Abstract
Disruption of Dictyostelium rasC, encoding a Ras subfamily protein, generated cells incapable of aggregation. While rasC expression is enriched in a cell type-specific manner during post-aggregative development, the defect in rasC– cells is restricted to aggregation and fully corrected by application of exogenous cAMP pulses. cAMP is not produced in rasC– cells stimulated by 2′-deoxy-cAMP, but is produced in response to GTPγS in cell lysates, indicating that G-protein-coupled cAMP receptor activation of adenylyl cyclase is regulated by RasC. However, cAMP-induced ERK2 phosphorylation is unaffected in rasC– cells, indicating that RasC is not an upstream activator of the mitogen-activated protein kinase required for cAMP relay. rasC– cells also exhibit reduced chemotaxis to cAMP during early development and delayed response to periodic cAMP stimuli produced by wild-type cells in chimeric mixtures. Furthermore, cAMP-induced Akt/PKB phosphorylation through a phosphatidylinositide 3-kinase (PI3K)-dependent pathway is dramatically reduced in rasC– cells, suggesting that G-protein-coupled serpentine receptor activation of PI3K is regulated by RasC. Cells lacking the RasGEF, AleA, exhibit similar defects as rasC– cells, suggesting that AleA may activate RasC.
Keywords: adenylyl cyclase/Akt-PKB/cAMP relay/chemotaxis/Dictyostelium
Introduction
The ras subfamily genes encode monomeric GTPases that function as molecular switches in cellular signal transduction by cycling between an active GTP-bound or an inactive GDP-bound state (Bourne et al., 1991). A variety of extracellular stimuli potentiate the activation of Ras by exchanging GDP for GTP (Campbell et al., 1998; Gutkind, 1998), a process catalyzed by guanine nucleotide exchange factors (GEFs) (Boguski and McCormick, 1993). GTPase activating proteins increase the intrinsic GTPase activity of Ras, hydrolyzing the bound GTP to GDP (Boguski and McCormick, 1993). In the active state, Ras proteins activate multiple cellular signaling pathways including mitogen-activated protein kinase (MAPK) cascades, the phosphatidylinositide 3-kinase (PI3K)-regulated pathways and RalGDS-dependent activation of Ral (Campbell et al., 1998). These Ras-mediated responses to membrane receptor stimuli regulate a wide range of cellular processes, including proliferation, cytoskeletal functions and differentiation.
The discovery of a large number of Ras subfamily homologs in mammals and in the model organisms Drosophila melanogaster, Caenorhabditis elegans and Dictyostelium discoideum (Reuther and Der, 2000; Wilkins and Insall, 2001) has raised important questions regarding the specific functions of individual Ras proteins that cannot be readily resolved by biochemical analysis. To understand Ras function in a multicellular context, it is necessary to analyze organisms that are genetically disrupted in the ras gene of interest. The tractability of the Dictyostelium haploid genome facilitates the functional characterization of strains with specifically targeted gene disruptions and, furthermore, its unique biology allows studies of growth and differentiation as distinct processes (Parent and Devreotes, 1996; Aubry and Firtel, 1999). Nutrient deprivation triggers the developmental program whereby the secretion and chemotactic response to the chemoattractant cAMP result in the aggregation of up to 105 cells. The aggregate then elongates to become a phototactic and thermotactic migrating slug. Cells within the slug differentiate and sort into prestalk or prespore cells that segregate into spatially separated populations, which, upon culmination, form a stalk consisting of dead vacuolated cells supporting a sorus of spores. Underlying this deceptively simple developmental program is a complex of cellular signaling pathways that are highly conserved relative to mammalian systems.
Six Dictyostelium Ras subfamily proteins with at least 50% amino acid identity to the mammalian H-, N- and K-Ras proteins have been described (Reymond et al., 1984; Daniel et al., 1995). Several lines of evidence have indicated that a Ras signaling pathway is involved in the regulation of the cAMP relay and in the chemotactic response to cAMP during aggregation. Disruption of aleA, the gene encoding a putative Ras GEF (Insall et al., 1996), and rip3, encoding a Ras interacting protein (Lee et al., 1999), resulted in cells that cannot aggregate due to defects in both the cAMP relay and chemotaxis. Furthermore, disruption of genes homologous to components of metazoan Ras-activated effector pathways also resulted in cells incapable of aggregation, suggesting a possible upstream role for a Ras protein. For example, the MAPK homolog ERK2 was shown to be essential for cAMP relay, but not necessary for chemotaxis (Segall et al., 1995), while cells lacking both PI3K1 and PI3K2, and cells lacking Akt/PKB, a downstream effector of PI3K activity, exhibit defects in chemotaxis but not in cAMP relay (Zhou et al., 1998; Meili et al., 1999).
Three of the Dictyostelium ras genes, rasS, rasG and rasD, have been disrupted, but these disruptions were found to have negligible effects on the aggregation process (Tuxworth et al., 1997; Chubb et al., 2000; Wilkins et al., 2000). In the studies described here, we present genetic and biochemical evidence that RasC is the Ras protein that had been previously implicated in the aggregation processes. Dictyostelium cells in which the rasC gene had been disrupted by targeted gene replacement failed to aggregate. RasC appears to be a central regulatory molecule acting downstream of serpentine receptor stimulation by cAMP that is required for two distinct effector pathways: the activation of Akt/PKB through PI3K and the activation of adenylyl cyclase. The discovery that the RasC protein is essential for aggregation provides evidence for a novel role for a Ras subfamily protein in the sensing of and response to chemotactic signals.
Results
Growth and developmental expression of rasC
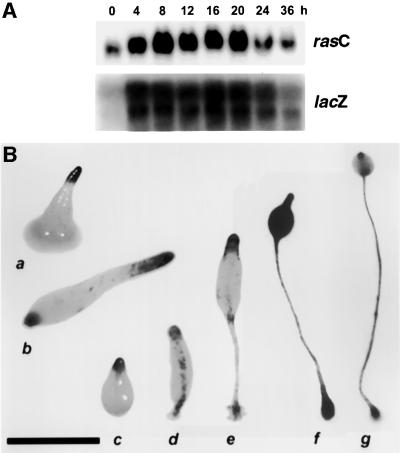
In order to analyze the spatial expression of rasC during development, the 0.55 kb DNA fragment upstream of the 5′ rasC coding region (Daniel et al., 1994) was isolated by PCR and ligated to the hislacZ gene, a variant of lacZ that encodes a short half-life β-galactosidase (Detterbeck et al., 1994). The resulting rasC::hislacZ fusion construct was transformed into the wild-type AX2 strain. A northern blot of RNA isolated from different developmental stages of this transformant showed a lacZ expression pattern that was almost identical to that for the rasC transcript (Figure 1A), indicating that the 0.55 kb DNA fragment encodes sufficient promoter sequence to reproduce the normal regulation of the rasC gene.
Fig. 1. (A) rasC and lacZ gene expression during development. rasC::hislacZ-transformed AX2 cells were plated for development on nitrocellulose filters and total RNA harvested at the times indicated for northern blot analysis. Duplicate blots were probed with either rasC or lacZ cDNA. (B) Spatial expression of lacZ driven from the rasC promoter during development. rasC::hislacZ-transformed AX2 cells were plated for development on nitrocellulose filters, fixed at various developmental stages and stained in situ for β-galactosidase activity. Shown are representative stained structures from the following developmental stages: first finger (a); slug (b); early to mid culmination (c–e); late culmination (f); and terminal fruiting body (g). All were stained for 2 h (a–f), except (g), which was stained overnight. Bar = 0.5 mm.
When the rasC::hislacZ transformant was developed on nitrocellulose filters, staining for β-galactosidase activity was observed predominantly in the tip during the transition from tipped mound to slug, and by the slug stage there was also some staining in the posterior (Figure 1B). These results indicate that rasC expression is enriched in the prestalk cell population, and this pattern of expression was maintained through early and mid culmination, as demonstrated by staining in the tip, stalk tube and basal disk. The whole population became equally stained during late culmination; however, in terminally differentiated fruiting bodies, staining was predominantly in the stalk tube, the upper cup and the lower cup, indicating a reversion to the enriched prestalk pattern of expression (Figure 1B). The transient change in rasC spatial expression during late culmination correlated with the peak of mRNA at 20 h of development (Figure 1A). This spatial and temporal expression pattern is suggestive of functional roles for RasC during slug formation and culmination.
Generation of a rasC null strain
In order to generate a rasC– strain, a rasC disruption vector was transformed into AX2 cells, and blasticidin S-resistant colonies were screened by western blotting using the RasC antibody. Out of 70 independently isolated clones analyzed, three exhibited no detectable RasC protein (one of the three is shown in Figure 2A). Southern blotting, using a rasC cDNA probe, verified that all three transformants contained a simple targeted disruption at the rasC locus (Figure 2B). Subsequent rehybridization with the bsr gene probe revealed a single insertion of bsr into the genome for all three isolates (data not shown), indicating that the null strains were the result of rasC disruption and not attributable to secondary effects resulting from random integration. All three rasC– cell lines exhibited identical phenotypes under all assay conditions and, therefore, all subsequent data presented are for one of the three transformants.
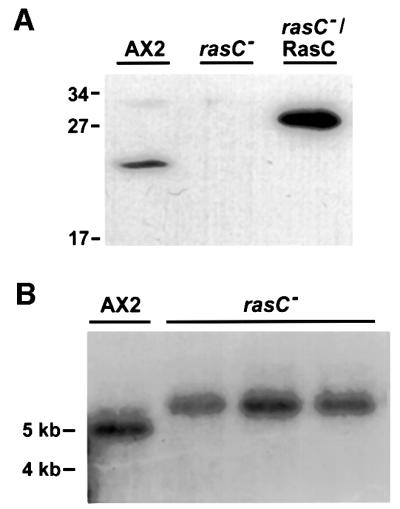
Fig. 2. Disruption of the rasC gene. (A) Western blot of cell lysates from the parental AX2, rasC– and rasC–/RasC, a rasC– transformant expressing RasC from the rasC promoter, probed with a highly specific RasC antibody. The higher molecular weight of the RasC protein in the rasC–/RasC strain is due to the fact that the expression construct encoded a RasC protein with an additional 17 amino acids at the N-terminus. Molecular weight markers in kilodaltons are as indicated. (B) Southern blot of genomic DNA from parental AX2 and three independent rasC– isolates. Genomic DNA was digested with DdeI, separated in 0.7% agarose gel, blotted onto nylon and probed with rasC cDNA. DdeI restriction sites flank the entire rasC genomic locus and are not present within the disruption construct used for homologous recombination. Approximate sizes in kilobases are as indicated.
Phenotype of the rasC– cells
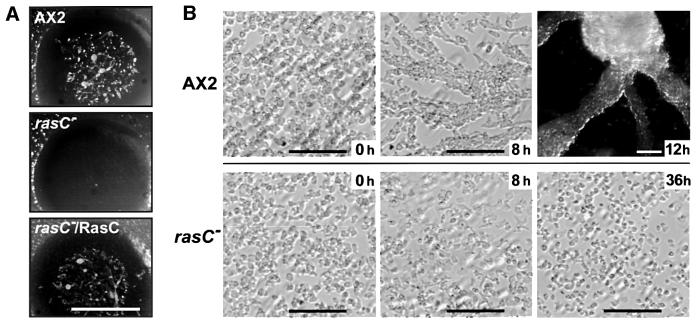
When plated on a lawn of bacteria, Dictyostelium cells grow by ingesting the bacteria, resulting in plaque formation. Cells at the plaque periphery continue to feed, expanding plaque size, while starving cells toward the center initiate multicellular development and eventually form fruiting bodies. When rasC– cells were clonally grown on a bacterial lawn, initial plaque formation was indistinguishable from that of the parental AX2, indicating normal growth and phagocytosis. However, rasC– cells failed to aggregate, appearing as clear plaques 5 days after initial plating (Figure 3A). At that time, all phases of multicellular development were observed in the AX2 plaques. Ectopic expression of the RasC protein from the rasC promoter restored multicellular development in the null strain (Figures 2A and 3A), confirming that the observed phenotype was a functional consequence of rasC gene disruption.
Fig. 3. Developmental phenotypes of parental AX2 and rasC– strains. (A) Clonal plaques of parental AX2, rasC– and rasC–/RasC strains after 5 days growth on a bacterial lawn. Bar = 5 mm. (B) AX2 and rasC– cells were seeded as monolayers at a density of 5 × 105 cells/cm2 in Nunc tissue culture dishes and submerged under Bonner’s salts solution. Images were taken at the times indicated following plating. Bars = 100 µm.
The aggregation process can also be observed by incubating cell monolayers on plastic surfaces submerged under non-nutrient buffer. Under these conditions, aggregation streams were observed for AX2 after 8 h, and distinct aggregates formed by 12 h (Figure 3B, top panels). rasC– cells did not form aggregation streams or centers, even after prolonged starvation for 36 h (Figure 3B, bottom panels). The aggregation defects observed in the rasC– cells were similar to those reported for the aleA– strain (Insall et al., 1996), suggesting the possibility that AleA is the GEF responsible for regulating RasC activation.
Exogenous cAMP pulses circumvent the block in aggregation of rasC– cells
Dictyostelium cells are unable to aggregate if they are deficient in the cAMP relay, a process whereby cells synthesize and secrete cAMP in response to an extracellular cAMP stimulus. When cells in suspension were pulsed every 6 min for 5 h with 50 nM cAMP (herein referred to as ‘cAMP-pulsed cells’) and then plated on nitrocellulose filters, development of rasC– cells was indistinguishable from that of AX2 cells (data not shown). cAMP-pulsed rasC– cells produced spores in equal numbers to cAMP-pulsed AX2 cells, and these spores exhibited the same viability (data not shown). Furthermore, when developed in chimeric mixtures with equal numbers of AX2 cells, rasC– cells completed development, producing equal numbers of spores (data not shown). The rasC– spores germinated to form aggregation-negative plaques when plated on bacterial lawns (data not shown). Contrary to the spatial expression results suggesting functional roles for RasC during mid to late development, the development of rasC– cells is fully restored if the block in aggregation is circumvented by exogenous application of cAMP pulses, an indication that RasC function is necessary for the cAMP relay.
cAMP receptor and heterotrimeric G-protein-dependent activation of adenylyl cyclase is mediated by RasC
To confirm that the rasC– cells were defective in the cAMP relay, cAMP-pulsed cells were stimulated in vivo with 2′-deoxy-cAMP, and cell lysates assayed for cAMP accumulation at various time points following stimulation. There was negligible accumulation of cAMP in the rasC– cells relative to that observed for AX2 cells (Figure 4A), indicating that RasC is required for cAMP-stimulated activation of adenylyl cyclase A (ACA), the predominant adenylyl cyclase present during early Dictyostelium development (Parent and Devreotes, 1996). A northern blot of mRNA isolated from cAMP-pulsed cells showed that the rasC– cells overexpress the cAMP receptor carA and the heterotrimeric G-protein gα2, relative to AX2 cells (Figure 4B). While the significance of this increased expression is not apparent, it is clear that the inability of the rasC– cells to activate ACA is not due to insufficient expression of required signaling components such as cAR1 and Gα2 (Parent and Devreotes, 1996).
Fig. 4. Adenylyl cyclase assays and expression of aggregation phase genes. (A) cAMP-pulsed AX2 (triangles) and rasC– (circles) cells were stimulated with 10 µM 2′-deoxy-cAMP, and cell lysates assayed for total cAMP accumulation at the indicated times. Plotted values are the means ± SD for three independent experiments. (B) AX2 and rasC– cells were shaken in suspension in KK2, with or without the application of 50 nM cAMP pulses every 6 min. Total RNA was prepared from cells harvested at the times indicated and duplicate northern blots probed with either carA or gα2 cDNA. (C) cAMP-pulsed AX2, rasC– and aleA– cell-free lysates were assayed for adenylyl cyclase activity in the presence of either 5 mM MnSO4 (hatched bar), 40 µM GTPγS (black bar) or no additional component (white bar) (see Materials and methods). Plotted values are normalized relative to the unstimulated activity obtained in the absence of MnSO4 or GTPγS. Values for AX2 and rasC– cell lysates are the means ± SD for three independent experiments. Values for aleA– cell lysates are from a single experiment.
To further assess the nature of the signaling defect, GTPγS-mediated activation of ACA was assayed in vitro using extracts prepared from cAMP-pulsed cells. Since aleA– cells also exhibit negligible cAMP synthesis when stimulated in vivo with cAMP (Insall et al., 1996), we included aleA– cell-free extracts in this assay for comparison. GTPγS is thought to stimulate ACA activity by uncoupling the Gβγ subunit from the heterotrimeric G-protein, thus bypassing the need for receptor activation in cell-free lysates (Parent and Devreotes, 1996). The addition of GTPγS stimulated the ACA activity of AX2 lysates ∼24-fold over the basal level, and the ACA activity of rasC– and aleA– cell lysates ∼12-fold (Figure 4C). Thus, cell lysates lacking RasC or AleA were capable of generating appreciable amounts of cAMP in the presence of GTPγS, although the levels were not as high as those obtained with wild-type cells. The lower levels of in vitro cAMP synthesis in the rasC– and aleA– cell lysates were not due to reduced expression of ACA, since the Mn2+-activated levels were similar for AX2, rasC– and aleA– cell lysates (Figure 4C).
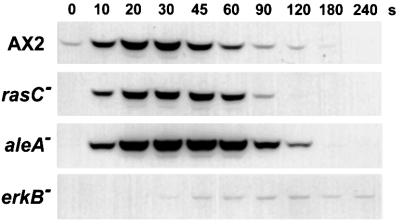
RasC is not necessary for activation of ERK2
Dictyostelium ERK2 is transiently activated in response to a cAMP stimulus (Maeda et al., 1996) and cells lacking ERK2 are unable to aggregate due to a defective cAMP relay (Segall et al., 1995). Since ERK proteins are important downstream effectors for Ras in metazoan signaling, the possibility that RasC is an upstream activator of ERK2 was tested. cAMP-pulsed cells were stimulated with cAMP and cell lysates were analyzed for ERK2 phosphorylation in western blots using a phospho-MAPK-specific antibody. Following cAMP stimulation, a 42 kDa protein corresponding to the predicted molecular weight of ERK2 was transiently phosphorylated in wild-type cells (Figure 5) with kinetics similar to that reported previously for ERK2 activation (Maeda et al., 1996). An erkB– strain assayed similarly showed only a very faint phosphorylation of the 42 kDa component (Figure 5). Since this particular erkB– strain (HS174) expresses low levels of ERK2 due to insertional disruption of a plasmid in the 3′ untranslated region of the gene (Segall et al., 1995), our results verify that the phosphorylated protein detected in the immunoblots is indeed ERK2. When rasC– cells were stimulated with cAMP, ERK2 was phosphorylated to similar levels and with similar kinetics to those seen for wild-type cells. This demonstrates that the activation of ERK2 is not downstream of RasC in the classical Ras–MAPK signaling cascade observed in metazoan systems.
Fig. 5. cAMP-induced stimulation of ERK2 phosphorylation. cAMP-pulsed AX2, rasC–, aleA– and erkB– cells were stimulated with cAMP to a final concentration of 100 nM, and cells lysed directly in SDS gel loading buffer at the indicated times. Protein samples of 10 µg were fractionated by SDS–PAGE, and western blots probed with a phospho-MAPK-specific antibody to assay for ERK2 phosphorylation. Results shown are representative of at least three independent experiments for each strain.
Since there had been a correlation between the phenotypes of the rasC– and aleA– cells, we repeated the ERK2 activation assay with the aleA– cells. Phosphorylation of ERK2 in aleA– cells reached a higher level, peaked at a later time point and was more persistent compared with wild-type cells (Figure 5), confirming the results of an earlier experiment using an in-gel kinase assay to measure ERK2 activity (Aubry et al., 1997). The results indicate that AleA may activate another Ras (Aubry et al., 1997; Kosaka et al., 1998), in addition to RasC.
rasC– cells exhibit an altered chemotactic response to cAMP
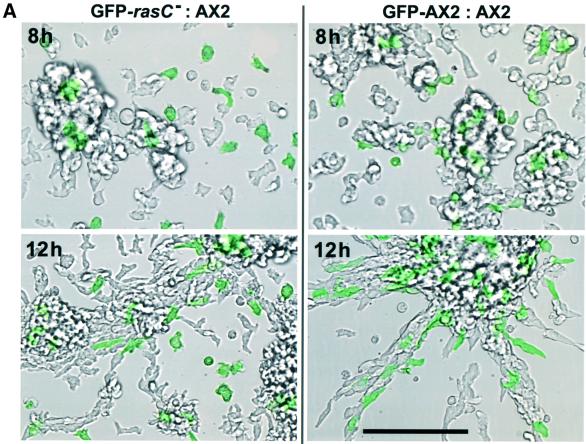
Dictyostelium aggregation is also dependent on the cell’s ability to sense and respond chemotactically to cAMP. To examine the chemotactic behavior of cells lacking RasC, green fluorescent protein (GFP)-labeled rasC– cells were mixed with unlabeled AX2 cells and allowed to aggregate on plastic submerged under buffer. The fluorescent label allowed tracking of individual rasC– cells in response to the natural cAMP oscillations produced by the wild-type cells. At 8 h of development, there were very few labeled rasC– cells in the small aggregates that had formed, while, in contrast, there were numerous labeled rasC– cells in the aggregates and aggregation streams by 12 h (Figure 6A, left panels). When GFP-labeled AX2 cells were mixed with unlabeled AX2 cells as a control, numerous labeled cells were observed both within the small aggregates at 8 h and within the aggregation streams by 12 h (Figure 6A, right panels), indicating that the GFP label had no deleterious effect on chemotactic behavior. Further analysis of the mixed population at 6 h following starvation by time-lapse microscopy revealed that synchronous, pulsatile movements of AX2 cells could be observed, whereas GFP-labeled rasC– cells exhibited no such response (data not shown). In contrast, by 12 h, the rasC– cells within the aggregation streams exhibited the same pulsatile responses as the AX2 cells, while the rasC– cells that remained outside the streams remained unresponsive and non-polarized.
Fig. 6. cAMP-mediated chemotaxis. (A) Aggregation of rasC– cells in chimeric mixtures with AX2 cells. GFP-labeled rasC– or GFP-labeled AX2 cells were mixed with unlabeled AX2 cells in a ratio of 1:4 and seeded at 5 × 105 cells/cm2 in Nunc tissue culture dishes submerged under Bonner’s salts solution. Shown are the images of GFP-labeled fluorescent cells (in green) that have been overlayed on phase-contrast images of the total aggregating population, at 8 and 12 h of development. (B) AX2 and rasC– cells seeded on Nunc dishes at 105 cells/cm2 submerged under Bonner’s salts were starved for 6 h and subjected to a micropipet filled with 100 µM cAMP at T = 0. Shown are phase-contrast images of the cells in the vicinity of the micropipet tip at the indicated times. (C) AX2 and rasC– cells that had been pulsed with cAMP for 5 h were seeded onto Nunc dishes and subjected to a cAMP filled micropipet as described in (B). Bars = 100 µm.
AX2 cells that had been starved for 6 h without exogenous cAMP pulsing responded within 20 min to an artificial cAMP gradient released from a micropipet (Figure 6B, top panels). In contrast, under these conditions, rasC– cells underwent chemotaxis poorly and did not exhibit the characteristic clustering of cells around the micropipet tip even after 40 min (Figure 6B, bottom panels). AX2 cells that had been pulsed for 5 h with cAMP polarized and underwent chemotaxis toward the cAMP source within 20 min of micropipet tip application (Figure 6C, top panels). However, equivalently treated rasC– cells polarized and underwent chemotaxis toward the tip within 6 min of application of the micropipet (Figure 6C, bottom panels). This enhanced chemotaxis of the rasC– cells might be the result of overexpression of carA, gα2 (Figure 4B) and possibly other components involved in mediating chemotaxis. Thus, rasC– cells are initially slow in undergoing chemotaxis to cAMP, but underwent chemotaxis rapidly after being pulsed. These results are consistent with the behavior of the rasC– cells in mixtures with AX2 described above.
Akt/PKB phosphorylation through a PI3K-dependent pathway requires RasC
pi3k1–/pi3k2– and pkbA– cells exhibit aggregation defects despite having normal cAMP relays, suggesting that the chemotactic response to cAMP requires a PI3K-mediated signaling pathway (Zhou et al., 1998; Meili et al., 1999). Consistent with this model, cAMP-stimulated activation of Akt/PKB does not occur in pi3k1–/pi3k2– cells, but is fully restored in pi3k1–/pi3k2– cells constitutively expressing PI3K1 (Meili et al., 1999). Dictyostelium Akt/PKB activation requires phosphorylation at conserved threonine residues in the kinase domain and at the C-terminus (Meili et al., 1999). When cell lysates of cAMP-stimulated AX2 cells were analyzed by western blotting using a phospho-threonine-specific antibody, a protein with the predicted molecular weight of Dictyostelium Akt/PKB (51 kDa) was transiently phosphorylated following cAMP stimulation (Figure 7A, left panels). When the blot was stripped and re-probed with an Akt/PKB-specific antibody, a 51 kDa protein was also detected (Figure 7A, right panels), consistent with the idea that the phospho-threonine-specific antibody had detected the transient phosphorylation of Akt/PKB. Pre-treatment of AX2 cells with 12.5 µM LY294002, a PI3K inhibitor (Vlahos et al., 1995), caused an ∼90% reduction in threonine phosphorylation of the 51 kDa protein, indicating that the observed phosphorylation was PI3K dependent (Figure 7A). Finally, the phosphorylation kinetics of the 51 kDa protein observed here was consistent with the activation of Akt/PKB reported previously (Meili et al., 1999). We are therefore confident that the transiently phosphorylated 51 kDa component is the Akt/PKB protein encoded by pkbA.
Fig. 7. cAMP-induced stimulation of Akt/PKB phosphorylation. (A) cAMP-pulsed AX2 cells were either not treated (Control) or pre-treated with 12.5 µM LY294002 for 1 min, before stimulation with 100 nM cAMP. At the indicated times, cells were lysed directly in SDS gel loading buffer, 10 µg of protein fractionated by SDS–PAGE and western blots probed with a phospho-threonine-specific antibody (left panels). The blots were subsequently stripped of bound antibodies and re-probed with an Akt/PKB-specific antibody (right panels). Molecular weight markers in kilodaltons are as indicated beside each blot. (B) cAMP-pulsed AX2, rasC– and aleA– cells were stimulated with 100 nM cAMP and analyzed as described in (A). Results shown are representative of at least three independent experiments for each strain. (C) Ten micrograms of protein from cAMP-pulsed AX2, rasC– and aleA– cells were analyzed by western blotting with the Akt/PKB specific-antibody to demonstrate equal Akt/PKB expression levels in all three strains. Closed arrowheads indicate phosphorylated Akt/PKB and open arrowheads indicate total Akt/PKB protein.
cAMP-pulsed rasC– cells exhibit dramatically reduced levels of phosphorylated Akt/PKB at 10 s following cAMP stimulation (Figure 7B), indicating that RasC is a major upstream effector of Akt/PKB phosphorylation. cAMP-stimulated Akt/PKB phosphorylation in aleA– cells was also reduced to levels similar to those observed for rasC– cells (Figure 7B), indicating that AleA also functions upstream of Akt/PKB, consistent with the possibility that it is a GEF for RasC. The trace of Akt/PKB phosphorylation at 10 s observed in the rasC– and aleA– cells suggests that low levels of activation not involving RasC or AleA can occur. The lower levels of Akt/PKB phosphorylation were not due to reduced expression of Akt/PKB in rasC– and aleA– cells, since western blots probed with an Akt/PKB-specific antibody revealed identical levels to those in AX2 (Figure 7C).
Discussion
A role for a Ras protein in aggregation has been suggested by several lines of evidence (Segall et al., 1995; Insall et al., 1996; Aubry et al., 1997; Lee et al., 1999; Meili et al., 1999; Firtel and Chung, 2000). Gene disruption of rasG produced cells that exhibited reduced motility and cytokinesis defects (Tuxworth et al., 1997); however, their ability to aggregate was only slightly delayed (our unpublished observations). Similarly, there is also no evidence for an involvement of RasD or RasS since disruption of the respective genes generated cells that aggregated normally (Chubb et al., 2000; Wilkins et al., 2000). In this report, we have presented results which clearly demonstrated a role for RasC in aggregation. We have generated a rasC– strain in which expression of RasC is completely abolished, and have shown that rasC– cells fail to aggregate. RasC appears to function as a regulatory molecule for both the cAMP relay and the chemotactic response to cAMP.
rasC– cells exhibited no accumulation of cAMP following in vivo stimulation by a cAMP analog, indicating that RasC is necessary for G-protein-coupled cAMP receptor-mediated activation of ACA. This process requires the release of the Gβγ subunit from the trimeric Gα2βγ complex (Wu et al., 1995). In mammalian cells, Ras is activated downstream of Gβγ in G-protein-coupled serpentine receptor activation (Gutkind, 1998). In order to determine whether RasC activation is also downstream of Gβγ in Dictyostelium, we measured the effect of GTPγS on ACA activity in cell-free extracts. The addition of GTPγS releases Gβγ subunits from G-proteins, thus bypassing the need for receptor activation (Parent and Devreotes, 1996). Since ACA activity was stimulated in rasC– cell lysates, it is clear that RasC is not essential for ACA activation in the same manner that Gβ (Wu et al., 1995) and the cytosolic regulatory proteins CRAC and Pia are required (Insall et al., 1994; Chen et al., 1997). The block to ACA activation in rasC– cells must lie upstream of Gα2βγ. A possible explanation of our results is that RasC is essential for the dissociation of the Gα2βγ complex both in vivo and in vitro, and that the partial activity observed in the rasC– cell-free extracts in vitro was provided by release of Gβγ from the remaining G-complexes (Gα1,3–8βγ) (Parent and Devreotes, 1996). This would be similar to the situation for gα2– cells, where ACA activation by GTPγS occurs in vitro, but not in intact cells stimulated through cAMP receptors (Pupillo et al., 1992).
Dictyostelium ERK2 is essential for ACA activity (Segall et al., 1995) and is transiently activated following receptor stimulation by cAMP (Maeda et al., 1996). Although both erkB– and rasC– cells exhibited a similar defect in cAMP relay, cAMP stimulation induced normal ERK2 phosphorylation in rasC– cells (Figure 5), indicating that RasC is not involved in ERK2 activation. Since ERK2 activation is cAR1-dependent but Gα2 and Gβ independent (Maeda et al., 1996), our results are consistent with the contention that RasC signaling is specifically routed through the Gα2βγ-dependent pathway. Despite the fact that ERKs are downstream signaling components of Ras activation in metazoans, there is still no evidence that Ras-mediated signaling is required for ERK2 activation in Dictyostelium. In fact, studies using strains overexpressing activated or dominant-negative forms of RasG or RasD suggest a negative regulatory role for a Ras protein in ERK2 activation (Aubry et al., 1997; Kosaka et al., 1998). It has been postulated that ERK2 function is important for the adaptation response, inhibiting degradation of intracellular cAMP when activated (Loomis, 1998; Aubry and Firtel, 1999). Cells lacking AleA exhibit increased and prolonged ERK2 phosphorylation when stimulated with cAMP (Figure 5) (Aubry et al., 1997), suggesting that AleA may regulate two Ras-mediated responses during cAMP relay: the activation of RasC for synthesis of cAMP and the activation of another Ras for down-regulation of ERK2 during adaptation, allowing the degradation of intracellular cAMP.
It has been shown that PI3K activity is necessary for the activation of Dictyostelium Akt/PKB, since Akt/PKB is not activated in pi3k1–/pi3k2– cells stimulated with cAMP (Meili et al., 1999). Akt/PKB activation is greatly reduced in rasC– and aleA– cells, strongly suggesting that both RasC and AleA play a role in activating PI3K upon cAMP stimulation. The mechanism for this RasC-dependent activation of PI3K remains to be determined, but since both PI3K1 and PI3K2 appear to possess putative Ras binding domains in their N-terminal regions (Zhou et al., 1995), RasC might be directly involved in the activation of either protein. Since RasC also appears to be required for Gα2βγ dissociation, and Gα2 and Gβ are necessary for Akt/PKB activation (Meili et al., 1999), RasC could be required for both direct and G-protein-dependent activation of the PI3Ks. While it is evident that Akt/PKB is necessary for efficient chemotaxis (Meili et al., 1999), our results indicate that this process does not require full Akt/PKB phosphorylation. The dramatic reduction in Akt/PKB phosphorylation in the rasC– cells produces only a slight deleterious effect on cAMP-mediated chemotaxis. It is likely that the trace of Akt/PKB phosphorylation observed in cAMP-stimulated rasC– cells, which had been cAMP-pulsed for 5 h, is sufficient to achieve efficient chemotaxis.
Several lines of evidence point to AleA as the GEF that activates RasC during aggregation. Genetic disruption of aleA was the only one of five ras gef gene disruptions that resulted in a clear aggregation-negative phenotype similar to that of rasC– cells (Wilkins and Insall, 2001; R.Insall, personal communication), and both rasC– and aleA– cells are defective in the cAMP relay (Insall et al., 1996). GTPγS was shown to be capable of partially activating ACA in cell-free extracts of aleA– and rip3– (Figure 4C; Lee et al., 1999). These results are consistent with the idea that AleA is the GEF that activates RasC during aggregation, and suggest the possibility that RasC, AleA and RIP3 form part of a complex that is necessary for optimum activation of ACA. aleA– cells also exhibited reduced phosphorylation of Akt/PKB in response to cAMP stimulation, indicating that AleA acts upstream of PI3K activity in a manner similar to RasC.
Despite the enriched expression of rasC in prestalk cells of slugs and the transient expression of rasC in prespore cells during late culmination, there is no evidence for a requirement for RasC beyond the aggregation stage of development. rasC– cells that had been pulsed with cAMP to circumvent the aggregation defect proceeded to complete development to form normally proportioned slugs and fruiting bodies. Furthermore, the fact that rasC– cells in chimeric mixtures were initially non-responsive to pulsatile cAMP signals from surrounding wild-type cells, but eventually became chemotactically competent after prolonged association, suggests that RasC is important during early aggregation, but is not necessary for later developmental events. Late in aggregation, cAMP signaling is coupled through cAR2 and cAR4 receptors (Parent and Devreotes, 1996), possibly leading to activation of PKB-R1, an Akt/PKB-related kinase that is cAMP stimulated in a PI3K-independent manner (Meili et al., 2000). Thus, while cells during early aggregation require RasC-mediated activation of PI3K for maximum phosphorylation of Akt/PKB, cAMP receptor stimulation during late aggregation could activate PKB-R1 directly.
It is possible that an important role for a Ras protein during late development is masked by functional redundancy due to the presence of one or more Ras homologs. The spatial expression of rasC throughout most of development is remarkably similar to that described previously for rasD (Esch and Firtel, 1991), suggesting the possibility that RasC and RasD might perform similar functions. We generated rasC–/rasD– double disruptant strains and found that these cells had exactly the same phenotype as rasC– single disruptants, including an aggregation defect that could be circumvented by cAMP pulsing (data not shown). Furthermore, the fruiting bodies that formed as a result of cAMP pulsing contained spores that exhibited no apparent defects. rasC–/rasD– cells also exhibited the same wild-type-like transient ERK2 phosphorylation in response to cAMP stimulation as did rasC– or rasD– single disruptants (data not shown). It had previously been shown that rasD– strains had no developmental phenotype other than an inability to perform slug phototaxis or thermotaxis (Wilkins et al., 2000). The available evidence therefore suggests that the role of RasC in development is restricted to the early aggregation stage.
Our data suggest that RasC regulates cAMP synthesis during aggregation and is important for optimum chemotactic cell movement during early development. There is striking conservation in the signaling components required for chemosensing and chemotaxis between Dictyostelium and human neutrophils. In both systems, chemoattractant signals are transmitted through G-protein-coupled serpentine receptors, resulting in transient activation of PI3K, and spatial activation and translocation of Akt/PKB to the membrane (Firtel and Chung, 2000; Rickert et al., 2000). Our results provide the first direct link between a Ras protein and PI3K in cells undergoing chemotaxis. The recent demonstration that human PI3Kγ activity is stimulated when co-expressed with activated H-Ras and Gβγ in COS cells (Pacold et al., 2000) suggests the possibility that the Gβγ-dependent activation of PI3K during neutrophil chemotaxis will also involve a Ras protein.
Materials and methods
Cell culture and development
Dictyostelium discoideum AX2 and transformed strains were grown in HL5 medium supplemented with 10 µg/ml blasticidin S, 25 µg/ml hygromycin (Calbiochem) or 10 µg/ml G418 (Gibco-BRL), as appropriate. To prepare cAMP-pulsed cells, vegetative cells were washed twice and resuspended to 5.0 × 106 cells/ml in KK2 (20 mM potassium phosphate pH 6.1), shaken at 160 r.p.m. for 1 h and pulsed every 6 min for 5 h with 50 nM cAMP. Washed vegetative or cAMP-pulsed cells were spread on nitrocellulose filters (Millipore) resting on KK2-saturated support pads to observe multicellular development. To observe aggregation streams, washed vegetative cells were seeded at ∼5 × 105 cells/cm2 in Nunclon tissue culture dishes submerged under Bonner’s salts (10 mM NaCl, 10 mM KCl, 2 mM CaCl2). For growth and development on bacterial lawns, Dictyostelium cells were clonally plated in association with Klebsiella oxytoca on rich nutrient agar plates. Spores were treated in 1% Triton X-100 in KK2 prior to plating on bacterial lawns.
Transformations
Twenty micrograms of the rasC disruption vector were linearized with PvuII, and electroporated into AX2 cells as described in Tuxworth et al. (1997), except that the MgCl2, CaCl2 healing step was omitted. All other transformations utilized the CaPO4 DNA precipitation method as described previously (Nellen et al., 1987). Following 7–14 days of selection, transformants were subcultured and clonally re-isolated as plaques on bacterial lawns.
Molecular cloning and vectors
The rasC promoter was isolated using a PCR-based method of chromosome walking from the known rasC coding region towards the non-coding 5′ flanking region, as described previously (Min and Powell, 1998). Based on sequences derived from the chromosomal walk, the nested primers 5′-AAAACCTCAAACACACATATTTAC-3′ (RC10) and 5′-AAGATCTTAATAATTTTGACATTGTGTATTTTC-3′ were used to PCR amplify a 0.55 kb DNA fragment from AX2 genomic DNA, which was ligated into pGEM-T Easy (Promega) to generate pJLW12. The rasC::hislacZ reporter plasmid was generated by directionally ligating the 0.55 kb SpeI–BglII rasC promoter fragment from pJLW12 into the XbaI–BglII sites of the psa::hislacZ plasmid (Detterbeck et al., 1994), replacing the psa promoter. The rasC::rasC expression plasmid was generated by directionally ligating the 0.67 kb BamHI–XhoI full-length rasC cDNA into the BglII–XhoI sites of the rasC::hislacZ plasmid, replacing the lacZ gene.
The rasC disruption vector, containing both genomic and cDNA rasC sequences flanking a blasticidin S resistance (bsr) selectable marker, was constructed as follows. First, a 1.6 kb genomic fragment encompassing the promoter and the complete rasC coding region was PCR amplified from AX2 DNA using RC10 and 5′-TTACAATATAATACATCCCCTTTTCTTTG-3′, and ligated into pGEM-T Easy to generate pJLW15. The terminal 5′ 0.84 kb AflIII (blunted)–EcoRI fragment, encompassing the promoter to the AflIII site within the second rasC exon, was excised from pJLW15 and directionally ligated into the SmaI–EcoRI sites of the bsr expression vector, pRHI119, to generate pJLW24. A 0.46 kb AflIII (blunted)–NotI fragment from the 3′ terminal end of a rasC cDNA plasmid was then directionally ligated into the SpeI (blunted)–NotI sites of pJLW24. This construct selectively omits use of the second rasC intron, which contains repetitive sequences, since earlier attempts to generate homologous recombinants using the full-length 1.6 kb genomic sequence were not successful. All plasmid constructions were verified by restriction mapping and DNA sequencing.
The pDHGABD(S65T) plasmid, which expresses a fusion protein comprising GFP and the actin binding domain from ABP-120, was provided by Dr D.A.Knecht. This is a variant that carries a hygromycin resistance selectable marker of the one described previously (Pang et al., 1998).
In situ β-galactosidase assays
AX2 cells transformed with the rasC::hislacZ vector were developed on nitrocellulose filters, fixed in situ and stained for β-galactosidase activity as described previously (Detterbeck et al., 1994). Cells transformed with the more labile ilelacZ vector provided poor expression; therefore, the less labile hislacZ variant was used. Staining reactions were conducted for 2 h or overnight at 22°C, and terminated by washing with Z-buffer.
Adenylyl cyclase assays
cAMP-pulsed cells were washed twice and resuspended to 5.0 × 107 cells/ml in KK2, and gently vortexed to maintain a uniform cell suspension. Cells were stimulated with 10 µM 2′-deoxy-cAMP in the presence of 5 mM dithiothreitol (DTT), and at the indicated time points 100 µl samples were lysed in 100 µl of 3.5% perchloric acid. The samples were then neutralized with 50 µl of 50% saturated KHCO3, and cAMP levels in the supernatants were measured using a cAMP binding protein assay kit (Amersham TRK432).
The in vitro adenylyl cyclase assay has been described elsewhere (Pupillo et al., 1992; Chen et al., 1997). Briefly, cAMP-pulsed cells were treated with 2 mM caffeine for 30 min and washed three times with ice-cold KK2. Cells were then resuspended to 5 × 107 cells/ml in ice-cold 2 mM MgSO4/KK2, mixed in equal volumes with lysis buffer (20 mM Tris–HCl pH 8.0, 2 mM MgSO4) and immediately lysed by filtration through two 5.0 µm TMTP membranes (Millipore). For stimulation by GTPγS, 80 µM GTPγS and 2 µM cAMP were included in the lysis buffer. Lysates were kept chilled on ice for 5 min, 200 µl samples were then incubated for 2.0 min at 22°C in a reaction mix containing 10 mM Tris–HCl pH 8.0, 0.1 mM ATP, 1.0 mM cAMP, 10 mM DTT and 106 c.p.m. [α32P]ATP (Amersham). Mn2+-stimulated activity was measured by inclusion of 5 mM MnSO4 in the reaction mix. Reactions were terminated with 100 µl of 1% SDS containing 1 mM cAMP and 9 mM ATP. cAMP was purified by sequential chromatography through Dowex-50 and alumina, as previously described (Salomon, 1979), and the eluted [32P]cAMP measured by scintillation counting.
ERK2 and Akt/PKB phosphorylation assays
cAMP-pulsed cells were washed twice and resuspended to 5.0 × 107 cells/ml in KK2, and vortexed gently. Before and after addition of cAMP to a final concentration of 100 nM,100 µl aliquots were removed at the indicated time points and immediately mixed with 20 µl of 6× SDS gel loading buffer (Sambrook et al., 1989) supplemented with 300 mM NaF, 1.2 mM Na3VO4, 12 mM EDTA and protease inhibitors (Roche Complete). For the PI3K inhibitor experiment, AX2 cell suspensions were treated for 1 min with 12.5 µM LY294002 (Cell Signaling Technologies) prior to cAMP addition. Protein samples of 10 µg were fractionated by SDS–PAGE, blotted onto nitrocellulose (Amersham), blocked with non-fat milk and probed with the appropriate antibody following the manufacturer’s instructions. Equal sample loading was verified by staining a duplicate gel with Coomassie Blue. To account for possible variations between multiple blots, standard cell extracts were included in each blot and the ECL autoradiogram signals verified by densitometric analysis (GeneQuant; Molecular Dynamics).
Chemotaxis assays
For the chimeric aggregation experiments, rasC– and AX2 cells transformed with pDHGABD(S65T) were mixed with non-labeled AX2 cells in a 1:4 ratio and seeded in Nunclon tissue culture dishes at ∼5 × 105 cells/cm2 submerged under Bonner’s salts. Micropipet assays were carried out using cells starved for 6 h under Bonner’s salts or 5 h cAMP-pulsed cells that were dispersed and seeded in Nunclon dishes. At T = 0, a micropipet (Eppendorf Femtotip) filled with 100 µM cAMP was positioned in the field of view and cell movements monitored by time-lapse microscopy. Phase-contrast and epifluorescent images were captured through an Olympus IX-70 inverted microscope equipped with a DAGE CCD camera using a Scion frame grabber and Scion Image 4.0.
Antibodies
The C-terminal 168- to 185-amino acid peptide of RasC (Daniel et al., 1994) was synthesized by the NAPS facility at the University of British Columbia. Rabbit polyclonal antiserum was raised against the peptide conjugated to keyhole limpet hemocyanin (Pierce). RasC antibodies were further purified by affinity chromatography against the peptide covalently coupled to AffiGel10 (Bio-Rad) according to the manufacturer’s protocols. The purified antibody exhibited no cross-reactivity when tested in western blots against bacterially expressed glutathione S-transferase fusion proteins of Dictyostelium RasG, RasD, RasS, RasB and Rap1.
The Akt/PKB-specific antibody was a gift from F.Jiang and Dr R.Dottin (Hunter College, New York). Phospho-MAPK antibody (Cat#9101) and phospho-threonine antibody (Cat#9381) were from Cell Signaling Technologies.
Southern and northern hybridization analysis
Fifteen micrograms of total RNA extracted using TRIzol reagent (Gibco BRL) were size fractionated in 1.25% agarose–formaldehyde gels. Equal loading of samples was checked by observing the intensities of ethidium bromide-stained rRNA bands. Genomic DNA was salt/ethanol precipitated from the nuclear fraction of cells that had been lysed in 40 mM Tris–HCl pH 7.8, 1.5% sucrose, 0.1 mM EDTA, 6 mM MgCl2, 40 mM KCl, 5 mM DTT and 0.4% NP-40. Ten micrograms of DNA were digested with the indicated restriction enzyme, size fractionated in 0.7% agarose/TBE gel, blotted onto Hybond N+ (Amersham) and hybridized to random primed 32P-labeled probes as described previously (Sambrook et al., 1989). Probes used were: (i) 0.6 kb complete rasC cDNA fragment; (ii) 2.1 kb ClaI–NdeI lacZ gene fragment; (iii) 0.5 kb NcoI–NdeI carA gene fragment; and (iv) 1.1 kb complete gα2 cDNA fragment.
Acknowledgments
Acknowledgements
We thank R.H.Insall for providing the aleA– strain, RI-19, and the pRHI119 plasmid; J.E.Segall for the erkB– strain, HS174; F.Jiang and R.Dottin for the Akt/PKB-specific antibody; and D.A.Knecht for the pDHGABD plasmid. C.J.L. was a recipient of a Medical Research Council (MRC) of Canada student fellowship. This work was supported by a grant to G.W. from MRC of Canada.
References
- Aubry L. and Firtel,R. (1999) Integration of signaling networks that regulate Dictyostelium differentiation. Annu. Rev. Cell Dev. Biol., 15, 469–517. [DOI] [PubMed] [Google Scholar]
- Aubry L., Maeda,M., Insall,R., Devreotes,P.N. and Firtel,R.A. (1997) The Dictyostelium mitogen-activated protein kinase ERK2 is regulated by ras and cAMP-dependent protein kinase (PKA) and mediates PKA function. J. Biol. Chem., 272, 3883–3886. [DOI] [PubMed] [Google Scholar]
- Boguski M.S. and McCormick,F. (1993) Proteins regulating Ras and its relatives. Nature, 366, 643–654. [DOI] [PubMed] [Google Scholar]
- Bourne H.R., Sanders,D.A. and McCormick,F. (1991) The GTPase superfamily: conserved structure and molecular mechanism. Nature, 349, 117–127. [DOI] [PubMed] [Google Scholar]
- Campbell S.L., Khosravi-Far,R., Rossman,K.L., Clark,G.J. and Der,C.J. (1998) Increasing complexity of Ras signaling. Oncogene, 17, 1395–1413. [DOI] [PubMed] [Google Scholar]
- Chen M.Y., Long,Y. and Devreotes,P.N. (1997) A novel cytosolic regulator, pianissimo, is required for chemoattractant receptor and G protein-mediated activation of the 12 transmembrane domain adenylyl cyclase in Dictyostelium. Genes Dev., 11, 3218–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb J.R., Wilkins,A., Thomas,G.M. and Insall,R.H. (2000) The Dictyostelium RasS protein is required for macropinocytosis, phagocytosis and the control of cell movement. J. Cell Sci., 113, 709–719. [DOI] [PubMed] [Google Scholar]
- Daniel J., Bush,J., Cardelli,J., Spiegelman,G.B. and Weeks,G. (1994) Isolation of two novel ras genes in Dictyostelium discoideum—evidence for a complex, developmentally regulated ras gene subfamily. Oncogene, 9, 501–508. [PubMed] [Google Scholar]
- Daniel J., Spiegelman,G.B. and Weeks,G. (1995) Dictyostelium ras genes. In Zerial,M. and Huber,L.A. (eds), Guidebook to the Small GTPases. Oxford University Press, New York, NY, pp. 100–104.
- Detterbeck S., Morandini,P., Wetterauer,B., Bachmair,A., Fischer,K. and MacWilliams,H.K. (1994) The ‘prespore-like cells’ of Dictyostelium have ceased to express a prespore gene: analysis using short-lived β-galactosidases as reporters. Development, 120, 2847–2855. [DOI] [PubMed] [Google Scholar]
- Esch R.K. and Firtel,R.A. (1991) cAMP and cell sorting control the spatial expression of a developmentally essential cell-type-specific ras gene in Dictyostelium. Genes Dev., 5, 9–21. [DOI] [PubMed] [Google Scholar]
- Firtel R.A. and Chung,C.Y. (2000) The molecular genetics of chemotaxis: sensing and responding to chemoattractant gradients. BioEssays, 22, 603–615. [DOI] [PubMed] [Google Scholar]
- Gutkind J.S. (1998) Cell growth control by G protein-coupled receptors: from signal transduction to signal integration. Oncogene, 17, 1331–1342. [DOI] [PubMed] [Google Scholar]
- Insall R., Kuspa,A., Lilly,P.J., Shaulsky,G., Levin,L.R., Loomis,W.F. and Devreotes,P. (1994) CRAC, a cytosolic protein containing a pleckstrin homology domain, is required for receptor and G protein-mediated activation of adenylyl cyclase in Dictyostelium. J. Cell Biol., 126, 1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insall R.H., Borleis,J. and Devreotes,P.N. (1996) The aimless RasGEF is required for processing of chemotactic signals through G-protein-coupled receptors in Dictyostelium. Curr. Biol., 6, 719–729. [DOI] [PubMed] [Google Scholar]
- Kosaka C., Khosla,M., Weeks,G. and Pears,C. (1998) Negative influence of RasG on chemoattractant-induced ERK2 phosphorylation in Dictyostelium. Biochim. Biophys. Acta, 1402, 1–5. [DOI] [PubMed] [Google Scholar]
- Lee S., Parent,C.A., Insall,R. and Firtel,R.A. (1999) A novel Ras-interacting protein required for chemotaxis and cyclic adenosine monophosphate signal relay in Dictyostelium.Mol. Biol. Cell, 10, 2829–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis W.F. (1998) Role of PKA in the timing of developmental events in Dictyostelium cells. Microbiol. Mol. Biol. Rev., 62, 684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda M., Aubry,L., Insall,R., Gaskins,C., Devreotes,P.N. and Firtel,R.A. (1996) Seven helix chemoattractant receptors transiently stimulate mitogen-activated protein kinase in Dictyostelium—role of heterotrimeric G proteins. J. Biol. Chem., 271, 3351–3354. [DOI] [PubMed] [Google Scholar]
- Meili R., Ellsworth,C., Lee,S., Reddy,T.B.K., Ma,H. and Firtel,R.A. (1999) Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J., 18, 2092–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meili R., Ellsworth,C. and Firtel,R.A. (2000) A novel Akt/PKB-related kinase is essential for morphogenesis in Dictyostelium. Curr. Biol., 10, 708–717. [DOI] [PubMed] [Google Scholar]
- Min G. and Powell,J.R. (1998) Long distance genome walking using the long and accurate polymerase chain reaction. Biotechniques, 24, 398–400. [DOI] [PubMed] [Google Scholar]
- Nellen W., Datta,S., Reymond,C., Sivertsen,A., Mann,S., Crowley,T. and Firtel,R.A. (1987) Molecular biology in Dictyostelium: tools and applications. Methods Cell Biol., 28, 67–100. [DOI] [PubMed] [Google Scholar]
- Pacold M.E. et al. (2000) Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase γ. Cell, 103, 931–943. [DOI] [PubMed] [Google Scholar]
- Pang K.M., Lee,E. and Knecht,D.A. (1998) Use of a fusion protein between GFP and an actin-binding domain to visualize transient filamentous-actin structures. Curr. Biol., 8, 405–408. [DOI] [PubMed] [Google Scholar]
- Parent C.A. and Devreotes,P.N. (1996) Molecular genetics of signal transduction in Dictyostelium. Annu. Rev. Biochem., 65, 411–440. [DOI] [PubMed] [Google Scholar]
- Pupillo M., Insall,R., Pitt,G.S. and Devreotes,P.N. (1992) Multiple cyclic AMP receptors are linked to adenylyl cyclase in Dictyostelium.Mol. Biol. Cell, 3, 1229–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuther G.W. and Der,C.J. (2000) The Ras branch of GTPases: Ras family members don’t fall far from the tree. Curr. Opin. Cell Biol., 12, 157–165. [DOI] [PubMed] [Google Scholar]
- Reymond C.D., Gomer,R.H., Mehdy,M.C. and Firtel,R.A. (1984) Developmental regulation of a Dictyostelium gene encoding a protein homologous to mammalian Ras protein. Cell, 39, 141–148. [DOI] [PubMed] [Google Scholar]
- Rickert P., Weiner,O.D., Wang,F., Bourne,H.R. and Servant,G. (2000) Leukocytes navigate by compass: roles of PI3Kγ and its lipid products. Trends Cell Biol., 10, 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y. (1979) Adenylate cyclase assay. In Brooker,G., Greengard,P. and Robison,G.A. (eds), Advances in Cyclic Nucleotide Research. Vol. 10. Raven Press, New York, NY, pp. 35–55. [PubMed]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning. A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Segall J.E., Kuspa,A., Shaulsky,G., Ecke,M., Maeda,M., Gaskins,C. and Firtel,R.A. (1995) A MAP kinase necessary for receptor-mediated activation of adenylyl cyclase in Dictyostelium. J. Cell Biol., 128, 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuxworth R.I., Cheetham,J.L., Machesky,L.M., Spiegelman,G.B., Weeks,G. and Insall,R.H. (1997) Dictyostelium RasG is required for normal motility and cytokinesis, but not growth. J. Cell Biol., 138, 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahos C.J. et al. (1995) Investigation of neutrophil signal transduction using a specific inhibitor of phosphatidylinositol 3-kinase. J. Immunol., 154, 2413–2422. [PubMed] [Google Scholar]
- Wilkins A. and Insall,R.H. (2001) Small GTPases in Dictyostelium: lessons from a social amoeba. Trends Genet., 17, 41–48. [DOI] [PubMed] [Google Scholar]
- Wilkins A., Khosla,M., Fraser,D.J., Spiegelman,G.B., Fisher,P.R., Weeks,G. and Insall,R.H. (2000) Dictyostelium RasD is required for normal phototaxis, but not differentiation. Genes Dev., 14, 1407–1413. [PMC free article] [PubMed] [Google Scholar]
- Wu L.J., Valkema,R., van Haastert,P.J.M. and Devreotes,P.N. (1995) The G protein β subunit is essential for multiple responses to chemoattractants in Dictyostelium. J. Cell Biol., 129, 1667–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K., Pandol,S., Bokoch,G. and Traynor-Kaplan,A.E. (1998) Disruption of Dictyostelium PI3K genes reduces [32P]phosphatidyl inositol 3,4 bisphosphate and [32P]phosphatidylinositol trisphosphate levels, alters F-actin distribution and impairs pinocytosis. J. Cell Sci., 111, 283–294. [DOI] [PubMed] [Google Scholar]
- Zhou K.M., Takegawa,K., Emr,S.D. and Firtel,R.A. (1995) A phosphatidylinositol (PI) kinase gene family in Dictyostelium discoideum: biological roles of putative mammalian p110 and yeast Vps34p PI 3-kinase homologs during growth and development. Mol. Cell. Biol., 15, 5645–5656. [DOI] [PMC free article] [PubMed] [Google Scholar]