Abstract
In October 2002, the U.S. Food and Drug Administration approved buprenorphine-naloxone (Suboxone®) sublingual tablets as an opioid dependence treatment available for use outside traditionally licensed opioid treatment programs. The NIDA Center for Clinical Trials Network (CTN) sponsored two clinical trials assessing buprenorphine-naloxone for short-term opioid detoxification. These trials provided an unprecedented field test of its use in twelve diverse community-based treatment programs. Opioid-dependent men and women were randomized to a thirteen-day buprenorphine-naloxone taper regimen for short-term opioid detoxification. The 234 buprenorphine-naloxone patients averaged 37 years old and used mostly intravenous heroin. Direct and rapid induction onto buprenorphine-naloxone was safe and well tolerated. Most patients (83%) received 8 mg buprenorphine-2 mg naloxone on the first day and 90% successfully completed induction and reached a target dose of 16mg buprenorphine-4 mg naloxone in three days. Medication compliance and treatment engagement was high. An average of 81% of available doses was ingested, and 68% of patients completed the detoxification. Most (80.3%) patients received some ancillary medications with an average of 2.3 withdrawal symptoms treated. The safety profile of buprenorphine-naloxone was excellent. Of eighteen serious adverse events reported, only one was possibly related to buprenorphine-naloxone. All providers successfully integrated buprenorphine-naloxone into their existing treatment milieus. Overall, data from the CTN field experience suggest that buprenorphine-naloxone is practical and safe for use in diverse community treatment settings, including those with minimal experience providing opioid-based pharmacotherapy and/or medical detoxification for opioid dependence.
Opioid dependence and its associated disorders represent a serious public health problem in the United States and many other countries. Less than 20% of an estimated 898,000 U.S. heroin users1 currently receive agonist treatment with methadone or LAAM. Further, sales and distribution of LAAM in the United States were discontinued in August 2003 as a result of severe cardiac-related adverse events associated with its use. Even fewer users receive antagonist treatment with naltrexone or some type of medical detoxification, followed by additional treatment such as that provided through a therapeutic community, short-term residential program, or drug-free outpatient program. Although each of these treatments can be effective, their availability and attractiveness to patients vary and are not sufficient to meet the current demand for treatment. The reasons are complex but include inadequate resources for patients to pay for current care, community resistance to new drug treatment programs (especially methadone), patient preference, and regulatory barriers that limit access to treatment.
Buprenorphine hydrochloride (HCl), a derivative of the morphine alkaloid the-baine, is a synthetic opioid and promising new pharmacotherapy for treating opioid dependence. Generally described as a mixed agonist-antagonist opioid, buprenorphine has been characterized as a partial agonist at the mu receptor and an antagonist at the kappa receptor.2 Buprenorphine’s partial mu-agonist pharmacology is unique, and comprehensive reviews are available regarding its clinical pharmacology and application to opioid dependence treatment.3,4 Buprenorphine’s low intrinsic activity at the mu-receptor results in a “ceiling” effect, such that higher doses of buprenorphine do not increase its agonist activity but lengthen its duration of action.5–7 As a result, buprenorphine can be dispensed on a daily or less-than-daily basis.8–11 High-dose treatment is relatively safe and affords a greater margin of safety from death by respiratory depression relative to full mu-agonist opioids such as heroin or methadone.12 Overdose with buprenorphine is uncommon. In France, where a sublingual buprenorphine tablet has been available by prescription since 1996, buprenorphine overdose is almost always associated with the injection of buprenorphine combined with benzodiazepines, alcohol, or other sedative-type substances.13–15
The half-life of buprenorphine in plasma is about 4–6 hours. Its duration of action, however, is much longer because of its exceptionally high affinity binding to mu receptors and a correspondingly slow dissociation.2,16 Slow receptor dissociation may reduce the magnitude of withdrawal symptoms associated with buprenorphine dose reduction.17 Many studies have shown that abstinence symptomology upon discontinuation of buprenorphine appears to be relatively mild,10,16,17–21 rendering buprenorphine a promising agent for opioid detoxification.
Different formulations of buprenorphine were examined during product development for the treatment of opioid dependence: a sublingual solution formulation, a sublingual tablet containing buprenorphine-only (Subutex®), and a combination tablet containing buprenorphine HCl and naloxone HCl in a 4 : 1 ratio (Suboxone®).22–25 Each formulation received extensive testing. The clinical efficacy of buprenorphine for maintenance treatment episodes of one year or less is clearly established.11,26–34 Since injection of the opioid antagonist naloxone will precipitate withdrawal in physically dependent individuals, the buprenorphine-naloxone tablet should mitigate, but not entirely eliminate, potential buprenorphine abuse through injection.25,35–46 Therefore, use of the buprenorphine-naloxone tablet is expected to be advantageous as clinical use of buprenorphine evolves in the United States.
Buprenorphine-only sublingual tablets are now available in 27 countries worldwide with approvals in place in 34 countries (Chris Chapleo, Reckitt Benckiser, personal communication, September 11, 2003). Both the buprenorphine-only and buprenorphine-naloxone tablets are available for use in the United States, although the buprenorphine-naloxone tablet is the focus of U.S. marketing efforts. Buprenorphine-only and buprenorphine-naloxone tablets were approved by the U.S. Food and Drug Administration (FDA) for opioid dependence treatment on October 8, 2002, and categorized as Schedule III narcotics by the U.S. Drug Enforcement Agency. Their approval followed more than two decades of research, countless setbacks, and combined legislative, governmental, pharmaceutical, and scientific efforts that culminated in a significant shift in U.S. treatment approaches for narcotic addiction.47
Buprenorphine and buprenorphine-naloxone sublingual tablets are the first agonist-based therapies in recent U.S. history available for use by certified physicians outside the traditional narcotic treatment delivery system and the strict requirements of the Narcotic Addict Treatment Act of 1974 (P.L. 93–281). This was made possible by the Drug Addiction Treatment Act of 2000 (DATA).48 DATA amended the Controlled Substances Act and established a waiver for qualified physicians to use Schedule III, IV, or V medications that are approved for the indication of narcotic addiction treatment outside the context of opioid dependence treatment programs (ie, in office-based treatment49,50 ). Physicians prescribing under the waiver are limited to treating a maximum of thirty patients with buprenorphine tablets at a time. Buprenorphine and buprenorphine-naloxone tablets are also available for use in a range of other treatment milieus, including licensed opioid treatment programs (OTPs). Use in OTPs became possible under an Interim Rule Change to current federal regulations [42 CFR section 8.12(h)(2)(i) and (ii)] effective May 22, 2003, that added buprenorphine to the list of approved pharmacotherapies available to these programs. As such, OTPs can dispense buprenorphine tablets much as they do methadone. It is anticipated that (1) the availability of buprenorphine-naloxone in multiple treatment milieus will increase the number of patients in treatment, and (2) its availability as an office-based treatment will bring the management of opioid dependence into the practice of mainstream medicine.
Sparse data have been gathered regarding the shorter-term use of buprenorphine-naloxone for detoxification from opiates, a strategy that mirrors much of the opiate detoxification currently practiced outside of licensed OTPs. In particular, studies are needed in the community-based clinics likely to use the marketed buprenorphine-naloxone tablet under DATA as well as in the community-based OTPs that now have access to this product under the revised federal regulations. Development of the NIDA’s National Drug Abuse Treatment Center for Clinical Trials Network (CTN) now permits such studies to occur.
Established in 1999, the NIDA CTN is a national U.S. initiative designed to integrate research and clinical practice and ultimately bridge existing gaps between the scientific and clinical treatment communities.51 A large, geographically diverse network of university-based addiction researchers and associated community-based treatment programs are partnering to conduct multisite clinical trials of established or promising treatments for substance abuse in real-life settings. Two parallel and open-label, randomized clinical trials comparing buprenorphine-naloxone and clonidine for short-term opioid detoxification were initiated across twelve CTN community treatment programs. Clonidine, an alpha-2 adrenergic agonist, is a safe, non-narcotic medication used to assist in the withdrawal of individuals from opioids.52 However, hypotensive and sedative side effects53,54 and an inability to manage the craving and general bodyaches associated with opioid withdrawal55 have limited clonidine’s treatment acceptability. The current studies were developed to help optimize strategies for detoxifying opioid-dependent individuals using buprenorphine-naloxone in non-research settings while enhancing clinical experience with the buprenorphine-naloxone tablet. The diversity of clinics in the CTN provided an unparalleled opportunity to assess field response to this new medication.
This report describes the field experience gained from the two buprenorphine-naloxone studies conducted within the NIDA CTN and may provide useful guidance for many providers considering this pharmacotherapy. Attention is focused on the induction procedure, treatment compliance and engagement, use of ancillary medications, compliance with urine drug testing, and patient safety. Only data from patients randomized to the buprenorphine-naloxone tablet are included. Descriptive data are discussed from a practical perspective that both encourages adoption and clarifies policy and implementation challenges. The outcomes of the randomized clinical trials and the assessment of factors affecting outcomes will be reported elsewhere.
METHODS
Study Design Overview
Two multi-center studies of similar design were conducted using a randomized, open-label, parallel groups design to compare buprenorphine-naloxone to clonidine in thirteen-day detoxification regimens. In study NIDA CTN-001, the detoxification regimen was initiated in residential settings, and participants were permitted as necessary to continue detoxification as outpatients for the full thirteen days. In NIDA CTN-002, the detoxification process was conducted in outpatient settings throughout the thirteen-days. In each study, a total of 360 participants were planned for randomization, of which 240 were to be assigned to buprenorphine-naloxone and 120 to clonidine after screening and baseline assessments. The 2 : 1 randomization scheme increased the number of potential participants exposed to buprenorphine-naloxone and maximized opportunities for community practitioners to work with this new treatment medication. Recruitment occurred from January 5, 2001, to February 26, 2002, at six sites in each study and was facilitated by newspaper and poster advertisements, word-of-mouth, and referrals from local treatment programs. After detoxification, participants were followed up at one, three, and six months from the date of randomization. The standard counseling procedures used at each clinic, along with self-help detoxification handbooks,56 were offered to all study participants.
Eligibility Criteria
Treatment-seeking males and non-pregnant and non-lactating females at least fifteen years old were eligible to participate. To be included in the studies, participants had to be in good general health, meet DSM-IV criteria for opioid dependence, be physically dependent on opioids, report symptoms of opioid withdrawal, and request medical treatment for these symptoms. Eligibility was determined through a comprehensive 2–3 hour intake interview completed before admission into the study. The interview included the psychoactive substance abuse disorder sections of the DSM-IV Criteria Checklist (modified from the DSM-III-R Checklist57) and a revised 1997 version of the Addiction Severity Index 5th Edition (ASI Lite58). Additional questionnaires were completed to provide information about demographics, drug history, and HIV risk. A medical history, physical exam, and laboratory evaluation assessed health status.
Exclusion criteria included evidence of acute severe psychiatric condition (eg, active psychosis, manic-depressive illness), imminent suicidality, serious medical illness that would make participation medically hazardous (eg, severe liver or cardiovascular disease, clinically significant abnormalities in ECG), or known allergy or sensitivity to buprenorphine, naloxone, or clonidine. Individuals who participated in another investigational drug study within thirty days before study enrollment, or who were receiving beta-blockers, calcium channel blockers, tricyclics, digitalis, or any other medication that could interact adversely with clonidine, were also excluded. Methadone or LAAM maintenance or detoxification within thirty days of enrollment, or pending legal actions or other reasons that might prevent an individual from remaining in the area for the duration of the active phase of treatment were also grounds for exclusion. Co-dependence on other drugs (eg, cocaine, alcohol, or benzodiazepines, or other depressants or stimulants) did not exclude individuals from participation unless immediate medical attention was required to manage these disorders.
Each study was approved by the Friends Research Institute West Coast Institutional Review Board for human research, the UCLA Office for Protection of Research Subjects, and the Institutional Review Boards for human research associated with each participating university group and/or community treatment program. Participants provided written, informed consent after receiving a full explanation of the procedures and before engaging in any research activities and inclusion in the study. All minors (except for those emancipated legally) were required to have parental/guardian consent and provide their assent to participate in the studies. Participants received $25 in gift certificates for completion of the intake interview and each of the three follow-up assessments. An additional $25 in gift certificates was provided to any participant completing all three follow-up visits. Participants did not receive any compensation during the thirteen-day detoxification with buprenorphine-naloxone.
Settings and Training Requirements
The twelve participating community treatment programs (CTP) included drug-free clinics, therapeutic communities, university-based programs, a community mental health center, a health maintenance organization, and opioid treatment programs (Tables 1 and 2). CTP and physicians’ clinical experience with opioid users and narcotic treatment medications varied. Some settings were inexperienced using narcotic medications and had limited experience with opioid detoxification, while others primarily treated opioid-dependent patients with a full range of approved narcotic and non-narcotic pharmacotherapies. Nine of the twelve study physicians (75%) were certified by the American Society for Addiction Medicine and all actively treated substance abusers at the clinics. However, training backgrounds ranged from addiction psychiatry to internal or family medicine to pediatrics or anesthesiology, and lengths of professional experience in the direct medical management of substance abuse ranged from one to twenty years.
TABLE 1.
Overview of Participating NIDA CTN-0001 Residential Programs (N = 77)
| Site (number randomized) | Location | Type | Program Involved in Study | Overall Services Provided* | Medications Historically Used for Opioid Dependence Treatment |
|---|---|---|---|---|---|
| Betty Ford Center (n = 12) | Rancho Mirage, CA | Private, for-profit, free- standing chemical dependency recovery hospital | Residential (intensive 4–6 week program) | C, IR, IOP, F | Non-narcotic only |
| Center for Drug Free Living (n = 20) | Orlando, FL | Public, non-profit integrated addiction and mental health services organization; includes opiate treatment program (OTP) | Residential detoxification unit | C, IR, IOP, D | Methadone and non-narcotic |
| Maryhaven (n = 13) | Columbus, OH | Private, non-profit corporation with integrated addiction and mental health services | Residential detoxification unit; ambulatory detoxification | C, IOP, D, O | Primarily drug-free except for the use of medications (including opiates) for opioid detoxification, and for medical and psychiatric treatment |
| Operation PAR (n = 18) | St. Petersburg, FL | Public, non-profit integrated addiction and mental health services organization; includes OTP | Residential therapeutic community | C, IR, IOP, D | Methadone and non- narcotic |
| Phoenix House (n = 2) | New York, NY | Public, non-profit therapeutic community | Residential | C, LTR | Non-narcotic; primarily drug-free except for use of medications for mental illness |
| Self-Help Addiction Recovery [SHAR] (n = 12) | Detroit, MI | Public, non-profit multimodality, providing addiction and mental health services | Residential therapeutic community | C, IOP, IR, A, AD | Non-narcotic; primarily drug-free except for use of medications for mental illness |
IOP = intensive outpatient; O = outpatient; IR = intensive residential; LTR = long-term residential; A = aftercare; AD = ambulatory detoxification; D = day treatment, C = comprehensive service; F = family services.
TABLE 2.
Overview of Participating NIDA CTN-0002 Outpatient Programs (N = 157)
| Site (number randomized) | Location | Type | Program Involved in Study | Overall Services Provided* | Medications Historically Used for Opioid Dependence Treatment |
|---|---|---|---|---|---|
| Addiction Research and Treatment Corporation (n = 30) | Brooklyn, NY | Public, non-profit opiate treatment program (OTP) | Large, outpatient OTP | C, O | Methadone |
| Aegis Medical Systems (n = 29) | Oxnard, CA | Public, for-profit organization of licensed OTPs | Large, outpatient OTP | C, O | Methadone, LAAM, naltrexone |
| Bellevue Hospital (n = 20) | New York, NY | Public, non-profit, hospital-based substance abuse service with OTP | Large, outpatient OTP | C, O, IOP | Methadone |
| Kaiser-Permanente Northwest (n = 19) | Portland, OR | Non-profit HMO | Department of Addiction Medicine within a large primary care setting | C, O, IOP, AD | Clonidine |
| Mercer Trenton Addiction Science Center/Robert Wood Johnson Medical School (n = 27) | Trenton, NJ | Public, non-profit, university-based with OTP | Outpatient OTP | C, O, IOP, AD | Methadone |
| Midtown Community Mental Health Center (n = 32) | Indianapolis, IN | Community Mental Health Center, public, non-profit, integrated mental health and addictions with OTP | Outpatient Detoxification Clinic at Wishard Hospital | C, O, IOP, A, AD, IR, D, F | Methadone, LAAM, non-narcotic |
IOP = intensive outpatient; O = outpatient; IR = intensive residential; LTR = long-term residential; A = aftercare; AD = ambulatory detoxification; D = day treatment, C = comprehensive service; F = family services.
CTP and physician research experience and staffing patterns also varied. While some programs, clinical and support staff, and physicians had years of experience participating in research, others had never conducted a medication study. Similarly, some programs had been newly staffed with experienced research personnel by associated university partners while others worked with their university partners to train existing clinical and support personnel to conduct the research.
Several national two-day protocol training sessions facilitated protocol implementation, provided all personnel with specific training in the clinical use of buprenorphine-naloxone and clonidine, reviewed protocol specifics and standard operating procedures, and addressed any previously unforeseen practical or logistical concerns. Additionally, all research personnel completed training in Good Clinical Practice and the Protection of Human Subjects. Throughout the studies, biweekly national protocol implementation calls addressed ongoing questions and/or concerns and provided study updates to participating clinics. Some of the participating university partners also held local biweekly teleconferences with their associated community treatment programs to discuss study issues.
Buprenorphine-Naloxone Induction and Daily Dosing Procedures
To facilitate a comfortable transition onto buprenorphine-naloxone, participants were instructed not to use any heroin or other opiates for at least six hours before receiving their first dose of the buprenorphine-naloxone tablet and to be in mild withdrawal before taking their first dose in accordance with published reports.3,4,8,9 Physicians or study coordinators at each site took a history from the patient before administering the first dose of buprenorphine to document the time and date of last drug use and verify patient withdrawal status using established observer (Clinical Opiate Withdrawal Scale [COWS])59 and self-report (Adjective Rating Scale for Withdrawal [ARSW])8,60,61 scales of opiate withdrawal.
Two added precautions were taken to help ensure a smooth transition onto the buprenorphine-naloxone tablet and mitigate potential adverse drug reactions. First, participants in both studies had to provide a methadone-negative urine sample on Day 1 before randomization and dosing. This was done to mitigate buprenorphine-precipitated withdrawal due to the presence of a long-acting opioid. Second, in response to reports of potential adverse drug reactions between benzodiazepines and buprenorphine in outpatient settings,13–15 participants in the outpatient protocol were also required to provide a urine sample negative for benzodiazepines on Day 1 before randomization and dosing. An on-site, rapid urine drug screen was used for these purposes (Accutest® 10-MultiDrug Screen, JANT Pharmacal, Encino, CA). If the result of this drug screen was methadone-and/or benzodiazepine-positive, the participant was not randomized, and the test was repeated at a later date using the same procedures as outlined above until all requirements for induction were met.
Participants randomized to buprenorphine-naloxone received daily doses for thirteen days with sublingual administration of 2 mg buprenorphine-0.5 mg naloxone tablet(s) and/or an 8 mg buprenorphine-2.0 mg naloxone tablet(s). Take-home doses for self-administration at home were provided for Saturday and Sunday and scheduled holidays, but only in the outpatient protocol. A modified, three-day, rapid, buprenorphine-naloxone induction procedure was used in accordance with published reports.8,9 On Day 1, patients received two sublingual tablets that each contained 2 mg of buprenorphine and 0.5 mg of naloxone, comprising a total dose of 4 mg of buprenorphine and 1.0 mg of naloxone. An additional two tablets (or 4 mg of buprenorphine and 1.0 mg of naloxone) were provided 1–2 hours following the initial 4 mg dose unless clinically contraindicated (eg, if the patient refused an additional dose or displayed obvious agonist effects). Physicians were encouraged to provide all participants the full 8/2 mg divided dose of buprenorphine-naloxone on Day 1. Table 3 lists the dosing schedule for the first and remaining buprenorphine-naloxone administrations over the course of the thirteen-day detoxification. Doses escalated in a step-wise manner to 16/4 mg on Day 3 and tapered to 2/0.5 mg by Days 12 to 13. The taper schedule was based on results from an earlier study18 and modified to represent an intermediate step between more rapid and gradual detoxification schedules that reflect the usual and customary length of opioid detoxification delivered in community-based drug treatment.
TABLE 3.
Buprenorphine-Naloxone Thirteen-day Detoxification Schedule
| Study Day | Buprenorphine-Naloxone Dose (expressed as mg of buprenorphine) |
|---|---|
| 1 | 4 + additional 4 as needed |
| >2 | 8 |
| 3 | 16 |
| 4 | 14 |
| 5 | 12 |
| 6 | 10 |
| 7 | 8 |
| 8 | 6 |
| 9 | 6 |
| 10 | 4 |
| >11 | 4 |
| 12 | 2 |
| 13 | 2 |
Buprenorphine-naloxone sublingual tablets were provided in child-proof blister packs from Reckitt Benckiser (Hull, UK) and supplied through the NIDA and Research Triangle Institute. Tablets were then pre-packaged for treatment sites by McKesson HBOC (Rockville, MD) into patient dosing kits containing each of the thirteen individual daily doses. These dosing kits were designed to minimize the burden on treatment staff and reduce the probability of dosing deviations involving buprenorphine-naloxone. Participants were instructed to hold the tablet(s) under their tongue until dissolved, which typically took about 5–8 minutes. Dissolution of tablets was monitored by personnel at each clinic as necessary.
Ancillary Medications
A range of prescription and over-the-counter ancillary medications to assist with specific withdrawal symptoms commonly encountered during opioid detoxification were made available throughout the thirteen-day treatment regimen. Supplementary medications were used to manage anxiety and restlessness (ie, oxazepam, lorazepam, phenobarbital, and hydroxyzine HCL), bone pain and arthralgias (ie, methocarbamol, ibuprofen, and acetaminophen), nausea (ie, trimethobenzamide), diarrhea (ie, loperamine and donnatal), and insomnia (ie, zolpidem tartrate, trazadone HCl, doxepin HCl, and diphenhydramine). The use of ancillary medications during opioid detoxification is fairly common, especially when using non-narcotic agents such as clonidine. Their availability helped ensure that these studies better reflected standard clinical care of opioid-dependent patients during detoxification at most community-based drug treatment programs.
Ancillary medications were provided in bulk supply to each study site in accordance with physician request. Physicians were not required to dispense each ancillary medication but rather to provide them according to their personal preference, practice, and patients’ clinical need but within protocol dosing guidelines.
Protocol dosing guidelines dictated that only one type of ancillary medication for any given symptom was to be dispensed on any given day. That is, physicians could not dispense multiple ancillary medications for a given symptom on a given day, although across days they could elect to try different medications. For outpatient programs, participants received the ancillary medications in a child-proof bottle for self-administration at home in accordance with instructions listed on the bottle. At the start of the detoxification, patients were instructed on the use of each medication. Refills were available to all participants during each scheduled clinic visit.
Dependent Measures
Descriptive data from patients randomized to buprenorphine-naloxone pertaining to general demographics, compliance with buprenorphine-naloxone, use of ancillary medications, and compliance with urine drug testing during the thirteen-day detoxification are presented in this report. The extent of serious adverse events is also described over the course of the entire trial (including follow-up). Treatment compliance and retention was calculated as the number of days each patient received detoxification medication. Missed visits or missed doses were recorded and counted as doses not given. Data are reported as the mean ±1 standard deviation unless otherwise indicated.
RESULTS
Demographic Characteristics of Patients Randomized to Buprenorphine-Naloxone
Two-hundred-thirty-four (234) opioid-dependent men and women were randomized to receive buprenorphine-naloxone. Demographic characteristics of the study population are listed in Table 4. Patients averaged 37 years old (range 19–65) and were mostly male (69%), White (44.9%) or African-American (30.8%), and employed at the time of study enrollment (74%). Patients averaged about three prior drug treatments in their lifetime (range 0–35). Twenty-one percent had no prior treatment, 19% had only one prior treatment, 23% had two prior treatments, and 37% had three or more prior drug treatments. Most patients (90%) had been regular heroin users for an average of 8.7 years (range 0–38) and most (65%) used heroin intravenously. Eight percent reported exclusive use of other opiates, and only one person reported exclusive use of black market methadone during the thirty days prior to enrollment. Dependence on cocaine and nicotine were the most frequent types of substance use co-morbidity.
TABLE 4.
Demographic Characteristics of Patients Randomized to Buprenorphine-Naloxone (N = 234)*
| Variable | |
|---|---|
| Age (yrs) | 37.4 ± 10.5 |
| Gender | |
| Male | 69% |
| Female | 31% |
| Race/Ethnicity | |
| White | 44.9% |
| African-American | 30.8% |
| Latino | 19.2% |
| Multiethnic | 3.8% |
| American Indian | 0.4% |
| Other | 0.9% |
| Employment Status | |
| Full-time | 53.8% |
| Part-time | 20.1% |
| Unemployed | 19.2% |
| Other | 6.9% |
| Duration of Opioid Use (yrs) | 9.7 ± 9.3 |
| Duration of Heroin Use (yrs) | 8.7 ± 9.2 |
| Route of Heroin Use | |
| IV injection | 65.5% |
| Non-IV injection | 3.8% |
| Intranasal | 30.0% |
| Smoke | 1.4% |
| Lifetime Prior Drug Treatments | 2.7 ± 3.7 |
| DSM-IV Other Current Dependence Diagnoses | |
| Nicotine | 29.1% |
| Cocaine | 19.2% |
| Alcohol | 6.0% |
| Cannabis | 5.6% |
| Sedative | 1.7% |
| Amphetamine | 1.3% |
| Hallucinogen | 0.4% |
| Inhalant | 0.4% |
| PCP | 0.0% |
Values represent means and standard deviations unless otherwise indicated.
Buprenorphine-Naloxone Induction
All 234 patients took at least one of the two allowable doses on Day 1 and most (82.9%) also received the second 4 mg dose. Most patients (90.1%) received a dose of medication on Days 1, 2, and 3, successfully completing the direct buprenorphine-naloxone induction.
Buprenorphine-Naloxone Compliance and Engagement across the Thirteen-day Detoxification
Fig. 1 compares the percent of patients taking the expected dose and any dose of buprenorphine-naloxone over the thirteen-day detoxification period and shows that adherence to the delivery of protocol-prescribed doses each day was excellent. There were eighteen occasions in which a dose slightly greater or lower than proscribed by protocol was provided. Although compliance decreased slightly over time, overall medication adherence was high, with a mean of 10.5 ± 3.8 doses taken (80.7%; range 1–13) of the thirteen doses possible. Slightly over half of the patient sample (52.9%) complied perfectly, completing the taper and taking a dose of medication on all thirteen days. Sixty-eight percent of the patients completed the thirteen-day taper program.
FIGURE 1.
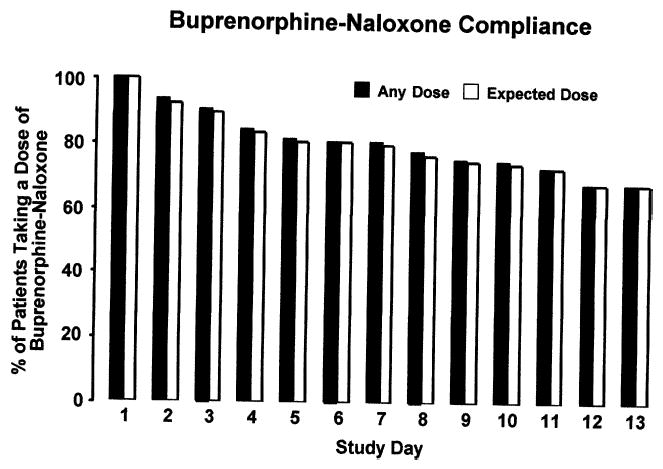
Percent of randomized patients taking any (solid bars) and the expected (open bars) milligram dose on each day of the buprenorphine-naloxone detoxification.
Types and Frequency of Ancillary Medications Used
Most (80.3%) patients received at least one ancillary medication during the study. An average of 2.3 withdrawal symptoms (range 0–5) were treated. Fig. 2 shows the percentage of patients receiving a dose of buprenorphine-naloxone who were also provided ancillary medication during each day of the dose taper. Insomnia (61.5%), anxiety and restlessness (52.1%), and bone pain and arthralgias (53.8%) were the most common symptom complaints; they were each treated with ancillary medications and managed at about the same frequency. Treatment for nausea (34.6%) and diarrhea (25.2%) was comparatively infrequent and occurred mostly during the first few days of the dose taper. By the second week of treatment, only about 5–10% of patients were treated for these latter complaints. The dispensing of all ancillary medications declined over time, regardless of the symptom treated.
FIGURE 2.
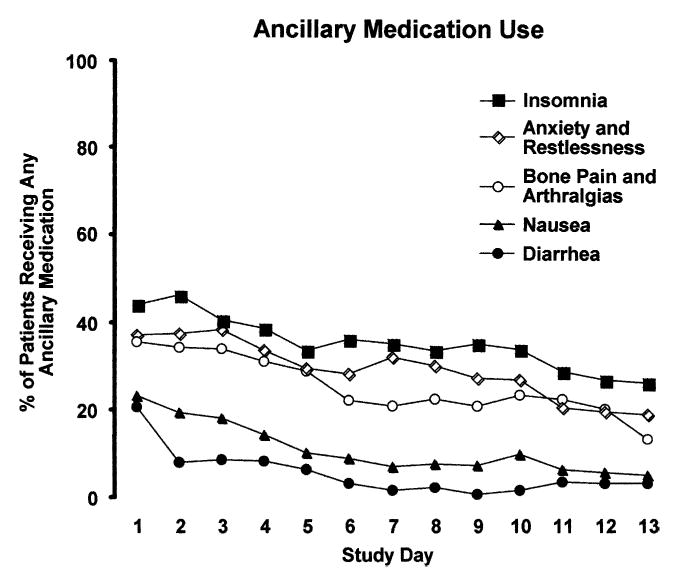
Percent of randomized patients who received a dose of buprenorphine-naloxone and any type of ancillary medication on each day of the dose taper for Insomnia (closed square); Anxiety and Restlessness (open diamond); Bone Pain and Arthralgias (open circle); Nausea (closed triangle); or Diarrhea (closed circle).
Physician preferences in dispensing certain types of ancillary medications were evident. The anxiolytics lorazepam and oxazepam were favored for managing anxiety and restlessness. Ibuprofen was used most frequently for managing bone pain and arthralgias. The oral formulation of trimethobenzamide was used almost exclusively for managing nausea. Loperamide was solely used to manage diarrhea, and zolpidem tartrate and trazadone were favored for managing insomnia.
Compliance with Urine Drug Screening
Most (3.1 ± 1.3; range 0–4) of the four required urine samples were obtained during the thirteen-day buprenorphine-naloxone detoxification.
Safety Profile
Generally, side effects encountered among patients receiving buprenorphine-naloxone were expected and primarily related to signs and symptoms of opioid withdrawal. Eighteen serious adverse events were reported over the course of the entire clinical trial (including follow-up). Seventeen of these events resulted in hospitalization, and one resulted in death. Sixty-one percent were expected events associated primarily with hospitalization for drug relapse or similarly related treatment, and most of these events (83%) transpired during the follow-up period. The death that transpired was unexpected, discovered at the six-month follow-up evaluation, and was associated with respiratory failure from a massive heart attack. The heart attack occurred four months after the patient had completed the taper with buprenorphine-naloxone and was not buprenorphine-naloxone related. Only one unexpected event was possibly related to buprenorphine-naloxone, and it was associated with hospitalization for vomiting blood (hematemesis), presumably due to bleeding from an esophageal tear. The relative contribution of the various factors causing the esophageal tear was hard to determine, but it was thought that the hematemesis was related to excessive hiccuping, which irritated the patient’s gastro-esophageal mucosa and may possibly have been related to buprenorphine-naloxone.
General Experience Bringing Buprenorphine-Naloxone to Community Treatment Programs, Protocol Compliance, and Regulatory Issues
A principal mission of the NIDA CTN is to conduct clinical trials in community-treatment programs and promote an integration of research and practice. Because many community practitioners and their associated medical personnel did not have experience conducting controlled research, we anticipated that there would be issues regarding protocol implementation and compliance, similar to most pivotal medication trials even when conducted by experienced research personnel in research-based settings.
Implementation across the community treatment programs went smoothly and the programs generally complied with the study protocol and adhered to well-accepted standards of good research practice. There were logistical issues to resolve at all study sites, and these related mostly to the physical space requirements for the separate storage of clinical versus research records and identifying appropriate storage locations for the study medications in the drug-free treatment settings. Discussion and visits to the study sites resolved these concerns and did not negatively impact participating programs.
The reported protocol violations were typical of those encountered during most multi-site clinical trials, including relatively minor medication dosing deviations, failure to document an adverse event, or including information obtained outside the permitted time frame for data capture. These types of violations were also expected, especially when taking into consideration the diversity of treatment sites involved and the fact that the two research protocols were the first pharmacotherapy trials to be conducted in the NIDA CTN.
Interestingly, medical staff had a tendency to exceed the protocol dosing guidelines for ancillary medications. None of the dosing deviations was serious from the standpoint of well-established standards of clinical practice and likely benefited patients. In hindsight, these dosing deviations probably reflect a weakness in the protocol for use of ancillary medications. For example, the protocol could have allowed physicians to treat withdrawal symptoms more aggressively with ancillary medications. Additional training during conference calls and ongoing discussion helped eliminate ancillary dosing deviations.
On February 26, 2002, one of the national Institutional Review Boards (IRB) suspended new enrollments in both study protocols due to their concerns regarding protocol violations and their desire for additional information before allowing the study to continue further. The IRB lifted the suspension on June 4, 2002. The NIDA Data Safety and Monitoring Board (DSMB) and the National Principal Investigator, however, recommended closing the two protocols. The NIDA CTN Steering Committee finalized the decision on August 14, 2002. The decision to close the study was not related to any activities related to the IRB suspension. Rather, the decision was based on the DSMB’s review of the data that had been collected prior to the suspension, the large enrollment status across both studies, the consistency of findings to date, and the fact that additional subject recruitment was unlikely to yield meaningful new information.
DISCUSSION
Field experiences in two clinical trials with a diversity of treatment settings suggest that short-term opioid detoxification using the buprenorphine-naloxone combination is feasible, practical, safe, and adaptable. Even programs without prior experience using opioid agonist therapies implemented study procedures with little difficulty. Community-based physicians, nurses, physician assistants, and counselors were trained in the use of buprenorphine-naloxone, and patients were detoxified safely. Four key findings emerged related to medication induction, patient compliance and engagement, use of ancillary medications, and patient safety. These findings are reviewed here, followed by discussion of the challenges and limitations, lessons learned, and final conclusions about the CTN field experience.
Induction
The induction procedure was more rapid than the procedures used in most prior studies of buprenorphine but was effective and safe. Ninety percent of the patients completed the induction schedule and reached the target dose of 16 mg buprenorphine—4 mg naloxone within three days. Patients safely tolerated 8 mg of buprenorphine—2 mg naloxone tablet on the first day of treatment. This buprenorphine starting dose is 2–4 times greater than previously reported in most studies using either the solution,11,29,34,62–65 buprenorphine tablet,26,32,66–68 or buprenorphine-naloxone tablet8,9 preparations and is consistent with current practice guidelines.69 Importantly, the higher buprenorphine-naloxone starting doses may have in part accounted for the excellent early treatment compliance. These data also suggest that when managing street heroin or oral opiate users, office-based physicians and/or practitioners from other treatment milieus can initiate buprenorphine treatment directly with the buprenorphine-naloxone tablet.
This finding has important pragmatic implications. The ability to use buprenorphine-naloxone tablets for dose induction with a geographically diverse group of street heroin and oral opioid users may further mitigate any potential for misuse or abuse of buprenorphine during the early stages of treatment and be beneficial to both providers and patients in that regard. Procedures for transferring patients from long-acting opioids will necessitate slightly different approaches.3,70,71
Compliance and Engagement
Most patients were compliant with the medication regimen and completed the short-term buprenorphine-naloxone detoxification. Compliance was similar in residential and outpatient settings and was unaffected by the type of treatment setting or its particular treatment philosophy. The 68% treatment retention rate was higher than those reported in the scant literature on similar buprenorphine taper schedules.72,73 In fact, this retention rate was comparable to or better than that seen after six to 24 weeks of daily buprenorphine maintenance using at least 8 mg of sublingual solution or tablet.29,32,34,68 Given this successful outcome, the length of the taper regimen used in these field trials may be of considerable importance from both a practical and clinical perspective.
On a practical level, although reimbursement for services varies by the payor, state, and the type of treatment program, the buprenorphine taper schedule was within the usual length of opiate detoxification in outpatient settings (21 days) and many residential settings (typically 5–7 days). Coupled with the high rate of treatment compliance and patient acceptability, the taper regimen appears to be suitable for use in a variety of treatment settings, regardless of program type or location. Moreover, although in some cases special arrangements were required, the majority of residential sites allowed patients to attend the clinic as outpatients when third-party payers would not tolerate longer stays (ie, those in excess of five days). These data suggest that even under such scenarios, residential programs can integrate a short-term course of buprenorphine-naloxone therapy into ongoing clinical programming.
On a clinical level, patients entering community-based treatment programs, or even patients that plan to pursue office-based buprenorphine therapy, do not necessarily approach providers for maintenance substitution treatment or other forms of long-term treatment. Rather, many patients simply seek immediate medical treatment to manage their opioid withdrawal. If managed satisfactorily, even short-term treatments can establish a therapeutic alliance critical to a patient’s receptivity to other modes of care, including those of longer duration. Although many patients in the current studies had attempted treatment previously, 21% had never received prior treatment for their drug dependence. Anecdotal reports also indicate that many patients continued or eventually returned to treatment (the follow-up data from the current study will address this point directly). However, subsequent clinical experiences of two treatment partners in the residential protocol (Phoenix House and Maryhaven) show that short-term buprenorphine detoxification can function as a gateway to longer-term treatment, as has been reported previously.74
Phoenix House, a therapeutic community, had not previously used opioid agonist medications in the medical management of opioid-dependent patients. They hoped buprenorphine-naloxone might serve as an adjunct to ongoing programming to reduce early treatment attrition among patients entering their program in withdrawal from heroin and/or methadone. After the study, Phoenix House augmented programming and developed an outpatient enhancement to the usual care provided during residential drug treatment known as First Step (T. Horton and E. Collins, Phoenix House, Personal Communications, August 6, 2003, and November 17, 24, and 26, 2003). First Step opened on May 5, 2003, and provides outpatient medical withdrawal from opioids with buprenorphine-naloxone using induction and tapering schedules similar to those developed for the study. Patients entering First Step are members of the treatment community from their first day of admission, enjoying complete continuity of care as Phoenix House focuses on engagement and transitioning these patients into long term treatment. As of October 31, 2003, First Step admitted 100 patients. The completion rate has been approximately 80%, and most patients (approximately 75%) started long-term residential treatment. Anecdotally, the admissions department reported that prior to the First Step program, only 40–50% of referrals to outside detoxification programs ever returned to Phoenix House to begin residential treatment.
Maryhaven also viewed the use of buprenorphine-naloxone as a possible tool for addressing high dropout rates as well as other treatment complications commonly observed during clonidine-assisted opiate detoxification. The staff ’s clinical observations and the patient’s feedback regarding the buprenorphine-naloxone field trial were both very positive, and Maryhaven began seeking support to implement buprenorphine/naloxone-facilitated opiate withdrawal shortly after the October 2002 FDA approval (G. Brigham, Personal Communications, October 16, 2003, and December 1, 2003). Maryhaven secured county and state level support for implementing a buprenorphine-naloxone program, trained two additional physicians who then received waivers for prescribing buprenorphine, and adopted the residential thirteen-day buprenorphine-naloxone taper with some modifications to broaden patient eligibility. All patients are counseled on the potential dangers of relapse and encouraged to continue with an intensive level of care. Patients are inducted on buprenorphine-naloxone in the residential detoxification unit and then transferred after a period of stabilization (usually seven days) to either short-term community residential treatment (usually fourteen to 35 days) or ambulatory detoxification (seven days of day treatment). The first patient received buprenorphine-naloxone on August 25, 2003, and by October 15, 2003, Maryhaven admitted 24 patients to the buprenorphine-naloxone program. Of the 24 patients started on buprenorphine-naloxone, 75% completed the buprenorphine-naloxone taper, and 58% entered ongoing treatment beyond detoxification. Six patients left against medical advice. Prior to Maryhaven’s introduction of the buprenorphine-naloxone program, their detoxification protocol completion rate was only 40%.
Ancillary Medications
Unlike most clinical trials of buprenorphine, including the limited number of controlled studies assessing buprenorphine detoxification,3 ancillary non-narcotic medications were available to patients for the duration of the dose taper to assist in the management of withdrawal symptoms. These ancillary medications were provided to the majority of patients and may have influenced the success of the short-term buprenorphine/naloxone detoxification regimen. While insomnia, anxiety and restlessness, and bone pain and arthralgias were the most frequently treated symptoms, these types of complaints are notoriously common among patients withdrawing from opioids, regardless of the medication used to help manage abstinence symptoms. Their appearance during a buprenorphine/naloxone detoxification is therefore not surprising. Although it is not possible to know whether the use of ancillary medications was necessary to maintain treatment compliance and retention, allowing physicians the latitude to combine compatible medications in an effort to enhance patient management is consistent with viewing addiction as a complex medical disease.
Safety
Few serious adverse events were encountered during the investigations, and only one was possibly related to the use of buprenorphine-naloxone. This side effects profile is consistent with other reports confirming the safety of buprenorphine in solution and tablet forms. Buprenorphine has been well tolerated with no apparent significant side effects in studies involving more than 5,000 patients in the United States.3 The experience from these field trials further underscores the safety of using buprenorphine-naloxone in a wide range of community treatment settings. As is the case with methadone, buprenorphine is safe and well tolerated when used as recommended. Precautions were taken in these studies to mitigate adverse drug reactions, such as those related to the combined misuse of buprenorphine and sedatives and/or other depressants13–15,75–77 and the co-administration of buprenorphine with long-acting opioids.78,79 Patients in our studies were carefully screened for dependence on benzodiazepines and were required to provide a benzodiazepine-negative urine sample before starting buprenorphine-naloxone. As with any agonist-based pharmacotherapy, educating patients about the potential lethality of abusing respiratory depressants (especially benzodiazepines) while taking buprenorphine, or abusing benzodiazepines and buprenorphine in combination, is extremely important. Although prescribing benzodiazepines to patients being treated with buprenorphine should not necessarily be avoided, such prescriptions should be carefully monitored, especially in patients at risk for benzodiazepine abuse. Patients being treated with buprenorphine should be cautioned regarding the danger of misusing buprenorphine with benzodiazepines and other central nervous system depressants (eg, alcohol). Moreover, patients recently maintained on long-acting opioids, primarily methadone or LAAM, were excluded from participation. This was done because the direct transfer of such patients onto buprenorphine-naloxone requires additional safeguards. Since this field trial represented the first experience with buprenorphine for the community treatment providers, we kept matters as simple as possible.
Challenges and Limitations
A few challenges in conducting these field trials and some study design limitations warrant discussion. The overall diversity of treatment settings participating in the field trials translated into unique implementation complexities.80 Community treatment programs expressed frustrations associated with participation in the clinical trials especially inadequate staffing and turnover, training and cross-training, and the volume of paperwork and regulatory requirements. However, now that buprenorphine is available for general clinical use, these burdens should lighten considerably.81
There were also limitations to the study design. Although criteria for inclusion in the study were less restrictive than in many other prior pivotal trials of buprenorphine, patients with recent histories of methadone maintenance or detoxification treatment, major medical problems, or serious psychiatric comorbidity were excluded from participation. While these sample characteristics somewhat limit the generalizability of the findings to the larger population of opioid users entering treatment programs, the study population was remarkably similar to other large samples of patients receiving opioid dependence treatment.82,83 Thus, potential differences in pretreatment characteristics between the CTN sample and other opioid-dependent patient cohorts are not likely to significantly affect the response to buprenorphine treatment.
Counseling procedures in existence at each community treatment program were used rather than trying to standardize the delivery of psychosocial services across study sites in order to maintain the CTN goal of conducting research in “real-life” clinic settings. While this may have contributed to increased variability in treatment response, it also underscores the compatibility of buprenorphine with a range of different treatment approaches and service environments. Self-help handbooks assured a basic platform of detoxification education for all participants.
Lastly, the experience with the protocol violations around the use of ancillary medications is important because it demonstrated that some physicians relied heavily on their own clinical experience with widely available medications, even when given guidelines for their use. In hindsight, we believe that this feature of the study protocol was flawed and should have been less restrictive, permitting physicians to practice within clinically established guidelines as opposed to limiting their ability to effectively manage patients.
Lessons Learned
Other lessons learned from this experience inform clinical practice with buprenorphine-naloxone. Importantly, the field experience with buprenorphine-naloxone met two of three key goals for achieving optimal patient success during opioid detoxification: maintaining medication compliance and facilitating treatment retention. Although not reviewed in the present report, a third goal, maintaining opioid abstinence, was also achieved, with almost half (43%) of the patients opioid-free on the last day of the buprenorphine taper. This latter finding is examined along with the outcomes of the randomized clinical trials.84
The field experience met many of the treatment providers’ goal of enhancing retention of opioid-dependent patients. Many of the community providers were initially interested in the protocol because they wanted to learn more about potential strategies that might reduce the high rate of early attrition among opioid-addicted patients. This was especially true for the therapeutic community organizations and drug-free clinics that provide long term care to drug-addicted patients. Many patients entering treatment in these settings undergo detoxification before arriving at the facility, receive less than effective detoxification at the site, or continue to have unpleasant withdrawal symptoms. These conditions may contribute to patients tending to drop out of treatment rapidly to resume street heroin use. Because many providers view buprenorphine as fundamentally different from traditional narcotic treatments (eg, methadone) due to its partial agonist pharmacology, clinics are more amenable to using it and are now able to do so under DATA. Moreover, logistical considerations such as medication security and procedures for drug accountability are much less of an issue. Providers at most of these programs are accustomed to following DEA rules for the management and storage of medications in Schedule III. The adaptability of the short-term taper regimen across so many treatment settings and the high rate of patient acceptability may therefore improve treatment retention and success of long-term residential or drug-free treatment for opioid dependence.
Patient interest in these buprenorphine-naloxone treatment programs was high enough that some programs had to develop wait lists for interested research participants. Dire predictions about the buprenorphine-naloxone tablet’s potential unpopularity85 may be unfounded. Demand for this treatment may escalate in accordance with its availability through office-based practice and other treatment programs. Hopefully, the restrictions imposed by DATA on the number of patients that can be treated at any one time under the waiver program will be balanced by an increased number of physicians and community treatment programs using buprenorphine, including licensed opioid treatment programs where such restrictions are not applicable.
All patients in these studies had on-site access to psychosocial services and were expected to attend counseling regularly. The extent to which these counseling requirements impacted outcomes in this study cannot be determined, although the positive relationship between the dose of psychosocial services received and opioid treatment outcomes is well established86 and confirmed in direct evaluations of buprenorphine detoxification.19 DATA requires that physicians have the capacity to refer patients to ancillary services during buprenorphine treatment, and it is hoped that all providers will directly provide or actively encourage patients to seek such services.
Finally, although urine testing is not a requirement for office-based buprenorphine treatment, it is a fairly common practice in community treatment programs, and urine test results are a useful marker of treatment response. The relative ease with which urine samples were obtained in the field trial, even though patients were not compensated for providing them during the thirteen-day taper, speaks to the viability of urine drug screening during buprenorphine treatment regardless of the setting for service delivery.
Conclusions
Although long-term outcomes associated with heroin detoxification are historically poor,87,88 data from these field trials suggests that a short-term intervention strategy using buprenorphine-naloxone may have merit. Patients were retained in treatment, and in 21% of the cases, the detoxification program served as a point of first contact with the treatment system. Moreover, two of the community treatment providers added short-term buprenorphine-naloxone detoxification into their standard clinical program following study participation and found that this addition is reducing early program attrition and facilitating patient transition into long-term treatment. These findings suggest that buprenorphine’s clinical pharmacology may render it uniquely well suited for opioid detoxification and allow what has traditionally been an unsuccessful treatment approach to serve as a possible gateway to long-term care. The findings also underscore how the research experience for our community partners met a major goal of the NIDA CTN initiative in that the use of buprenorphine-naloxone was successfully disseminated into real-life clinical practice.
Safe and effective short-term intervention strategies can provide incremental relief from a chronic disease while helping to establish an alliance with treatment providers. Treatment engagement is especially salient when viewed in light of disturbing findings from a longitudinal cohort study of heroin addicts spanning over three decades.83 Severe, lifelong, personal and social consequences associated with heroin addiction were evident, and the likelihood of permanent heroin abstinence was low. The findings point to a need for drug abuse treatment programs to focus more attention on fostering incremental and more realistic improvements in the lives of heroin addicts,83 such as those that might be obtained from short-term detoxification with buprenorphine-naloxone.
Of course, when viewing current findings from this perspective, it should not be forgotten that decades of addiction research have shown that agonist maintenance treatment has the best track record of controlling opioid use and saving lives.82,89,90 Access and easy transfer to such care should be part of any medically supervised withdrawal program.
Acknowledgments
This publication was supported by a series of grants from NIDA as part of the Cooperative Agreement on CTN (University of California, Los Angeles: U10 DA13045; Oregon Health Sciences Center: U10 DA13036; New York University School of Medicine: U10 DA13046; University of Pennsylvania: U10 DA13043; Wayne State University: U10 DA13710; University of Cincinnati: U10 DA13732; University of Miami Center for Family Studies: U10 DA13720; Research Foundation for Mental Hygiene, Inc., New York State Psychiatric Institute Division: U10 DA13035). We thank the NIDA CTN staff, especially Betty Tai, Ph.D., Jack Blaine, M.D., Ming Shih, Ph.D., and Carmen Rosa. We thank Nora Chiang, Ph.D., and Moo Park, Ph.D., of NIDA’s Division of Treatment Research & Development for their patience and help bringing this project to life. We also thank the members of the CTN Publications Subcommittee and Jonathan B. Kamien, Ph.D., for their helpful comments on earlier versions of this manuscript.
The following individuals are thanked for their invaluable assistance with these studies: Joe Hass and Ben Weltin from McKesson HBOC; Clare Keany, Mindy Blum, Ph.D., Frank Flammino, Ph.D., Luna Yojay, Dave Bennet, and Richard Rawson, Ph.D., from the UCLA Integrated Substance Abuse Programs; Nancy Waite-O’Brien, Ph.D., Michelle Buckman, R.N., and Steven Ey, M.D. from the Betty Ford Center; Steve Ruh, M.D., and Suzette Gelacio from Aegis Medical Systems, Inc.; Lynn Kunkel, Sara Lamb, and Anna Sosnowski from the Oregon Health and Science University; Bradley M. Anderson, M.D., and Lucile Gauger, PA-C, from the Kaiser Permanente Northwest Department of Addiction Medicine; Joseph Kann, PA-C, Reesa Laws, Frances Lynch, and Suzanne E. Gillespie from the Kaiser Center for Health Research; Robert Maslansky, M.D., from Bellevue Hospital; Svetlana Brodsky, M.D., Ph.D., and Rhonda Wade, R.N., from the NYU School of Medicine; Melissa Chu, M.S., Larry Brown, M.D., Sidiki Dabo, M.D., Anthony McLeod, B.A., Michael Blizzard, B.A., and A.T.M. Yousuf, M.D., from the Addiction Research and Treatment Corporation; Trish Dooley, M.A., L.P.C., Ivy Pearlstein, M.S.N., A.P.N., Jeff Berman, M.D., Glenda Torres, R.N., Sylvia Atdjian, M.D., Marc Steinberg, M.A., Anna O’Kinsky, A.P.N., Ava Stanley, M.D., Mary Joan Barr, A.P.N., Michael Centrella, D.O., Alina Vrinceanu, M.D., and Donna Drummond, M.A., from the Mercer Trenton Addiction Science Center/Robert Wood Johnson Medical School; Eric Pihlgren, Ph.D., James Pierre, M.D., John Hopper, M.D., Adrianne Haggins, B.S., Tanya Paul, B.S., and Luanne Beamer, Ph.D., from Wayne State University; Alan Bray, M.HS., Virginia Ryan, Ph.D., Anne Benion, and Vickie Seeley, R.N., from SHAR House; Eugene Somoza, M.D., Ph.D., Judy Harrer, Ph.D., Rebecca Defevers, Jeff Goldsmith, M.D., Peggy Somoza, M.S., and Julie Jansen from the University of Cincinnati; Stephanie Kapp, B.A., Mary Ann Crawford, R.N., Steve Fekete, M.D., and Leela Rau, M.D., from Midtown Community Mental Health Center; Ruth Ann Holzhauser, M.D., Cynthia Kohl, R.N., and Cookie Hart from Maryhaven; Jos3 Szapocznik, Ph.D., Daniel Santisteban, Ph.D., and Roberto Dominguez, M.D., from the Center for Family Studies, University of Miami; Fred Sanchez, R.N., and Michael Sheehan, M.D., from Operation PAR; Deborah Orr, Ph.D., Judy Ruiz, R.N., and Hector Barreto, M.D., from the Center for Drug-Free Living; Eric Collins, M.D., from Columbia University College of Physicians and Surgeons, Department of Psychiatry; Sahadeo Ramnauth, R.P.A.C., and Timothy Wallace, R.P.A.C., from Phoenix House; and Lois Levy, L.C.S.W., from the New York State Psychiatric Institute.
We are especially grateful for the support from the Phamaceutical Division of the firm Reckitt Benckiser. We thank Reckitt Benckiser for providing buprenorphine-naloxone for these studies and sponsoring it for this indication, and for their unwavering dedication to buprenorphine’s development as a treatment for narcotic addiction.
We extend our gratitude to all of the individuals who participated in these studies and placed their care into our hands. Without them, this work would not have been possible.
References
- 1.Office of National Drug Control Policy. National Drug Control Strategy Washington, DC: Office of National Drug Control Policy; 2002.
- 2.Lewis JW. Buprenorphine. Drug Alcohol Depend. 1985;14:363–372. doi: 10.1016/0376-8716(85)90067-5. [DOI] [PubMed] [Google Scholar]
- 3.Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(2 suppl):S59–S77. doi: 10.1016/s0376-8716(03)00060-7. [DOI] [PubMed] [Google Scholar]
- 4.Bickel WK, Amass L. Buprenorphine treatment of opiate dependence: a review. Exp Clin Psychopharmacol. 1995;3:477–489. [Google Scholar]
- 5.Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- 6.Walsh SL, Preston KL, Bigelow GE, Stitzer ML. Acute administration of buprenorphine in humans: partial agonist and blockade effects. J Pharmacol Exp Ther. 1995;274:361–372. [PubMed] [Google Scholar]
- 7.Chawarski MC, Schottenfeld RS, O’Conner PG, Pakes J. Plasma concentrations of buprenorphine 24 to 72 hours after dosing. Drug Alcohol Depend. 1999;55:157–163. doi: 10.1016/s0376-8716(98)00192-6. [DOI] [PubMed] [Google Scholar]
- 8.Amass L, Kamien JB, Mikulich SK. Efficacy of daily and alternate-day dosing regimens with the combination buprenorphine-naloxone tablet. Drug Alcohol Depend. 2000;58:143–152. doi: 10.1016/s0376-8716(99)00074-5. [DOI] [PubMed] [Google Scholar]
- 9.Amass L, Kamien JB, Mikulich SK. Thrice-weekly supervised dosing with the combination buprenorphine-naloxone tablet is preferred to daily supervised dosing by opioid-dependent humans. Drug Alcohol Depend. 2001;61:173–181. doi: 10.1016/s0376-8716(00)00141-1. [DOI] [PubMed] [Google Scholar]
- 10.Fudala PJ, Jaffe JH, Dax EM, Johnson RE. Use of buprenorphine in the treatment of opiate addiction: II. physiologic and behavioral effects of daily and alternate-day administration and abrupt withdrawal. Clin Pharmacol Ther. 1990;47:525–534. doi: 10.1038/clpt.1990.67. [DOI] [PubMed] [Google Scholar]
- 11.Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- 12.Lange WR, Fudala PJ, Dax EM, Johnson RE. Safety and side effects of buprenorphine in the clinical management of heroin addiction. Drug Alcohol Depend. 1990;26:19–28. doi: 10.1016/0376-8716(90)90078-s. [DOI] [PubMed] [Google Scholar]
- 13.Kintz P. Deaths involving buprenorphine: a compendium of French cases. Forensic Sci Int. 2001;121:65–69. doi: 10.1016/s0379-0738(01)00454-6. [DOI] [PubMed] [Google Scholar]
- 14.Kintz P. Buprenorphine-related deaths. In: Kintz P, Marquet P, eds. Buprenorphine Therapy of Opiate Addiction Totowa, NJ: Humana Press; 2002:109–118.
- 15.Kintz P. A new series of 13 buprenorphine-related deaths. Clin Biochem. 2002;35:513–516. doi: 10.1016/s0009-9120(02)00304-1. [DOI] [PubMed] [Google Scholar]
- 16.Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine. Arch Gen Psychiatry. 1978;35:501–516. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- 17.Rance MJ, Dickens JN. The influence of drug-receptor kinetics on the pharmacological and pharmaco-kinetic profiles of buprenorphine. In: Van Ree JM, Pereniums L., eds. Characteristics and Function of Opioids Amsterdam: Elsevier/North-Holland Biomedical Press; 1978: 65–66.
- 18.Amass L, Bickel WK, Higgins ST, Hughes JR. A preliminary investigation of outcome following gradual or rapid buprenorphine detoxification. J Addict Dis. 1994;13:33–45. doi: 10.1300/j069v13n03_04. [DOI] [PubMed] [Google Scholar]
- 19.Bickel WK, Amass L, Higgins ST, Badger GJ, Esch RA. Effects of adding behavioral treatment to opiate detoxification with buprenorphine. J Consult Clin Psychol. 1997;65:803–810. doi: 10.1037//0022-006x.65.5.803. [DOI] [PubMed] [Google Scholar]
- 20.Cheskin LJ, Fudala PJ, Johnson RE. A controlled comparison of buprenorphine and clonidine for acute detoxification from opiates. Drug Alcohol Depend. 1994;36:115–121. doi: 10.1016/0376-8716(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 21.Seow SSW, Quigley AJ, Ilett KF, et al. Buprenorphine: a new maintenance opiate? Med J Aust. 1986;144:407–411. doi: 10.5694/j.1326-5377.1986.tb128412.x. [DOI] [PubMed] [Google Scholar]
- 22.Chiang CN, Hawks R. Development of a buprenorphine-naloxone combination drug for the treatment of drug addiction [abstract]. In: Harris LS, ed. Problems of Drug Dependence NIDA Research Monograph Series, No. 141. Rockville, Md.: National Institute on Drug Abuse;1994:458.
- 23.Chiang CN, Hawks RL. Pharmacokinetics of the combination tablet of buprenorphine and naloxone. Drug Alcohol Depend. 2003;70(2 suppl):S39–S47. doi: 10.1016/s0376-8716(03)00058-9. [DOI] [PubMed] [Google Scholar]
- 24.Chiang CN, Bridge P, Hawks RL, et al. The development of buprenorphine-naloxone products for treating opiate dependence [abstract]. In Harris LS, ed. Problems of Drug Dependence NIDA Research Monograph Series, No. 162. Rockville, Md.: National Institute on Drug Abuse; 1996:117.
- 25.Fudala PJ, Yu E, Macfadden W, Boardman C, Chiang NC. Effects of buprenorphine and naloxone in morphine-stabilized opiate addicts. Drug Alcohol Depend. 1998;50:1–8. doi: 10.1016/s0376-8716(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 26.Ahmadi J. A controlled trial of buprenorphine treatment for opium dependence: the first experience from Iran. Drug Alcohol Depend. 2002;66:111–114. doi: 10.1016/s0376-8716(01)00202-2. [DOI] [PubMed] [Google Scholar]
- 27.Amass L, Kamien JB, Branstetter SA, Mikulich SK. A controlled comparison of the buprenorphine-naloxone tablet and methadone for opioid maintenance treatment: interim results [abstract]. In: Harris LS, ed. Problems of Drug Dependence NIDA Research Monograph Series, No. 180. NIH Publication No. 00–4737. Washington D.C.: U.S. Government Printing Office; 2000:161.
- 28.Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349:949–958. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- 29.Johnson RE, Jaffe JH, Fudala PJ. A controlled trial of buprenorphine treatment for opiate dependence. JAMA. 1992;267:2750–2755. [PubMed] [Google Scholar]
- 30.Kakko J, Svanborg KD, Kreek MJ, Heilig M. One-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. The Lancet. 2003;361:662–668. doi: 10.1016/S0140-6736(03)12600-1. [DOI] [PubMed] [Google Scholar]
- 31.Mattick RP, Ali R, White J, O’Brien S, Wolk S, Danz C. Buprenorphine versus methadone maintenance therapy: a randomized double-blind trial with 405 opioid-dependent patients. Addiction. 2003;98:441–452. doi: 10.1046/j.1360-0443.2003.00335.x. [DOI] [PubMed] [Google Scholar]
- 32.Pani PP, Maremmani I, Pirastu R, Tagliamonte A, Gessa GL. Buprenorphine: a controlled clinical trial in the treatment of opioid dependence. Drug Alcohol Depend. 2000;60:39–50. doi: 10.1016/s0376-8716(99)00140-4. [DOI] [PubMed] [Google Scholar]
- 33.Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. Buprenorphine versus methadone maintenance for concurrent opiate dependence and cocaine abuse. Arch Gen Psychiatry. 1997;54:713–720. doi: 10.1001/archpsyc.1997.01830200041006. [DOI] [PubMed] [Google Scholar]
- 34.Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Comparison of buprenorphine and methadone in the treatment of opiate dependence. Am J Psychiatry. 1994;151:1025–1030. doi: 10.1176/ajp.151.7.1025. [DOI] [PubMed] [Google Scholar]
- 35.Pickworth WB, Johnson RE, Holicky BA, Cone EJ. Subjective and physiologic effects of intravenous buprenorphine in humans. Clin Pharmacol Ther. 1993;53:570–576. doi: 10.1038/clpt.1993.72. [DOI] [PubMed] [Google Scholar]
- 36.Strain EC, Walsh SL, Preston KL, Liebson IA, Bigelow GE. The effects of buprenorphine in buprenorphine-maintained volunteers. Psychopharmacology. 1997;129:329–338. doi: 10.1007/s002130050199. [DOI] [PubMed] [Google Scholar]
- 37.Comer SD, Collins ED, Fischman MW. Intravenous buprenorphine self-administration by detoxified heroin abusers. J Pharmacol Exp Ther. 2002;301:266–276. doi: 10.1124/jpet.301.1.266. [DOI] [PubMed] [Google Scholar]
- 38.Comer SD, Collins ED. Self-administration of intravenous buprenorphine and the buprenorphine/naloxone combination by recently detoxified heroin abusers. J Pharmacol Exp Ther. 2002;303:695–703. doi: 10.1124/jpet.102.038141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendelson J, Jones RT, Fernandez I, Welm S, Melby AK, Baggott MJ. Buprenorphine and naloxone interactions in opiate-dependent volunteers. Clin Pharmacol Ther. 1996;60:105–114. doi: 10.1016/S0009-9236(96)90173-3. [DOI] [PubMed] [Google Scholar]
- 40.Mendelson J, Jones RT, Welm S, Melby AK, Brown J, Batki SL. Buprenorphine and naloxone interactions in methadone maintenance patients. Biol Psychiatry. 1997;41:1095–1101. doi: 10.1016/S0006-3223(96)00266-1. [DOI] [PubMed] [Google Scholar]
- 41.Mendelson J, Jones RT, Welm S, et al. Buprenorphine and naloxone combinations: the effects of three dose ratios in morphine-stabilized, opiate-dependent volunteers. Psychopharmacology. 1999;141:37–46. doi: 10.1007/s002130050804. [DOI] [PubMed] [Google Scholar]
- 42.Mendelson J, Jones RT. Clinical and pharmacological evaluation of buprenorphine and naloxone combinations: why the 4 : 1 ratio for treatment? Drug Alcohol Depend. 2003;70(2 suppl):S29–S37. doi: 10.1016/s0376-8716(03)00057-7. [DOI] [PubMed] [Google Scholar]
- 43.Stoller KB, Bigelow GE, Walsh SL, Strain EC. Effects of buprenorphine/naloxone in opioid-dependent humans. Psychopharmacology. 2001;154:230–242. doi: 10.1007/s002130000637. [DOI] [PubMed] [Google Scholar]
- 44.Harris DS, Jones RT, Welm S, Upton RA, Lin E, Mendelson J. Buprenorphine and naloxone co-administration in opiate-dependent patients stabilized on sublingual buprenorphine. Drug Alcohol Depend. 2000;61:85–94. doi: 10.1016/s0376-8716(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 45.Strain EC, Stoller K, Walsh SL, Bigelow GE. Effects of buprenorphine versus buprenorphine/naloxone tablets in non-dependent opioid abusers. Psychopharmacology. 2000;148:374–383. doi: 10.1007/s002130050066. [DOI] [PubMed] [Google Scholar]
- 46.Amass L, Kamien JB, Reiber C, Branstetter SA. Abuse liability of IV buprenorphine-naloxone, buprenorphine and hydromorphone in buprenorphine-naloxone maintained volunteers. Drug Alcohol Depend. 2000;60:S6. [Google Scholar]
- 47.Jaffe JH, O’Keeffe CO. From morphine clinics to buprenorphine: regulating opioid agonist treatment of addiction in the United States. Drug Alcohol Depend. 2003;70(2 suppl):S3–S11. doi: 10.1016/s0376-8716(03)00055-3. [DOI] [PubMed] [Google Scholar]
- 48.Drug Addiction Treatment Act, 42 USC§3502a (2000).
- 49.Boatwright DE. Buprenorphine and addiction: challenges for the pharmacist. Journal of American Pharmacological Association. 2002;42:432–438. doi: 10.1331/108658002763316860. [DOI] [PubMed] [Google Scholar]
- 50.Fiellin DA, O’Connor PG. New federal initiatives to enhance the medical treatment of opioid dependence. Ann Intern Med. 2002;137:688–692. doi: 10.7326/0003-4819-137-8-200210150-00014. [DOI] [PubMed] [Google Scholar]
- 51.Hanson GR, Leshner AI, Tai B. Putting drug abuse research to use in real-life settings. J Subst Abuse Treat. 2002;23:69–70. doi: 10.1016/s0740-5472(02)00269-6. [DOI] [PubMed] [Google Scholar]
- 52.Gold MS, Pottash AC, Sweeney DR, Kleber HD. Opiate withdrawal using clonidine. a safe, effective and rapid nonopiate treatment. JAMA. 1980;243:343–346. [PubMed] [Google Scholar]
- 53.Charney DS, Sternberg DE, Kleber HD, Heninger GR, Redmond DE. The clinical use of clonidine in abrupt withdrawal from methadone: effects on blood pressure and specific signs and symptoms. Arch Gen Psychiatry. 1981;38:273–277. doi: 10.1001/archpsyc.1981.01780360089010. [DOI] [PubMed] [Google Scholar]
- 54.Gossup M. Clonidine and the treatment of the opiate withdrawal syndrome. Drug Alcohol Depend. 1988;21:253–259. doi: 10.1016/0376-8716(88)90078-6. [DOI] [PubMed] [Google Scholar]
- 55.Rounsaville BJ, Kosten T, Kleber H. Success and failure at outpatient opioid detoxification: evaluating the process of clonidine- and methadone- assisted withdrawal. J Nerv Ment Dis. 1985;173:103–110. doi: 10.1097/00005053-198502000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Ling W, Obert JL. Handbook for Recovery from Opiate Dependence Adapted from: The Matrix Model. Vers. 1. 16th Nov. 2000.
- 57.Hudziak J, Helzer JE, Wetzel MW, et al. The use of the DSM-III-R checklist for initial diagnostic assessments. Compr Psychiatry. 1993;34:375–383. doi: 10.1016/0010-440x(93)90061-8. [DOI] [PubMed] [Google Scholar]
- 58.McLellan AT, Luborsky L, Cacciola J, et al. New data from the Addiction Severity Index: reliability and validity in three centers. J Nerv Ment Dis. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Wesson D, Ling W. The Clinical Opiate Withdrawal Scale (COWS) J Psychoactive Drugs. 2003;35:253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- 60.Bickel WK, Stitzer MI, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. A clinical trial of buprenorphine: comparison with methadone in the detoxification of heroin addicts. Clin Pharmacol Ther. 1988;43:72–78. doi: 10.1038/clpt.1988.13. [DOI] [PubMed] [Google Scholar]
- 61.Bickel WK, Stitzer MI, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. Buprenorphine: dose-related blockade of opioid challenge effects in opioid-dependent humans. J Pharmacol Exp Ther. 1988;247:47–53. [PubMed] [Google Scholar]
- 62.Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, Bigelow GE. A placebo-controlled clinical trial of buprenorphine as a treatment for opiate dependence. Drug Alcohol Depend. 1995;40:17–25. doi: 10.1016/0376-8716(95)01186-2. [DOI] [PubMed] [Google Scholar]
- 63.O’Connor PG, Oliveto AH, Shi JM, et al. A randomized trial of buprenorphine maintenance for heroin dependence in a primary care clinic for substance users versus a methadone clinic. Am J Med. 1998;105:100–105. doi: 10.1016/s0002-9343(98)00194-6. [DOI] [PubMed] [Google Scholar]
- 64.Ling W, Wesson DR, Charuvastra C, Klett CJ. A controlled trial comparing buprenorphine and methadone maintenance in opiate dependence. Arch Gen Psychiatry. 1996;54:401–407. doi: 10.1001/archpsyc.1996.01830050035005. [DOI] [PubMed] [Google Scholar]
- 65.Ling W, Charuvastra C, Collins JF, et al. Buprenorphine maintenance treatment of opiate dependence: a multicenter, randomized clinical trial. Addiction. 1998;93:475–486. doi: 10.1046/j.1360-0443.1998.9344753.x. [DOI] [PubMed] [Google Scholar]
- 66.Uehlinger C, Déglon J-J, Livoti S, Petitjean S, Waldvogel D, Ladewing D. Comparison of buprenorphine and methadone in the treatment of opioid dependence. Eur Addict Res. 1998;4:13–18. doi: 10.1159/000052036. [DOI] [PubMed] [Google Scholar]
- 67.Fischer G, Gombas W, Eder H, et al. Buprenorphine versus methadone maintenance for the treatment of opioid dependence. Addiction. 1999;94:337–347. doi: 10.1046/j.1360-0443.1999.94913376.x. [DOI] [PubMed] [Google Scholar]
- 68.Petitjean S, Stohler R, Deglon J-J, et al. Double-blind randomized trial of buprenorphine and methadone in opiate dependence. Drug Alcohol Depend. 2001;62:97–104. doi: 10.1016/s0376-8716(00)00163-0. [DOI] [PubMed] [Google Scholar]
- 69.Clinical Guidelines for Buprenorphine Treatment Rockville, Md.: Center for Substance Abuse Treatment Administration; Forthcoming. Treatment Improvement Protocol Series.
- 70.Law FD, Bailey JE, Allen DS, et al. The feasibility of abrupt methadone-buprenorphine transfer in British opiate addicts in an outpatient setting. Addict Biol. 1997;2:191–200. doi: 10.1080/13556219772732. [DOI] [PubMed] [Google Scholar]
- 71.Bouchez J, Beauverie P, Touzeau D. Substitution with buprenorphine in methadone- and morphine sulfate-dependent patients. Eur Addict Res. 1998;4:8–12. doi: 10.1159/000052035. [DOI] [PubMed] [Google Scholar]
- 72.Seifert L, Metzner C, Paetzold W, et al. Detoxification of opiate addicts with multiple drug abuse: a comparison of buprenorphine and methadone. Pharmacopsychiatry. 2002;35:159–164. doi: 10.1055/s-2002-34115. [DOI] [PubMed] [Google Scholar]
- 73.Vignau J. Preliminary assessment of a 10-day rapid detoxification programme using high dosage buprenorphine. Eur Addict Res. 1998;4:29–31. doi: 10.1159/000052039. [DOI] [PubMed] [Google Scholar]
- 74.Lintzeris N, Bell J, Bammer G, Jolley D, Rushworth L. A randomized controlled trial of buprenorphine in the management of short-term ambulatory heroin withdrawal. Addiction. 2002;97:1395–1404. doi: 10.1046/j.1360-0443.2002.00215.x. [DOI] [PubMed] [Google Scholar]
- 75.Reynaud M, Tracqui A, Petit G, Potard D, Courty P. Six deaths linked to misuse of buprenorphine-benzodiazepine combinations. Am J Psychiatry. 1998;155:448–449. [PubMed] [Google Scholar]
- 76.Tracqui A, Kintz P, Ludes B. Buprenorphine-related deaths among drug addicts in France: a report on 20 fatalities. J Anal Toxicol. 1998;22:430–434. doi: 10.1093/jat/22.6.430. [DOI] [PubMed] [Google Scholar]
- 77.Tracqui A, Tournoud C, Flesch F, et al. Buprenorphine poisoning in drug abusers on substitution therapy: 29 non-fatal and 20 fatal cases. La Presse Md. 1998;27:557–561. [PubMed] [Google Scholar]
- 78.Walsh SL, June HL, Schuh KJ, Preston KL, Bigelow GE, Stitzer ML. Effects of buprenorphine and methadone in methadone-maintained subjects. Psychopharmacology. 1995;119:268–276. doi: 10.1007/BF02246290. [DOI] [PubMed] [Google Scholar]
- 79.Strain EC, Preston KL, Liebson IA, Bigelow GE. Buprenorphine effects in methadone-maintained volunteers: effects at two hours after methadone. J Pharmacol Exp Ther. 1995;272:628–638. [PubMed] [Google Scholar]
- 80.Amass L. Bringing buprenorphine to community providers: the NIDA Clinical Trials Network experience [abstract]. In: Amass L, Ling W. Ready, set, go: bringing buprenorphine to the U.S. treatment market. In: Harris LS, ed. Problems of Drug Dependence NIDA Research Monograph Series, No. 182. NIH Publication No. 02–5097. Washington D.C.: U.S. Government Printing Office;2002:27–37.
- 81.Ling W, Smith D. Buprenorphine: blending practice and research. J Subst Abuse Treat. 2002;23:87–92. doi: 10.1016/s0740-5472(02)00257-x. [DOI] [PubMed] [Google Scholar]
- 82.Ball JC, Ross A. The Effectiveness of Methadone Maintenance Treatment New York: Springer-Verlag; 1991.
- 83.Hser YI, Hoffman V, Grella CE, Anglin MD. A 33-year follow-up of narcotics addicts. Arch Gen Psychiatry. 2001;58:503–508. doi: 10.1001/archpsyc.58.5.503. [DOI] [PubMed] [Google Scholar]
- 84.Ling W, Amass L, et al. Bupenorphine-naloxone and clonidine for short-term opioid withdrawal: results from the NIDA Clinical Trials Network multi site evaluation. Forthcoming.
- 85.Agar M, Bourgois P, French J, Murdoch O. Buprenorphine: field trials of a new drug. Qualitative Health Research. 2001;11:69–84. doi: 10.1177/104973201129118948. [DOI] [PubMed] [Google Scholar]
- 86.McLellan AT, Arndt IO, Metzger DS, Woody GE, O’Brien CP. The effects of psychosocial services in substance abuse treatment. JAMA. 1993;269:1953–1959. [PubMed] [Google Scholar]
- 87.Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000;283:1303–1310. doi: 10.1001/jama.283.10.1303. [DOI] [PubMed] [Google Scholar]
- 88.Gossop M, Green L, Phillips G, Bradley B. Lapse, relapse, and survival among opiate addicts after treatment: a prospective follow-up study. Br J Psychiatry. 1989;154:348–353. doi: 10.1192/bjp.154.3.348. [DOI] [PubMed] [Google Scholar]
- 89.Hser YI, Yamaguchi K, Chen J, Anglin MD. Effects of interventions on relapse to narcotics addiction: an event-history analysis. Evolutional Review. 1995;19:123–140. [Google Scholar]
- 90.McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]


