Abstract
Uveal melanoma (UVM) is the most common eye cancer in adults, with 50% of patients developing overt metastasis that often proves fatal. The majority of UVM harbor mutations in GNAQ or GNA11, encoding constitutively active Gαq proteins. Combined inhibition of MEK and FAK downstream of Gαq has shown promising effects in UVM cells by inducing apoptotic cell death, but resistance to this strategy can occur in the clinic. Here, we aimed to identify new targets to overcome resistance to MEK + FAK inhibition (FAKi + MEKi). Reverse-phase protein array (RPPA) analysis in UVM cells treated with FAKi + MEKi showed increased levels of pro-apoptotic proteins, such as PUMA and BIM, which promoted cell death. However, we observed an adaptive increase in anti-apoptotic proteins, including BCL2, upon FAK + MEK blockade. We generated UVM cells resistant to FAKi + MEKi by prolonged exposure. Whole-exome sequencing did not reveal relevant acquired mutations; instead, resistant cells exhibit increased BCL2 levels. Moreover, expression of a stable BCL2 mutant confers resistance to both FAKi + MEKi and FAKi+“RAF-MEK clamp” (avutometinib) treatment. Of direct translational relevance, we found that an approved BCL2 inhibitor (venetoclax) displays synergistic efficacy with FAK + MEK blockade and overcomes acquired resistance, including when combined with darovasertib, a dual PKC/PKN inhibitor limiting MEK and FAK signaling that is under clinical evaluation. Our findings suggest that resistance to FAKi + MEKi in UVM cells can be driven by an adaptive upregulation of the anti-apoptotic protein BCL2, and that, in turn, BCL2 inhibitors represent a promising precision-targeted strategy to overcome FAKi + MEKi treatment resistance and improve therapeutic outcomes.
Keywords: resistance, uveal melanoma, FAK, MEK, BCL2, venetoclax, avutometinib, darovasertib
Uveal melanoma (UVM) is the most common primary intraocular malignancy in adults and the second most prevalent form of melanoma after cutaneous melanoma (1). It presents significant clinical challenges due to its high metastatic potential and limited effective therapeutic options. In the United States, approximately 2500 individuals are diagnosed with UVM each year. UVM often remains asymptomatic until advanced stages, complicating early detection and timely intervention (2). While localized treatments such as radiation therapy or enucleation can manage the primary tumor, nearly 50% of patients develop metastatic uveal melanoma (mUVM), predominantly involving the liver (3). UVM metastases often remain asymptomatic for years, complicating early detection of disseminated disease and timely intervention. The prognosis for mUVM is poor, with a median survival of less than 1 year, underscoring the urgent need for more effective therapeutic strategies (4). Kimmtrak/tebentafusp, which is a bispecific fusion protein, was recently granted FDA approval for treating individuals diagnosed with unresectable or mUVM, but its use is limited by the requirement of a specific HLA haplotype in about half of patients with UVM and a modest objective response rate (<10%) (5).
At the molecular level, UVM is characterized by a remarkably low mutation burden but exhibits a high frequency of specific oncogenic mutations. Over 93% of UVM cases harbor activating mutations in either GNAQ or GNA11, genes encoding the Gαq subunits of heterotrimeric G proteins, which play pivotal roles in intracellular signal transduction (6). These mutations result in the constitutive activation of downstream signaling cascades, notably the Gαq/11-PLCβ-PKC-MAPK pathway, promoting tumor growth and survival (7). This oncogenic signaling axis represents a compelling therapeutic target, and efforts have been made to inhibit it using MEK inhibitors (MEKi) such as selumetinib and trametinib (8). However, clinical trials have demonstrated that the efficacy of these inhibitors is limited due to suboptimal responses, adverse toxicity profiles, and the development of resistance mechanisms within tumor cells (9, 10).
Recent studies using computational approaches and CRISPR-Cas9 screens to identify synthetic lethal interactions with GNAQ oncogenes showed that Gαq promotes UVM cell survival through focal adhesion kinase (FAK) activation, and that in turn, combining MEKi with FAK inhibitors (FAKi) enhances antitumor effects in UVM (11, 12). Specifically, the combination of MEKi + FAKi exhibits synergistic effects, leading to increased tumor cell apoptosis and reduced proliferation. These findings suggest that dual targeting of MEK and FAK may address some limitations associated with MEKi monotherapy and offer a more efficacious therapeutic strategy. In addition to MEK and FAK inhibitors, the combination of the dual protein kinase C (PKC)/protein kinase N (PKN) inhibitors, such as darovasertib (LXS-196), with FAKi has demonstrated synergism against UVM cells (13, 14). These combined therapeutic approaches highlight the potential of targeting multiple nodes within the oncogenic signaling network of UVM to enhance antitumor efficacy.
However, the use of targeting agents for cancer treatment often results in the acquisition of treatment resistance (15). This includes the use of MEKi + FAKi in UVM in a recent clinical trial (16). In this study, we investigated the mechanisms underlying the resistance of UVM cells to combined FAK and MEK inhibition. We observed that specific anti-apoptotic proteins are upregulated in response to this combination therapy, with BCL2 prominently involved. Members of the BCL2 family, particularly BCL2 itself, are known to promote cell survival by inhibiting apoptosis (17, 18). By knocking down BCL2 and employing venetoclax (19), a selective BCL2 inhibitor, we assessed whether inhibiting BCL2 could reverse resistance and enhance the efficacy of the FAKi + MEKi. Our findings provide new insights into the adaptive resistance mechanisms employed by UVM cells and suggest that targeting BCL2 represents a viable strategy to overcome resistance to combined MEK and FAK inhibition. This approach can potentially improve therapeutic outcomes for patients with advanced UVM, addressing a critical unmet medical need.
Results
A reverse-phase protein array (RPPA) analysis reveals that FAK + MEK inhibition increases the levels of pro-apoptotic and anti-apoptotic molecules
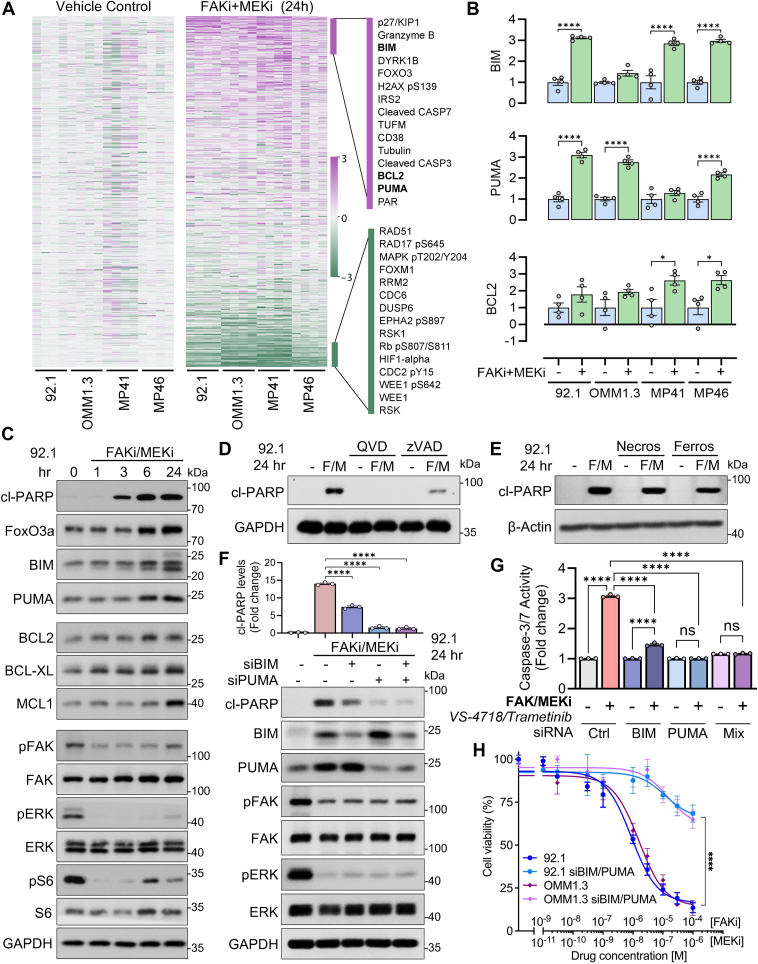
Combined inhibition of MEK and FAK pathways induces significant alterations in the proteomic landscape of UVM cells. To elucidate these changes, we conducted a comprehensive proteomic analysis using reverse-phase protein array (RPPA) technology on lysates from representative UVM cells harboring GNAQ mutations (92.1, OMM1.3, MP41, and MP46). Cells were treated with the FAKi (VS-4718, 1 μM) in combination with MEKi (trametinib, 10 nM) (Fig. 1A). Bioinformatic analysis of RPPA data revealed that, 24 h post-treatment, there was a significant upregulation of DNA damage, cell stress, and pro-apoptotic proteins, including p27/KIP1, granzyme B, FOXO3, H2AX, BIM, and PUMA (Figs. 1B and S1, A and B). Simultaneously, anti-apoptotic proteins such as BCL2 were significantly upregulated (Fig. 1B). To validate these findings, we performed time-course Western blot analyses of 92.1 cells treated with the combination of FAKi and MEKi. The results demonstrated increased expression of pro-apoptotic proteins FOXO3a, BIM, and PUMA, along with elevated levels of cleaved poly(ADP-ribose) polymerase (cl-PARP), a marker of apoptosis (20, 21) (Figs. 1C and S1, C–F). Consistent with these observations, treatment with MEKi in FAK knockdown (KD) 92.1 cells resulted in similar increases in BIM, PUMA, and cl-PARP levels (Fig. S1G), validating previously reported synergistic effects of MEKi + FAKi in promoting apoptosis (12). Notably, we also observed the upregulation of anti-apoptotic proteins, including BCL2, BCL-XL, and MCL1 (Figs. 1C and S1, E–F). These proteins belong to the BCL2 family, which represent candidate anti-apoptotic proteins that might promote the survival of persisting cells against treatment. The concurrent upregulation of both pro-apoptotic and anti-apoptotic proteins suggests a complex cellular response to dual FAK and MEK inhibition. While apoptotic pathways are activated, the cells may simultaneously initiate survival mechanisms by upregulating anti-apoptotic proteins to counteract cell death signals.
Figure 1.
Combined FAK and MEK inhibition increases pro-apoptotic and anti-apoptotic protein levels in uveal melanoma cells.A, Heat map from RPPA analysis of uveal melanoma (UVM) cell lines (92.1, OMM1.3, MP41, and MP46) treated with FAKi (VS-4718, 1 μM) and MEKi (trametinib, 10 nM) for 24 h. B, RPPA analysis highlights changes in BCL2, PUMA, and BIM expression levels in treated cells. Data represents four different samples: ∗p < 0.05, ∗∗∗∗p < 0.0001, unpaired t test. C, Western blot validation showing the time course of pro-apoptotic (PUMA, BIM, FOXO3a, and cl-PARP) and anti-apoptotic (BCL2, BCL-XL, MCL1) proteins in 92.1 cells treated with the FAKi + MEKi combination, as well as total and phosphorylated FAK, ERK, and S6. The blots represent three independent experiments. D, Western blot indicating that caspase inhibitors (QVD-FMK and zVAD) prevent apoptosis induced by FAKi + MEKi treatment in 92.1 cells, as shown by reduced cl-PARP levels. The blots represent three independent experiments. E, Western blot demonstrates that necroptosis and ferroptosis inhibitors (necrostatin and ferrostatin, respectively) do not prevent apoptosis induced by FAKi + MEKi in 92.1 cells. All western blots were performed in at least 3 independent experiments; representative images are shown. F, knockdown of PUMA and BIM by siRNA reduces apoptosis (cl-PARP levels) induced by FAKi + MEKi in 92.1 cells. All the blots are representative of three independent experiments. ∗∗∗∗p < 0.0001, unpaired t test. G, knockdown of PUMA and BIM by siRNA decreases Caspase 3/7 activity induced by FAKi + MEKi in 92.1 cells. The graph represents three experiments; ns: no significant, ∗∗∗∗p < 0.0001, unpaired t test. H, knockdown of PUMA and BIM by siRNA diminishes the reduction in cell viability caused by FAKi + MEKi in 92.1 cells, as measured by a cell viability assay. ∗∗∗∗p < 0.0001, two-way ANOVA followed by Tukey test.
PUMA and BIM drive apoptosis mediated by FAK and MEK inhibition
To determine which pathway predominantly influences protein levels during apoptosis, we first assessed the effects of individual inhibitors. Notably, treatment with the MEKi alone induced both pro-apoptotic and anti-apoptotic proteins, while FAKi alone increased, especially PUMA and MCL1 levels (Fig. S1H). Based on these findings, we hypothesize that the combination of FAK and MEK inhibitors induces the death of UVM cells via apoptosis. To test this hypothesis, we demonstrated that caspase inhibitors (QVD and zVAD) effectively prevented cl-PARP and caspase activity increase in cells treated with FAKi + MEKi combination (Figs. 1D and S1I). In contrast, as negative controls, the inhibitors of necroptosis and ferroptosis (necrostatin-1 and ferrostatin, respectively) did not prevent the effect of FAK and MEK inhibition on cl-PARP levels (Fig. 1E) or cytotoxicity, as measured by the LDH assay (Fig. S1J). These cell death pathways, necroptosis and ferroptosis, are characterized as caspase-independent mechanisms. Together, all the results suggest that apoptosis is the primary cell death pathway activated by this treatment through BIM, PUMA, and caspase activity.
We then performed siRNA-mediated knockdown of the pro-apoptotic proteins PUMA and BIM and found significantly reduced apoptosis induced by FAKi + MEKi combination, as evidenced by decreased levels of cl-PARP (Figs. 1F and S1, K and L) and caspase activity (Fig. 1G). Additionally, silencing PUMA and BIM reduced sensitivity to FAKi + MEKi treatment in cell viability assays in 92.1 and OMM1.3 UVM cells (Fig. 1H). These results indicate that PUMA and BIM play crucial roles in mediating apoptosis under combined FAK and MEK inhibition. Consistent with this pro-apoptotic effect through BIM and PUMA, we found that cells harboring an active AKT pathway (via ectopic myr-PI3K and myr-AKT expression) were resistant to treatment, as evidenced by reduced cl-PARP levels (Fig. S1M). This observation supports the idea that AKT activity regulates apoptotic signaling, thereby conferring resistance to FAK + MEK inhibition.
Uveal melanoma cells develop adaptive resistance to combined MEK and FAK inhibitors
To prioritize molecular targets for potential therapeutic intervention, we generated spontaneous resistant UVM cell lines through chronic exposure to combined FAK and MEK inhibitors. This approach resulted in the development of multiple resistant populations (mass culture, MC) and individual clones (CL) (Fig. 2A). Specifically, UVM cells were cultured in the presence of two combinations of FAK and MEK inhibitors at low, medium, and high concentrations. We functionally validated the resistance of these cell lines by performing cell viability assays using both drug combinations of MEK and FAK inhibitors. Parental 92.1 UVM cells and the resistant cells were seeded and treated with increasing concentrations of trametinib (MEKi) and VS-4718 (FAKi) (Fig. 2B, left panel), as well as the combination of avutometinib (a “RAF/MEK clamp”) (22) and defactinib (FAKi) (Fig. 2B, right panel). To elucidate potential genetic mechanisms underlying resistance to combined FAK and MEK inhibition in UVM cells, we performed whole-exome sequencing on the resistance cell populations (Fig. 2C). Our analysis revealed that drug-resistant MCs and CLs exhibited distinct mutational profiles with no overlapping mutations that can be predictive of the emergence of pre-existing or acquired resistance-driving genetic alterations.
Figure 2.
Development of UVM cells resistant to combined FAK and MEK inhibition.A, experimental design for generating FAKi + MEKi-resistant clones (CL) and mass cultures (MC) through chronic drug exposure. The schematic illustrates continuous exposure of UVM cells to FAKi (VS-4718) and MEKi (trametinib or RAF/MEK clamp avutometinib). B, cell viability assays show that various resistant UVM cells (both clones and mass cultures) exhibit resistance to FAKi + MEKi compared to parental cells. We used the two FAKi: VS-4718 or defactinib; and two MEKi: trametinib or avutometinib. ∗∗∗∗p < 0.0001, two-way ANOVA followed by Tukey test. C, Whole-exome sequencing analysis reveals distinct mutational profiles in the resistant UVM cells (from B). D and E, Western blot analyses show reduced apoptosis (indicated by decreased levels of cl-PARP) in resistant clones (D) and mass cultures (E) upon treatment with FAKi (VS-4718) and MEKi (trametinib). All western blots were performed in at least 3 independent experiments; representative images are shown.
To confirm that these CL and MC are resistant to the FAKi + MEKi combination treatment, we measured cl-PARP levels (Fig. 2, D and E, upper blot) and observed reduced apoptosis, evidenced by their decreased levels relative to parental cells, consistent with a resistant phenotype. Further analysis showed that BCL2 protein levels increased slightly in resistant cells (CL and MC, Fig. S2, A and D) compared to parental cells, consistent with the data shown in Figure 1 during the treatment with the combination. Conversely, certain resistant cells, including MC1, MC2, MC3, CL2, and CL3, showed decreased levels of the pro-apoptotic protein BIM when treated with FAKi + MEKi (Figs. 2, D and E and S2, B and E). In parallel, in some of them, PUMA increases (Figs. 2, D and E and S2, C and F), likely as a compensation for the clear BIM reduction; however, it does not reach the levels required to trigger apoptosis progression. These findings prompted us to explore whether treatment resistance might be driven by non-genomic adaptive changes leading to the upregulation of anti-apoptotic proteins, such as BCL2.
BCL2 activity modulates apoptosis mediated by combined FAK and MEK inhibition
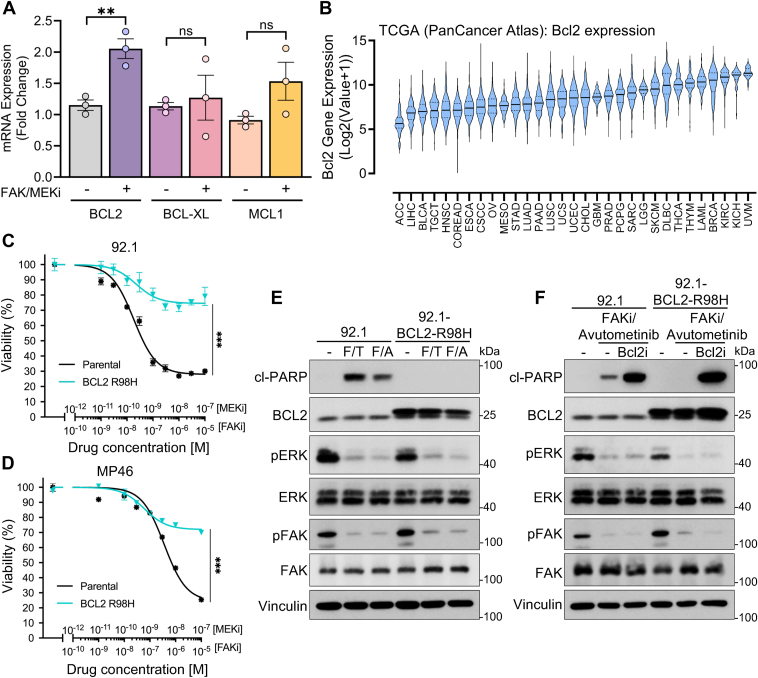
Given the potential pro-survival role of BCL2 in UVM cells, we analyzed its expression by quantitative real-time PCR (qPCR) and the induction during treatment. The analysis confirmed a significant increase in BCL2 mRNA levels after 24 h of treatment with the FAKi and MEKi combination in 92.1 UVM cells. In contrast, the mRNA levels of other BCL2 family members as BCL-XL and MCL1, did not show significant changes (Fig. 3A). Remarkably, by analyzing The Cancer Genome Atlas (TCGA) database across various cancer types, we found that UVM exhibits the highest expression levels of BCL2 compared to all other cancers (Fig. 3B). This suggests that BCL2 may play a crucial role in regulating apoptosis in UVM, potentially more so than other anti-apoptotic proteins.
Figure 3.
Expression of the BCL2 mutant confers resistance to FAK and MEK inhibitors.A, qPCR of BCL2, BCL-XL, and MCL1 expression in 92.1 cells treated with FAKi + MEKi for 24 h. The graph represents three experiments; ns: no significant, ∗∗p < 0.01, unpaired t test. B, analysis of BCL2 expression across different cancer types in TCGA, showing significantly elevated expression in uveal melanoma (UVM). C and D, cell viability assay in 92.1 (C) and MP46 (D) cells expressing the BCL2 R98H mutant and treated with MEKi (trametinib) + FAKi (VS-4718), demonstrating increased resistance compared to parental cells. ∗∗∗p < 0.001, two-way ANOVA followed by Tukey test. E, Western blot analysis of cl-PARP indicates that the BCL2-R98H mutant expression reduces apoptosis in cells treated with FAKi (F) (VS-4718) + trametinib (T) (MEKi) or avutometinib (A) (RAF/MEKi). All the blots are representative of three independent experiments. F, Western blot of cl-PARP in 92.1 cells expressing the BCL2-R98H mutant treated with FAKi + RAF/MEKi + BCL2i. All western blots were performed in at least 3 independent experiments; representative images are shown.
Building on our previous findings, we investigated the effect of this drug combination on 92.1 and MP46 UVM cells transduced to express an active mutant form of BCL2 (BCL2-R98H mutant). The R98H substitution in BCL2 affects a critical site within the BH3-binding groove, where Arg98 normally stabilizes interactions with pro-apoptotic BH3-only proteins through hydrogen bonding (PDB IDs: 4MAN and 6O0K) (23). Mutations in adjacent residues, such as G101V, have been reported to weaken BH3 domain binding and thereby enhance BCL2’s overall anti-apoptotic function (24). We performed cell viability assays using concentration-response curves for the FAKi + MEKi combination. The results demonstrated that cells expressing BCL2-R98H exhibited significant resistance to the treatment compared to the parental 92.1 (Fig. 3C) and parental MP46 cells (Fig. 3D). To further assess the impact of the active BCL2 mutant on apoptosis, we analyzed levels of cl-PARP in parental and BCL2-R98H 92.1 cells treated with FAKi + MEKi or FAKi + RAF/MEKi combination (Fig. 3E). The expression of the active BCL2 mutant effectively inhibited apoptosis induced by the drug combinations, as evidenced by the absence of cl-PARP in BCL2-R98H cells. This indicates that the active BCL2 mutant confers resistance by blocking apoptotic pathways activated by FAK and MEK inhibition.
Considering that the ectopic expression of active BCL2 mutant induces resistance to FAK and MEK inhibitors, we next evaluated whether inhibiting BCL2 could overcome this resistance. Among BCL2 family inhibitors (BCL2i), we selected the clinically approved venetoclax (ABT-199) (25) as a candidate to co-target with FAKi + MEKi the BCL2-R98H. Remarkably, venetoclax restored sensitivity to the FAK + RAF-MEKi (Fig. 3F) or FAK + MEKi combination (Fig. S3, A and B), overcoming the resistance induced by ectopic expression of BCL2-R98H, suggesting that targeting BCL2 activity can reverse resistance and enhance the efficacy of combined FAK and MEK inhibition in UVM.
Synergistic antiproliferative effect and apoptosis induction by combined FAKi + MEKi and BCL2i treatment in parental and drug-resistant uveal melanoma cells
Building upon the abovementioned results, we hypothesized that simultaneous inhibition of FAK, MEK, and BCL2 could synergistically induce apoptosis in uveal melanoma cells. To test this hypothesis, we conducted viability assays using a matrix (on 96-well plates) of increasing concentrations of the inhibitors venetoclax (BCL2i) co-targeting with FAKi (VS-4718) plus RAF/MEKi (avutometinib) in 92.1 and MP46 UVM cells. The latter was selected, given the recent use of this combination in the clinic (16). Heat maps of cell viability for 92.1 and MP46 (Fig. 4, A and B, left panels) demonstrated that the venetoclax combination treatment significantly reduced cell viability in both UVM cell lines compared with the lanes treated with the single agents. Synergy analysis of the viability data using the Highest Single Agent (HSA) model revealed a high synergy score for the combination of FAKi + RAF/MEKi with venetoclax in both cells (Fig. 4, A and B, right panels). These findings highlight the potential therapeutic benefit of the triple inhibition strategy in UVM.
Figure 4.
BCL2 inhibition enhances the effect of combined FAK + MEK inhibitors in both parental and resistant UVM cells.A and B, viability of MP46 (A) and 92.1 (B) cells after 72 h of treatment with FAKi (VS-4718) + RAF/MEKi (avutometinib) and the BCL2i (venetoclax). Synergy and its distribution in MP46 or 92.1 UVM cells treated. After 3 days, cell viability was analyzed by fluorescence. The Synergy Score (SS) was calculated with the HSA model (SS < −10 antagonism, −10 < SS < 10 additive, SS > 10 synergy). The heat map and synergy analysis represent at least three replicates. C–E, Western blot analysis of cl-PARP in MP46 parental (C), 92.1 parental (D), and a representative 92.1 resistant mass culture (MC3) (E) cell lines treated with FAKi + MEKi (trametinib) or RAF/MEKi (avutometinib) in combination with BCL2i (venetoclax). All Western blots were performed in at least 3 independent experiments; representative images are shown. The graph represents the quantification of three experiments with MC3 cells; ns: no significant, ∗∗∗∗p < 0.0001, unpaired t test. F–I. Caspase-3/7 activity assays in 92.1 parental (F and G) and 92.1 MC3-resistant cells (H and I) treated with FAKi + MEKi (trametinib) or RAF/MEKi (avutometinib) in combination with BCL2i (venetoclax). The graph represents three experiments: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, unpaired t test.
Consistent with the observed synergistic reduction in cell viability using the combination of FAKi + MEKi and BCL2 inhibitor (BCL2i, venetoclax), we investigated whether this triple inhibition could enhance apoptosis in both parental and resistant UVM cells, potentially overcoming resistance to FAK/MEK inhibition. We first assessed the apoptotic effects of increasing concentrations of venetoclax by measuring cl-PARP levels in MP46 and 92.1 UVM cell lines. Both cell lines exhibited dose-dependent increases in cl-PARP levels at 24 h upon venetoclax treatment (Fig. S3C), including resistant 92.1 cells (Fig. S3D), confirming that venetoclax effectively induces apoptosis in UVM cells. Building on these findings, we tested whether venetoclax could enhance the effect of FAKi + MEKi combinations in parental UVM cells and potentially overcome resistance in resistant cells. We found that the combination of BCL2i with FAKi (VS-4718) and MEKi (trametinib) or the RAF/MEKi clamp (avutometinib) enhanced apoptosis in BAP1-mutant MP46 (Figs. 4C and S4, A and B) and 92.1 cells (Figs. 4D and S4, C and D), as indicated by increased cl-PARP levels. This effect was further confirmed by a notable rise in caspase activity upon venetoclax treatment (Fig. 4, F and G), supporting the therapeutic potential of this combination.
We also assessed the effect of venetoclax on FAKi + RAF-MEKi-resistant UVM cells. All resistant cultures were sensitive to venetoclax, as evidenced by increased cl-PARP levels (Fig. S3D). Specifically, using representative resistant MC cells, venetoclax enhanced the apoptotic effects of FAKi + trametinib and restored the efficacy of FAKi + avutometinib, as demonstrated by increased cl-PARP levels, elevated caspase activity (MC3 cells: Fig. 4E, 4H-I, MC2 cells: Fig. S4, G and H), and reduced the viability in concentration-response curves (Fig. S4, E and F). These results suggest that BCL2 inhibition can overcome resistance to combined FAK and MEK inhibition. Collectively, these findings demonstrate that the triple combination enhances apoptosis in both parental and resistant UVM cells, supporting its potential as a strategy to overcome drug resistance.
BCL2 inhibitor displays a synergistic effect with the dual PKC/PKN inhibitor, darovasertib
Consistent with the observed synergistic effect of co-inhibiting FAK, MEK, and BCL2, we explored whether combining BCL2i with darovasertib, a dual PKC and PKN inhibitor (13), could enhance antitumor efficacy in UVM. Darovasertib is currently undergoing clinical trials for UVM and has shown therapeutic potential in the clinic (NCT05907954) (26), particularly when combined with a FAKi in experimental systems (13, 14). Since PKC and PKN are upstream regulators of both MEK and FAK pathways, respectively, rationally comparable to the MEKi + FAKi combination but with less toxicity than MEKi (trametinib), we hypothesized that inhibiting these kinases with darovasertib, in combination with BCL2 inhibition, would result in synergistic anticancer effects. To test this hypothesis, we performed cell viability assays using a matrix of increasing concentrations of darovasertib with venetoclax in 92.1 cells. The resulting heat map of cell viability and the synergism analysis revealed an additive interaction (synergy score 7.13) between darovasertib and venetoclax (Fig. 5A). The viability curves confirmed this trend, showing an enhancement of the effect of darovasertib when combined with venetoclax compared to the effects of the single agents (Fig. 5B).
Figure 5.
BCL2 inhibitor shows a synergistic effect with the PKC/PKN inhibitor darovasertib.A, synergy matrix of cell viability in 92.1 cells treated for 72 h with darovasertib (PKC/PKNi) in combination with BCL2i (venetoclax). The heat map and synergy analysis represent at least three replicates. Assessment of synergy and its distribution in 92.1 UVM cells treated (n = 3). After 3 days, cell viability was analyzed by fluorescence. B, concentration–response curves in 92.1 cells for venetoclax, darovasertib, or their combination. The graph represents three replicates normalized as a percentage change from the basal. Two-way ANOVA followed by Tukey test. C, synergy assay of cell viability in 92.1 cells after 72 h of darovasertib (PKC/PKNi) + FAKi (VS-4718) in combination with BCL2i (venetoclax). The heat map and synergy analysis represent at least three replicates. D, concentration–response curves in 92.1 cells for venetoclax, darovasertib + FAKi, or their combination. The graph represents three replicates normalized as a percentage change from the basal. Two-way ANOVA followed by Tukey test. E and F, Western blot analysis of cl-PARP in 92.1 (E) cells and MP46 (F) cells treated with darovasertib ± FAKi (VS-4718) and ± venetoclax (BCL2i). All Western blots were performed in at least 3 independent experiments; representative images are shown. G, caspase-3/7 activity assays in 92.1 UVM cells treated with darovasertib (PKC/PKNi) and FAKi (VS-4718) in combination with BCL2i (venetoclax). The graphs represent three independent experiments normalized (fold change) with control basal; ###p < 0.001 darovasertib vs darovasertib + FAKi, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, unpaired t test.
Building on our previous finding that FAKi combined with darovasertib exhibits a synergistic antitumor activity (13), we further investigated whether the addition of the FAKi could further enhance the antitumor activity of the darovasertib-venetoclax combination. We tested the triple combination of FAKi (VS-4718), darovasertib, and venetoclax. Remarkably, this combination demonstrated a highly synergistic interaction with a synergy score of 19.6 (Fig. 5C, Synergy Score > than 10 = synergistic). The viability curves of darovasertib + FAKi + BCL2i showed the best effect compared to the darovasertib + FAKi combination (Fig. 5D). To determine whether the reduction in cell viability was due to increased apoptosis, we measured levels of cl-PARP in 92.1 (Figs. 5E and S5A) and MP46 (Figs. 5F and S5B) UVM cells. Treatment with darovasertib alone induced apoptosis, as evidenced by an increase in cl-PARP levels; however, the addition of venetoclax significantly increased this effect in both cell lines. Similarly, when venetoclax was combined with darovasertib and two different FAKi (VS-4718 or defactinib), we observed a substantial increase in cl-PARP level and caspase activity with BCL2i + Darovasertib and with triple combination (Figs. 5, E–G and S5, C and D), indicating an enhanced induction of apoptotic cell death.
Discussion
Despite significant advancements in elucidating the molecular mechanisms of uveal melanoma, this malignancy remains a significant clinical challenge due to its high propensity for metastasis and limited effective therapeutic options. Targeting the Gαq pathway, which drives UVM through mutations in GNAQ or GNA11, has proven difficult, particularly given the lack of clinically relevant direct inhibitors and the limited efficacy of MEKi in clinical trials (9, 10). Consequently, identifying and exploiting key downstream survival mechanisms has emerged as a promising strategy to address these therapeutic challenges. In this study, we investigated the adaptive responses of UVM cells to combined MEK and FAK inhibition, a combination that showed promise in inducing apoptosis in UVM experimental models (12), and encouraging results in the clinic, albeit patients develop treatment resistance (16). Our findings reveal a dualistic cellular response: while MEK + FAK inhibition effectively activates pro-apoptotic signaling pathways, notably through upregulation of BH3-only proteins BIM and PUMA, it simultaneously induces an adaptive increase in anti-apoptotic proteins, including BCL2, BCL-XL, and MCL1. This suggests that UVM cells may deploy a compensatory mechanism to evade apoptosis, thereby conferring resistance to MEK + FAK-targeted therapies (Fig. 6). In fact, in cells resistant to MEKi + FAKi, we found a significant increase in BCL2 and low levels of BIM, promoting survival; these changes in the balance between anti- and pro-apoptotic proteins were consistent across cultures of resistant cells.
Figure 6.
BCL2 induces adaptive resistance to FAK and MEK inhibition in UVM cells. Proposed mechanism of action of MEK, RAF/MEK, FAK, and PKN/PKC inhibition, and the resulting compensatory increase in BCL2, thereby conferring resistance to MEK + FAK-targeted therapies and exposing a druggable target for combination therapies increasing therapeutic response (see text for details).
Analysis of data from TCGA revealed that BCL2 expression is elevated in UVM compared to all other cancer types, underscoring its critical role in tumor cell survival and resistance to therapy. This elevated expression may be attributed to the unique biology of UVM cells, which may rely heavily on BCL2-mediated survival pathways. Furthermore, the high BCL2 levels in UVM suggest a potential vulnerability that could be exploited therapeutically. Indeed, we found that venetoclax, a clinically approved selective BCL2 inhibitor, overcomes this resistance mechanism, resulting in UVM cell death. Venetoclax is currently used for hematological malignancies, such as chronic lymphocytic leukemia (27) and acute myeloid leukemia (28), and although the possibility of combining MEKi, FAKi, and venetoclax can be counterintuitive, given the potential of added toxicities, the quadruple combination of targeted agents, including venetoclax, has recently shown clinical benefit in relapsed diffuse large B-cell lymphoma (29). This suggests the potential applicability in solid tumors, such as in UVM, to synergize with MEK/FAK inhibitors or to overcome resistance to these targeted agents. Similarly, BCL2 inhibition can potentiate the pro-apoptotic effects of targeted therapies disrupting upstream signaling pathways critical for UVM cell survival, including darovasertib, a potent and selective dual inhibitor of PKC and PKN currently undergoing clinical trials for UVM (NCT05907954) (26). We further explored a triple combination therapy by adding a FAKi to the darovasertib and venetoclax regimen. Remarkably, this triple combination exhibited the highest synergy score among all other combinations, suggesting its superior therapeutic potential. This comprehensive strategy may effectively suppress the oncogenic drivers of this malignancy and the protective mechanisms that enable UVM cells to resist apoptosis.
Ultimately, by uncovering the adaptive upregulation of BCL2 as a resistance mechanism to MEK/FAK inhibition and demonstrating that BCL2 inhibition can overcome this resistance, we provide a strong rationale for the future clinical evaluation of combination therapies incorporating venetoclax. Finally, although further studies are required to determine its full therapeutic potential, the triple combination of darovasertib, FAK inhibitor, and venetoclax showed enhanced synergy (Fig. 6), suggesting that it may pave the way for more effective and personalized treatment options for patients with this aggressive ocular cancer.
Experimental procedures
Reverse-phase protein array (RPPA)
UVM cells were lysed for RPPA analysis in a buffer consisting of 50 mmol/L HEPES pH 7.4, 150 mmol/L NaCl, 1.5 mmol/L MgCl2, 1 mmol/L EGTA, 100 mmol/L NaF, 10 mmol/L Na pyrophosphate, 1 mmol/L Na3VO4, 10% glycerol, and 1% Triton X-100. Protease inhibitors (Roche, Basel, Switzerland) were added just before use. After 20 min of lysis, lysates were clarified by centrifugation. Protein concentration of the resultant supernatants was determined using Protein Assay Dye Reagent Concentrate (Bio-Rad, Hercules, CA). Protein lysates were collected from four independent experiments and sent to the MD Anderson (Houston, TX) Reverse Phase Protein Array Core Facility for analysis.
Whole-exome sequencing
DNA was isolated from cell pellets using the DNeasy Blood & Tissue Kit (Qiagen). Quantification of DNA was performed with the Qubit dsDNA HS Assay Kit and a Qubit 2.0 Fluorometer (Thermo Fisher Scientific). DNA sequencing was carried out by Novogene (Novogene Corporation). FASTQ files were analyzed using the bcbio workflow manager (version 1.2.9). In brief, reads were trimmed using atropos, aligned to the Novogene WES regions of GRCh37 using BWA, and variants were called using a 2 of 2 ensemble method with both vardict and mutect2. Parental cells were labeled as “normal” and the MEK/FAK-resistant cells as “cancer”. Resultant VCF mutation calls for all “cancer” lines were then filtered for QUAL > 30, AF > 0.04, and DP > 75, then loaded into R, and oncoprint plots were made using maftools.
Cell lines, culture procedures, and chemicals
Uveal melanoma cells (92.1, OMM1.3, human uveal melanoma cells) were cultured in RPMI-1640 (R8758, Sigma Aldrich Inc.) containing 10% FBS (F2442, Sigma-Aldrich Inc.), 1X antibiotic/antimycotic solution (A5955, Sigma-Aldrich Inc.), and 1X Plasmocin prophylactic (ant-mpp, InvivoGen). MP41 and MP46 human uveal melanoma cells were cultured in RPMI-1640 (R8758, Sigma Aldrich Inc.) containing 20% FBS (F2442, Sigma-Aldrich Inc.), 1X antibiotic/antimycotic solution, and 1X Plasmocin prophylactic. The drugs used were: Trametinib (GSK1120212), VS-4718 (S7653), darovasertib (LXS-196, S6723), avutometinib (VS-6766), and venetoclax (ABT-199, S8048) were purchased from SelleckChem.
Transfections of siRNAs
All knockdown experiments were performed using siRNAs purchased from Horizon Discovery Biosciences (Non-targeting Control: D-001810-10-05, BIM (BCL2L11): smartpool L-004383-00-0005, siRNA#1 J-004383-09-0002, siRNA#2 J-004383-10-0002; PUMA (BBC3): smartpool L-004380-00-0005, siRNA#1 J-004380-09-0002, siRNA#2 J-004380-10-0002. The transfection mix was prepared in OptiMEM media (Cat. 31985-070) and was added the siRNAs and Lipofectamine RNAiMAX Reagent (13778150, Thermo Fisher Scientific) were added according to the manufacturer’s instructions.
Cell viability assay
For viability curves and viability in the synergism protocol, the 92.1 and MP46 cells were seeded at a density of 3000 cells/well in 96-well white plates (CulturePlate: PerkinElmer Inc.). Up to 10 dilutions of each inhibitor were assayed in technical triplicate for 72 h. Later, we removed the media with treatments, the cells were washed with 1X PBS, and complete media was added. Then, AquaBluer Cell Viability Reagent (MultiTarget Pharmaceuticals, cat. 6015) was incubated for 3 h, and the cell viability was measured on a microplate reader (Spark, Tecan) according to the manufacturer’s instructions.
Synergy determination with the HSA method
The Highest Single Agent (HSA) synergy model (30) was used to determine the possible synergistic effects of MAPKi (avutometinib or Darovasertib) + FAKi + BCL2i combinations. Briefly, cells were seeded at a density of 3 × 103 to 5 × 103 cells/well in 96-well white plates (CulturePlate; PerkinElmer Inc). Cells were treated with either single inhibitors or combinations thereof using seven and five dilutions of each inhibitor, and in technical triplicate. We prepared a matrix of increasing concentrations to combine all of them in independent wells. for trametinib, we use the concentrations: 100, 10, 3, 1, 0.3, 0.1, and 0.01 nM. Meantime for avutometinib, darovasertib, defactinib, and VS-4718: 10, 1, 0.3, 0.1, 0.03, 0.01, and 0.001 μM. Finally, for venetoclax, we use 10, 3, 1, 0.3, and 0.1 μM in supplemented medium. After 72 h, we changed the medium, and the cell viability was measured with the AquaBluer Cell Viability Reagent on a Spark microplate reader (Tecan). The Synergy Score (SS) was calculated using the SynergyFinder + web application (31). SS < than −10 = antagonistic, SS from −10 to 10 = additive, SS > than 10 = synergistic.
Immunoblotting
Cells were deprived of serum overnight and subsequently treated as specified in the figure legend. To lyse the cells, they were washed twice with cold PBS and then lysed using 1X Cell Lysis buffer (Cell Signaling Technologies, catalog #9803) supplemented with Halt Protease and Phosphatase Inhibitor Cocktail (ThermoFisher Scientific, #78440) and 1 mM Sodium Orthovanadate (New England Biolabs, P0758S). The lysates were centrifuged at maximum speed at 4 °C, and protein concentrations were determined using DC Protein Assay (BioRad Laboratories, catalog #5000111). Subsequently, lysates were mixed with 4x Laemmli Sample Buffer (BioRad Laboratories, #1610747) containing β-mercaptoethanol and boiled for 5 min at 98 °C.
For immunoblotting, the cell lysates were separated by SDS/PAGE on 10% acrylamide gels and then transferred to PVDF membranes. Blocking and antibody incubation (both primary and secondary) were carried out in Tris-buffered saline with 0.1% Tween 20, supplemented with either 5% (w/v) BSA or 5% (w/v) skim milk. Primary antibodies from Cell Signaling Technologies were used at a 1:1000 dilution, including BIM (#2933), PUMA (#12450), BCL-XL (#2764), MCL1 (#94296), Foxo3A (#2497), cl-PARP (#5625), FAK (#71433), pY397-FAK (#8556), S6 (#2317), pS235/236 S6 (#4858), ERK1/2 (#9102), pT202/Y204-ERK1/2 (#4370), GAPDH (#5174), Beta-actin (#4970), Vinculin (#13901). For BCL2 detection, an antibody from Santa Cruz (sc-783) was used. HRP-conjugated goat anti-rabbit and anti-mouse IgG (Southern Biotech, AL) were applied at a 1:10,000 dilution. The specificity of the antibodies was validated using inhibitors or siRNAs to confirm the correct band. Immunoreactive bands were visualized using Immobilon Western Chemiluminescent HRP substrate (Millipore) following the manufacturer’s protocol. The blot images were developed in autoradiography film (Cat. No. 1141J52, labForce; Thomas Scientific) and with the iBright 1500 machine (Invitrogen). All representative Western blots were conducted in a minimum of 3 independent experiments. The blots were analyzed using ImageJ to obtain the densitometric values shown in the graphs, with normalization to GAPDH levels.
CaspaseGlo3/7 assay
Cells were plated at a density of 10,000 cells per well in 96-well white plates. Following a 24-h incubation, the cells were starved and treated with either drug or vehicle control, and the indicated assays were performed. Apoptosis was evaluated 24 h after treatment using the Caspase-Glo 3/7 Assay System (Promega G8090) according to the manufacturer's guidelines.
Cytotoxicity LDH assay
A cytotoxicity assay using LDH detection (Dojindo Kit, cat. CK12) was performed. The cells were plated at a density of 10,000 cells per well in 96-well white plates. The next day, the cells were starved and treated with MEKi + FAKi and caspase, ferroptosis, or necroptosis inhibitors (QVD, Ferrostatin, Necrostatin, respectively (32)) for 24 h, and the indicated assays were performed. LDH was evaluated 24 h after treatment in the supernatants, according to the manufacturer's guidelines.
RNA extraction, reverse transcription, and real-time PCR
Total RNA was extracted using QIAzol (Qiagen, cat 79306) following the manufacturer’s instructions. Prior to reverse transcription, 1 μg of total RNA was treated with DNase I (amplification grade, Invitrogen) according to the manufacturer’s protocol. Then, 0.25 μg of DNase-treated RNA was reverse transcribed using the iScript cDNA Synthesis Kit (Bio-Rad) on an S1000 Thermal Cycler (Bio-Rad). Gene expression analysis by qPCR was carried out using 1 μl of 1:5 diluted cDNA, SsoAdvanced Universal Supermix (Bio-Rad), and specific primers on a CFX96 Real-Time System (Bio-Rad). The reaction conditions consisted of an initial step at 98 °C for 30 s, followed by 40 cycles of 98 °C for 3 s, 58 °C for 20 s, and 72 °C for 10 s. A melting-curve analysis (65 °C to 95 °C, 0.5 °C/s increment) was performed after each PCR to verify specificity. All reactions were conducted in triplicate, and the average Ct value of the triplicates was used to determine transcript expression via the 2−ΔΔCt method. GAPDH served as the reference gene.
qPCR Primers:
BCL2 qPCR Fw: TGACTGAGTACCTGAACCGG, Rv: GCCAAACTGAGCAGAGTCTT.
Bcl-XL qPCR Fw: GTAAACTGGGGTCGCATTGT, Rv: TGCTGCATTGTTCCCATAGA.
Mcl1 qPCR-Fw: AGAAAGCTGCATCGAACCAT, Rv: CCAGCTCCTACTCCAGCAAC.
GAPDH qPCR-Fw: CGCTCTCTGCTCCTCCTGTT, Rv: CCATGGTGTCTGAGCGATGT.
Statistical analysis
All data analysis was performed using GraphPad Prism Version 10.0.1 for Mac (GraphPad Software; www.graphpad.com). The data were analyzed by the normality Shapiro-Wilk test, and by one-way ANOVA or two-way ANOVA followed by Tukey, and by unpaired t test as appropriate and described in figure legends (asterisks denote: ns, no significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
Data availability
All data relevant to this study are included in the article. For further details or requests for resources and reagents, please contact the lead author, Dr J. Silvio Gutkind (sgutkind@health.ucsd.edu).
Supporting information
This article contains supporting information.
Conflict of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: J. Silvio Gutkind reports consulting fees from Radionetics Oncology, BTB Therapeutics, Pangea Therapeutics, and io9 and is the founder of Kadima Pharmaceuticals. J. S. G. and NA hold a patent US11679113B2 related in part to this work. A. E. Aplin holds an ownership interest in patent number 9880150 and has a pending patent application, PCT/US22/76492. Other authors declare no competing financial interests.
Acknowledgments
Author contributions
S. L., R. D. C. V., N. A., and J. S. G. conceptualization; S. A. G., A. O., and A. E. A. methodology; S. L., R. D. C. V., S. A. G., and G. C. M. validation; S. L. and R. D. C. V. formal analysis; S. L., R. D. C. V., N. A., G. C. M., and J. S. G. investigation; S. L., R. D. C. V., N. A., and J. S. G. visualization; S. L., N. A., A. E. A., and J. S. G. funding acquisition; J. S. G. supervision; S. L., R. D. C. V., and J. S. G. writing–original draft; S. L., R. D. C. V., A. E. A., and J. S. G. writing–review & editing.
Funding and additional information
Support was provided by the National Institute of Health R01CA257505 (J. S. G., A. E. A.), Department of Defense W81XWH2110821 (J. S. G., A. E. A.), Melanoma Research Alliance MRA827624 (J. S. G., A. E. A.), and National Institutes of Health 5U54CA209891 (J. S. G.). A. E. A. was also supported by an award from the Dr Miriam and Sheldon G. Adelson Medical Research Foundation. The RPPA studies were performed at the Functional Proteomics Core Facility at The University of Texas MD Anderson Cancer Center, which is supported by an NIH/NCI Cancer Center Support Grant (P30 CA16672). European Union Horizon 2020 Marie Skłodowska-Curie no. 101027731 (S. L.), AIRC and the European Union’s Horizon 2020 Marie Skłodowska-Curie no. 800924 (to S. L.).
Reviewed by members of the JBC Editorial Board. Edited by Kirill Martemyanov
Contributor Information
Simone Lubrano, Email: lubrano.simone16@gmail.com.
J. Silvio Gutkind, Email: sgutkind@health.ucsd.edu.
Supporting information
References
- 1.Singh A.D., Turell M.E., Topham A.K. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118:1881–1885. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 2.Robertson A.G., Shih J., Yau C., Gibb E.A., Oba J., Mungall K.L., et al. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer Cell. 2017;32:204–220.e215. doi: 10.1016/j.ccell.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodman S.E. Metastatic uveal melanoma: biology and emerging treatments. Cancer J. 2012;18:148–152. doi: 10.1097/PPO.0b013e31824bd256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvajal R.D., Schwartz G.K., Tezel T., Marr B., Francis J.H., Nathan P.D. Metastatic disease from uveal melanoma: treatment options and future prospects. Br. J. Ophthalmol. 2017;101:38–44. doi: 10.1136/bjophthalmol-2016-309034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan P., Hassel J.C., Rutkowski P., Baurain J.F., Butler M.O., Schlaak M., et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N. Engl. J. Med. 2021;385:1196–1206. doi: 10.1056/NEJMoa2103485. [DOI] [PubMed] [Google Scholar]
- 6.Van Raamsdonk C.D., Bezrookove V., Green G., Bauer J., Gaugler L., O'Brien J.M., et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shoushtari A.N., Carvajal R.D. GNAQ and GNA11 mutations in uveal melanoma. Melanoma Res. 2014;24:525–534. doi: 10.1097/CMR.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 8.Carvajal R.D., Piperno-Neumann S., Kapiteijn E., Chapman P.B., Frank S., Joshua A.M., et al. Selumetinib in combination with dacarbazine in patients with metastatic uveal melanoma: a phase III, multicenter, randomized trial (SUMIT) J. Clin. Oncol. 2018;36:1232–1239. doi: 10.1200/JCO.2017.74.1090. [DOI] [PubMed] [Google Scholar]
- 9.Carvajal R.D., Sosman J.A., Quevedo J.F., Milhem M.M., Joshua A.M., Kudchadkar R.R., et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. Jama. 2014;311:2397–2405. doi: 10.1001/jama.2014.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steeb T., Wessely A., Ruzicka T., Heppt M.V., Berking C. How to MEK the best of uveal melanoma: a systematic review on the efficacy and safety of MEK inhibitors in metastatic or unresectable uveal melanoma. Eur. J. Cancer. 2018;103:41–51. doi: 10.1016/j.ejca.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Feng X., Arang N., Rigiracciolo D.C., Lee J.S., Yeerna H., Wang Z., et al. A platform of synthetic lethal gene interaction networks reveals that the GNAQ uveal Melanoma oncogene controls the Hippo pathway through FAK. Cancer Cell. 2019;35:457–472.e455. doi: 10.1016/j.ccell.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paradis J.S., Acosta M., Saddawi-Konefka R., Kishore A., Gomes F., Arang N., et al. Synthetic lethal screens reveal cotargeting FAK and MEK as a multimodal precision therapy for GNAQ-Driven uveal melanoma. Clin. Cancer Res. 2021;27:3190–3200. doi: 10.1158/1078-0432.CCR-20-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arang N., Lubrano S., Ceribelli M., Rigiracciolo D.C., Saddawi-Konefka R., Faraji F., et al. High-throughput chemogenetic drug screening reveals PKC-RhoA/PKN as a targetable signaling vulnerability in GNAQ-driven uveal melanoma. Cell Rep. Med. 2023;4 doi: 10.1016/j.xcrm.2023.101244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarin M., Nemati F., Decaudin D., Canbezdi C., Marande B., Silva L., et al. FAK inhibitor-based combinations with MEK or PKC inhibitors trigger synergistic Antitumor effects in uveal melanoma. Cancers (Basel) 2023;15 doi: 10.3390/cancers15082280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin H., Wang L., Bernards R. Rational combinations of targeted cancer therapies: background, advances and challenges. Nat. Rev. Drug Discov. 2023;22:213–234. doi: 10.1038/s41573-022-00615-z. [DOI] [PubMed] [Google Scholar]
- 16.Seedor R.S., Terai M., Majeed A., Tanaka R., Aplin A.E., Orloff M., et al. Abstract CT260: a phase II trial of defactinib combined with avutometinib in patients with metastatic uveal melanoma. Cancer Res. 2024;84:CT260. [Google Scholar]
- 17.Kaloni D., Diepstraten S.T., Strasser A., Kelly G.L. BCL-2 protein family: attractive targets for cancer therapy. Apoptosis. 2023;28:20–38. doi: 10.1007/s10495-022-01780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarosiek K.A., Wood K.C. Endogenous and imposed determinants of apoptotic vulnerabilities in Cancer. Trends Cancer. 2023;9:96–110. doi: 10.1016/j.trecan.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts A.W., Davids M.S., Pagel J.M., Kahl B.S., Puvvada S.D., Gerecitano J.F., et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic Leukemia. N Engl J Med. 2016;374:311–322. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green D.R. Apoptotic pathways: the roads to ruin. Cell. 1998;94:695–698. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- 21.Chaitanya G.V., Steven A.J., Babu P.P. PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. 2010;8:31. doi: 10.1186/1478-811X-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lubrano S., Cervantes-Villagrana R.D., Faraji F., Ramirez S., Sato K., Adame-Garcia S.R., et al. FAK inhibition combined with the RAF-MEK clamp avutometinib overcomes resistance to targeted and immune therapies in BRAF V600E melanoma. Cancer Cell. 2025;43:428–445.e426. doi: 10.1016/j.ccell.2025.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petros A.M., Medek A., Nettesheim D.G., Kim D.H., Yoon H.S., Swift K., et al. Solution structure of the antiapoptotic protein bcl-2. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3012–3017. doi: 10.1073/pnas.041619798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petros A.M., Olejniczak E.T., Fesik S.W. Structural biology of the Bcl-2 family of proteins. Biochim. Biophys. Acta. 2004;1644:83–94. doi: 10.1016/j.bbamcr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Croce C.M., Reed J.C. Finally, an apoptosis-targeting therapeutic for cancer. Cancer Res. 2016;76:5914–5920. doi: 10.1158/0008-5472.CAN-16-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joshua A.M., O'day R., Glasson W., Sia D., McGrath L., Ameratunga M., et al. A phase 2 safety and efficacy study of neoadjuvant/adjuvant darovasertib for localized ocular melanoma. J. Clin. Oncol. 2024;42:9510. [Google Scholar]
- 27.Eichhorst B., Niemann C.U., Kater A.P., Furstenau M., von Tresckow J., Zhang C., et al. First-Line venetoclax combinations in chronic lymphocytic leukemia. N. Engl. J. Med. 2023;388:1739–1754. doi: 10.1056/NEJMoa2213093. [DOI] [PubMed] [Google Scholar]
- 28.DiNardo C.D., Jonas B.A., Pullarkat V., Thirman M.J., Garcia J.S., Wei A.H., et al. Azacitidine and venetoclax in previously untreated acute Myeloid Leukemia. N Engl J Med. 2020;383:617–629. doi: 10.1056/NEJMoa2012971. [DOI] [PubMed] [Google Scholar]
- 29.Melani C., Lakhotia R., Pittaluga S., Phelan J.D., Huang D.W., Wright G., et al. Combination targeted therapy in relapsed diffuse large B-Cell lymphoma. N. Engl. J. Med. 2024;390:2143–2155. doi: 10.1056/NEJMoa2401532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loewe S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung. 1953;3:285–290. [PubMed] [Google Scholar]
- 31.Zheng S., Wang W., Aldahdooh J., Malyutina A., Shadbahr T., Tanoli Z., et al. SynergyFinder plus: toward better interpretation and annotation of drug combination screening datasets genomics. Proteomics Bioinform. 2022;20:587–596. doi: 10.1016/j.gpb.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koshizuka K., Wu X., Sato K., Vo P.T.T., Murawska G.M., Ishikawa T., et al. Genome-Wide CRISPR screening reveals that mTOR inhibition initiates ferritinophagy and ferroptosis in head and neck. Cancer Cancer Res. 2025;85:3032–3051. doi: 10.1158/0008-5472.CAN-24-3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to this study are included in the article. For further details or requests for resources and reagents, please contact the lead author, Dr J. Silvio Gutkind (sgutkind@health.ucsd.edu).