Abstract
Transgenic crops producing Bacillus thuringiensis (Bt) toxins kill some key insect pests and thus can reduce reliance on insecticides. Widespread planting of such Bt crops increased concerns that their usefulness would be cut short by rapid evolution of resistance to Bt toxins by pests. Pink bollworm (Pectinophora gossypiella) is a major pest that has experienced intense selection for resistance to Bt cotton in Arizona since 1997. We monitored pink bollworm resistance to Bt toxin for 8 years with laboratory bioassays of strains derived annually from 10-17 cotton fields statewide. Bioassay results show no net increase from 1997 to 2004 in the mean frequency of pink bollworm resistance to Bt toxin. A synthesis of experimental and modeling results suggests that this delay in resistance can be explained by refuges of cotton without Bt toxin, recessive inheritance of resistance, incomplete resistance, and fitness costs associated with resistance.
Keywords: Bacillus thuringiensis, resistance management, transgenic crops
To reduce reliance on insecticides, cotton and corn have been genetically engineered to produce proteins from Bacillus thuringiensis (Bt) that kill some key insect pests. First grown on a large scale in 1996, transgenic Bt crops covered >20 million hectares worldwide in 2004 (1). Rapid evolution of resistance by target pests is considered the primary threat to their continued success (2-9). In particular, worst-case scenarios yielded predictions that pests would evolve resistance to Bt crops in as little as 3 years (7-9). Although field-evolved resistance to Bt crops has not yet been documented, resistance to Bt toxins has evolved in laboratory-selected strains of many insects (2, 5, 6). Furthermore, resistance to Bt sprays has evolved in greenhouse populations of cabbage looper (Trichoplusia ni) and in field populations of diamondback moth (Plutella xylostella) (2, 5, 6, 10).
Although several approaches have been proposed for delaying pest resistance to Bt crops, the refuge strategy has been the primary tactic used in the field (3, 4, 11-13). In the United States and elsewhere, farmers must grow refuges of crop plants that do not make Bt toxins to promote the survival of susceptible insects. Ideally, most of the rare resistant pests emerging from Bt crops mate with the relatively abundant susceptible pests emerging from refuges. In principle, if resistance is recessive, the heterozygous progeny produced by such matings are killed by the Bt crop, and resistance evolution is greatly delayed. Results from models and short-term, small-scale experiments suggest that refuges can delay resistance substantially (3, 14-16), but long-term, large-scale field tests of the refuge strategy have proved problematic.
Here, we report results of a long-term study of resistance to Bt cotton in field populations of pink bollworm (Pectinophora gossypiella) across the state of Arizona. Pink bollworm is a major lepidopteran pest (17) that has experienced particularly intense selection for resistance. In Arizona, pink bollworm larvae feed almost exclusively on cotton. Transgenic cotton producing Bt toxin Cry1Ac has accounted for more than half of cotton planted statewide since 1997 (18). Unselected populations of pink bollworm are highly susceptible to Cry1Ac, and Bt cotton kills nearly100% of pink bollworm larvae that eat it (19).
Laboratory selection of pink bollworm strains derived from Arizona cotton fields in 1997 quickly increased their resistance to Cry1Ac and survival on Bt cotton (19, 20). This rapid response to laboratory selection confirmed the unexpectedly high frequency of resistance detected in 1997 from field populations of pink bollworm (19). Pink bollworm resistance to Bt cotton in at least four laboratory-selected strains is a recessive trait associated with mutations in a gene encoding a cadherin protein that binds Cry1Ac (21-24).
In light of extensive use of Bt cotton in Arizona and pink bollworm's quick response to laboratory selection, rapid field-evolved resistance might have occurred. However, bioassay data reported here show no net increase from 1997 to 2004 in pink bollworm resistance to Bt toxin Cry1Ac in Arizona. We elucidate this outcome using a synthesis of experimental and modeling results. The lack of field-evolved resistance despite extensive use of Bt cotton and rapid resistance evolution in the laboratory suggests that the refuge strategy has helped to delay pink bollworm resistance to Bt cotton.
Materials and Methods
Bioassays. To monitor pink bollworm resistance to Cry1Ac, we used previously described field sampling procedures and laboratory bioassays (19). We tested an average of 2,680 larvae per year from 10-17 cotton fields in Arizona per year. The progeny of field-collected individuals from each site were reared and tested separately (i.e., 10-17 strains tested per year). Neonates were tested individually for 21 days on artificial diet without toxin (control) or on diet with 10 μg of Cry1Ac per ml of diet, which kills susceptible homozygotes and heterozygotes but not resistant homozygotes (19, 24). Based on recessive inheritance of resistance, the resistance allele frequency for each site was estimated as the square root of the frequency of survivors after adjustment for control mortality. Although resistance allele frequency calculations were based on Hardy-Weinberg equilibrium, the observed pattern in the frequency of resistant survivors does not depend on this assumption. We used the bootstrap method with 10,000 repetitions to calculate the 95% confidence interval for each yearly statewide mean resistance allele frequency. Whereas Bayesian inference is preferred for estimating credibility intervals for a single population (25), the confidence intervals reported here reflect the variation in estimated resistance allele frequency among the 10-17 populations sampled each year. Bioassay data from 1997 to 2001 were reported previously (6, 19).
Population Genetic Model. We analyzed a single-locus population genetic model (26, 27) with R and S alleles conferring resistance and susceptibility, respectively. This is a simplified, but reasonable representation of pink bollworm resistance to Bt toxin Cry1Ac, which is tightly linked to a single cadherin locus at which three mutant alleles associated with resistance have been identified (20-23). Larvae with any combination of two resistance alleles are resistant to Cry1Ac, whereas those with one or none are susceptible. We assumed that mating occurred randomly among adults of all genotypes and eggs were distributed randomly between refuges and Bt cotton (28) according to the proportion of the habitat they each occupied.
We used the model to determine combinations of values for key parameters that cause increases, decreases, or stability in resistance allele frequency. The three key parameters are refuge percentage, fitness costs, and incomplete resistance. Refuge percentage is the percentage of cotton acreage planted with non-Bt cotton. Fitness costs occur when the fitness in refuges is lower for insects with resistance alleles than insects without resistance alleles. Fitness costs select against resistance in refuges. Based on experimental data from pink bollworm (29-31), we assumed that fitness costs are recessive. Incomplete resistance occurs when resistant insects suffer a disadvantage on Bt crop plants relative to non-Bt crop plants, reducing selection for resistance on Bt crop plants (6).
The direction of evolution is determined by the net effect of selection for resistance in Bt crop fields and selection against resistance in refuges. The change in the frequency of the R allele is given by
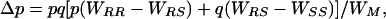 |
[1] |
where, respectively, p and q are the frequencies of the R and S alleles; and WRR, WRS, WSS, and WM are the fitnesses of RR, RS, SS, and the mean of all three genotypes. Thus, resistance allele frequency is stable when
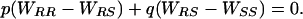 |
[2] |
Because pink bollworm resistance to Bt cotton and fitness costs of resistance are recessive, WRS = WSS and Eq. 2 simplifies to
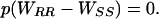 |
[3] |
The fitnesses of resistant and susceptible homozygotes are
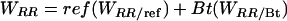 |
[4] |
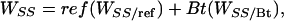 |
[5] |
where, respectively, ref and Bt (= 1 - ref) are the proportions of the cotton acreage planted to refuges and Bt cotton; WRR/ref and WRR/Bt are the fitnesses of RR in refuges and in Bt cotton fields; and WSS/ref and WSS/Bt are the fitnesses of SS in refuges and in Bt cotton fields. Note that WSS/Bt = 0 and p > 0. Thus, substitution for WRR and WSS in Eq. 3 and rearrangement indicates that resistance is unchanged when
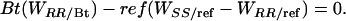 |
[6] |
Therefore, resistance is stable when the net effect of selection for resistance in Bt cotton fields favoring RR balances the net effect of fitness costs selecting against RR in refuges. Resistance increases if selection favoring resistance [Bt(WRR/Bt)] is greater than selection against resistance [ref(WSS/ref - WRR/ref)] and decreases if the opposite occurs. To evaluate potential outcomes in the field, we defined WSS/ref as 1 and estimated ranges of the three other parameters in Eq. 6 (ref, WRR/Bt, and WRR/ref), as described below.
Empirical Estimates of Refuge Percentage, Fitness Costs, and Incomplete Resistance. We used data from nine previously published field and greenhouse studies of pink bollworm to estimate refuge percentage, fitness costs, and incomplete resistance. Because empirical estimates show considerable variation in each of these parameters, we emphasize the ranges rather than single estimates for each factor.
Refuge percentage for six cotton-growing counties of Arizona was estimated for 1998-2003 by using reports from growers that were mapped and analyzed with methods of Geographical Information Systems (18). Leaves from cotton plants from a subset of fields were checked for Bt toxin with ELISA to verify the accuracy of the maps (18).
We calculated fitness costs and incomplete resistance from previously reported experimental data using units that could be incorporated readily in the model described above. To estimate fitness costs, we used results from previous studies comparing the performance of resistant and susceptible strains in field and greenhouse experiments. To reduce the influence of differences between strains unrelated to resistance, we focused here on comparisons between resistant and susceptible strains that shared a common genetic background (i.e., the resistant strain was derived from the susceptible strain via laboratory selection). We did not include data from comparisons between unrelated resistant and susceptible strains or comparisons of larval survival on artificial diet. Fitness cost (%) was calculated as
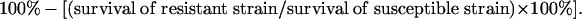 |
For example, with larval survival on non-Bt cotton of 0.15 for a resistant strain (APHIS-98R) and 0.29 for its susceptible parent strain (APHIS-S), the survival of RR relative to SS is estimated to be 0.52 (0.15/0.29), and the fitness cost is 48% (29).
To estimate the combined fitness cost per generation of the larval survival and overwintering costs, we assumed that they act independently. For simplicity, we distributed the effect of the overwintering cost equally among generations, assuming five generations of pink bollworm per year (17). In field experiments, the mean overwintering cost was 71% (30). This cost reflects an overwintering survival ratio of 0.29 for the resistant strain relative to the susceptible strain. Distributing this overwintering survival cost equally over five generations yields a ratio per generation of 0.78 (= 0.291/5) and an overwintering cost per generation of 22% (100% - 78%). The combined fitness cost per generation affecting larval survival on non-Bt cotton plants and overwintering (OW) survival was calculated as
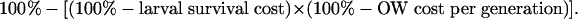 |
For example, a larval survival fitness cost of 41% and an overwintering survival cost per generation of 22% yields a combined fitness cost per generation of 54% (100% - [0.59 × 0.78]). Assuming that no other differences in fitness occur between resistant (RR) and susceptible (SS) insects, WRR/ref = [100% - fitness cost(%)]. In this example, WRR/ref = 0.46 (100% - 54%).
Results from greenhouse experiments in which a resistant strain was compared on Bt versus non-Bt cotton plants were used to calculate the larval survival component of incomplete resistance as
 |
For example, survival of the resistant strain AZP-R was 3.1% on Bt cotton and 7.8% on non-Bt cotton (19), yielding a larval survival component of 0.40. One study also measured effects of incomplete resistance on adult sex ratio (proportion female), fecundity, and egg hatch rate (32). Measurement of these life history traits enabled an estimate of effects of incomplete resistance on net reproductive rate (i.e., population growth per generation) as (32)
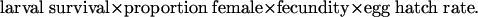 |
To estimate the mean and range for net reproductive rate, we used the mean and range for the larval survival component of incomplete resistance and multiplied them by the ratios for Bt vs. non-Bt cotton for proportion female (0.75), fecundity (0.56), and egg hatch rate (1.06) (32). By using a single estimate for each of the latter three traits, this approach underestimates variation in incomplete resistance.
In estimating fitness costs and incomplete resistance, we assumed fixed genotypes for resistant strains (RR) and susceptible strains (SS). Although resistant strains were fixed or nearly fixed for RR, some susceptible strains used in fitness cost comparisons were heterogeneous, which could cause underestimation of fitness costs (30, 31, 33). The presence of RS or SS individuals in resistant strains could cause overestimation of effects of incomplete resistance because RS and SS perform better on non-Bt cotton than on Bt cotton. However, this effect was probably minimal because of the high proportion of RR individuals in resistant strains.
Results
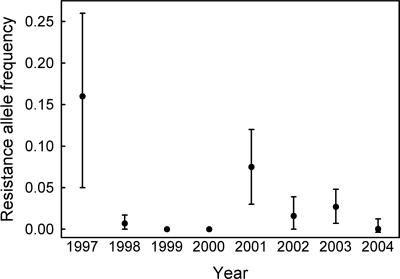
Resistance Allele Frequency in Field Populations. Bioassay results show that the highest mean resistance allele frequency for pink bollworm in Arizona occurred in 1997, the first year of the study (Fig. 1). The seven means for resistance allele frequency from 1998 to 2004 are lower than the mean for 1997 (sign test, P = 0.016). The mean resistance allele frequency (95% confidence limits) decreased from 0.16 (0.05-0.26) in 1997 to 0.007 (0-0.02) in 1998. Although some variation occurred from 1999 to 2003, the mean resistance allele frequency did not differ significantly between 1998 and 2004 (0.004, 95% confidence limits = 0-0.01).
Fig. 1.
The mean resistance allele frequency (with 95% confidence intervals) for pink bollworm in Arizona from 1997 to 2004. The frequency of resistance to Cry1Ac, the toxin in Bt cotton, was estimated with laboratory diet bioassays testing an average of 2,680 pink bollworm larvae per year from 10-17 sites per year (see Materials and Methods). In 1999 and 2000, no larvae survived on diet treated with a diagnostic concentration of Cry1Ac (10 μg of toxin per ml diet). Data from 1997 to 2001 were reported previously (6, 19).
Empirical Estimates of Refuge Percentage, Fitness Costs, and Incomplete Resistance. Field and greenhouse data show substantial variation in refuge percentage, fitness costs, and incomplete resistance (Table 1). In the six major cotton-growing counties of Arizona, non-Bt cotton refuges accounted for 14-78% of the cotton acreage per county from 1998 to 2003 (Table 1). The combined effects of fitness costs affecting larval and overwintering survival of resistant relative to susceptible pink bollworm averaged 54% per generation with a range from 19% to 68% (Table 1). The mean for incomplete resistance, reflecting a disadvantage for resistant pink bollworm on Bt cotton relative to non-Bt cotton, was 0.35 (range of 0.18-0.67; Table 1).
Table 1. Empirical estimates of refuge percentage, fitness costs, and incomplete resistance.
| Parameter | Values* | Reference |
|---|---|---|
| Refuge, % | 14—78 | 18 |
| Fitness costs, %, per generation (resistant relative to susceptible) | ||
| Larval survival on non-Bt cotton plants | 41 (0—55)† | 20, 29, 31 |
| Overwintering survival | 22 (19—29) | 30, see Materials and Methods |
| Larval/overwintering survival | 54 (19—68) | See Materials and Methods |
| Incomplete resistance (resistant on Bt cotton relative to non-Bt cotton) | ||
| Larval survival | 0.78 (0.40—1.5) | 19–21, 32, 48 |
| Proportion female | 0.75 | 32 |
| Fecundity (eggs per female) | 0.56 | 32 |
| Egg hatch rate | 1.06 | 32 |
| Net reproductive rate | 0.35 (0.18—0.67) | See Materials and Methods |
For estimates of fitness cost and incomplete resistance, values are means with ranges in parentheses. For traits estimated in more than one study (survival of resistant larvae relative to susceptible larvae on non-Bt cotton and survival of resistant larvae on Bt vs. non-Bt cotton), the means are the average of the means from all studies, and the ranges are the lowest and highest individual estimates from all studies
Includes one estimate with anomalously low survival on non-Bt cotton of a susceptible strain (20). Excluding this estimate, the mean is 48%, and the range is 37—55%
Variation in refuge percentage occurs because the perceived advantage of Bt cotton to growers varies as a function of expected pink bollworm damage and economic factors such as the price of cotton, the extra cost of seed for Bt cotton versus non-Bt cotton, and the cost of insecticides. Factors contributing to variation in estimated fitness costs and incomplete resistance include environmental variation, genetic background, and experimental error.
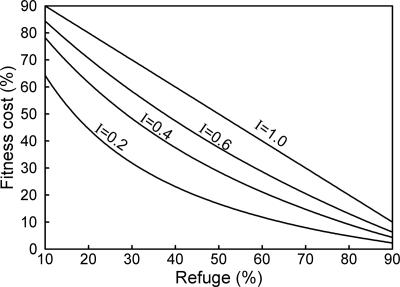
Synthesis of Experimental and Modeling Results. To understand the pattern observed in field populations of pink bollworm, we incorporated the aforementioned empirical estimates of key parameters into a population genetic model. This enabled analysis of thresholds for conditions that can cause increases, decreases, or stability in resistance. The modeling results show that resistance is more likely to decrease with increases in refuge percentage, fitness costs, and the disadvantage for resistant insects on Bt cotton relative to non-Bt cotton (i.e., incomplete resistance) (Fig. 2).
Fig. 2.
Thresholds for conditions causing increases or decreases in resistance based on a population genetic model. Table 1 gives empirical estimates of refuge percentage, fitness cost, and incomplete resistance for pink bollworm in Arizona. Incomplete resistance (I) indicates the fitness of homozygous resistant insects on Bt cotton relative to non-Bt cotton. For each of the four values of I examined (0.2, 0.4, 0.6, and 1.0), combinations of refuge percentage and fitness cost above each threshold line cause decreases in resistance, and those below each line cause increases in resistance. For example, with 50% refuges (close to the statewide mean) and I = 0.6, fitness costs of >37.5% cause decreased resistance, whereas fitness costs <37.5% cause increased resistance.
Because pink bollworm resistance to Bt cotton is recessive, modeling results indicate no increases in resistance across a wide range of combinations of realistic values for refuges, fitness costs, and incomplete resistance (Table 1 and Fig. 2). For example, with the mean empirical values for fitness costs (54%) and incomplete resistance (0.35), the model predicts decreases in resistance with >23% refuges. With 50% refuges (close to the statewide mean) and incomplete resistance of 0.40, the model predicts decreased resistance with fitness costs >29% (Fig. 2). However, with only 14% refuges, resistance is expected to increase unless the combined effect of fitness cost and incomplete resistance is strong (e.g., fitness cost of 60% and incomplete resistance of <0.24). Thus, some realistic parameter combinations yield predicted increases in resistance.
Discussion
Contrary to expectations of rapid evolution of pest resistance to Bt crops under worst-case scenarios (7-9), bioassay data (Fig. 1) indicate no net increase from 1997 to 2004 in pink bollworm resistance to Bt toxin Cry1Ac in Arizona. The observed delay in resistance occurred despite widespread use of Cry1Ac-producing Bt cotton during this period. In addition to the bioassay data, this conclusion is supported by results from laboratory selection experiments and field efficacy tests. The relatively high frequency of resistance alleles in pink bollworm field populations in 1997 was confirmed by rapid responses to laboratory selection with Cry1Ac in three Arizona strains derived from the field in 1997 (19, 20). In contrast, similar selection in subsequent years did not yield any resistant strains from Arizona. Field tests comparing pink bollworm infestations in paired fields of Bt and non-Bt cotton indicate that the efficacy of Bt cotton has remained >99% (34). Also, despite a widely publicized, grower-sponsored hotline and rapid response team for addressing potential resistance problems (35), no cases of reduced efficacy related to resistance have been documented. Consistent with the lack of field-evolved resistance, long-term suppression of pink bollworm has occurred in regions of Arizona with high adoption of Bt cotton (36).
The modeling results incorporating empirical estimates of key parameters for pink bollworm show that resistance is not expected to increase across a wide range of realistic values for refuges, fitness costs, and incomplete resistance (Fig. 2). However, the modeling results also suggest that resistance could increase with some realistic parameter combinations (i.e., small refuges and moderate to weak effects of fitness costs and incomplete resistance). Thus, with spatial and temporal variation in refuge percentage, fitness costs, and incomplete resistance, the direction of resistance evolution may also vary in space and time. The observed pattern in the field shows that the combined effects of all factors did not cause net, statewide increases in resistance from 1997 to 2004.
The model used here, rather than a complete description, is a simplified representation intended to give a theoretical framework for interpreting field observations. For example, the model assumes discrete generations, random movement between Bt cotton and refuges, and random mating among adults. Results from a more complex model of pink bollworm and Bt cotton that includes age structure and spatial structure also show that increases in resistance may be delayed or prevented with realistic values for fitness costs and other factors (37).
We did not attempt to incorporate potential fitness costs affecting development time and reproductive success. In most cases, tests on non-Bt cotton plants showed no fitness costs affecting development time or pupal weight (29, 31). In no-choice laboratory cage experiments, resistant males were not at a disadvantage relative to susceptible males when mating with susceptible, virgin females (38). However, in choice experiments, males from one of the two resistant strains tested mated less often than susceptible males. Moreover, compared with susceptible males, resistant males mating first with susceptible, virgin females sired a lower proportion of progeny, which indicates a fitness cost affecting paternity (38).
We also did not incorporate an observed developmental delay of resistant pink bollworm on Bt cotton relative to non-Bt cotton, which is a component of incomplete resistance (32, 39). The tendency of this developmental delay to slow resistance by lowering the reproductive rate of resistant pink bollworm on Bt cotton may be countered by assortative mating associated with developmental asynchrony between resistant adults from Bt cotton and susceptible adults from refuges (32, 39, 40).
Perhaps the most intriguing observation in this long-term study of pink bollworm is the relatively high frequency of resistance seen in 1997, which was the second year of widespread use of Bt cotton in Arizona. Five of the 10 Arizona cotton field populations sampled in 1997 yielded survivors of exposure to diet with 10 μg of Cry1Ac per ml of diet, which kills susceptible homozygotes and heterozygotes but not resistant homozygotes (19, 24). Two rounds of laboratory selection with survivors pooled from statewide samples yielded a resistant strain (AZP-R), with larvae capable of survival on Bt cotton (19).
Subsequently, laboratory selection was conducted with two of the strains derived from the field in 1997 that contributed individuals to AZP-R. This selection produced a resistant strain from a single site in western Arizona (MOV97-R) and another resistant strain from a single site in eastern Arizona (SAF97-R) (20). Although recessive mutations in a cadherin gene are associated with resistance in all three strains, each strain has a distinctive frequency of the three identified resistance alleles (r1, r2, and r3). AZP-R has all three alleles (21, 23), which is consistent with the multiple origins of individuals pooled to create this strain. MOV97-R has only alleles r1 and r3, and SAF97-R has only r1 and r2 (20). Although the possibility of mutations in the laboratory cannot be excluded, the quick response to selection and distinctive resistance allele frequencies of each strain support the conclusion that the resistance alleles were present in individuals sampled from the field in 1997.
We do not know why the frequency of resistance was so high in 1997, but we can infer that one or more key factors influencing resistance (Fig. 2) changed from 1997 to 1998, causing the observed decline in resistance frequency (Fig. 1). One factor that might have changed is the concentration of Cry1Ac in Bt cotton. In 1997, some problems with Bt cotton were observed in field evaluations, but it is not clear whether these were caused by moderate concentrations of Cry1Ac or putative Bt cotton bolls that produced no Cry1Ac (35). If Bt cotton grown during 1996 and 1997 produced enough Cry1Ac to kill homozygous susceptible larvae but not most heterozygous larvae, nonrecessive inheritance of resistance could have quickly raised resistance allele frequency (41). If increased Cry1Ac concentration in 1998 and thereafter killed all or nearly all heterozygous larvae, this shift to recessive inheritance could have favored delays or even decreases in resistance. Another possibility is that the effects of fitness costs and incomplete resistance, which varied substantially in experiments (Table 1), increased after 1997. Such changes could be associated with changes in agronomic practices, weather, or other environmental factors (33). A third possibility is a decreased effect from 1997 to 1998 of one or more factors other than Bt cotton favoring the resistance alleles.
Setting aside the unusually high frequency of pink bollworm resistance to Cry1Ac detected in 1997, the bioassay results provide evidence that pink bollworm resistance to Bt cotton has not increased substantially since 1998. Although pink bollworm's response to Bt cotton in Arizona is one of the most closely monitored cases, evidence from other pests and other regions also indicates no field-evolved increases in resistance to Bt crops so far (6). The continued efficacy of Bt crops after 9 years refutes the worst-case scenarios predicting pest resistance to Bt crops in as little as 3 years (7-9).
In contrast to modeling results showing the potential for long delays in resistance (3, 16, 27), the most pessimistic predictions (7-9) do not incorporate recessive inheritance, large refuges, or large effects of fitness costs and incomplete resistance. In some cases, one or more of these less optimistic assumptions are reasonable. For example, resistance to Bt crops may not be recessive in some pests (42). Furthermore, field-evolved resistance to Cry1A toxins in diamondback moth, which occurred in response to Bt sprays, does not necessarily entail fitness costs or incomplete resistance on Bt plants (43, 44). Unlike laboratory-selected resistance to Cry1A toxins in some strains of pink bollworm, tobacco budworm (Heliothis virescens) (45), and cotton bollworm (Helicoverpa armigera) (46), field-evolved resistance to Cry1A toxins in some strains of diamondback moth is not linked with cadherin mutations (47). Thus, in pink bollworm and other pests, resistance could evolve via selection for alleles that are not recessive, have lower fitness costs, or completely overcome disadvantages on Bt plants.
Acknowledgments
We thank Aaron Gassmann, David Onstad, David Heckel, Allison Snow, Mark Sisterson, David Crowder, Jeffrey Fabrick, Shai Morin, and Robert Smith for helpful suggestions and Sarah Brink, Brook Wood, and the staff of the Extension Arthropod Resistance Management Laboratory for technical assistance. This work was supported by U.S. Department of Agriculture (USDA) National Research Initiative Grants 2001-35302-09976 and 2003-01469, USDA Biotechnology Risk Assessment Grant 2003-4371, the Arizona Cotton Research and Protection Council, Monsanto, Cotton Inc., and the University of Arizona.
Author contributions: B.E.T., T.J.D., and Y.C. designed research; B.E.T. performed modeling; T.J.D. performed field research; Y.C. analyzed data; and B.E.T., T.J.D., and Y.C. wrote the paper.
Conflict of interest statement: The authors received funding from three organizations that could be affected financially by this article, the Arizona Cotton Research and Protection Council, Cotton Inc., and Monsanto.
Abbreviation: Bt, Bacillus thuringiensis.
References
- 1.Lawrence, S. (2005) Nat. Biotechnol. 23, 281. [DOI] [PubMed] [Google Scholar]
- 2.Tabashnik, B. E. (1994) Annu. Rev. Entomol. 39, 47-79. [Google Scholar]
- 3.Gould, F. (1998) Annu. Rev. Entomol. 43, 701-726. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Environmental Protection Agency (2001) www.epa.gov/pesticides/biopesticides/pips/bt_brad.htm. [PubMed]
- 5.Ferré, J. & Van Rie, J. (2002) Annu. Rev. Entomol. 47, 501-533. [DOI] [PubMed] [Google Scholar]
- 6.Tabashnik, B. E., Carrière, Y., Dennehy, T. J., Morin, S., Sisterson, M. S., Roush, R. T., Shelton, A. M. & Zhao, J.-Z. (2003) J. Econ. Entomol. 96, 1031-1038. [DOI] [PubMed] [Google Scholar]
- 7.Harris, M. K. (1991) Science 253, 1075. [DOI] [PubMed] [Google Scholar]
- 8.Gould, F., Anderson, A., Jones, A., Sumerford, D., Heckel, D. G., Lopez, J., Micinski, S., Leonard, R. & Laster, M. (1997) Proc. Natl. Acad. Sci. USA 94, 3519-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roush, R. T. (1997) Pestic. Sci. 51, 328-334. [Google Scholar]
- 10.Janmaat, A. F. & Myers, J. H. (2003) Proc. R. Soc. London Ser. B 270, 2263-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shelton, A. M., Zhao, J.-Z. & Roush, R. T. (2002) Annu. Rev. Entomol. 47, 845-881. [DOI] [PubMed] [Google Scholar]
- 12.Mehlo, L., Gahakwa, D., Nghia, P. T., Loc, N. T., Capell, T., Gatehouse, J. A., Gatehouse, A. M. R. & Christou, P. (2005) Proc. Natl. Acad. Sci. USA 102, 7812-7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao, J.-Z., Cao, J., Collins, H. L., Bates, S. L., Roush, R. T., Earle, E. D. & Shelton, A. M. (2005) Proc. Natl. Acad. Sci. USA 102, 8426-8430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu, Y. B. & Tabashnik, B. E. (1997) Proc. R. Soc. London Ser. B 264, 605-610. [Google Scholar]
- 15.Shelton, A. M., Tang, J. D., Roush, R. T., Metz, T. D. & Earle, E. D. (2000) Nat. Biotechnol. 21, 1493-1497. [DOI] [PubMed] [Google Scholar]
- 16.Carrière, Y., Sisterson, M. S. & Tabashnik, B. E. (2004) in Insect Pest Management: Field and Protected Crops, eds. Horowitz, A. R. & Ishaaya, I. (Springer, Berlin), pp. 65-95.
- 17.Henneberry, T. J. & Naranjo, S. E. (1998) Integr. Pest Manag. Rev. 3, 31-52. [Google Scholar]
- 18.Carrière, Y., Ellers-Kirk, C., Kumar, K., Heuberger, S., Whitlow, M., Antilla, L., Dennehy, T. J. & Tabashnik, B. E. (2005) Pest Manag. Sci. 61, 327-330. [DOI] [PubMed] [Google Scholar]
- 19.Tabashnik, B. E., Patin, A. L., Dennehy, T. J., Liu, Y. B., Carrière, Y., Sims, M. A. & Antilla, L. (2000) Proc. Natl. Acad. Sci. USA 97, 12980-12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabashnik B. E., Biggs, R. W., Higginson, D. M., Henderson, S., Unnithan, D. C., Unnithan, G. C., Ellers-Kirk, C., Sisterson, M. S., Dennehy, T. J., Carrière, Y. & Morin, S. (2005) J. Econ. Entomol. 98, 635-644. [DOI] [PubMed] [Google Scholar]
- 21.Morin, S., Biggs, R. W., Sisterson, M. S., Shriver, L., Ellers-Kirk, C., Higginson, D., Holley, D., Gahan, L. J., Heckel, D. G., Carrière, Y., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 5004-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morin, S., Henderson, S., Fabrick, J. A., Carrière, Y., Dennehy, T. J., Brown, J. K. & Tabashnik, B. E. (2004) Insect Biochem. Mol. Biol. 34, 1225-1233. [DOI] [PubMed] [Google Scholar]
- 23.Tabashnik, B. E., Liu, Y. B., Unnithan, D. C., Carrière, Y., Dennehy, T. J. & Morin, S. (2004) J. Econ. Entomol. 97, 721-726. [DOI] [PubMed] [Google Scholar]
- 24.Tabashnik, B. E., Liu, Y.-B., Dennehy, T. J., Sims, M. A., Sisterson, M. S., Biggs, R. W. & Carrière, Y. (2002) J. Econ. Entomol. 95, 1018-1026. [DOI] [PubMed] [Google Scholar]
- 25.Andow, D. A. & Alstad, D. N. (1998) J. Econ. Entomol. 91, 572-578. [Google Scholar]
- 26.Hartl, D. L. & Clark, A. G. (1989) Principles of Population Genetics (Sinauer, Sunderland, MA), 2nd Ed.
- 27.Carrière, Y. & Tabashnik, B. E. (2001) Proc. R. Soc. London Ser. B 268, 1475-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, Y. B., Tabashnik, B. E., Dennehy, T. J., Carrière, Y., Sims, M. A. & Meyer, S. K. (2002) J. Econ. Entomol. 95, 143-148. [DOI] [PubMed] [Google Scholar]
- 29.Carrière, Y., Ellers-Kirk, C., Liu, Y.-B., Sims, M. A., Patin, A. L., Dennehy, T. J. & Tabashnik, B. E. (2001) J. Econ. Entomol. 94, 1571-1576. [DOI] [PubMed] [Google Scholar]
- 30.Carrière, Y., Ellers-Kirk, C., Patin, A. L., Sims, M. A., Meyer, S., Liu, Y.-B., Dennehy, T. & Tabashnik, B. E. (2001) J. Econ. Entomol. 94, 935-941. [DOI] [PubMed] [Google Scholar]
- 31.Carrière, Y., Ellers-Kirk, C., Biggs, R., Degain, B., Holley, D., Yafuso, C., Evans, P., Dennehy, T. J. & Tabashnik, B. E. (2005) J. Econ. Entomol. 98, 947-954. [DOI] [PubMed] [Google Scholar]
- 32.Liu, Y.-B., Tabashnik, B. E., Dennehy, T. J., Patin, A. L., Sims, M. A., Meyer, S. & Carrière, Y. (2001) J. Econ. Entomol. 94, 1237-1242. [DOI] [PubMed] [Google Scholar]
- 33.Carrière, Y., Ellers-Kirk, C., Biggs, R., Higginson, D. M., Dennehy, T. J. & Tabashnik, B. E. (2004) J. Econ. Entomol. 97, 1710-1718. [DOI] [PubMed] [Google Scholar]
- 34.Dennehy, T. J., Unnithan, G. C., Brink, S. A., Wood, B. D., Carrière, Y., Tabashnik, B. E., Antilla, L. & Whitlow, M. (2004) Update on Pink Bollworm Resistance to Bt Cotton in the Southwest (Univ. of Arizona Cooperative Extension, Tucson), Publ. No. P-138; http://cals.arizona.edu/pubs/crops/az1335/az13357b.pdf.
- 35.Simmons, A. L., Dennehy, T. J., Tabashnik, B. E., Bartlett, A., Gouge, D. & Staten, R. (1998) Proc. Beltwide Cotton Conf. 2, 1025-1030. [Google Scholar]
- 36.Carrière, Y., Ellers-Kirk, C., Sisterson, M., Antilla, L., Whitlow, M., Dennehy, T. J. & Tabashnik, B. E. (2003) Proc. Natl. Acad. Sci. USA 100, 1519-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sisterson, M. S., Antilla, L., Ellers-Kirk, C., Carrière, Y. & Tabashnik, B. E. (2004) J. Econ. Entomol. 97, 1413-1424. [DOI] [PubMed] [Google Scholar]
- 38.Higginson, D. M., Nyboer, M. E., Biggs, R. W., Morin, S., Tabashnik, B. E. & Carrière, Y. (2005) Evolution (Lawrence, Kans.) 59, 915-920. [PubMed] [Google Scholar]
- 39.Liu, Y. B., Tabashnik, B. E., Dennehy, T. J., Patin, A. L. & Bartlett, A. C. (1999) Nature 400, 519. [DOI] [PubMed] [Google Scholar]
- 40.Peck, S. L., Gould, F. & Ellner, S. P. (1999) J. Econ. Entomol. 92, 1-16. [Google Scholar]
- 41.Tabashnik, B. E., Gould, F. & Carrière, Y. (2004) J. Evol. Biol. 17, 904-912. [DOI] [PubMed] [Google Scholar]
- 42.Bird, L. J. & Akhurst, R. J. (2005) J. Econ. Entomol. 98, 1311-1319. [DOI] [PubMed] [Google Scholar]
- 43.Ramachandran, S., Buntin, G. D., All, J. N., Tabashnik, B. E., Raymer, P. L., Adang, M. J., Pulliam, D. A. & Stewart, C. N., Jr. (1998) J. Econ. Entomol. 91, 1239-1244. [Google Scholar]
- 44.Tang, J. D., Collins, H. L., Roush, R. T., Metz, T. D., Earle, E. D. & Shelton, A. M. (1999) J. Econ. Entomol. 92, 47-55. [DOI] [PubMed] [Google Scholar]
- 45.Gahan, L. J., Gould, F. & Heckel, D. G. (2001) Science 293, 857-860. [DOI] [PubMed] [Google Scholar]
- 46.Xu, X., Yu, L. & Wu, Y. (2005) Appl. Environ. Microbiol. 71, 948-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baxter, S. W., Zhao, J.-Z., Gahan, L. J., Shelton, A. M., Tabashnik, B. E. & Heckel, D. G. (2005) Insect Mol. Biol. 14, 327-334. [DOI] [PubMed] [Google Scholar]
- 48.Tabashnik, B. E., Dennehy, T. J., Sims, M. A., Larkin, K., Head, G. P., Moar, W. J. & Carrière, Y. (2002) Appl. Environ. Microbiol. 68, 3790-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]