H. & Goldman, R. D. (2000) J. Cell Biol. 149, 1179-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spann, T. P., Moir, R. D., Goldman, A. E., Stick, R. & Goldman, R. D. (1997) J. Cell Biol. 136, 1201-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy, B. K., Barbie, D. A., Classon, M., Dyson, N. & Harlow, E. (2000) Genes Dev. 14, 2855-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dessev, G., Iovcheva-Dessev, C., Bischoff, J. R., Beach, D. & Goldman, R. (1991) J. Cell Biol. 112, 523-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heald, R. & McKeon, F. (1990) Cell 61, 579-589. [DOI] [PubMed] [Google Scholar]
- 15.Haas, M. & Jost, E. (1993) Eur. J. Cell Biol. 62, 237-247. [PubMed] [Google Scholar]
- 16.Dessev, G. & Goldman, R. (1988) Dev. Biol. 130, 543-550. [DOI] [PubMed] [Google Scholar]
- 17.Glass, J. R. & Gerace, L. (1990) J. Cell Biol. 111, 1047-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson, L. J., Bollen, M. & Fields, A. P. (1997) J. Biol. Chem. 272, 29693-29697. [DOI] [PubMed] [Google Scholar]
- 19.Wiese, C., Goldberg, M. W., Allen, T. D. & Wilson, K. L. (1997) J. Cell Sci. 110, 1489-1502. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Soler, R. I., Moir, R. D., Spann, T. P., Stick, R. & Goldman, R. D. (2001) J. Cell Biol. 154, 61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonne, G., Mercuri, E., Muchir, A., Urtizberea, A., Becane, H. M., Recan, D., Merlini, L., Wehnert, M., Boor, R., Reuner, U., et al. (2000) Ann. Neurol. 48, 170-180. [PubMed] [Google Scholar]
- 22.Goldman, R. D., Gruenbaum, Y., Moir, R. D., Shumaker, D. K. & Spann, T. P. (2002) Genes Dev. 16, 533-547. [DOI] [PubMed] [Google Scholar]
- 23.Worman, H. J. & Courvalin, J. C. (2004) J. Clin. Invest. 113, 349-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eriksson, M., Brown, W. T., Gordon, L. B., Glynn, M. W., Singer, J., Scott, L., Erdos, M. R., Robbins, C. M., Moses, T. Y., Berglund, P., et al. (2003) Nature 423, 293-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Sandre-Giovannoli, A., Bernard, R., Cau, P., Navarro, C., Amiel, J., Boccaccio, I., Lyonnet, S., Stewart, C. L., Munnich, A., Le Merrer, M., et al. (2003) Science 300, 2055. [DOI] [PubMed] [Google Scholar]
- 26.Favreau, C., Dubosclard, E., Ostlund, C., Vigouroux, C., Capeau, J., Wehnert, M., Higuet, D., Worman, H. J., Courvalin, J. C. & Buendia, B. (2003) Exp. Cell Res. 282, 14-23. [DOI] [PubMed] [Google Scholar]
- 27.Vigouroux, C., Auclair, M., Dubosclard, E., Pouchelet, M., Capeau, J., Courvalin, J. C. & Buendia, B. (2001) J. Cell Sci. 114, 4459-4468. [DOI] [PubMed] [Google Scholar]
- 28.Raharjo, W. H., Enarson, P., Sullivan, T., Stewart, C. L. & Burke, B. (2001) J. Cell Sci. 114, 4447-4457. [DOI] [PubMed] [Google Scholar]
- 29.Goldman, R. D., Shumaker, D. K., Erdos, M. R., Eriksson, M., Goldman, A. E., Gordon, L. B., Gruenbaum, Y., Khuon, S., Mendez, M., Varga, R., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 8963-8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muchir, A., van Engelen, B. G., Lammens, M., Mislow, J. M., McNally, E., Schwartz, K. & Bonne, G. (2003) Exp. Cell Res. 291, 352-362. [DOI] [PubMed] [Google Scholar]
- 31.Östlund, C., Bonne, G., Schwartz, K. & Worman, H. J. (2001) J. Cell Sci. 114, 4435-4445. [DOI] [PubMed] [Google Scholar]
- 32.Gruenbaum, Y., Goldman, R. D., Meyuhas, R., Mills, E., Margalit, A., Fridkin, A., Dayani, Y., Prokocimer, M. & Enosh, A. (2003) Int. Rev. Cytol. 226, 1-62. [DOI] [PubMed] [Google Scholar]
- 33.Goebel, H. H. & Warlo, I. (2000) Mol. Genet. Metab. 71, 267-275. [DOI] [PubMed] [Google Scholar]
- 34.Burke, B., Mounkes, L. C. & Stewart, C. L. (2001) Traffic (Oxford) 2, 675-683. [DOI] [PubMed] [Google Scholar]
- 35.Newmeyer, D. D. & Wilson, K. L. (1991) Methods Cell Biol. 36, 607-634. [DOI] [PubMed] [Google Scholar]
- 36.Philpott, A., Leno, G. H. & Laskey, R. A. (1991) Cell 65, 569-578. [DOI] [PubMed] [Google Scholar]
- 37.Murray, A. W. (1991) Methods Cell Biol. 36, 581-605. [PubMed] [Google Scholar]
- 38.Brandenberger, R., Kammerer, R. A., Engel, J. & Chiquet, M. (1996) J. Cell Biol. 135, 1583-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coverley, D., Downes, C. S., Romanowski, P. & Laskey, R. A. (1993) J. Cell Biol. 122, 985-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stick, R. (1988) EMBO J. 7, 3189-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melchior, F., Guan, T., Yokoyama, N., Nishimoto, T. & Gerace, L. (1995) J. Cell Biol. 131, 571-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laemmli, U. K. (1970) Nature 227, 680-685. [DOI] [PubMed] [Google Scholar]
- 43.Moir, R. D., Donaldson, A. D. & Stewart, M. (1991) J. Cell Sci. 99, 363-372. [DOI] [PubMed] [Google Scholar]
- 44.Zackroff, R. V. & Goldman, R. D. (1979) Proc. Natl. Acad. Sci. USA 76, 6226-6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lohka, M. J. & Masui, Y. (1984) J. Cell Biol. 98, 1222-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newport, J. (1987) Cell 48, 205-217. [DOI] [PubMed] [Google Scholar]
- 47.Erber, A., Riemer, D., Hofemeister, H., Bovenschulte, M., Stick, R., Panopoulou, G., Lehrach, H. & Weber, K. (1999) J. Mol. Evol. 49, 260-271. [DOI] [PubMed] [Google Scholar]
- 48.Bonne, G., Di Barletta, M. R., Varnous, S., Becane, H. M., Hammouda, E. H., Merlini, L., Muntoni, F., Greenberg, C. R., Gary, F., Urtizberea, J. A., et al. (1999) Nat. Genet. 21, 285-288. [DOI] [PubMed] [Google Scholar]
- 49.Shumaker, D. K., Kuczmarski, E. R. & Goldman, R. D. (2003) Curr. Opin. Cell Biol. 15, 358-366. [DOI] [PubMed] [Google Scholar]
- 50.Holaska, J. M., Wilson, K. L. & Mansharamani, M. (2002) Curr. Opin. Cell Biol. 14, 357-364. [DOI] [PubMed] [Google Scholar]
- 51.Spann, T. P., Goldman, A. E., Wang, C., Huang, S. & Goldman, R. D. (2002) J. Cell Biol. 156, 603-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonne, G., Capeau, J., De Visser, M., Duboc, D., Merlini, L., Morris, G. E., Muntoni, F., Recan, D., Sewry, C., Squarzoni, S., et al. (2002) Neuromuscul. Disord. 12, 187-194. [DOI] [PubMed] [Google Scholar]