Abstract
Virus-specific memory T lymphocytes traffic to sites of viral infection. Herpes simplex virus (HSV) type 2–specific CD4+ and CD8+ T lymphocytes differ with regard to their homing kinetics to infected tissues. We studied the expression of cutaneous lymphocyte–associated antigen (CLA) and E-selectin ligand (ESL) by HSV-2–specific CD4+ T lymphocytes. Virus-reactive T lymphocytes were identified ex vivo by CD154 or interferon-γ up-regulation. We detected selective expression of CLA by HSV-2–reactive CD4+ T lymphocytes, but at levels lower than those we previously observed for CD8+ T lymphocytes. Short-term HSV-2–reactive CD4+ lines generated from peripheral-blood mononuclear cells preferentially express CLA, compared with cytomegalovirus- or influenza-specific cells. CLA is expressed by HSV-2–reactive cells that are initially CLA negative before restimulation. Short-term culture-expanded HSV-2–specific CD4+ T lymphocytes also selectively express ESL. These findings have implications for the optimization of vaccines for HSV and other cutaneous pathogens.
Memory T lymphocytes can display tissue-specific trafficking that matches the pathogenesis of their cognate microbial antigen. For example, CD4+ T lymphocytes specific for the gastrointestinal pathogen rotavirus preferentially express α4β7 integrin, an adhesion molecule for gastrointestinal-tract endothelial cells [1]. CD4+ T lymphocytes specific for cutaneous allergens preferentially express cutaneous lymphocyte–associated antigen (CLA), a complex fucose-containing carbohydrate moiety recognized by the monoclonal antibody (MAb) HECA-452 [2]. To date, no studies of the expression of CLA by CD4+ T lymphocytes specific for cutaneous infectious pathogens have been conducted. CLA is related, but not identical, to an E-selectin ligand (ESL). ESL is thought to be functionally important in the mediation of T lymphocyte rolling adhesion via binding to E-selectin, a transmembrane protein that occurs on the lumenal surface of dermal vascular endothelial cells and that is up-regulated during inflammation [3–5]. CLA is normally expressed by 5%–15% of circulating CD4+ and CD8+ T lymphocytes, occurring as a posttranslational modification of P-selectin glycoprotein ligand 1 [6], whereas ESL is expressed by a somewhat lower frequency of circulating CD4+ and CD8+ lymphocytes [5]. We have previously shown that E-selectin is up-regulated in human genital herpes simplex virus (HSV) type 2 lesions and that infiltrating cells express CLA [7].
In the immunocompetent host, replication of HSV-2 is generally limited to the epidermis and innervating sensory neurons [8]. A dense dermal lymphocytic in-filtrate occurs subjacent to herpetic erosions. The early infiltrate is CD4 predominant, and HSV-2–specific CD4+ T lymphocytes are consistently recovered from biopsy samples on day 2 after the onset of symptoms. CD8+ T lymphocytes traffic into lesions a few days later, and HSV-2–specific CTL activity is temporally correlated with the clearance of live virus [9]. Thus, the kinetics of the recruitment of HSV-2–specific CD4+ and CD8+ T lymphocytes may differ. Interactions between antigen-presenting cells (APCs) and naive T lymphocytes appear to program the homing characteristics of resultant memory cells [10, 11]. In murine models, different dendritic cell (DC) populations prime HSV-specific CD4+ and CD8+ T lymphocytes [12, 13]. These observations indicate that the homing characteristics of HSV-2–specific CD4+ and CD8+ T lymphocytes may differ. The partial efficacy of an HSV-2 vaccine that stimulates CD4+ but not CD8+ T lymphocyte responses [14] also motivates research into the homing of HSV-2–specific CD4+ T lymphocytes.
The present study of the expression of CLA and ESL by HSV-2–specific memory CD4+ T lymphocytes complements previous CD8 studies [7, 15]. Circulating HSV-2–specific CD8+ T lymphocytes from chronically infected persons, defined by tetramer binding, generally show 50%–70% expression of CLA, compared with <15% for CD8+ T lymphocytes specific for Epstein-Barr virus (EBV) or cytomegalovirus (CMV), both herpesviruses that seldom infect the skin. As direct tetramer staining is not routinely available for virus-specific CD4+ T lymphocytes, the present study uses alternative ex vivo methods to identify virus-specific cells. The phenotypic difference between HSV-2–and EBV-specific CD8+ T lymphocytes was stable in vitro, as only HSV-2–specific T lymphocytes expressed CLA and ESL after restimulation and expansion with cognate peptide [7]. Our new data also suggest that, during in vitro expansion, HSV-2–specific CD4+ T lymphocytes selectively express CLA and ESL. We propose that vaccination strategies for cutaneous pathogens should consider optimization of CD4+ effector cell localization to sites of challenge.
SUBJECTS, MATERIALS, AND METHODS
Subjects and specimens
HSV-1 and HSV-2 immune status and CMV immune status were assayed by HSV type–specific immunoblot assay [16] and ELISA (Abbott), respectively. Peripheral-blood mononuclear cells (PBMCs) were cryopreserved after ficoll-hypaque (Invitrogen) centrifugation. No subject was experiencing a clinical outbreak of HSV or receiving antiviral medication at the time of blood sampling. Informed consent was obtained from subjects, and the human-experimentation guidelines of the US Department of Health and Human Services and the University of Washington were followed.
Antigens and mitogens
HSV-2 strain 333 [17] was grown and titrated as described elsewhere [18]. Cell-associated virus was UV irradiated for 30 min. For mock virus, the process used uninfected Vero cells. Tetanus toxoid was obtained from Corixa, and CMV antigen was obtained from Advanced Bio-technologies. The influenza antigen used was 2002 vaccine (Aventis) dialyzed against PBS. HSV-2 VP16 peptides 369–379, restricted by HLA-DQB1*0602, and 465–484, restricted by HLA-DRB1*0401, were prepared as described elsewhere [7, 19–21].
Flow-cytometry reagents
Anti-CD4 were obtained from CalTag. Biotin- and fluorescein isothiocyanate (FITC)–labeled anti-CLA, phycoerythrin (PE)–labeled anti-CD40L (CD154), PE- and APC-labeled anti–interferon (IFN)–γ, isotype control–PE, and anti–E-selectin were obtained from Pharmingen. Chimeric human E-selectin–Fc, obtained from R&D Systems, was detected by use of PE-F(ab′)2–labeled goat anti–human Fc (Coulter). Secondary reagents used were PE-labeled goat anti–mouse IgG (Biomeda) and PE-labeled streptavidin (Pharmingen). PE-labeled tetramers DQ6-369 and DR4-485, which label CD4+ T lymphocytes specific for peptides in HSV-2 VP16 beginning with the above-indicated amino acids, have been described elsewhere [20, 22].
Ex vivo assays
Virus-reactive T lymphocytes were identified by intracellular cytokine cytometry (ICC) or surface CD154 up-regulation. For ICC, PBMCs (1–3 million cells/tube) were incubated in 1 mL of T lymphocyte medium [23]. Stimuli used were UV-killed HSV-2 (1:1000), CMV antigen (1:1000), or phorbol myristate acetate (PMA; 50 ng/mL; Sigma) plus ionomycin (1 μg/mL; Sigma). Anti-CD28–specific and anti-CD49d–specific MAb (1 μg/mL; Pharmingen), at time 0, and brefeldin A (5 μg/mL; Sigma), at 1 h, were added, and tubes were maintained at 37°C in 5% CO2. Five hours later, cells were shifted to 4°C. Cells were recovered, washed in fluorescence-activated cell sorter (FACS) buffer (PBS, 1% fetal calf serum [FCS], and 0.1% Na azide), and stained with anti–CLA-FITC and anti–CD4-PECy5. Cells were washed, treated with FACS-Lyse and Cytoperm-Cytofix, washed in Perm-Wash (all from Pharmingen), split, stained with anti–human IFN-γ–PE or iso-type control for 30 min at 4°C, washed again, and fixed. For CD154, live HSV-2 (MOI, 10) was used, brefeldin A was omitted, and monensin was added to some cultures. After 6 h, cells were washed, split, and stained with anti–CLA-FITC, anti–CD4-PECy5, and either anti–CD154-PE or isotype control.
In vitro assays
Proliferation assays were conducted in triplicate in 96-well U-bottom plates in 200 μL of TCM. Immunomagnetic depletion of CLA+ PBMCs was done by use of bio-tinylated anti-CLA and streptavidin (MACS; Miltenyi). Cells eluted with PBS and 4 mmol/L EDTA composed the CLA-depleted fraction. PBMCs (CLA depleted or unmanipulated) were plated at 1 ×105 cells/well and were incubated at 37°C in 5% CO2. Antigens used were UV-killed HSV-2 or mock Vero antigen (1:100), tetanus toxoid (1:10,000), and phytohemagglutinin (PHA; 1.6 μg/mL; Remel). Autologous irradiated (3300 rad) PBMCs (5 × 104 cells/well) were added as APCs. [3H]-thymidine incorporation was performed as described elsewhere [23].
To study CLA expression among bulk T lymphocyte responder populations, 2 × 106 cells/well were incubated in 2 mL of T lymphocyte medium in 24-well plates. Interleukin (IL)–2 (32 U/mL; Hemagen) was added starting on day 5 or 6. For short-term assays, PBMCs were labeled with 1 μmol/L 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE; Molecular Probes), in accordance with the manufacturer’s instructions; were plated at 1 × 106 cells/well in 1 mL of TCM in 48-well plates; and were studied after 5 days of culture without exogenous cytokines. For studies of soluble E-selectin binding, similar cultures without CFSE, supplemented with 50 U/mL recombinant human IL-2 on day 5, were tested on day 7. Stimuli used were UV-killed HSV-2 or mock Vero antigen (1:100), PHA (1.6 μg/mL), or CMV or influenza (1:1000). In separate stimulations, PBMCs were also incubated with peptides (1 μg/mL), by use of the same densities and conditions listed above. On day 3, 20 ng/mL human IL-7 (gift from Corixa) and 50 U/mL human IL-2 (Chiron) were added, and cells were fed with similar cytokines every other day. In some cultures, human natural IL-2 (32 U/mL) was substituted.
Flow cytometry
MAbs were added to cells for 30 min at 4°C in 100 μL of FACS buffer. Tetramers were used in ~100 μL of T lymphocyte medium for 1 h at 37°C before the addition of MAbs. Cells were washed twice with FACS buffer or PBS, fixed, and analyzed on a FACScan instrument (Beckton Dickinson). Analysis was conducted by use of WinMDI (version 2.8; available at http://facs.scripps.edu/software.html). For 6-h ex vivo assays, the proportions of CD4+ T lymphocytes (forward/side scatter dot-plot) that stained with isotype control were subtracted from the proportion that stained with CD154 or IFN-γ, to give net staining. For 9–19 day–bulk cultures, analyses were gated on large CD4+ lymphoblasts (forward/side scatter dot-plot). For CFSE tests, the gates were CD4+, CFSE-low (divided) lymphoblasts. To detect ESL, cells were incubated in 100 μL of TCM with 1 μg of E-selectin–Fc (R&D Systems) for 30 min at 37°C, washed twice, and stained with anti–CD4-PECy5, anti–CLA-FITC, and 1 μL of anti–Fc-PE for 30 min at 4°C. EDTA (10 mmol/L) was used during E-selectin–Fc incubation and the first wash.
E-selectin adhesion
CHO cells expressing human E-selectin (CHO-E) were cultured in methotrexate [7]. Control CHO transformed with pBJ vector [24] were maintained in 400 μg/mL G418. Cells plated (3 × 105 cells/well) in 6-well plates were used at 48 h. CFSE-labeled responders on day 5 were washed and plated (3 × 105 cells/well) in TCM on CHO/CHO-E monolayers and were rocked at 60 tilts/min for 60 min at 37°C in 5% CO2. Unbound cells were collected and pooled with cells from a single additional rinse with TCM. Bound cells were collected by pipetting with cold PBS and 4 mmol/L EDTA. Fractions were collected by centrifugation, washed, stained with anti-CD4, washed again, and fixed. The entire volume of each specimen was analyzed to completion in the flow cytometer, and the number of CD4+, CFSE-low lymphoblasts were recorded. The ratio of bound:unbound cells was calculated.
Statistical analyses
Comparison of surface antigen expression was performed by use of the Mann-Whitney U test or paired Wilcoxon rank sum test (both 2-tailed). GraphPad InStat software (version 3.00; GraphPad Software) was used.
RESULTS
Expression of CLA by virus-reactive CD4+ T lymphocytes ex vivo
To compare the expression of CLA, subjects seropositive for both CMV and HSV-2 were selected. Expression of CLA by virus-reactive CD4+ T lymphocytes was compared after a 6-h stimulation (figure 1A). CMV, a non–skin-tropic herpesvirus that causes chronic infections, was used as a control. Surface expression of CD154 by CD4+ lymphocytes, as previously reported for other recall antigens [25, 26], also appears useful for identifying HSV-2– and CMV-reactive cells. The detailed results (table 1) show that the mean ± SD net proportions of virus-specific cells were 0.70% ± 0.45% and 0.82% ± 0.68% for HSV-2 and CMV antigens, respectively. HSV-2 seronegative donors had low responses. Control responses to PMA/ionomycin were brisk. Addition of monensin did not affect the results (data not shown).
Figure 1.

Expression of cutaneous lymphocyte–associated antigen (CLA) by CD4+ T lymphocytes after ex vivo restimulation for 6 h. A, Peripheral-blood mononuclear cells (PBMCs) stimulated as indicated were stained with anti-CD4 and isotype control (left) or anti-CD154 monoclonal antibody (right). Gates for CD4+CD154+ T lymphocytes are shown. B, Expression of CLA by gated CD4+CD154+ T lymphocytes from dot-plots 6–8 in panel A. Nos. are percentages of positive cells. C, Summary of expression of CLA by CD4+ lymphocytes from the 8 subjects seropositive for herpes simplex virus (HSV) type 2 and 6 subjects seropositive for cytomegalovirus (CMV) (detailed in table 1) that were either CD154+ or CD154− after the indicated stimuli. D, Expression of interferon (IFN)–γ by PBMCs in response to stimulation with HSV-2 or CMV. CD4+ T lymphocytes positive or negative for IFN-γ were analyzed for expression of CLA. Nos. in the dot-plots are percentages of CD4+ T lymphocytes that express IFN-γ. Nos. in the histograms are the percentages of the gated cells—either CD4+IFN-γ + or CD4+IFN-γ−—that express CLA. Mock ag, mock antigen; PE, phycoerythrin; PMA/iono, phorbol myristate acetate plus ionomycin.
Table 1.
Expression of CD154 by herpes simplex virus (HSV) type 2–reactive CD4+ lymphocytes, in a 6-h ex vivo stimulation assay.
| Mock antigen
|
HSV-2
|
CMV
|
PMA + iono
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Category, subject | Iso | CD154 | Net | Iso | CD154 | Net | Iso | CD154 | Net | Iso | CD154 | Net |
| HSV-2–infected subjects | ||||||||||||
| 10 | 0.33 | 0.22 | −0.11 | 0.32 | 0.66 | 0.34 | 0.43 | 1.42 | 0.99 | 0.35 | 50.87 | 50.34 |
| 11 | 0.40 | 0.34 | −0.06 | 0.44 | 1.16 | 0.72 | 0.54 | 0.85 | 0.31 | 0.92 | 46.81 | 45.89 |
| 12 | 0.36 | 0.37 | 0.01 | 0.52 | 1.23 | 0.71 | 0.34 | 1.29 | 0.95 | 0.51 | 40.51 | 40.00 |
| 4 | 0.55 | 0.51 | −0.04 | 0.32 | 2.05 | 1.73 | 0.50 | 0.38 | −0.12 | 1.96 | 54.56 | 52.60 |
| 5 | 0.33 | 0.25 | −0.08 | 0.43 | 1.00 | 0.57 | 0.40 | 2.28 | 1.88 | 0.63 | 65.45 | 64.82 |
| 6 | 0.48 | 0.40 | −0.08 | 0.42 | 1.02 | 0.60 | 0.38 | 1.29 | 0.91 | 0.73 | 12.42 | 11.69 |
| 13 | 0.23 | 0.17 | −0.06 | 0.20 | 0.48 | 0.28 | ND | ND | ND | 0.24 | 82.00 | 81.76 |
| 14 | 0.27 | 0.13 | 0.14 | 0.15 | 0.81 | 0.66 | ND | ND | ND | 0.30 | 72.20 | 71.90 |
| Mean ± SD | … | … | −0.07 ± 0.04 | … | … | 0.70 ± 0.45 | … | … | 0.82 ± 0.68 | … | … | 52.4 ± 21.6 |
| HSV-seronegative control subjects | ||||||||||||
| 15 | 0.11 | 0.06 | −0.05 | 0.06 | 0.12 | 0.06 | ND | ND | ND | 0.20 | 47.60 | 47.40 |
| 16 | 0.16 | 0.16 | 0 | 0.38 | 0.32 | −0.06 | ND | ND | ND | 0.27 | 77.00 | 76.73 |
NOTE. Data are the proportion of CD4+ T lymphocytes that stained with isotype or anti-CD154 and the proportion of the net specific CD154 expression. Peripheral-blood mononuclear cells stimulated with the indicated substances were stained with isotype (iso) or anti-CD154 monoclonal antibody, anti-CD4, and anti–cutaneous lymphocyte–associated antigen. CMV, cytomegalovirus; iono, ionomycin; ND, not done; PMA, phorbol myristate acetate.
After exposure to HSV-2, the expression of CLA was higher among CD4+CD154+ T lymphocytes (mean ± SD, 18.3% ± 9.6%) than among bystander CD4+CD154− T lymphocytes (mean ± SD, 5.8% ± 3.9%) (representative subject, figure 1B; summary of 6 subjects, figure 1C). CD4+CD154+ T lymphocytes detected after exposure to either CMV or PMA/ionomycin had lower levels of CLA expression. CLA expression after exposure to HSV-2 was significantly higher (P < .05, for HSV-2–stimulated CD4+CD154+ T lymphocytes vs. HSV-2–stimulated CD4+CD154− T lymphocytes and for CD4+CD154+ T lymphocytes vs. CD4+/CD154− T lymphocytes after both CMV and PMA/ionomycin stimulation).
The specific accumulation of IFN-γ observed after stimulation of PBMCs with HSV-2 antigens was similar to that reported in previous studies from our group [27] and others [28]. Selective gating again showed higher expression of CLA by HSV-reactive cells than by CMV-reactive CD4+ T lymphocytes or by nonreactive CD4+ T lymphocytes (figure 1D). Similar results were obtained for 3 other dually seropositive subjects (data not shown).
Expression of CLA by CD4+ T lymphocytes restimulated with HSV-2 peptides
In our hands, direct HLA-peptide fluorescent tetramer detection of HSV-2–specific CD4+ T lymphocytes ex vivo has not worked for HSV-2, likely because of the very low abundance of peptide-specific cell populations [20]. To examine expression of CLA by HSV-2–specific cells at the level of defined epitopes, we therefore used tetramers [23] after short-term peptide stimulation. PBMC specimens from subjects with suitable HLA class II alleles were stimulated in vitro with suitable known antigenic HSV-2 peptides [21, 22, 29]. Expression of CLA by CD4+tetramer+ T lymphocytes was higher than for CD4+tetramer+ T lymphocytes through day 14 of culture (P<.05, for each time point; paired Wilcoxon rank sum test) (figure 2).
Figure 2.
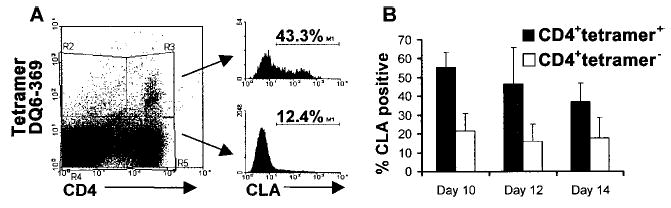
Expression of cutaneous lymphocyte–associated antigen (CLA) by CD4+ T lymphocytes specific for herpes virus simplex (HSV) type 2 peptides. Peripheral-blood mononuclear cells (PBMCs) from 6 subjects were stimulated with an HLA-appropriate HSV-2 peptide and were stained with a tetramer, anti-CD4, and anti-CLA. A, Example of CLA expression among HSV-2–specific CD4+tetramer+ or CD4+tetramer− T lymphocytes at day 10. B, Summary of data from 6 cultures tested at different time points; data are mean ± SD.
Expression of CLA by bulk herpesvirus-reactive CD4+ T lymphocytes in vitro
The tetramer experiments did not compare HSV-2–specific cells with other proliferating cells and left open the possibility that CLA was expressed as an activation marker. We therefore compared expression of CLA among CD4+ lymphoblasts after restimulation of whole PBMCs. For 5 HSV-2 seropositive subjects, gating on CD4+ lymphoblasts from cultures showed that HSV-2 stimulation led to consistently higher levels of CLA expression than did PHA stimulation, for up to 19 days of culture (P < .05, for each time point; paired Wilcoxon rank sum test) (table 2). In agreement with previous findings [30], expression of CLA does not seem to increase greatly in response to polyclonal stimulation in RPMI 1640–human serum–based medium.
Table 2.
Expression of cutaneous lymphocyte–associated antigen (CLA) by gated CD4+ lympoblasts after restimulation of peripheral-blood mononuclear cells from herpes simplex virus (HSV) type 2–seropositive subjects with whole HSV-2 antigen or phytohemagglutinin (PHA).
| Stimulated with
|
||||||
|---|---|---|---|---|---|---|
| Category | Whole HSV-2 antigen | PHA | ||||
| Time in culture, range, days | 9–10 | 12–15 | 17–19 | 9–10 | 12–15 | 17–19 |
| CD4+ lympoblasts expressing CLA, mean ± SD, % | 26.1 ± 12.9 | 36.5 ± 9.2 | 32.2 ± 5.3 | 8.1 ± 2.0 | 13.6 ± 6.7 | 10.8 ± 5.0 |
To compare various recall antigens, stimulation was performed for 5 days in the absence of exogenous cytokines. CFSE labeling was combined with lymphoblast cell size and granularity to better define dividing cells. Very few CD4+, CFSE-low lymphocytes in the lymphoblast gate or total lymphoblasts were noted in response to mock antigen (figure 3A, column 1) or in response to CMV or HSV-2 in subjects seronegative for these agents (figure 3A, columns 6 and 7). Larger cells in the forward/side scatter dot-plots in the seronegative subjects represent residual monocytes, as they stain CD3−CD14+ T lymphocytes (data not shown). In contrast, abundant CFSE-low cells were present in the lymphoblast gate in CMV- and HSV-2–driven cultures for seropositive subjects, as well as in most influenza-driven cultures from seropositive subjects (figure 3A, all columns other than 6 and 7). Twelve of 16 subjects had highly selective expression of CLA on CD4+ T lymphocytes responding to HSV-2, whereas 4 (subjects A–D) of the 16 did not (figure 3B). Group analysis of data (mean ± SD) from all 16 subjects shows that 53.8% ± 17.8% of HSV-2–reactive CD4+ lymphoblasts (n = 12) expressed CLA after 5 days in culture, compared with 14.7% ± 10.7% for CMV (n = 13 ), 8.7% ± 3.7% for influenza (n = 11 ), and 15.2% ± 7.1% for the nonspecific mitogen PHA (n = 16) (P < .05, for HSV-2 vs. other stimuli; Mann-Whitney U test). In the medium conditions used, CLA expression after exposure to recall antigen appears to be higher for HSV-2 whole viral antigen than for the other stimuli tested.
Figure 3.
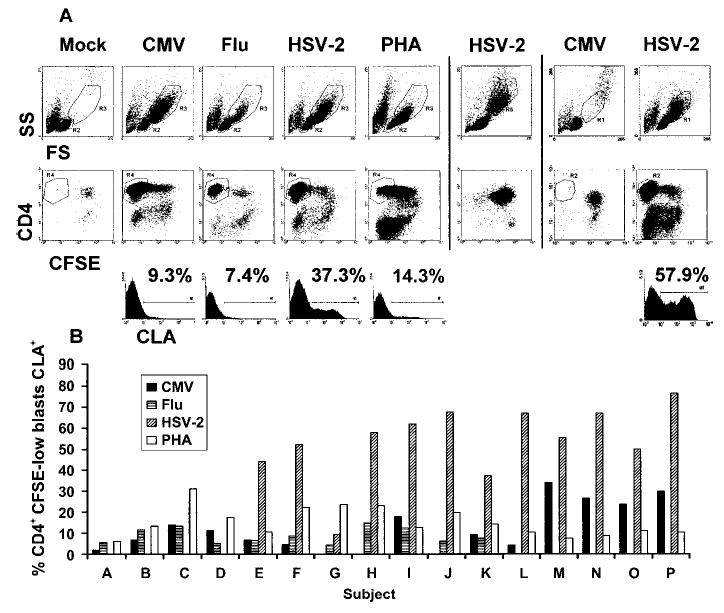
Expression of cutaneous lymphocyte–associated antigen (CLA) by CD4+ lymphoblasts responding to whole viral antigens. 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE)–labeled peripheral-blood mononuclear cells (PBMCs) were stimulated for 5 days without exogenous cytokines. A, Examples of forward/side scatter dot-plot (top row, showing gates used for lymphoblasts or monocytes) and CFSE and CD4 staining of gated lymphoblasts (middle row). Stimuli are indicated. Subjects are separated by vertical bars. Subject 1 (columns 1–5) is seropositive for herpes simplex virus (HSV) type 2 and cytomegalovirus (CMV), subject 2 (column 6) is seronegative for HSV-1 and HSV-2, and subject 3 (columns 7 and 8) is seronegative for CMV and seropositive for HSV-2. Row 3 shows CLA expression by gated CD4+, CFSE-low lymphoblasts and percentages of positive cells. B, CLA expression of CD4+, CFSE-low lymphoblasts from 16 HSV-2–seropositive subjects. Only subjects known to be seropositive for CMV were tested with CMV antigen. Flu, influenza; FS, forward scatter; PHA, phytohemagglutinin; SS, side scatter.
Response of CLA-depleted PBMCs to HSV-2 restimulation
The CD154 and IFN-γ ex vivo studies of HSV-2–reactive CD4+ T lymphocytes detected specific, but relatively low, expression of CLA by HSV-2–reactive CD4+ T lymphocytes (figure 1). The large proportion of CLA-negative cells among the cells reacting to HSV-2 antigen with CD154 and IFN-γ up-regulation leads us to hypothesize that CLA-low cells would also retain proliferative potential. To test this, we depleted CLA+ cells from PBMCs. CD4+ lymphocytes in unmanipulated PBMCs contained a mean ± SD of 7.3% ± 2.2% of CLA+ cells, which is consistent with the results of published reports [31]. CLA expression was a mean ± SD of 0.24% ± 0.15% after depletion for 97% removal (figure 4A). We observed no significant difference in the proliferative responses of unmanipulated PBMCs and CLA-depleted PBMCs for UV-killed HSV-2 (P > .99, paired Wilcoxon rank sum test) (figure 5). Proliferation to tetanus toxoid (P = .38) was not affected, which is consistent with the results of published results [32]. Our results suggest that the circulating HSV-2–specific cells that do not express CLA in vivo can still respond to HSV-2 antigen with proliferation in vitro. Because expression of CLA has been linked to the regulatory T lymphocyte phenotype among CD4+ T lymphocytes [33], removal of CLA+ cells may have complex effects by reducing both the number of antigen-specific cells and the number of suppressive cells.
Figure 4.

Effect of cutaneous lymphocyte–associated antigen (CLA) depletion on expression of CLA by CD4+ T lymphocytes responding to antigens or mitogens. A, Examples of peripheral-blood mononuclear cells (PBMCs) on day 0, both before and after depletion of CLA-expressing cells by use of immunomagnetic beads. Unmanipulated or depleted populations were then stimulated with antigens or mitogens for 5 days. B, Expression of CLA in gated lymphoblasts after in vitro restimulation with the indicated substances. The percentages of CD4+ T lymphocytes expressing CLA are shown. Cultures in each column are derived from the starting PBMC populations above them in row A. CMV, cytomegalovirus; HSV, herpes simplex virus; PHA, phytohemagglutinin.
Figure 5.
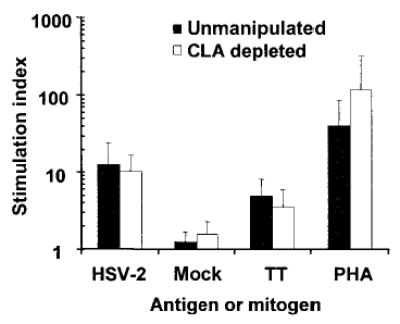
Effect of cutaneous lymphocyte–associated antigen (CLA) depletion on lymphoproliferative responses. Peripheral-blood mononuclear cells (PBMCs) (unmanipulated or depleted of CLA-expressing cells) from 8 herpes simplex virus (HSV)–2 seropositive subjects were costimulated with autologous cells and mitogen or antigen for 5 days, followed by routine [3H]-thymidine incorporation assays. Data are mean ± SD of stimulation indices. PHA, phytohemagglutinin; TT, tetanus toxoid.
Compared with the results of the direct ex vivo tests (figure 1), HSV-2–stimulated cells in vitro displayed higher levels of CLA expression (figures 2 and 3). The previous experiment indicated that HSV-2–specific CD4+ T lymphocytes lacking CLA could proliferate in vitro. To examine the stability of the CLA-low phenotype on antigen restimulation, we stained CLA-depleted PBMCs (figure 4A) after 5–6 days of stimulation with HSV-2. Without CLA depletion, a mean ± SD of 54.7% ± 22.5% of CD4+ lymphoblasts expressed CLA (table 3 and figure 4B). With initial depletion of CLA+ cells from the responder population, a mean ± SD of 38.5% ± 26.4% of CD4+ lymphoblasts expressed CLA. This 30% reduction was not statistically significant (P = .56, Mann-Whitney U test; P = .125, paired Wilcoxon rank sum test). Some person-to-person variability was noted, with subject 6 (table 3) having lower CLA expression in response to all stimuli. CLA expression by CMV-reactive and PHA-driven cultures was generally low, regardless of the presence or absence of CLA+ cells in the responder population, with the exception of 1 culture (for subject 4, unsplit PBMCs). In our defined culture conditions, HSV-2–specific, but not CMV-specific, CLA+CD4+ T lymphocytes expressed CLA after recognition of cognate antigens.
Table 3.
Expression of cutaneous lymphocyte–associated antigen (CLA) by gated CD4+ lymphoblasts after 5 days of restimulation with inactivated viruses or phytohemagglutinin (PHA).
| CD4+ lymphoblasts expressing CLA, %
|
||||||
|---|---|---|---|---|---|---|
| Unmanipulated PBMCs
|
CLA-depleted PBMCs
|
|||||
| Subject | HSV-2 | CMV | PHA | HSV-2 | CMV | PHA |
| 4 | 80.90 | 18.68 | 8.08 | 65.14 | 2.35 | 8.64 |
| 5 | 64.40 | 4.94 | 6.99 | 53.52 | 2.28 | 6.90 |
| 6 | 29.90 | 3.45 | 7.28 | 5.60 | 0.58 | 2.70 |
| 7 | 43.60 | 5.94 | 8.26 | 29.90 | 2.00 | 6.88 |
| Mean ± SD | 54.7 ± 22.5 | 8.3 ± 7.0 | 7.7 ± 0.6 | 38.5 ± 26.4 | 1.8 ± 0.8 | 6.3 ± 2.5 |
NOTE. Responder cells were unmanipulated peripheral-blood mononuclear cells (PBMCs) or PBMCs depleted of CLA-expressing cells. CMV, cytomegalovirus; HSV, herpes simplex virus.
Binding of HSV-2–specific CD4+ T lymphocytes to E-selectin
CLA is not molecularly identical to the ESL present on some circulating lymphocytes [5]. We therefore evaluated the binding of stimulated PBMCs to E-selectin using subjects seropositive for HSV-2 and CMV. CHO cells transfected with E-selectin stained 89% positive with anti–E-selectin, compared with 0% for untransfected CHO cells (data not shown). Overall, the binding of CD4+, CFSE-low lymphoblasts was significantly higher for HSV-2 than for either CMV (P = .015, Mann-Whitney U test) or PHA (P = .015, Mann-Whitney U test) (table 4). Similar analyses failed to distinguish differences in binding to control CHO monolayers (P > .10, for each comparison between HSV-2, PHA, and CMV; Mann-Whitney U test). Binding of HSV-2– or CMV-reactive cells to CHO-E also was stronger than binding of the same cells to CHO (P = .015 and P = .004, respectively, Mann-Whitney U test), whereas PHA-reactive CD4+ lymphoblasts bound equally well to both substrates (P = .31).
Table 4.
Binding of human CD4+, 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE)–low (divided) lymphoblasts to CHO cells expressing cell-surface human E-selectin (CHO-E) and to control CHO cells after 5 days of restimulation with herpes simplex virus (HSV) type 2 or cytomegalovirus (CMV) antigen or with phytohemagglutinin (PHA).
| CHO-E
|
Control CHO cells
|
|||||
|---|---|---|---|---|---|---|
| Subject | HSV-2 | CMV | PHA | HSV-2 | CMV | PHA |
| 13 | 1.24 | 1.07 | 0.65 | 0.38 | 0.20 | 0.03 |
| 17 | 4.70 | 1.08 | 1.01 | 0.57 | 0.46 | 0.50 |
| 18 | 2.37 | 0.56 | 0.35 | 0.35 | 0.28 | 0.34 |
| 7 | 5.07 | 0.98 | 0.89 | 0.56 | 0.24 | 0.62 |
| 19 | 28.14 | 4.06 | 3.54 | 3.93 | 0.42 | 3.53 |
| 5 | 5.08 | 1.69 | 2.17 | 1.47 | 0.77 | 1.07 |
NOTE. Data are the ratio of bound:unbound CD4+, CFSE-low lymphoblasts.
ESL activity was tested by use of soluble E-selectin and day-7 PBMC cultures. ESL was detected in HSV-2–stimulated cultures but not in CMV- or PHA-stimulated cultures (figure 6C). As expected, inclusion of EDTA during E-selectin incubation abrogated binding [34]. Omission of soluble E-selectin eliminated staining (figure 6A and 6B). Similar results were obtained with 3 other subjects (data not shown).
Figure 6.

Expression of E-selectin ligand (ESL). Peripheral-blood mononuclear cells (PBMCs) were stimulated for 5 days with the indicated antigens or mitogens. Dot-plots are gated on CD4+ T lymphoblasts. A, Cells stimulated with UV-killed herpes simplex virus (HSV) type 2 were stained only with phosphatidylethanolamine-F (ab′)2–labeled goat anti–human Fc (Coulter), the secondary detection reagent for ESL detection. Little staining was noted. B, Chimeric E-selectin–Fc protein was incubated with the same target population and EDTA, followed by incubation with detection reagent and anti-CLA. High CLA expression but little ESL staining was detected. C–E, EDTA was omitted; CLA and ESL were detected for HSV-2 stimulation but not for cytomegalovirus (CMV) or phytohemagglutinin (PHA) stimulation.
DISCUSSION
Models of leukocyte trafficking include rolling adhesion, in which circulating cells interact with venular endothelium [35]. Biopsies have shown that HSV-2–specific CD4+ T lymphocytes localize by day 2 after the onset of symptoms [9]. Our experiments were conducted to address the mechanism(s) of CD4 localization, with the focus on CLA and ESL.
Ex vivo assays using CD154 or IFN-γ up-regulation showed that HSV-2–specific CD4+ T lymphocytes express higher levels (~20%) of CLA than do CMV-specific or bystander cells (figure 1). Ideally, we would have used HLA class II tetramers to detect HSV-2–specific CD4+ T lymphocytes ex vivo, which, unlike the CD154 and IFN-γ tests, do not require restimulation with antigen for 6 h. However, the frequency of HSV peptide–specific CD4+ T lymphocytes is quite low and approaches the background levels seen with HLA class II tetramers [36]. Even the 6-h stimulation with antigen could initiate the expression of CLA through a cytokine-mediated or other indirect effect, as discussed below. However, several days are required to detect cytokine influences on the expression of CLA by stimulated T lymphocytes [30, 37, 38].
The low levels of expression of CLA on HSV-2–specific CD4+ T lymphocytes contrast with the 50%–70% expression of CLA by circulating HSV-2–specific CD8+ T lymphocytes [7]. Functional [9] and histologic [8] data show that CD4+ T lymphocytes traffic more quickly to HSV-2 lesions than do CD8+ T lymphocytes. The apparent dissociation between the rapidity of in vivo trafficking and the ex vivo expression of CLA requires further investigation. Some data have indicated that DCs and tissue microenvironments may influence the homing phenotype of memory cells [11]. Recent reports, albeit in different murine models, have indicated that distinct DC subsets are involved in priming HSV-specific CD4+ vs. CD8+ T lymphocytes after peripheral inoculation [12, 13]. Future animal work in the HSV system may reveal mechanisms for the control of homing phenotypes of HSV-specific CD4+ and CD8+ T lymphocytes, but murine models, in contrast to human infection, generally involve only a single episode of lytic HSV replication, without the periodic recurrences typically observed in humans that may continue to influence T lymphocyte trafficking.
CLA has been associated with the Th2 CD4 phenotype, especially in atopic persons [39]. Our focus on IFN-γ + cells could bias away from the detection of Th2-like CLA+ cells. However, most researchers do not detect IL-4+ cells with CMV antigen [40–42]. We excluded subjects with atopic dermatitis. In addition, CD154 up-regulation, independent of cytokine profile, showed preferential expression of CLA by HSV-2–reactive cells. CD154 is an emerging marker for antigen-specific CD4+ T lymphocytes, one that identifies cells with a physiologically important effector function, modulation of DC function [43]. In the present study, CD154 up-regulation was associated with CD69 up-regulation (data not shown), a more widely used marker for T lymphocyte activation.
We examined a cross-sectional set of specimens from persons with chronic (>1 year) HSV-2 infection that was obtained between clinically evident outbreaks of HSV-2. If CLA+ cells preferentially traffic to skin, it is possible that the abundance of HSV-2–reactive cells, or the proportion of circulating HSV-2–reactive cells expressing CLA or ESL, could change over time. Reported low PBMC proliferative responses to HSV during reactivations could be due to exodus from blood, although the original report implicated CD8+ suppressors [44]. Patient-reported recurrence is poorly correlated with both objective lesions [45] and replicating HSV-2 [46]. Future temporal studies will use intensive serial physical examinations and sampling for virus by polymerase chain reaction and culture [47]. The study of lesion-derived HSV-2–specific CD4+ T lymphocytes could potentially address selective trafficking of CLA+ or ESL+ cells. However, few lymphocytes can be isolated from human biopsy specimens [21].
In agreement with the direct ex vivo data indicating that only a minority of HSV-2–reactive CD4+ T lymphocytes in PBMCs express CLA, CLA-depleted PBMCs still proliferate well in response to HSV-2 (figure 4). Our data do not address the relative replication potential of CLA+ vs. CLA− HSV-2–specific CD4+ T lymphocytes. CLA expression has been linked to the CD25+ regulatory T lymphocyte phenotype and to skin homing [33]. CD4+CD25+ T lymphocytes influence HSV-1 pathogenesis in animals [48, 49]. Therefore, the depletion of all CLA+ cells within PBMCs may have had complex effects on proliferative responses to recall antigens; we are presently addressing this possibility in depletion and reconstitution experiments.
The expression of CLA by CD4+ T lymphocytes increased during HSV-2 restimulation in vitro (tables 2 and 3; figures 2–4 and 6), to ~30%–70%. Up-regulation of CLA was not universal but was noted in most subjects (figure 3). The reason for this heterogeneity is unknown. The link between HSV-2 and CLA was maintained during restimulation, as HSV-2 stimulated higher levels of CLA than did CMV, influenza, or a mitogen. Selective expression of ESL was also documented after stimulation with HSV-2 (figure 6). Unexpectedly, on stimulation with HSV-2, cells that were initially CLA negative gained expression of CLA (figure 4).
There are 2 general explanations for this increase. First, cytokines known to increase the expression of CLA during T lymphocyte activation in vitro include IL-12, IFN-α, and transforming growth factor (TGF)–β [37, 50, 51]. HSV preparations can stimulate secretion of IL-12, IFN-α, and TGF-β from PBMCs, including DCs and monocytes [52–54]. Thus, cytokine effects may increase the levels of CLA and ESL in HSV-2–stimulated cultures. These cytokines are up-regulated by HSV-2 in vivo [55–57] and therefore may have pathophysiologic significance. We are presently testing this possibility by stimulating memory CD4+ T lymphocytes in the presence of HSV-2 antigen in HSV-seronegative persons; preliminary results (data not shown) are consistent with a cytokine-mediated or other indirect effect. Cytokines or other factors in the HSV-2 preparation also could directly up-regulate CLA. This could be addressed with purified HSV-2 and with HSV-2 and CMV antigens from the same producer cell type.
Second, it is possible that HSV-2–specific CD4+ memory T lymphocytes are programmed to express CLA on antigenic re-stimulation. The specific expression of CLA ex vivo (see above) supports some level of specific programming even before re-stimulation. The results of our peptide experiments are consistent with this notion, because peptides, which are unlikely to drive cytokine responses or to induce expression of CLA, still result in high expression of CLA by tetramer-positive progeny. We found that CLA-negative precursor cells give rise to CLA-positive cells after stimulation through T cell receptors with HSV-2 antigen. A supportive cytokine milieu, programming for CLA expression, or both factors could be operative.
Cell culture conditions were closely regulated in the present experiments, as most lymphocytes express CLA if expanded in specific media [30]. We used an RPMI–human serum–based medium that has been shown to minimize nonspecific expression of CLA [30]. We confirmed [30] that FCS-based media are permissive for CLA (data not shown). In our experiments, neither recombinant nor biologically derived IL-2 induced non-specific expression of CLA; cryopreserved PBMCs were used throughout. Strong proliferative responses were nonetheless detected, indicating that antigen presenting and responder cell populations were intact. Changes in the expression of CLA by responder cells related to freeze and thaw cannot be ruled out.
CLA and ESL expression are closely associated in inhibitor [58] and single-cell studies [51]. Fucosyltransferase VII (FTVII) expression up-regulates both CLA and ESL [59]. The precise structures that bind anti-CLA and that form ESL are undefined. Anti-CLA inhibition of functional E-selectin binding is controversial [3, 60], and dual staining of CD4+ T lymphocytes with soluble E-selectin and anti-CLA reveals populations that are singly positive (figure 6). In the present study, using cell-cell binding and flow-cytometry assays, we have shown that HSV-2–specific T lymphocytes express ESL. In single-cell analyses, there was a correlation between a high expression of CLA and a high expression of ESL, in agreement with the results of Takahashi et al. [5].
It is not known whether CLA or ESL is required for successful T lymphocyte homing to skin in vivo. Modifiers of CLA attenuate cutaneous inflammation in animals [61]. Persons deficient in IL-12 or IL-12 receptors (who might have low levels of CLA) have not been reported to have severe HSV or cutaneous infections [62]. Persons deficient in fucose transport (leukocyte adhesion deficiency–II) [63] or with mutations in FTVII [64] can have reduced CLA expression [65] but have not been reported to have severe cutaneous infections.
The present study is the first to document the expression of CLA and ESL by pathogen-reactive CD4+ T lymphocytes. Torres et al. examined the expression of CLA with respect to erythema multiforme or Stevens-Johnson syndrome, which are generally felt to be immunopathologic reactions; there was no difference in the expression of CLA by PBMCs between the patients with virus-associated systemic dermatitis and the control subjects [66]. HSV-related generalized syndromes such as HSV-associated erythema multiforme (HAEM) were not specifically evaluated. We used CMV and influenza as controls. Rarely do persons have rash during initial CMV infection [67], but this is immunopathologic and is not infectious. Cutaneous disease with recurrent CMV is very unusual, even in immunocompromised persons [68]; it would be of interest to study CLA expression during HAEM and among cells specific for other cutaneous pathogens.
In summary, circulating HSV-2–specific CD4+ T lymphocytes in persons with chronic HSV-2 infection selectively express CLA on their surface. Compared with that of circulating HSV-2–specific CD8+ T lymphocytes, the percentage of CD4+ T lymphocytes that directly express CLA ex vivo is lower, on the order of 20%. HSV-2–specific CD4+ T lymphocytes include a population of cells that circulate in a CLA-negative state but that can divide and express CLA on restimulation. This permissiveness could involve cell-resident transcriptional or chromatin mechanisms, exogenous cytokine/APC factors, or possibly both. Future studies of these issues may assist in the design of strategies to control T lymphocyte homing during vaccination or of immunotherapy for infectious, autoimmune, or malignant disorders.
Acknowledgments
We thank the subjects; Lawrence Corey, for helpful advice; Rhoda Ashley-Morrow, for diagnostic virologic testing; and Stacy Selke, for data management.
Footnotes
Presented in part: 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, 27–30 September 2002 (abstract V-916).
Financial support: National Institutes of Health (grants AI50132 and AI30731); the present work was conducted while J.C.G. was a Pfizer Postdoctoral Fellow.
References
- 1.Rott LS, Rose JR, Bass D, Williams MB, Greenberg HB, Butcher EC. Expression of mucosal homing receptor A4B7 by circulating CD4+ cells with memory for intestinal rotavirus. J Clin Invest. 1997;100:1204–8. doi: 10.1172/JCI119633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duijavestijn AM, Horst E, Pals ST, et al. High endothelial differentiation in human lymphoid and inflammatory tissues defined by monoclonal antibody HECA-452. Am J Pathol. 1988;130:147–55. [PMC free article] [PubMed] [Google Scholar]
- 3.Berg EL, Yoshino T, Rott LS, et al. The cutaneous lymphocyte antigen is a skin homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461–6. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansson EL, Rudin A, Wassen L, Holmgren J. Distribution of lymphocytes and adhesion molecules in human cervix and vagina. Immunology. 1999;96:272–7. doi: 10.1046/j.1365-2567.1999.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi R, Mizukawa Y, Yamazaki Y, et al. In vitro differentiation from naive to mature E-selectin binding CD4 T cells: acquisition of skin-homing properties occurs independently of cutaneous lymphocyte antigen expression. J Immunol. 2003;171:5769–77. doi: 10.4049/jimmunol.171.11.5769. [DOI] [PubMed] [Google Scholar]
- 6.Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymhocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389:978–81. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- 7.Koelle DM, Liu Z, McClurkan CM, et al. Expression of cutaneous lymphocyte–associated antigen by CD8+ T cells specific for a skin-tropicvirus. J Clin Invest. 2002;110:537–48. doi: 10.1172/JCI15537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham AL, Turner RR, Miller AC, Para MF, Merigan TC. Evolution of recurrent herpes simplex lesions: an immunohistologic study. J Clin Invest. 1985;75:226–33. doi: 10.1172/JCI111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koelle DM, Posavad CM, Barnum GR, Johnson ML, Frank JM, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest. 1998;101:1500–8. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. 2002;195:135–41. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mora JR, Bono MR, Manjunath N, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 12.Zhao X, Deak E, Soderberg K, et al. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus–2. J Exp Med. 2003;197:153–62. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allan RS, Smith CM, Belz GT, et al. Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–8. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 14.Stanberry LR, Spruance S, Cunningham AL, et al. Prophylactic vaccination against genital herpes with adjuvanted recombinant glycoprotein D vaccine: two randomized contolled trials. N Engl J Med. 2002;347:1652–61. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 15.Koelle DM, Liu Z, McClurkan CL, et al. Immunodominance among herpes simplex virus–specific CD8 T-cells expressing a tissue-specific homing receptor. Proc Natl Acad Sci USA. 2003;100:12899–904. doi: 10.1073/pnas.2131705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G–specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988;26:662–7. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kit S, Kit M, Qavi H, Trkula D, Otsuka H. Nucleotide sequence of the herpes simplex virus type 2 (HSV-2) thymidine kinase gene and predicted amino acid sequence of thymidine kinase polypeptide and its comparison with the HSV-1 thymidine kinase gene. Biochim Biophys Acta. 1983;741:158–70. doi: 10.1016/0167-4781(83)90056-8. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt NJ. Cell culture proceedures for diagnostic virology. In: Schmidt NJ, ed. Diagnostic proceedures for viral, rickettsial and chlamydial infections. 6th ed. Washington, DC: American Public Health Association, 1989:51–100.
- 19.Novak EJ, Liu AW, Gebe JA, et al. Tetramer-guided epitope mapping: rapid identification and characterization of immunodominant CD4+ T cell epitopes from complex antigens. J Immunol. 2001;166:6665–70. doi: 10.4049/jimmunol.166.11.6665. [DOI] [PubMed] [Google Scholar]
- 20.Kwok WW, Liu AW, Novak EJ, et al. HLA-DQ tetramers identify epitope-specific T cells in peripheral blood of herpes simplex virus type 2–infected individuals: direct detection of immunodominant antigen-responsive cells. J Immunol. 2000;164:4244–9. doi: 10.4049/jimmunol.164.8.4244. [DOI] [PubMed] [Google Scholar]
- 21.Koelle DM, Chen HB, Gavin MA, Wald A, Kwok WW, Corey L. CD8 CTL from genital herpes simplex lesions: recognition of viral tegument and immediate early proteins and lysis of infected cutaneous cells. J Immunol. 2001;166:4049–58. doi: 10.4049/jimmunol.166.6.4049. [DOI] [PubMed] [Google Scholar]
- 22.Kwok WW, Gebe JA, Liu A, et al. Rapid epitope identification from complex class-II–restricted T-cell antigens. Trends Immunol. 2001;22:583–8. doi: 10.1016/s1471-4906(01)02038-5. [DOI] [PubMed] [Google Scholar]
- 23.Koelle DM, Corey L, Burke RL, et al. Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J Virol. 1994;68:2803–10. doi: 10.1128/jvi.68.5.2803-2810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamata T, Puzon W, Takada Y. Identification of putative ligand-binding sites of the integrin α4β1 (VLA-4, CD49d/CD29) Biochem J. 1995;305:945–51. doi: 10.1042/bj3050945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaur A, Hale CL, Noren B, Kassis N, Simon MA, Johnson RP. Decreased frequency of cytomegalovirus (CMV)–specific CD4+ T lymphocytes in simian immunodeficiency virus–infected rhesus macaques: inverse relationship with CMV viremia. J Virol. 2002;76:3646–58. doi: 10.1128/JVI.76.8.3646-3658.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bitmansour AD, Waldrop SL, Pitcher CJ, et al. Clonotypic structure of the human CD4+ memory T cell response to cytomegalovirus. J Immunol. 2001;167:1151–63. doi: 10.4049/jimmunol.167.3.1151. [DOI] [PubMed] [Google Scholar]
- 27.Posavad CM, Wald A, Hosken N, Huang M-L, Koelle DM, Corey L. T cell immunity to herpes simplex virus in seronegative persons: silent infection or acquired immunity? J Immunol. 2003;170:4380–8. doi: 10.4049/jimmunol.170.8.4380. [DOI] [PubMed] [Google Scholar]
- 28.Asanuma H, Sharp M, Maecker HT, Maino VC, Arvin AM. Frequencies of memory T cells specific for varicella-zoster virus, herpes simplex virus and cytomegalovirus determined by intracellular detection of cytokine expression. J Infect Dis. 2000;181:859–66. doi: 10.1086/315347. [DOI] [PubMed] [Google Scholar]
- 29.Koelle DM, Frank JM, Johnson ML, Kwok WW. Recognition of herpes simplex virus type 2 tegument proteins by CD4 T cells infiltrating human genital herpes lesions. J Virol. 1998;72:7476–83. doi: 10.1128/jvi.72.9.7476-7483.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armerding D, Kupper TS. Functional cutaneous lymphocyte antigen can be induced in essentially all peripheral blood T lymphocytes. Int Arch Allergy Immunol. 1999;119:212–22. doi: 10.1159/000024197. [DOI] [PubMed] [Google Scholar]
- 31.Picker LJ, Michie SA, Rott LS, Butcher EC. A unique phenotype of skin-associated lymphocytes in humans: preferential expression of the HECA-452 epitope by benign and malignant T cells at cutaneous sites. Am J Pathol. 1990;136:1053–68. [PMC free article] [PubMed] [Google Scholar]
- 32.Jung T, Schulz S, Zachmann K, Neumann C. Expansion and proliferation of skin-homing T cells in atopic dermatitis as assessed at the single cell level. Int Arch Allergy Immunol. 2003;130:143–9. doi: 10.1159/000069010. [DOI] [PubMed] [Google Scholar]
- 33.Cavani A, Nasorri F, Ottaviani C, Sebastiani S, De Pita O, Girolomoni G. Human CD25+ regulatory T cells maintain immune tolerance to nickel in healthy, nonallergic individuals. J Immunol. 2003;171:5760–8. doi: 10.4049/jimmunol.171.11.5760. [DOI] [PubMed] [Google Scholar]
- 34.Anostario M, Jr, Li SH, Huang KS. A ligand binding assay for E-selectin. Anal Biochem. 1994;221:317–22. doi: 10.1006/abio.1994.1419. [DOI] [PubMed] [Google Scholar]
- 35.Robert C, Kupper TS. Inflammatory skin diseases, T cells, and immune surveillance. N Engl J Med. 1999;341:1817–27. doi: 10.1056/NEJM199912093412407. [DOI] [PubMed] [Google Scholar]
- 36.Kwok WW, Liu AW, Novak EJ, et al. HLA–DQ tetramers identify epitope-specific T-cells in peripheral blood of herpes simplex virus–2–infected individuals: direct detection of immunodominant antigen responsive cells. J Immunol. 2000;164:4244–9. doi: 10.4049/jimmunol.164.8.4244. [DOI] [PubMed] [Google Scholar]
- 37.Picker LJ, Treer JR, Ferguson-Darnell B, Collins PA, Bergstresser PR, Terstappen LWMM. Control of lymphocyte recirculation in man. II. Differential regulation of the cutaneous lymphocyte–associatedantigen, a tisue-selective homing receptor for skin-homing T cells. J Immunol. 1993;150:1122–36. [PubMed] [Google Scholar]
- 38.Tsuchiyama J, Yoshino T, Toba K, et al. Induction and characterization of cutaneous lymphocyte antigen on natural killer cells. Br J Haematol. 2002;118:654–62. doi: 10.1046/j.1365-2141.2002.03608.x. [DOI] [PubMed] [Google Scholar]
- 39.Akdis M, Trautmann A, Klunker S, et al. T helper (Th) 2 predominance in atopic diseases is due to preferential apoptosis of circulating memory/effector Th1 cells. FASEB J. 2003;17:1026–35. doi: 10.1096/fj.02-1070com. [DOI] [PubMed] [Google Scholar]
- 40.Rentenaar RJ, Gamadia LE, van DerHoek N, et al. Development of virus-specific CD4+ T cells during primary cytomegalovirus infection. J Clin Invest. 2000;105:541–8. doi: 10.1172/JCI8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kallas EG, Reynolds K, Andrews J, et al. Cytomegalovirus-specific IFNγ and IL-4 are produced by antigen expanded human blood lymphocytes from seropositive volunteers. Immunol Lett. 1998;64:63–9. doi: 10.1016/s0165-2478(98)00080-7. [DOI] [PubMed] [Google Scholar]
- 42.Hensel N, Melenhorst JJ, Bradstock K, et al. Flow cytometric quantitation and characterization of the T-lymphocyte memory response to CMV in healthy donors. Cytotherapy. 2002;4:29–40. doi: 10.1080/146532402317251509. [DOI] [PubMed] [Google Scholar]
- 43.Subauste CS, Wessendarp M, Portilllo JA, et al. Pathogen-specific induction of CD154 is impaired in CD4+ T cells from human immunodeficiency virus–infected patients. J Infect Dis. 2004;189:61–70. doi: 10.1086/380510. [DOI] [PubMed] [Google Scholar]
- 44.Vestey JP, Norval M, Howie SE. Lymphoproliferative responses in recrudescent orofacial herpetic infections. J R Soc Med. 1990;83:308–11. doi: 10.1177/014107689008300510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koutsky LA, Stevens CE, Holmes KK, et al. Underdiagnosis of genital herpes by current clinical and viral-isolation procedures. N Engl J Med. 1992;326:1533–9. doi: 10.1056/NEJM199206043262305. [DOI] [PubMed] [Google Scholar]
- 46.Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342:844–50. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]
- 47.Koelle DM, Wald A. Herpes simplex virus: the importance of asymptomatic shedding. J Antimicrob Chemother. 2000;45:1–8. doi: 10.1093/jac/45.suppl_4.1. [DOI] [PubMed] [Google Scholar]
- 48.Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol. 2004;172:4123–32. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 49.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akdis M, Klunker S, Schliz M, Blaser K, Akdis CA. Expression of cutaneous lymphocyte–associated antigen on human CD4+ and CD8+ Th2 cells. Eur J Immunol. 2000;30:3533–41. doi: 10.1002/1521-4141(2000012)30:12<3533::AID-IMMU3533>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 51.Teraki Y, Picker LJ. Independent regulation of cutaneous lymphocyte–associated antigen expression and cytokine synthesis phenotype during human CD4+ memory T cell differentiation. J Immunol. 1997;159:6018–29. [PubMed] [Google Scholar]
- 52.Malmgaard L, Paludan SR, Mogensen SC, Ellerman-Eriksen S. Herpes simplex virus type 2 induces secretion of IL-12 by macrophages through a mechanism involving NF-κB. J Gen Virol. 2000;81:3011–20. doi: 10.1099/0022-1317-81-12-3011. [DOI] [PubMed] [Google Scholar]
- 53.Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principle type 1 interferon–producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 54.Mendez-Samperio P, Hernandez M, Ayala HE. Induction of transforming growth factor–β1 production in human cells by herpes simplex virus. J Interferon Cytokine Res. 2000;20:273–80. doi: 10.1089/107999000312405. [DOI] [PubMed] [Google Scholar]
- 55.Van Voorhis WC, Barrett LK, Koelle DM, Nasio JM, Plummer FA, Lukehart SA. Primary and secondary syphillis lesions contain mRNA for Th1 cytokines and activated cytolytic T cells. J Infect Dis. 1996;173:491–5. doi: 10.1093/infdis/173.2.491. [DOI] [PubMed] [Google Scholar]
- 56.Saitoh-Ishibashi K, Ishibashi K, Azumi A, Negi A. Analysis of cytokine mRNAs in murine herpes simplex virus type 1 retinitis. Jpn J Ophthalmol. 2003;47:166–72. doi: 10.1016/s0021-5155(02)00700-1. [DOI] [PubMed] [Google Scholar]
- 57.Hendricks RL, Weber PC, Taylor JL, Koumbis A, Tumpey TM, Glorioso JC. Endogenously produced interferon-α protects mice from herpes simplex virus type 1 corneal disease. J Gen Virol. 1991;72:1601–10. doi: 10.1099/0022-1317-72-7-1601. [DOI] [PubMed] [Google Scholar]
- 58.Dimitroff CJ, Bernacki RJ, Sackstein R. Glycosylation-dependent inhibition of cutaneous lymphocyte–associated antigen expression: implications in modulating lymphocyte migration to skin. Blood. 2003;101:602–10. doi: 10.1182/blood-2002-06-1736. [DOI] [PubMed] [Google Scholar]
- 59.Knibbs KN, Craig RA, Maly P, et al. α(1,3)-Fucosyltrasferase VII-dependent synthesis of P- and E-selectin ligands on cultured T lympho-blasts. J Immunol. 1998;161:6305–15. [PubMed] [Google Scholar]
- 60.Wagers AJ, Stoolman LM, Craig R, Knibbs RN, Kansas GS. An sLex-deficient variant of HL60 cells exhibits high levels of adhesion to vascular selectins: further evidence that HECA-452 and CSLEX1 monoclonal antibody epitopes are not essential for high avidity binding to vascular selectin. J Immunol. 1998;160:5122–9. [PubMed] [Google Scholar]
- 61.Dimitroff CJ, Kupper TS, Sackstein R. Prevention of leukocyte migration to inflamed skin with a modified fluorosugar modifier of cutaneous lymphocyte–associated antigen. J Clin Invest. 2003;112:1008–18. doi: 10.1172/JCI19220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fieschi C, Casanova JL. The role of interleukin-12 in human infectious diseases: only a faint signature. Eur J Immunol. 2003;33:1461–4. doi: 10.1002/eji.200324038. [DOI] [PubMed] [Google Scholar]
- 63.Luhn K, Wild MK, Eckhardt M, Gerardy-Schahn R, Vestweber D. The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat Genet. 2001;28:69–72. doi: 10.1038/ng0501-69. [DOI] [PubMed] [Google Scholar]
- 64.Schottelius AJ, Hamann A, Asadullah K. Role of fucosyltransferases in leukocyte trafficking: major impact for cutaneous immunity. Trends Immunol. 2003;24:101–4. doi: 10.1016/s1471-4906(03)00024-3. [DOI] [PubMed] [Google Scholar]
- 65.Kuijpers TW, Etzioni A, Pollack S, Pals ST. Antigen-specific immune responsiveness and lymphocyte recruitment in leukocyte adhesion deficiency type II. Int Immunol. 1997;9:607–13. doi: 10.1093/intimm/9.4.607. [DOI] [PubMed] [Google Scholar]
- 66.Torres MJ, Corzo JL, Leyva L, et al. Differences in the immunological responses in drug- and virus-induced cutaneous reactions in children. Blood Cells Mol Dis. 2003;30:124–31. doi: 10.1016/s1079-9796(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 67.Oskay T, Karademir A, Kutluay L. Vesicular and pustular eruption related to cytomegalovirus in an immunocompetent patient. Clin Exp Dermatol. 2003;28:610–2. doi: 10.1046/j.1365-2230.2003.01404.x. [DOI] [PubMed] [Google Scholar]
- 68.Khoshnevis M, Tyring SK. Cytomegalovirus infections. Dermatol Clin . 2002;20(vii):291–9. doi: 10.1016/s0733-8635(01)00007-9. [DOI] [PubMed] [Google Scholar]


