Abstract
Gram-negative bacterial proteins secreted by ABC exporters carry a secretion signal in their carboxylic extremities. This characteristic suggests that the polypeptide needs to be fully synthesized before it can be secreted and, therefore, presumably may fold at least in part before its secretion. We investigated the relationship between folding and secretion using HasA, a hemoprotein of Serratia marcescens secreted into the extracellular medium by a dedicated Has ABC exporter. We first demonstrated that when HasA is sequestered in the cytoplasm it can acquire its tertiary structure, as assessed from its capacity to bind heme. The cytoplasmic pool of HasA cannot be secreted and inhibits the secretion of newly synthesized molecules. HasA folding in the cytoplasm was independent of either its capacity to bind heme or the presence of SecB, although SecB is essential for HasA secretion. Our findings indicate a strong coupling between synthesis and secretion in the type I secretion pathway.
Keywords: ABC exporter/folding/hemophore/SecB/ type I secretion
Introduction
In Gram-negative bacteria, proteins are targeted to various compartments: cytoplasm, inner and outer membranes, periplasm and extracellular medium. Work with reporter proteins active in one compartment and not in others (e.g. β-galactosidase and alkaline phosphatase), and the existence of inactive cytoplasmic precursors indicate that extracytoplasmic proteins are not necessarily active before they reach their final localization. In some cases, e.g. proteases and toxins, proteins must be kept inactive in the cytoplasm. To reach their extracytoplasmic localization, proteins have to cross one or two membranes. There are various secretion pathways facilitating this process (for recent reviews see Koster et al., 2000; Stathopoulos et al., 2000; Harper and Silhavy, 2001).
Two pathways have been described for crossing the inner membrane: the Sec and the Tat pathways. Only unfolded proteins can be translocated to the periplasm by the Sec machinery. N-terminal signal sequences and cytoplasmic chaperones ensure that proteins remain competent for translocation (Rosemond et al., 1994; Wild et al., 1996; Randall et al., 1998). In contrast, the Tat pathway translocates proteins that are at least partially folded and often carrying a cofactor (Berks et al., 2000). Two mechanisms for crossing the outer membrane have been described. One is a two-step process involving the Sec pathway and then transport of the protein substrate from the periplasm to the extracellular medium. The other allows secretion from the cytoplasm to the extracellular medium in a single step by pathways unrelated to Sec and Tat. When substrate proteins reside, even briefly, in the periplasm, they may fold and disulfide bonds may form (Pugsley et al., 2001). It has also been reported that some proteins need to fold before they can cross the outer membrane (Peek and Taylor, 1992; Hardie et al., 1995; El Khattabi et al., 1999). Several pathways have also been shown to allow folded proteins to cross membranes in eukaryotes (Teter and Klionsky, 1999).
Two independent secretion pathways (type I and type III) use the single-step strategy. Some proteins that use the type III machinery are synthesized with specific cytoplasmic chaperones; however, the folding of these proteins during secretion has not been studied (Wattiau et al., 1996). The type I secretion pathway uses a simple secretion apparatus consisting of only three proteins: the inner membrane ABC protein (ATP binding cassette), which hydrolyses ATP, the membrane fusion protein (MFP) (which is also in the inner membrane), and a specific outer membrane protein (OMP), of which the best known member is TolC from Escherichia coli (Wandersman and Delepelaire, 1990). This secretion apparatus is known as an ABC exporter. The secretion signal carried by the protein substrate is at the C-terminus of the protein, implying that the protein is fully synthesized before its secretion (Nicaud et al., 1986). The folding state competent for secretion has not been studied. The crystal structure of TolC shows that its trimeric state forms a pore of ∼35 Å in diameter and ∼140 Å in length, suggesting that most secreted proteins have to be unfolded (Koronakis et al., 2000).
HasA is a small hemoprotein (188 amino acids) of Serratia marcescens, which is secreted by the dedicated Has ABC exporter. Its function, once secreted into the extracellular medium in the apo form, is to acquire heme from other hemoproteins and to transfer it to a specific outer membrane receptor, HasR (Létoffé et al., 1994a; Ghigo et al., 1997). Both the secretion and heme acquisition systems have been successfully reconstituted in E.coli (Létoffé et al., 1994b; Ghigo et al., 1997). The ABC exporter, Has, is composed of HasD (the ABC protein), HasE (the MFP protein) and HasF or TolC (the OMP protein). The hemoprotein interacts with the ABC protein and induces the association of the three exporter components (Binet and Wandersman, 1996; Létoffé et al., 1996). Hemin–agarose affinity chromatography was successfully used to isolate HasA associated with its ABC exporter from solubilized cell envelope preparations (Létoffé et al., 1996). As none of the three exporter proteins bind to hemin–agarose, this experiment showed that the hemoprotein retained the ability to interact with the exporter when bound to hemin–agarose. This interaction was shown to be dependent on the ABC protein, which implies that folded HasA can interact with the ABC protein (Létoffé et al., 1996). This interaction is probably driven by the C-terminal extremity, which is highly disordered, is independent of the rest of the globular protein and is on the opposite side to the heme-binding pocket (Wolff et al., 1997; Arnoux et al., 1999). HasA secretion nevertheless requires the SecB protein, a specific chaperone of the Sec pathway, which maintains cytoplasmic precursors in an unfolded export-competent state and targets them to the Sec machinery (Delepelaire and Wandersman, 1998; Randall et al., 1998). This SecB dependency suggested that HasA might be maintained in an unfolded state for its secretion. The exporter recognizes folded HasA in vitro, and a chaperone is required for efficient secretion of HasA in vivo. We therefore investigated the folding state of HasA during its secretion.
Many bacterial hemoproteins with non-covalently bound heme, such as hemoglobins or catalases, are active in the cytoplasm. If they stay soluble, foreign hemoproteins produced in the E.coli cytoplasm are loaded with heme (Verderber et al., 1997; Wang et al., 1999). Heme-loaded hemoproteins show a spectral characteristic called the Soret band. We used this spectral property to develop a simple technique to measure the heme-binding capacity of HasA in whole cells. We then studied the relationships between the cytoplasmic folding of HasA and its secretion by its own ABC exporter.
Results
HasA binds heme when sequestered in the cytoplasm
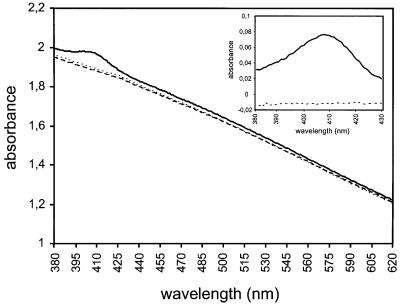
We wished to use the capacity for HasA to bind heme as a marker of the HasA folding state in vivo. To test for folded HasA in the cytoplasm, we expressed hasA in E.coli in the absence of its exporter. HasA accumulated in the cytoplasm, as detected by Coomassie Blue staining (Figure 1, lane 1) and confirmed by immunoblotting (not shown). No HasA was detected in the supernatant (Figure 1, lane 2). When we measured the absorption spectrum of whole cells (see Materials and methods), there was a peak centered at 407 nm (the Soret band) in strains containing cytoplasmic HasA. This indicated that HasA was heme loaded (Figure 2). There was no such peak either in strains containing a heme-binding defective mutant HasA (Létoffé et al., 2001) or strains not containing HasA (Figure 2).
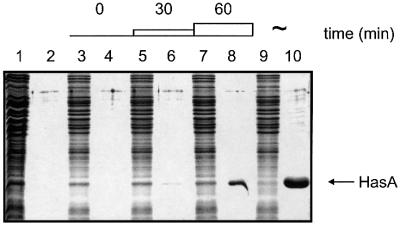
Fig. 1. Visualization of secreted and non-secreted HasA by the arabinose-regulated ABC exporter. Proteins from whole cells were loaded onto odd lanes and proteins present in the corresponding supernatants onto even lanes of a 14% polyacrylamide gel. Lanes 1 and 2, phasA/MC4100; lanes 3–10, phasA/LMD439, in the absence of arabinose (lanes 3 and 4), 30 min after addition of 0.02% arabinose (lanes 5 and 6), 60 min after addition of 0.02% arabinose (lanes 7 and 8) and continuously induced with 0.02% arabinose (lanes 9 and 10). An OD600 equivalent of 0.2 was loaded in each lane, with the exception of lanes 1 and 2 in which a 0.4 OD equivalent was loaded.
Fig. 2. Evidence for the accumulation of holo HasA in the cytoplasm of E.coli. The visible spectrum of MC4100 cells expressing the following plasmids, pAM238 (dotted line), phasA (solid line) and phasA(H32A-Y75A-H83A) (dashed line), was recorded as described in Materials and methods. The inset represents the 380–430 nm region after normalization by subtraction of the absorption of cells carrying pAM238.
The amplitude of the Soret band suggested that a high proportion of the cytoplasmic HasA was loaded with heme in the cytoplasm. We measured the heme content of cells containing HasA and those not expressing hasA (see Materials and methods). The total heme content of cells containing HasA was >20 times that of the control strain. The amount of HasA in extracts used to quantify the heme content was estimated by immunoblotting (not shown). With the stoichiometry of HasA for heme being 1, we calculated that >90% of the cytoplasmic HasA was loaded with heme (see Materials and methods).
Folded HasA is not secreted
We tested whether this folded cytoplasmic HasA could be secreted by the Has ABC exporter. To control the expression of the exporter, we cloned hasDE genes under an arabinose-inducible promoter and integrated this construction into the chromosome (see Materials and methods). In the absence of arabinose, no HasA was secreted into the culture supernatant, as assessed by Coomassie Blue staining (Figure 1, lanes 3 and 4). In the presence of 0.02% arabinose, a large amount of HasA was secreted (Figure 1, lanes 9 and 10). The amount of HasA secreted increased substantially 30 and 60 min after adding arabinose (Figure 1, lanes 5–8). Thus, the HasD and HasE proteins were satisfactorily induced by arabinose, and HasA secretion occurred rapidly after addition of the inducer.
We assessed the secretion of folded HasA by pulse– chase experiments using the strain carrying hasDE genes under the control of the arabinose promoter. We labeled cells for 1 min, and then added arabinose while simultaneously starting to chase the radioactivity. No pre-synthesized HasA protein was secreted either 1 or 60 min after induction (Figure 3A). A pulse–chase experiment was carried out with the same strain growing in the continuous presence of inducer. HasA secretion was then as efficient as the control strain constitutively expressing the three proteins HasA, HasD and HasE (Figure 3B and C). These results showed that the synthesis of HasA in the absence of its exporter led to the accumulation of a cytoplasmic HasA that is not competent to be secreted.
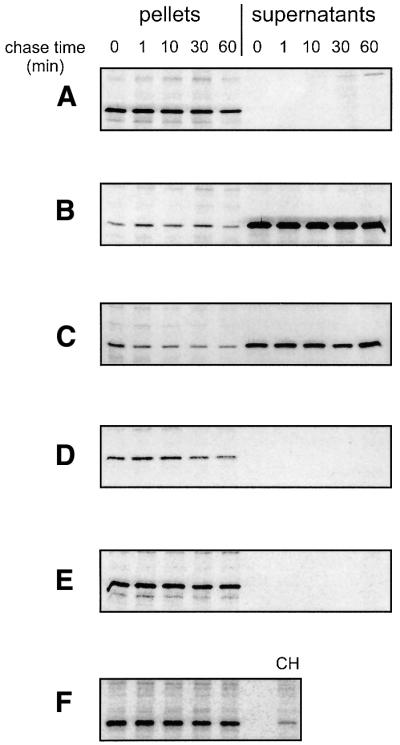
Fig. 3. Folded HasA is not secreted. Pulse–chase experiments were performed as described in Materials and methods on strain LMD439 carrying the plasmid phasA (A and B), phasADE (C), phasA-H32A-Y75A-H83A (D) or phasA and psecB (E and F). (A) Lack of secretion of HasA when synthesis of the HasD and HasE proteins is started after labeling by adding 0.02% arabinose at t = 0. (B) Secretion of HasA when synthesis of the HasD and HasE proteins is induced by 0.02% arabinose several hours prior to labeling. (C) Secretion of HasA when co-expressed with HasD and HasE proteins (from phasADE plasmid) as it is from the original genetic organization. (D) Lack of secretion of HasA-H32A-Y75A-H83A mutant protein as in (A). (E) Lack of secretion of HasA as in (A), but in the presence of a plasmid over expressing the SecB protein. (F) Overproduced SecB protein present in the same cell pellets as in (E). CH indicates the level of SecB detected in cell pellets when expressed from the chromosome.
Folded HasA inhibits its ABC exporter
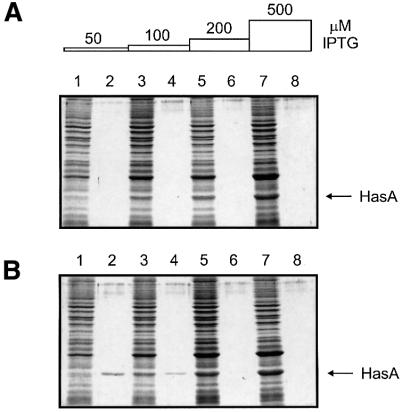
Létoffé et al. (1996) have shown that HasA in complex with its ABC exporter can be isolated using a hemin– agarose column. This demonstrated that the ABC exporter can interact with a folded form of HasA in vitro. Therefore, the pool of cytoplasmic and folded HasA that could not be secreted may be able to interact with its ABC exporter in vivo. One possible consequence of such an interaction could be the inhibition of HasA secretion. We compared HasA secretion efficiencies in the strain carrying the arabinose-inducible hasDE genes on the chromosome and in which we introduced a plasmid allowing the expression of hasA at different levels from an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter (see Materials and methods). In the absence of arabinose, addition of IPTG led to the accumulation of HasA in the cytoplasm (Figure 4A). In the presence of arabinose and low level induction of hasA, HasA was fully secreted into the medium (Figure 4B, lanes 1 and 2). However, when hasA induction was stronger, the amount of HasA secreted decreased, whereas cytoplasmic HasA accumulated (Figure 4B, lanes 3–8). An immunoblot to detect HasD and HasE proteins confirmed that the amount of exporter was identical in all cases (not shown). Thus, the accumulation of HasA in the cytoplasm inhibits its secretion apparatus.
Fig. 4. Folded HasA inhibits its own secretion. Strain LMD439 (hasDE under arabinose control) carrying the plasmid pLMD485 (hasA under IPTG control) was grown on LB (A) or LB with 0.02% arabinose (B). When the OD600 reached 0.1, these cultures were aliquoted into flasks containing various amounts of IPTG and grown for 3 h. Proteins present in supernatants (even lanes) and cell pellets (odd lanes) were visualized after separation on 14% polyacrylamide gels and staining with Coomassie Blue. An OD600 equivalent of 0.2 was loaded in each lane.
Increasing the amount of the ABC exporter relieves the inhibition
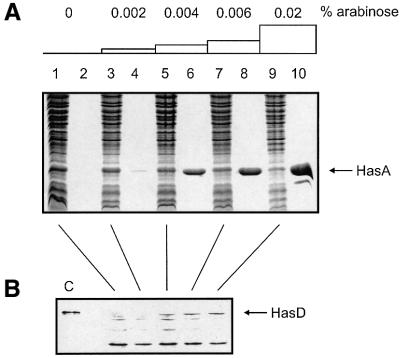
Accumulation of cytoplasmic HasA inhibited its ABC exporter. This may be due to titration of a cytoplasmic factor required for secretion. To test this possibility, we introduced a plasmid constitutively expressing HasA at a low level into the strain carrying the arabinose-inducible hasDE genes on the chromosome. At low arabinose concentration, most of the HasA produced was in the cytoplasm and only a small amount in the supernatant. Thus, secretion was functional but strongly inhibited (Figure 5A, lanes 3 and 4). The amount of HasA in the supernatant increased and that in the cytoplasm decreased with increasing arabinose concentration (Figure 5A, lanes 5–10). A western blot analysis with anti-HasD antibodies confirmed that the amount of exporter increased with increasing arabinose concentration (Figure 5B). This demonstrated that increasing the amount of exporter can relieve the inhibition.
Fig. 5. Increasing the exporter concentration can relieve the inhibition of secretion. Strain LMD439 (hasDE under arabinose control) carrying the plasmid phasA (constitutively expressing HasA) was grown in M9 medium with various amounts of arabinose for several hours. When the OD600 reached 0.6, cell pellets were separated from their super natants and both were precipitated using trichloroacetic acid (TCA). (A) Proteins present in supernatants (even lanes) and cell pellets (odd lanes) were visualized after separation on 14% polyacrylamide gels and staining with Coomassie Blue. An OD600 equivalent of 0.2 was loaded in each lane. (B) The same amounts of cell pellets as in (A) were separated on 10% polyacrylamide gels and transferred to a nitrocellulose membrane. The membrane was probed with anti-HasD antibodies. A purified His-tagged version of HasD (which has a slightly higher molecular weight than wild-type HasD) was loaded in lane C.
Heme-binding activity is not involved in the lack of HasA secretion
The HasA accumulating in the cytoplasm was mostly in the holo form. The holo HasA has a melting temperature (Tm) of 95°C and is more thermostable than the apo HasA with its Tm of 61°C (C.Deniau, personal communication). We investigated whether the presence of the heme prevented HasA secretion. We tested the secretion of a pre-synthesized HasA mutant unable to bind heme and having the same secondary structure content as the wild type (N.Wolff, personal communication) using the pulse– chase procedure described above (Figure 3D). Like the wild type, this HasA mutant was not secreted when synthesized prior to the ABC exporter.
SecB does not interfere with the cytoplasmic folding of HasA
SecB is a cytoplasmic chaperone required for HasA secretion. In a wild-type strain, 95% of HasA is immediately secreted, whereas in a secB strain only ∼10% of HasA is secreted (Delepelaire and Wandersman, 1998). We investigated whether the lack of SecB or the presence of an excess of SecB affected the proportion of folded HasA when expressed in the absence of its exporter. The cytoplasmic heme-loaded HasA pools, as assessed by spectrophotometry, in secB and wild-type strains were similar, suggesting that the folded state that HasA acquired in the absence of its exporter is not dependent on SecB (not shown). Using a plasmid overexpressing secB, we performed a pulse–chase experiment to determine whether HasA could be secreted under such conditions when synthesized prior to the ABC exporter. No HasA was detectable in the culture supernatant, demonstrating that the overexpression of SecB did not allow folded HasA to become competent for secretion (Figure 3E and F).
Discussion
In prokaryotes, protein translation and protein secretion are in most cases independent. Strikingly, in the case of protein secretion by ABC exporters, the location of the secretion signal within the last 50 amino acids implies that translation and secretion are uncoupled. However, the conformation of the polypeptide required for recognition by the exporter and secretion has never been studied in detail. We report evidence that the hemophore HasA synthesized prior to its ABC exporter inhibits its own secretion. This inhibition is exerted by the folded form of the protein. We propose a mechanism in which the secretion is strongly coupled to the translation.
Using the HasA hemophore produced in E.coli, from which it can be secreted by the Has ABC exporter of S.marcescens, we developed a simple method to assess its tertiary structure in situ. In its native conformation, extracellular HasA binds heme and displays a Soret band characteristic of hemoproteins. In the absence of its exporter, HasA accumulates in the cytoplasm in a fully active form, as demonstrated by its loading with heme. Accumulation was similarly observed in S.marcescens when using conditions in which the exporter expression was very low (not shown). The protein accumulated in the cytoplasm was relatively stable with a half-life of ∼60 min for the wild type and 30 min for the mutant lacking heme affinity. Moreover, we did not detect the formation of inactive aggregates that could be isolated as inclusion bodies (not shown).
Both fully synthesized polypeptides and nascent chains can fold. Although protein folding is a very complex process, two folding states can be defined for the purposes of protein secretion: the secretion-competent form and the secretion-incompetent form. To test whether the stable tertiary structure of HasA was compatible with its secretion, the hemophore was pulse labeled just before the exporter was set up using an E.coli strain expressing the exporter genes under a tightly regulated arabinose promoter. The labeled protein was not secreted, whereas >95% of the protein was secreted when the protein and the exporter were expressed at the same time. Similar results were obtained with the mutant version of HasA defective for heme binding, showing that the binding of heme per se was not preventing the secretion. This strongly suggested that the folded form of HasA was not competent for secretion.
The complete lack of secretion of the labeled folded protein, even after a long chase time, suggested either that the conformation adopted by the polypeptide was irreversible or that the folded polypeptide blocked the secretion. Heme binding by HasA may possibly account for the irreversibility of folding. However, since a heme mutant defective for heme binding is not secreted as the wild type, this seems unlikely. When the amount of HasA to be secreted was large, there was total inhibition of secretion. The inhibition may be an indirect consequence of the titration of a cytosolic factor by the folded form, or due to a direct interaction between the folded form and the exporter. Increasing the amount of exporter relieved the inhibition, indicating that the titration of a cytosolic factor was not involved. The three observations (the folded form is not secreted even after a long chase time, secretion is inhibited at high HasA concentration and inhibition is relieved by increasing the exporter concentration) clearly demonstrate that the folded protein has affinity for the ABC protein in vivo. This agrees with our previous observation that folded HasA isolated from cell envelope preparations is associated with its exporter via its ABC protein (Létoffé et al., 1996).
This complete block of the secretion machinery was unexpected, especially for a wild-type protein and its dedicated wild-type exporter. There have been no previous descriptions of this type of phenomenon. Many studies of protein secretion mechanisms involve the search for mutants affected in secretion processes. Among these mutants, some affect protein folding (e.g. secB-related mutants). In our study, the blockage strongly supports a mechanism for which the accumulation of a folded form is prevented.
HasA is the only known protein for which secretion by an ABC exporter is dependent on SecB. However, the overexpression of SecB did not prevent heme loading and did not increase the secretion of the cytoplasmic pool of HasA. Thus, SecB does not seem to have an effect on folded HasA molecules. It has never been shown that SecB is an unfolding factor in vivo. The presence of the signal peptide in proteins secreted by the Sec pathway favors SecB interactions, which in turn slow the folding kinetics (Kumamoto, 1989; Topping and Randall, 1997). Both antifolding activity and targeting to SecA define SecB as a secretion-specific chaperone (Randall et al., 1998).
Our results lead to a model for HasA secretion in which at least the first step is co-translational. First, the nascent polypeptide interacts with SecB and the ABC exporter. This crucial step requires the antifolding activity of SecB and targeting to the exporter (G.Sapriel, C.Wandersman and P.Delepelaire, in preparation). Secondly, as soon as translation is finished, the C-terminal secretion signal interacts with the ABC protein to allow secretion. In the absence of the ABC exporter, the polypeptide acquires its tertiary structure and thereby becomes incompetent for secretion. The intracytoplasmic folding is not dependent on either the presence or absence of SecB. This model is based on work with HasA, and may not apply to other ABC-secreted proteins. In cases for which SecB and other chaperones are not required, e.g. α-hemolysin, the secretion-competent form of the polypeptide may either have slow folding kinetics or be maintained unfolded by the ABC protein (Blight and Holland, 1994).
Materials and methods
Strains and media
We have previously shown that the Has ABC exporter system can be functionally reconstituted in E.coli using TolC instead of HasF (Binet and Wandersman, 1996). The expression of the three proteins, HasA, HasD and HasE, is sufficient to allow the secretion of HasA in the medium. Strains and plasmids used in this study are listed in Table I. Strains were grown at 30°C in rich medium (LB), or in minimal medium (M9) supplemented with vitamin B1 and glycerol as the carbon source. When necessary, ampicillin was added at 100 µg/ml, spectinomycin at 50 µg/ml and chloramphenicol at 50 µg/ml. IPTG was used to induce the expression of hasA from pLMD485 (see Table I).
Table I. Strains and plasmids used in this study.
| Strains | Relevant genotype | Source or reference |
|---|---|---|
| MC4100 | araD139 relA1 rpsL150 thi flbB5301 (lacU139) deo7 ptsF25 | laboratory collection |
| JP313 | MC4100 Δara714 | Pogliano et al. (1997) |
| LMD439 |
pLMD435 into JP313 via λInCh1 |
this work |
| Plasmids |
Descriptions |
|
| pAM238 | low-copy-number cloning vector | Binet and Wandersman (1995) |
| phasADE | hasADE in pACYC184 (named pSYCAC1 in reference) | Ghigo et al. (1997) |
| phasA | hasA in pAM238 (named pSYC134.238 in reference) | Binet and Wandersman (1995) |
| phasA(H32A-Y75A-H83A) | same as phasA but with three mutations H32A-Y75A-H83A | Létoffé et al. (2001) |
| pLMD433 | hasD under arabinose promoter | this work |
| pLMD435 | hasDE under arabinose promoter | this work |
| pLMD481 | hasA under plac promoter in pTrc99a (bla version) | this work |
| pLMD485 | hasA under plac promoter in pTrc99a (cat version) | this work |
| psecB | secB in pACYC184 | laboratory collection |
Plasmid and strain constructions
pLMD433 was constructed by cloning a PCR product containing the hasD coding sequence cut by NcoI and XbaI enzymes into pBAD33 (Guzman et al., 1995) cut by the same enzymes. This PCR product was obtained with primers #1 and #2 (see below) using pSYC34.238 (Binet and Wandersman, 1995) as the template. A BsrGI–HindIII fragment containing the 3′ end of the hasD coding sequence and the entire hasE coding sequence from pSYC34.238 was then subcloned in pLMD433 cut by the same enzymes to give pLMD435. The 5′ region of hasD from the PCR reaction was sequenced to check the absence of mutation. pLMD435 was integrated in the chromosome of the JP313 strain following the protocol described by Boyd et al. (2000) to give a final strain called LMD439, in which hasDE gene expression is under arabinose control.
pLMD481 was constructed by cloning a PCR product containing the hasA coding sequence cut by NcoI and XbaI enzymes into pTrc99a (Pharmacia) cut by the same enzymes. This PCR product was obtained with primers #3 and #4 using phasA as the template. The region corresponding to the hasA gene was sequenced to check the absence of mutation. A HindIII–HincII fragment from the plasmid pLMD48 containing the cat gene (coding for resistance to chloramphenicol) was cloned into pLMD481 cut by HindIII and FspI to give the plasmid pLMD485. pLMD48 was obtained by cloning a BsaAI–BsaAI fragment from pACYC184 (containing the cat gene) into pLMD27 (a version of pBR322 in which the EcoRI site was cut and end-filled with the Klenow fragment) cut by SspI and DraI. Restriction and modification enzymes were used following the manufacturer’s recommendations (NEB Biolabs).
Primers
#1: 5′-GAGGAATTCACCATGAAAAACGCCGTCAGG-3′
#2: 5′-CAGCTCTAGATCATACTTTTCGTTCACCGC-3′
#3: 5′-TTGCCACCATGGCATTTTCAGTCAATTATG-3′
#4: 5′-AAATGGATCCTCAGGCCGCCAGCAGTTCCG-3′
Electrophoresis and immunoblotting techniques
Protein samples from cell pellets were resuspended in 10 mM Tris–HCl, 1 mM EDTA pH 8 and then precipitated by adding TCA at 10% final concentration; supernatants were precipitated directly with TCA. After centrifugation and a wash using 1 vol. of 100% acetone, dry samples were resuspended in sample buffer and loaded onto 14% SDS–polyacrylamide gels. When required, gels were transferred to nitrocellulose membranes using a semi-dry apparatus. Membranes were probed with polyclonal anti-HasA, anti-HasD or anti-HasE antibodies.
HasA spectroscopy and heme quantification
To visualize HasA charged with heme in the cytoplasm of whole cells, 5 ml of cells grown in M9 medium to an OD600 of ∼0.5 were centrifuged and washed once in 10 mM Tris–HCl pH 8, and finally resuspended in 1.5 ml of 50% glycerol. The viscosity of this solution slowed down cell movement. One milliliter was introduced in a microcuvette and optical densities were adjusted to allow each cell suspension to have the same OD600 reading. Spectra were recorded from 380 to 620 nm on a Uvikon 930 spectrophotometer. The Soret band of holo HasA is centered on 407 nm (Izadi et al., 1997).
To quantify the heme content, 50 ml of cells grown in M9 media to an OD600 of ∼0.5 were centrifuged at 8000 r.p.m. for 10 min and resuspended in 3 ml of 10 mM Tris–HCl pH 8, 0.5 mM EDTA. Part of this suspension (1.5 ml) was broken by sonication. Sonicated cells were centrifuged for 10 min at 8000 r.p.m. and the heme content of the supernatant was quantified by the pyridine hemochrome assay described by Berry and Trumpower (1987). Visible spectra were recorded before and after adding dithionite. By subtracting the amount of heme present in cells that do not express HasA from that present in cells expressing HasA, we calculated the amount of heme specifically due to the presence of HasA in the cytoplasm (submitting a known amount of heme-loaded HasA to the same procedure for reference). To estimate the amount of HasA present in each sample, a sample of these extracts was loaded onto a polyacrylamide gel, followed by immunoblotting. A known amount of purified HasA was used as a reference.
Pulse–chase experiments
Cells were grown in M9 medium to early logarithmic phase. A volume of culture was then labeled with [35S]methionine (100 µCi/ml) for 1 min. Chasing started when an excess of cold methionine (0.1% final concentration) was added to the culture. After labeling, cells were centrifuged for 15 min at 15 000 r.p.m. An aliquot of the supernatant was taken and precipitated by adding TCA (10% final concentration), followed by centrifugation and washing with 100% acetone. Finally, the dry pellet was resuspended in sample buffer. The rest of the supernatant of the labeled culture was discarded and the cell pellets were resuspended in 50 µl of 10 mM Tris–HCl pH 8, 1 mM EDTA, 1% SDS and lysed by boiling for 5 min. After immunoprecipitation of HasA proteins with polyclonal antibodies, polyacrylamide gels were loaded with equivalent amounts of proteins from cell pellets and supernatants. Dry gels were scanned with a PhosphorImager (Molecular Dynamics).
Acknowledgments
Acknowledgements
We gratefully acknowledge members of C.W.’s laboratory for helpful discussions, particularly P.Delepelaire. We thank J.M.Clément and M.Marden for early suggestions that helped this work and J.M.Betton for the gift of anti-SecB antibodies.
References
- Arnoux P., Haser,R., Izadi,N., Lecroisey,A., Delepierre,M., Wandersman,C. and Czjzek,M. (1999) The crystal structure of HasA, a hemophore secreted by Serratia marcescens. Nature Struct. Biol., 6, 516–520. [DOI] [PubMed] [Google Scholar]
- Berks B.C., Sargent,F. and Palmer,T. (2000) The Tat protein export pathway. Mol. Microbiol., 35, 260–274. [DOI] [PubMed] [Google Scholar]
- Berry E.A. and Trumpower,B.L. (1987) Simultaneous determination of hemes a, b and c from pyridine hemochrome spectra. Anal. Biochem., 161, 1–15. [DOI] [PubMed] [Google Scholar]
- Binet R. and Wandersman,C. (1995) Protein secretion by hybrid bacterial ABC-transporters: specific functions of the membrane ATPase and the membrane fusion protein. EMBO J., 14, 2298–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet R. and Wandersman,C. (1996) Cloning of the Serratia marcescens hasF gene encoding the Has ABC exporter outer membrane component: a TolC analogue. Mol. Microbiol., 22, 265–273. [DOI] [PubMed] [Google Scholar]
- Blight M.A. and Holland,I.B. (1994) Heterologous protein secretion and the versatile Escherichia coli haemolysin translocator. Trends Biotechnol., 12, 450–455. [DOI] [PubMed] [Google Scholar]
- Boyd D., Weiss,D.S., Chen,J.C. and Beckwith,J. (2000) Towards single-copy gene expression systems making gene cloning physiologically relevant: λInCh, a simple Escherichia coli plasmid–chromosome shuttle system. J. Bacteriol., 182, 842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delepelaire P. and Wandersman,C. (1998) The SecB chaperone is involved in the secretion of the Serratia marcescens HasA protein through an ABC transporter. EMBO J., 17, 936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khattabi M., Ockhuijsen,C., Bitter,W., Jaeger,K.E. and Tommassen,J. (1999) Specificity of the lipase-specific foldases of Gram-negative bacteria and the role of the membrane anchor. Mol. Gen. Genet., 261, 770–776. [DOI] [PubMed] [Google Scholar]
- Ghigo J.M., Létoffé,S. and Wandersman,C. (1997) A new type of hemophore-dependent heme acquisition system of Serratia marcescens reconstituted in Escherichia coli. J. Bacteriol., 179, 3572–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman L.M., Belin,D., Carson,M.J. and Beckwith,J. (1995) Tight regulation, modulation and high-level expression by vectors containing the arabinose pBAD promoter. J. Bacteriol., 177, 4121–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie K.R., Schulze,A., Parker,M.W. and Buckley,J.T. (1995) Vibrio spp. secrete proaerolysin as a folded dimer without the need for disulphide bond formation. Mol. Microbiol., 17, 1035–1044. [DOI] [PubMed] [Google Scholar]
- Harper J.R. and Silhavy,T.J. (2001) Germ warfare: the mechanisms of virulence factor delivery. In Groisman,E.A. (ed.), Principles of Bacterial Pathogenesis. Academic Press, San Diego, CA, pp. 43–74.
- Izadi N., Henry,Y., Haladjian,J., Goldberg,M.E., Wandersman,C., Delepierre,M. and Lecroisey,A. (1997) Purification and characteriz ation of an extracellular heme-binding protein, HasA, involved in heme iron acquisition. Biochemistry, 36, 7050–7057. [DOI] [PubMed] [Google Scholar]
- Koronakis V., Sharff,A., Koronakis,E., Luisi,B. and Hughes,C. (2000) Crystal structure of the bacterial membrane protein TolC central to multi-drug efflux and protein export. Nature, 405, 914–919. [DOI] [PubMed] [Google Scholar]
- Koster M., Bitter,W. and Tommassen,J. (2000) Protein secretion mechanisms in Gram-negative bacteria. Int. J. Med. Microbiol., 290, 325–331. [DOI] [PubMed] [Google Scholar]
- Kumamoto C.A. (1989) Escherichia coli SecB protein associates with exported protein precursors in vivo. Proc. Natl Acad. Sci. USA, 86, 5320–5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Létoffé S., Ghigo,J.M. and Wandersman,C. (1994a) Iron acquisition from heme and hemoglobin by a Serratia marcescens extracellular protein. Proc. Natl Acad. Sci. USA, 91, 9876–9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Létoffé S., Ghigo,J.M. and Wandersman,C. (1994b) Secretion of the Serratia marcescens HasA protein by an ABC transporter. J. Bacteriol., 176, 5372–5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Létoffé S., Delepelaire,P. and Wandersman,C. (1996) Protein secretion in Gram-negative bacteria: assembly of the three components of ABC protein-mediated exporters is ordered and promoted by substrate binding. EMBO J., 15, 5804–5811. [PMC free article] [PubMed] [Google Scholar]
- Létoffé S., Deniau,C., Wolff,N., Dassa,E., Delepelaire,P., Lecroisey,A. and Wandersman,C. (2001) Haemophore-mediated bacterial haem transport: evidence for a common or overlapping site for haem-free and haem-loaded haemophore on its specific outer membrane receptor. Mol. Microbiol., 41, 439–450. [DOI] [PubMed] [Google Scholar]
- Nicaud J.M., Mackman,N., Gray,L. and Holland,I.B. (1986) The C-terminal, 23 kDa peptide of E.coli haemolysin 2001 contains all the information necessary for its secretion by the haemolysin (Hly) export machinery. FEBS Lett., 204, 331–335. [DOI] [PubMed] [Google Scholar]
- Peek J.A. and Taylor,R.K. (1992) Characterization of a periplasmic thiol:disulfide interchange protein required for the functional maturation of secreted virulence factors of Vibrio cholerae. Proc. Natl Acad. Sci. USA, 89, 6210–6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano J., Lynch,A.S., Belin,D., Lin,E.C. and Beckwith,J. (1997) Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev., 11, 1169–1182. [DOI] [PubMed] [Google Scholar]
- Pugsley A.P., Bayan,N. and Sauvonnet,N. (2001) Disulfide bond formation in secretion component PulK provides a possible explanation for the role of DsbA in pullulanase secretion. J. Bacteriol., 183, 1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L.L., Topping,T.B., Smith,V.F., Diamond,D.L. and Hardy,S.J. (1998) SecB: a chaperone from Escherichia coli. Methods Enzymol., 290, 444–459. [DOI] [PubMed] [Google Scholar]
- Rosemond M.J., Strobel,S.M., Ray,P.H. and Bassford,P.J. (1994) Ability of MBP or RBP signal peptides to influence folding and in vitro translocation of wild-type and hybrid precursors. FEBS Lett., 349, 281–285. [DOI] [PubMed] [Google Scholar]
- Stathopoulos C., Hendrixson,D.R., Thanassi,D.G., Hultgren,S.J., St Geme,J.W. and Curtiss,R.,III (2000) Secretion of virulence determinants by the general secretory pathway in Gram-negative pathogens: an evolving story. Microb. Infect., 2, 1061–1072. [DOI] [PubMed] [Google Scholar]
- Teter S.A. and Klionsky,D.J. (1999) How to get a folded protein across a membrane. Trends Cell Biol., 9, 428–431. [DOI] [PubMed] [Google Scholar]
- Topping T.B. and Randall,L.L. (1997) Chaperone SecB from Escherichia coli mediates kinetic partitioning via a dynamic equilibrium with its ligands. J. Biol. Chem., 272, 19314–19318. [DOI] [PubMed] [Google Scholar]
- Verderber E., Lucast,L.J., Van Dehy,J.A., Cozart,P., Etter,J.B. and Best,E.A. (1997) Role of the hemA gene product and δ-amino levulinic acid in regulation of Escherichia coli heme synthesis. J. Bacteriol., 179, 4583–4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C. and Delepelaire,P. (1990) TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc. Natl Acad. Sci. USA, 87, 4776–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Elliott,M. and Elliott,T. (1999) Conditional stability of the HemA protein (glutamyl-tRNA reductase) regulates heme biosynthesis in Salmonella typhimurium. J. Bacteriol., 181, 1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattiau P., Woestyn,S. and Cornelis,G.R. (1996) Customized secretion chaperones in pathogenic bacteria. Mol. Microbiol., 20, 255–262. [DOI] [PubMed] [Google Scholar]
- Wild J., Rossmeissl,P., Walter,W.A. and Gross,C.A. (1996) Involvement of the DnaK–DnaJ–GrpE chaperone team in protein secretion in Escherichia coli. J. Bacteriol., 178, 3608–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff N., Delepelaire,P., Ghigo,J.M. and Delepierre,M. (1997) Spectroscopic studies of the C-terminal secretion signal of the Serratia marcescens haem acquisition protein (HasA) in various membrane–mimetic environments. Eur. J. Biochem., 243, 400–407. [DOI] [PubMed] [Google Scholar]