Abstract
Objectives:
Balance and gait problems have repeatedly been mentioned in case descriptions of patients infected with Human Immunodeficiency Virus (HIV-1). Objective evidence of these problems has rarely been reported, however. Furthermore, the extent to which balance and gait are influenced by antiretroviral medications or comorbid psychiatric disorders has rarely been examined.
Design:
The study compared 78 HIV-1 seronegative volunteers to 28 HIV/AIDS patients receiving no antiretroviral therapy, 25 patients receiving only nucleoside analogue therapy, and 37 patients receiving Highly Active Antiretroviral Therapy (HAART).
Methods:
The dependent measures included Equilibrium Quotient scores recorded during 3 subtests of the Sensory Organization Test (SOT), the number of falls during each subtest, the functional base of support, gait speed and cadence, single leg balance time, proximal strength, and vibrotactile threshold of the foot. The analysis employed the number of alcohol and drug abuse problems, depression severity, and body mass index as covariates.
Results:
ANCOVAs revealed significant decrements in the 3 HIV-1 seropositive groups relative to the control group on Equilibrium Quotient scores during the most difficult of the SOT subtests (sway-referenced support surface with eyes-closed). HIV/AIDS patients also exhibited a smaller functional base of support and greater vibrotactile thresholds. Antiretroviral treatment did not affect balance; but, it did alter sensory threshold in a complex manner.
Conclusions:
HIV/AIDS is associated with reliable decrements in balance and peripheral sensory function which are variably sensitive to antiretroviral treatment. The implications of these findings for mobility, and workplace or operator safety, should be contemplated.
Keywords: musculoskeletal equilibrium, gait, sensory thresholds, sensory neuropathy, antiretroviral therapy, substance-related disorders
Introduction
HIV-1 may compromise balance or gait at multiple levels of the nervous system [1,2] with the potential for an additive effect and a pronounced mobility impairment. The factors which can independently or cumulatively contribute to balance and gait problems in HIV/AIDS patients include peripheral neuropathies [3–6], PML [7], muscle weakness [8], myalgia and fatigue [9], and cerebellar [10,11] and basal ganglia lesions [1,12].
Clinical evidence of balance or gait disturbance is reported in more than 57% of symptomatic HIV-1 seropositive patients and 25% of their asymptomatic peers [13,14]. Quantitative assessments of posture and stance stabilization have confirmed the clinical data. For example, both Arendt and colleagues [13] and Beckley and colleagues [15] examined balance and postural reflexes in a seronegative control group and in HIV-1 seropositive patients while they stood on a force-sensitive platform. Static ataxia, estimated by sway area, was significantly exaggerated in the HIV-1 seropositive group. Furthermore, the amplitude of the stance-stabilizing reflex following a sudden perturbation of the supporting platform was significantly exaggerated in that group.
Balance and gait problems in HIV/AIDS patients may be influenced by other factors. Although several studies have shown improved motor function following the introduction of antiretroviral treatment [16,17], adverse effects have also been observed. Co-morbid psychiatric disorders, including substance abuse/dependence and depression, may enhance the degree of disability [18–24].
The goal of the present study was to assess sensorimotor function in a large sample of HIV/AIDS patients and an HIV-1 seronegative control group. Importantly, both patient and control groups included a sufficient number of members to permit a powerful analysis of the effects of moderating and amplifying variables. Another unique feature of the study was its focus on medically-stable outpatients, for whom frailty originating from acute illness and opportunistic disease was less likely to be a confound. The list of dependent measures included quantitative posturographic indices recorded during the Sensory Organization Test, indices of gait speed and efficiency, tactile sensitivity, and proximal strength.
Methods
Subject Recruitment and Screening
Ninety HIV-1 seropositive subjects were recruited via advertisements posted within outpatient Infectious Disease Clinics in the greater Hartford, CT region. Interested individuals were invited to telephone a member of the research staff for eligibility screening. The telephone interview included questions about demographic characteristics, general medical status, substance use, and psychiatric symptoms. Individuals who passed the initial telephone screen were invited to visit the Health Center on a subsequent day, on which an IRB-approved consent form and a medical records release were signed. Additional eligibility screening, laboratory evaluations, and sensorimotor testing were performed on that day.
The most common method for recruiting the 78 members of the HIV-1 seronegative group was word-of-mouth advertising provided by the seropositive participants. HIV-1 seronegative volunteers were invited to telephone the research assistant for initial screening and were brought to the Health Center for further screening. They were subject to the same protocol as the outpatients.
After completing the informed consent and medical release documents, all subjects were asked to provide a blood sample for laboratory confirmation of HIV serostatus. The laboratory work-up also included CBC with differential, HIV RNA viral load, CD4 lymphocyte count and percent, VDRL, HBV screen, HCV, toxoplasmosis and cytomegalovirus antibody titers, renal and liver function, serum protein, albumin, and G-6-PD. Toxicological analyses for cocaine, opiates, amphetamine, and marijuana were performed on urine samples (Ontrak™, Varian Inc., Palo Alto, CA) and a breathalyzer was used to detect recent alcohol use. In addition, an Optec 2000 Vision Tester™ was used to confirm normal visual acuity (with correction).
A structured psychiatric interview, the Computerized Diagnostic Interview Schedule for DSM-IV [25,26], was then administered for the purpose of detecting DSM-IV Axis I and II disorders. Subjects also completed questionnaires or brief interviews assessing medical history, medication use, family history, demographics, psychiatric symptoms, alcohol and drug use, and cognitive status. The assessments included the Addiction Severity Index [27], Michigan Alcoholism Screening Test [28], Drug Abuse Screening Test [29], and Beck Depression Inventory Version II [30]. In addition, the Kaufman Brief Intelligence Test [31] was administered to derive an estimate of IQ.
Exclusion criteria included pregnancy, seizures, mental retardation, neurosurgery, and a history of head injury with loss of consciousness for greater than 10 minutes. In addition, subjects were required to have no acute illness, and no major neurological (i.e., epilepsy, seizure disorder), psychiatric (i.e., DSM-IV defined schizophrenia or bipolar disorder) or medical disorders unrelated to HIV/AIDS (i.e., chronic obstructive pulmonary disease, Type 1 diabetes, cirrhosis, hepatic encephalopathy, ocular disorders, etc.). Subjects were not required to abstain from drugs of abuse prior to testing, although urine and breath samples were assayed, and data regarding quantity, frequency and recency of use were collected.
Group Assignment
Subjects were assigned to groups based upon the results of the HIV screen and a review of medication usage. The 90 HIV-1 seropositive patients were subdivided into groups who reported: (1) no current use of antiretroviral medication or a failure to adhere to an antiretroviral medication regimen for more than 90% of days during the past month (n = 28); (2) current use and >90% adherence to a regimen of only nucleoside analogues (n = 25); or (3) current use and >90% adherence to HAART (n = 37).
Procedures
Sensory Organization Test
Balance was recorded with a system consisting of a force-sensitive platform, A/D converter, and computer (Equi-Test System, NeuroCom International, Inc.). The A/D converter digitized each voltage output from the platform at a rate of 100 Hz. Balance was measured while the subject stood erect in stocking feet.
A computerized Sensory Organization test was employed, consisting of three conditions. All of the conditions provided inaccurate somatosensory information in which the support platform was programmed to track changes in the subject’s center of gravity (i.e., sway-referenced). The differences across conditions related to the amount of visual information available to the subject regarding posture and sway. In the first condition, visual input was normal and, in the second condition, eyes were closed, i.e., visual input was absent. In the third condition, eyes were open but the visual horizon was sway-referenced, i.e., visual input was inaccurate. Three 20-second trials were completed for each condition. The dependent variables for each condition were the Equilibrium Quotient (EQ), number of falls, and time before a fall. The sum of falls across individual conditions was also examined.
The Equilibrium Quotient is a continuous measure of postural stability which can range between 0 and 100. Perfect postural stability, without sway, is defined by a score of 100. Data were retained if the subject completed at least one of the three 20-sec trials in each SOT condition without experiencing a fall. If the subject fell during a trial, the EQ data for that trial were deleted and the EQ score was the average of the remaining trials.
Time before a fall was expressed in seconds for each SOT condition. In the event that no fall occurred, subjects received a score of 20 seconds, indicating that postural stability was maintained throughout the condition.
Functional Base of Support (FBOS)
While standing on a fixed support surface and wearing a shoulder harness, subjects were instructed to lean as far forward as possible without losing balance. In a second condition, subjects were instructed to lean as far backward as possible without losing balance. Each condition was composed of two trials. An index of lean performance was calculated by adding the larger of the two forward lean values to the larger of the two backward lean values. This value represents the range over which the base of support remains functional. The lean performance index was then divided by foot length, and this ratio served as an estimate of the functional base of support.
Single Leg Stance Time
Subjects were instructed to stand for as long as possible on one leg with the arms crossed over the chest. Four trials were conducted, alternating between preferred and non-preferred legs. Rest breaks were permitted between trials. The dependent variable was the length of time that the single leg stance was maintained (maximum 30 seconds). The single best score was retained for preferred and nonpreferred leg trials.
Gait Speed and Cadence
To produce a measure of “normal” gait speed, subjects were asked to walk 8 meters at their usual pace. In a second condition, estimating “fast” gait speed, subjects were asked to walk 8 meters as quickly as possible without running. The times required to complete these conditions were recorded. Cadence, defined as the time to complete 5 footfalls using the leading foot, was derived for both “normal” and “fast” pace conditions. Two trials were conducted and averaged.
360° Turn
Subjects were instructed to complete a 360° turn as quickly as possible. Both a right turn and a reversal were executed and an average of the two trials was computed.
Repeated Chair Rises
Subjects were instructed to alternate rapidly between standing and sitting 5 times without pauses and with arms crossed. The time required to complete the task was recorded.
Vibrotactile Threshold
Subjects were instructed to rest the great toe of the right foot upon a buzzer-like device (Bio-Thesiometer; Biomedical Instrument company, Newbury, Ohio) which delivered a 120 Hz vibration of programmable amplitude. The sensory threshold for detecting a change in the amplitude of vibration was recorded. Thresholds were measured during an ascending and descending series and averaged.
Results
Background Characteristics
Group differences in background characterics were evaluated using univariate ANOVA for continuous measures and Pearson’s χ2 Test for categorical measures. A summary of the background characteristics of the four subject groups is shown in Table 1.
Table 1.
Background Characteristics of Subject Groups†.
| HIV-(n = 78) | HIV+, NO TX (n = 28) | HIV+, NRTI (n = 25) | HIV+, HAART (n= 37) | Test Result | |
|---|---|---|---|---|---|
| Age (SD) | 38(7.1) | 40(7.2) | 40(5.9) | 39(6.4) | F = 0.8 |
| % Male | 47.4 | 42.9 | 36.0 | 37.8 | χ2 = 1.5 |
| % White | 28.2 | 21.4 | 24.0 | 43.2 | χ2 = 4.6 |
| Yrs of Education | 12.6(2.2) | 11.5(1.6) | 11.6(1.7) | 12.1(2.6) | F = 2.6 |
| Estimated IQ | 94.6(13.3) | 92.6(11.3) | 91.2(10.7) | 93.4(12.8) | F = 0.6 |
| MAST Score | 3.7(5.9) | 5.7(6.4) | 6.8(7.3) | 4.4(5.7) | F = 1.8 |
| DAST-10 Score | 2.8(3.1) | 3.8(3.8) | 4.0(4.5) | 2.7(3.2) | F = 1.2 |
| BDI-II Score | 11.3(10.3) | 19.2(10.4) | 12.8(12.4) | 11.5(9.8) | F = 4.1* |
| % Alcohol Dependent Lifetime | 16.7 | 39.3 | 40.0 | 35.1 | χ2 = 9.4* |
| % Cocaine Dependent Lifetime | 41.0 | 67.9 | 68.0 | 51.4 | χ2 = 9.1* |
| % Opioid Dependent Lifetime | 29.5 | 64.3 | 40.0 | 37.8 | χ2 = 10.5* |
| % Major Depressive Disorder– Recurrent | 17.9 | 42.9 | 28.0 | 35.1 | χ2 = 7.9* |
| % Urine Positive for Amphetamine | 0 | 0 | 0 | 0 | – |
| % Urine Positive for Cocaine | 21.8 | 32.1 | 20.0 | 24.3 | χ2 = 1.4 |
| % Urine Positive for Opiates | 9.0 | 32.1 | 12.0 | 16.1 | χ2 = 9.4* |
| CD4 count in cells/μL | 915(358) | 351(282) | 457(375) | 320(200) | F = 41.1* |
| HIV Burden × 1000 copies/ml | – | 93.8(163) | 35.5(102) | 20.1(48.2) | F = 9.5* |
†Abbreviations: MAST, Michigan Alcoholism Screening Test; DAST-10, Drug Abuse Screening Test; BDI-II, Beck Depression Inventory Version II; IQ, Intelligence Quotient.
*p < 0.05.
The four subject group were similar in age, educational level, and intelligence. In general, the subjects were middle-aged, had completed 11–12 years of education, and possessed normal or below-normal intelligence. Most were female and members of a racial or ethnic minority.
A substantial percentage of the sample reported a history of substance abuse. Rates of substance dependence varied significantly across the groups. HIV-seropositive subjects who were not receiving or complying with antiretroviral treatment reported the highest rates of substance dependence and were more likely to provide a urine toxocology test positive for cocaine or opiates. The members of this group also reported more depression symptoms than the HIV-seronegative control group.
Analyses of HIV disease severity revealed the expected pattern. Viral load was greatest among HIV-seropositive not receiving antiretroviral treatment and lowest among HIV-seropositive patients receiving combined antiretroviral therapy (HAART). CD4 cell count was lower in the three HIV-1 seropositive groups than in the seronegative control group. However, CD4 cell count did not vary significantly as a function of the presence or type of antiretroviral treatment.
Sensorimotor Tests
Independent analyses of inter-related dependent measures can confer an increased of Type I error. In an attempt to control the experiment-wise error rate, all variables were first tested simultaneously within a multivariate analysis of variance. Univariate tests of the effect of Group on individual variables were performed only if the MANOVA yielded a significant result.
Within the MANOVA and subsequent ANOVAs, several covariates were specified. They included: the number of alcohol and drug use problems from the MAST and DAST-10, respectively; the number of depression symptoms from the BDI-II; and body mass index. Tukey post hoc tests were used to determine the significance of pairwise differences.
The MANOVA revealed significant effects of several of the covariates. Body mass index [MANOVA F(19,142) = 3.5, p < 0.01], the number of drug problems from the DAST-10 [MANOVA F(19,142) = 1.7, p < 0.04], and the number of depression symptoms from the BDI-II [MANOVA F(19,142) = 1.9, p < 0.02] were all related to the linear composite of sensorimotor tests. The MANOVA also revealed a significant effect of group membership [Wilks’ λ = 0.53, F(57,424) = 1.7, p < 0.002] on sensorimotor test performance.
Univariate ANOVAs evaluating individual measures revealed significant group differences in the functional base of support [F(3,160) = 4.86, p < 0.004], vibrotactile threshold [F(3,160) = 5.29, p < 0.003], and Equilibrium Quotient [F(3,160) = 3.18, p < 0.03] scores during the eyes-closed condition only. There were no significant group differences during other SOT subtests. There were also no significant differences on measures of proximal strength (viz., 360° turn time, time to complete 5 chair rises), gait speed and cadence, and on other measures of balance.
Tukey post hoc tests were employed to evaluate the source of the group differences (see Fig. 1). The tests revealed that the three HIV-1 seropositive groups differed significantly from the seronegative group on Equilibrium Quotient and the functional base of support. Differences related to the presence or form of antiretroviral treatment were not significant.
Fig. 1.
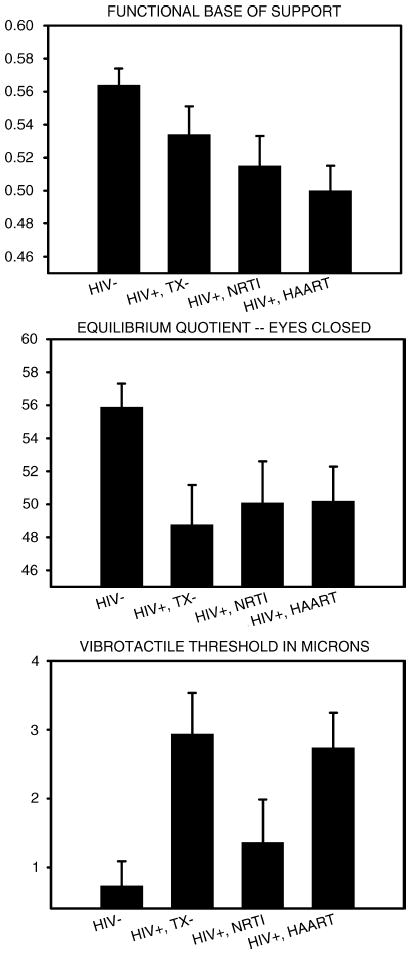
Effects of HIV and antiretroviral treatment on balance and vibrotactile threshold. Mean + 1 SE.
Post hoc analyses of vibrotactile threshold data revealed a more complex pattern of findings. Sensory thresholds were higher in the untreated (HIV+, TX−) and HAART (HIV+, HAART) groups in comparison to the HIV− and HIV+, NRTI groups. There were no significant differences among the other groups.
Secondary Analyses
Because the four subject groups differed in the prevalence of substance dependence and Major Depressive Disorder, a confound may exist which precludes the identification of the proximate cause of the sensorimotor differences. For the purpose of simplifying the interpretation of the results, a series of secondary analyses were performed. For all of these analyses, the HIV-1 seronegative control group was dropped.
In the first series of analyses, the 90 HIV-1 seropositive patients were regrouped based upon DSM-IV diagnoses of Alcohol, Cocaine, or Opiate Dependence, or Major Depressive Disorder-Recurrent. Separate analyses were performed for each of the diagnoses. For the substance dependence diagnoses, subjects could be assigned to one of three levels: never dependent, formerly dependent (i.e., prior to the past year), currently dependent (i.e., during the past year). The dependent measures were the three measures shown sensitive to the effects of HIV and antiretroviral treatment in the major analysis. Table 2 summarizes the results. ANOVAs revealed no significant effects (i.e., all p’s > 0.05) of substance dependence or Major Depressive Disorder on the Equilibrium Quotient score, vibrotactile threshold, or functional base of support.
Table 2.
Effects of Substance Dependence and Recurrent Major Depressive Disorder on Selected Measures.††
| Cocaine Dependence
|
Opioid Dependence
|
Alcohol Dependence
|
Major Depressive Disorder
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Never | Former | Current | Never | Former | Current | Never | Former | Current | Negative | Positive | |
| Vibrotactile Threshold in microns [M(SD)] | 3.1(5.4) | 2.1(3.6) | 1.8(1.8) | 2.6(4.6) | 2.7(4.2) | 1.3(1.4) | 2.4(4.3) | 2.6(4.2) | 2.0(2.3) | 2.5(3.9) | 2.2(4.6) |
| Equilibrium Score–eyes closed | 50.9(11) | 49.4(13) | 45.9(15) | 48.6(13) | 50.4(10) | 49.3(17) | 50.7(12) | 47.2(14) | 44.2(7) | 49.3(12) | 49.2(13) |
| Functional Base of Support | .53(.1) | .52(.1) | .49(.1) | .53(.1) | .52(.1) | .46(.1) | .53(.1) | .51(.1) | .46(.1) | .52(.1) | .51(.1) |
††HIV-1 seropositive subjects only. All effects are not significant at p < 0.05.
Another analysis series examined the association between markers of HIV disease severity and the three measures significantly affected by HIV-1 in the major analysis. Spearman rank-order correlations were computed between viral load, CD4 cell count, and the selected measures. Two of the correlations were significant. CD4 cell count was positively related to the functional base of support (rho = 0.38, p < 0.05). Viral load was positively correlated with vibrotactile threshold (rho = 0.32, p < 0.05).
Discussion
The present study evaluated balance, gait, strength, and sensory function in 90 HIV-1 infected outpatients and in a control group of 78 uninfected volunteers. The focus on medically-stable outpatients is noteworthy. If the present study had instead included inpatients, or outpatients experiencing an acute phase of illness, then questions would arise as to whether the group differences are merely reflecting frailty and an effect that might be found in any disease. The preservation of group differences when body mass index was entered as a covariate further discounts frailty as the proximate cause. Another finding which discounts frailty is the absence of significant group differences on measures of proximal strength (viz., 360° turn time and time-to-complete 5 chair rises).
The present study is relatively unique in ensuring that the HIV-1 seropositive and seronegative groups were similar in background characteristics. As noted previously, many of the subjects in the seronegative control group were recruited via word-of-mouth advertising provided by the outpatients. An advantage of this recruitment method, versus other methods, is the ability to sample the control group from a population demographically similar to that of the patients. Importantly, it is also thereby possible to recruit a large number of seronegative controls who resemble the outpatients in psychiatric and substance use characteristics. The results shown in Table 1 demonstrate that the recruitment method was successful in maintaining equivalence across groups with respect to the lifetime number of alcohol and drug use problems reported on the MAST and DAST-10, respectively. Some differences remained. For example, HIV/AIDS patients not receiving or complying with antiretroviral treatment reported more depression symptoms and were more likely to possess DSM-IV diagnoses of substance dependence or recurrent Major Depressive Disorder. Previous studies of antiretroviral medication adherence failure have reported similar findings [32–34]. To determine if the present findings regarding sensorimotor differences across groups were confounded with the effects of substance dependence or depression, a secondary analysis was performed. That analysis found no significant effects of the background variables and thereby discounted their role.
The pattern of group differences illustrated in Fig. 1 suggests a deleterious effect of HIV/AIDS on 2 related measures of balance: FBOS and Equilibrium Quotient. The results shown in Fig. 1 also suggest that antiretroviral treatment does not reverse the HIV/AIDS-associated deficits in balance. One must be cautious, however, in accepting the null hypothesis of no treatment effect. The present study examined the effects of antiretroviral treatment in a between-subjects design and may therefore lack power for detecting subtle effects. Future studies should examine the effects of antiretroviral treatment within a repeated measures design where patients are evaluated before and during treatment.
A higher sensory threshold was found among untreated HIV/AIDS patients in comparison to the seronegative control group. The presence of distal sensory neuropathy in HIV/AIDS patients is well-documented [e.g., 35]. It is clinically apparent in approximately 50% of cases not previously exposed to HAART [36]. These data, combined with the present demonstration of a significant correlation between viral load and vibrotactile threshold, suggest that HIV-1 can cause a distal neuropathy.
Treatment with a single NRTI appeared to normalize vibrotactile threshold and eliminate this sign of distal neuropathy in HIV/AIDS patients. Although NRTI treatment has typically been shown to have the opposite effect, it is worth noting that 13 of the 25 patients assigned to the NRTI group were receiving zidovudine. Zidovudine is arguably associated with a lower prevalence and more moderate degree of neuropathy [37–39] than other NRTIs, especially didanosine and stavudine. Thus, the positive effect of zidovudine in reducing HIV-1-associated peripheral neuropathy may have predominated over its own negative effects on peripheral nerve function.
Highly active antiretroviral therapy was associated a significant elevation in vibrotactile threshold. This finding is consistent with other data indicating that the incidence of distal neuropathy increases significantly with the number of drugs comprising the treatment regimen [40]. Patients receiving HAART within the present study were also more likely to be exposed to NRTIs other than zidovudine and could thereby incur greater neurotoxicity.
The present study revealed no relationship between distal neuropathy and balance abnormalities in patients with HIV/AIDS. For example, vibrotactile threshold was influenced by the type of antiretroviral therapy whereas balance was not. In addition, vibrotactile threshold was found to be uncorrelated with the balance measures (all r’s < 0.19, all p’s > 0.07). The explanation for balance abnormalities among HIV/AIDS in the present study may therefore relate more to central than peripheral nervous system dysfunction. The demonstration that eye closure was essential to demonstrating group differences in balance argues further in favor of an explanation rooted in the CNS. Future studies should directly test the hypothesis by comparing MRI, balance, and gait findings within the same group of patients. It was, unfortunately, not possible to perform MRI scans, or conduct clinical neurological exams, of the 168 participants in the present study because of limited funds.
The balance and vibrotactile threshold abnormalities demonstrated presently should be interpreted in a larger context. Although group differences were found, the abnormalities are, on average, not so severe as to pose a significant risk to mobility, or operator or workplace safety. The distributional properties of FBOS [41] and EQ scores [42], and vibrotactile threshold [43] have been described within large samples of healthy adults. When compared to a normal population of 40 y.o. adults, the present sample of ~40 y.o. HIV/AIDS patients exhibited modest decrements of 1.2 and 1.6 standard deviations, respectively, on FBOS and EQ scores. Untreated HIV/AIDS patients and those receiving HAART exhibited moderate elevations in vibrotactile threshold of 1.80 and 1.96 standard deviations, respectively, in comparison to the normal population.
Footnotes
Sponsorship: This study was supported by grant #R01MH61346 funded jointly by NIMH and NIDA. Additional support was provided by grants #T32AA07290 and #M01RR06192 funded by NIAAA and NCRR, respectively.
References
- 1.Cardoso F. HIV-related movement disorders: epidemiology, pathogenesis and management. CNS Drugs. 2002;16:663–668. doi: 10.2165/00023210-200216100-00002. [DOI] [PubMed] [Google Scholar]
- 2.Dal Pan GL, McArthur JC, Harrison MJG. Neurological symptoms in Human Immunodeficiency Virus Infection In: AIDS & The Nervous System Berger JR, Levy RM (editors). New York: Lippincott – Raven Publishers. 1997. pp. 141–172.
- 3.Brew BJ. The peripheral nerve complications of human immunodeficiency virus (HIV) infection. Muscle Nerve. 2003;28:542–552. doi: 10.1002/mus.10484. [DOI] [PubMed] [Google Scholar]
- 4.Gilmer WS. Neurologic problems of the lower extremity associated with HIV and AIDS. Clin Podiatr Med Surg. 1998;15:281–303. [PubMed] [Google Scholar]
- 5.Pardo CA, McArthur JC, Griffin JW. HIV neuropathy: insights in the pathology of HIV peripheral nerve disease. J Peripher Nerv Syst. 2001;6:21–27. doi: 10.1046/j.1529-8027.2001.006001021.x. [DOI] [PubMed] [Google Scholar]
- 6.Wulff EA, Wang AK, Simpson DM. HIV-associated peripheral neuropathy: epidemiology, pathophysiology and treatment. Drugs. 2000;59:1251–1260. doi: 10.2165/00003495-200059060-00005. [DOI] [PubMed] [Google Scholar]
- 7.Berger JR, Pall L, Lanska D, Whiteman M. Progressive multi-focal leukoencephalopathy in patients with HIV infection. J Neurovirol. 1998;4:59–68. doi: 10.3109/13550289809113482. [DOI] [PubMed] [Google Scholar]
- 8.Walsh K, Kaye K, Demaerschalk B, Stewart S, Crukley J, Hammond R. AZT myopathy and HIV-1 polymyositis: one disease or two? Can J Neurol Sci. 2002;29:390–393. doi: 10.1017/s0317167100002286. [DOI] [PubMed] [Google Scholar]
- 9.Masanes F, Pedrol E, Grau JM, Coll-Vincent B, Casademont J, Miro O, Mallolas J, Urbano-Marques A. Symptomatic myopathies in HIV-1 infected patients untreated with antiretroviral agents–a clinico-pathological study of 30 consecutive patients. Clin Neuropathol. 1996;15:221–225. [PubMed] [Google Scholar]
- 10.Sclar G, Kennedy CA, Hill JM, McCormack MK. Cerebellar degeneration associated with HIV infection. Neurology. 2000;54:1–12. doi: 10.1212/wnl.54.4.1012. [DOI] [PubMed] [Google Scholar]
- 11.Tagliati M, Simpson D, Morgello S, Clifford D, Schwartz RL, Berger JR. Cerebellar degeneration associated with human immunodeficiency virus infection. Neurology. 1998;50:244–251. doi: 10.1212/wnl.50.1.244. [DOI] [PubMed] [Google Scholar]
- 12.Von Giesen H-J, Wittsack H-J, Wenserki F, Koller H, Hefter H, Arendt G. Basal ganglia abnormalities in minor motor disorders associated with human immunodeficiency virus type 1. Arch Neurol. 2001;58:1281–1286. doi: 10.1001/archneur.58.8.1281. [DOI] [PubMed] [Google Scholar]
- 13.Arendt G, Maecker H, Purrmann J, Homberg V. Control of posture in patients with neurologically asymptomatic HIV infection and patients with beginning HIV-1 related encephalopathy. Arch Neurol. 1994;51:1232–1235. doi: 10.1001/archneur.1994.00540240076019. [DOI] [PubMed] [Google Scholar]
- 14.Trenkwalder C, Straube A, Paulus W, Krafcyk S, Scheike E, Einhaupl KM. Postural imbalance: an early sign in HIV-1 infected patients. Eur Arch Psychiatry Clin Neurosci. 1992;41:267–272. doi: 10.1007/BF02195975. [DOI] [PubMed] [Google Scholar]
- 15.Beckley DJ, Bastiaan BR, Martin EM, Panzer VP, Remler MP. Postural reflexes in patients with HIV-1 infection. Electroencephalogr Clin Neurophysiol. 1998;109:402–408. doi: 10.1016/s0924-980x(98)00040-x. [DOI] [PubMed] [Google Scholar]
- 16.Arendt G, Giesen VH, Hefter H, Theisen A. Therapeutic effects of nucleoside analogues on psychomotor slowing in HIV infection. AIDS. 2001;15:493–500. doi: 10.1097/00002030-200103090-00008. [DOI] [PubMed] [Google Scholar]
- 17.Arendt G, Hefter H, Buescher L, Hiperath F, Elsing C, Freund HJ. Improvement of motor performance of HIV-positive patients under AZT therapy. Neurology. 1992;42:891–896. doi: 10.1212/wnl.42.4.891. [DOI] [PubMed] [Google Scholar]
- 18.Bauer LO. Motoric signs of CNS dysfunction associated with alcohol and cocaine withdrawal. Psychiatry Res. 1993;47:69–77. doi: 10.1016/0165-1781(93)90056-m. [DOI] [PubMed] [Google Scholar]
- 19.Bauer LO. Psychomotor and electroencephalographic sequelae of cocaine dependence In: Neurotoxicity and Neuropathology Associated With Cocaine Abuse (NIDA Research Monograph #163) Majewska MD (editor). Washington, D.C.: National Institute on Drug Abuse; 1996. pp. 66–93. [PubMed]
- 20.Buchner DM, Cress ME, Esselman PC, Margherita AJ, de Lateur BJ, Campbell AJ, Wagner EH. Factors associated with changes in gait speed in older adults. J Gerontol A Biol Sci Med Sci. 1996;51:M297–M302. doi: 10.1093/gerona/51a.6.m297. [DOI] [PubMed] [Google Scholar]
- 21.Gerr F, Letz R. Covariates of human peripheral nerve function: III. Effects of reported drinking. Neurotoxicol Teratol. 1994;16:113–122. doi: 10.1016/0892-0362(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 22.Lemke MR, Wendorff T, Mieth B, Buhl K, Linnemann M. Spatiotemporal gait patterns during over ground locomotion in major depression compared with healthy controls. J Psychiatr Res. 2000;34:277–283. doi: 10.1016/s0022-3956(00)00017-0. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan EV, Desmond JE, Lim KO, Pfefferbaum A. Speed and efficiency but not accuracy or timing deficits of limb movements in alcoholic men and women. Alcohol Clin Exper Res. 2002;26:705–713. [PubMed] [Google Scholar]
- 24.Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000;24:611–621. [PubMed] [Google Scholar]
- 25.American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, DSM-IV 4th ed. Washington, D.C., American Psychiatric Press; 1994.
- 26.Robins LN, Cottler L, Bucholz K, Compton, W. The Diagnostic Interview Schedule, Version IV St. Louis: Washington University; 1995.
- 27.McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients the addiction severity index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Selzer ML. The Michigan Alcoholism Screening Test: The quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- 29.Skinner H. The Drug Abuse Screening Test. Addict Behav. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- 30.Beck AT, Steer RA, Brown GK. Beck Depression Inventory Version II Manual San Antonio, TX: Psychological Corporation/Harcourt Brace; 1996.
- 31.Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test Circle Pines, MN: American Guidance Services; 1990.
- 32.O’Connell JM, Braitstein P, Hogg RS, Yip B, Craib KJ, O’Shaughnessy MV, Montaner JS, Burdge DR. Age, adherence and injection drug use predict virological suppression among men and women enrolled in a population based antiretroviral drug treatment programme. Antivir Ther. 2003;8:569–576. doi: 10.1177/135965350300800601. [DOI] [PubMed] [Google Scholar]
- 33.Starace F, Ammassari A, Trotta MP, Murri R, DeLongis P, Izzo C, Scalzini A, d’Arminio Monforte A, Wu AW, Antinori A, AdICoNA Study Group. NeuroICoNA Study Group Depression is a risk factor for suboptimal adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31(Suppl):S136–S139. doi: 10.1097/00126334-200212153-00010. [DOI] [PubMed] [Google Scholar]
- 34.Wood E, Montaner JS, Yip B, Tyndall MW, Schechter MT, O’Shaughnessy MV, Hogg RS. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. CMAJ. 2003;169:656–661. [PMC free article] [PubMed] [Google Scholar]
- 35.Morgello S, Estanislao L, Simpson D, Geraci A, DiRocco A, Gerits P, Ryan E, Yakoushina T, Khan S, Mahboob R, Naseer M, Dorfman D, Sharp V, Manhattan HIV. HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: the Manhattan HIV Brain Bank. Arch Neurol. 2004;61:546–551. doi: 10.1001/archneur.61.4.546. [DOI] [PubMed] [Google Scholar]
- 36.Schifitto G, McDermott MP, McArthur JC, Marder K, Sacktor N, Epstein L, Kieburtz K, Dana Consortium on the Therapy of HIV and Related Cognitive Disorders Incidence of and risk factors for HIVassociated distal sensory polyneuropathy. Neurology. 2002;58:1764–1768. doi: 10.1212/wnl.58.12.1764. [DOI] [PubMed] [Google Scholar]
- 37.Bozzette SA, Santangelo J, Villasana D, Fraser A, Wright B, Jacobsen C, Hayden E, Schnack J, Spector SA, Richman DD. Peripheral nerve function in persons with asymptomatic or minimally symptomatic HIV disease: absence of zidovudine neurotoxicity. J Acquir Immune Defic Syndr. 1991;4:851–855. [PubMed] [Google Scholar]
- 38.Skowron G, Bozzettee SA, Lim L, Pettinelli CB, Schaumburg HH, Arezzo J, Fischl MA, Powderly WG, Gocke DJ, Richman DD. Alternating and intermittent regimens of zidovudine and dideoxycytidine in patients with AIDS or AIDS-related complex. Ann Intern Med. 1993;118:321–330. doi: 10.7326/0003-4819-118-5-199303010-00001. [DOI] [PubMed] [Google Scholar]
- 39.Dalakas MC. Peripheral neuropathy and antiretroviral drugs. J Peripher Nerv Syst. 2001;6:14–20. doi: 10.1046/j.1529-8027.2001.006001014.x. [DOI] [PubMed] [Google Scholar]
- 40.Scarsella A, Coodley G, Shalit P, Anderson R, Fisher RL, Liao Q, Ross LL, Hernandez JE. Stavudine associated peripheral neuropathy in zidovudine-naïve patients: effects of stavudine exposure and antiretroviral experience. Adv Ther. 2002;19:1–8. doi: 10.1007/BF02850013. [DOI] [PubMed] [Google Scholar]
- 41.King MB, Judge JO, Wolfson LI. Functional base of support decreases with age. J Gerontol. 1994;49:M258–M263. doi: 10.1093/geronj/49.6.m258. [DOI] [PubMed] [Google Scholar]
- 42.NeuroCom International, Inc. Equitest System Operator’s Manual Clackamas, OR: NeuroCom International Inc., 1989.
- 43.Arezzo JC. Quantitative sensory testing of vibration threshold: Vibratron II. Rationale and methods. Clifton, NJ: Physitemp Intruments, Inc. (unpublished report), 1993.


