Abstract
PROBLEM
Genital herpes simplex infections are generally limited to epithelia and neurons. Vaccines have had activity in herpes simplex virus (HSV)-seronegative women only. Understanding how HSV-specific T cells traffic to infected sites may assist in vaccine design.
METHOD OF STUDY
Herpes simplex virus epitopes recognized by HSV-specific CD8 T cells were identified and used to make fluorescent human leukocyte antigen (HLA)-peptide tetramers. Molecules related to lymphocyte rolling adhesion were studied by flow cytometry and cell binding. HSV-specific CD4 T cells identified ex vivo by cytokine accumulation or activation marker expression, or detected in vitro by 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE) dilution, were similarly investigated.
RESULTS
Herpes simplex virus-specific T cells are 10- to 100-fold more prevalent in lesional skin compared with blood and greatly enriched in lesions compared with normal skin. Diverse viral antigens are recognized by HSV-specific T cells. Functionally active E-selectin ligand, and cutaneous lymphocyte antigen (CLA), are expressed by circulating HSV-2-specific CD8 cells. CD4 cells display lower levels of CLA that are dramatically up-regulated upon re-stimulation with antigen.
CONCLUSIONS
Herpes simplex virus-2-specific CD8 and CD4 T cells differ in constitutive expression of skin homing molecules. Vaccines designed to induce proper homing are postulated to have increased efficacy.
Keywords: Cutaneous lymphocyte-associated antigen, E-selectin, herpes simplex virus, lymphocyte trafficking
INTRODUCTION
Some sexually transmitted infections (STI) are caused by non-invasive microbes that preferentially infect epithelia. Chlamydia trachomatis infects cervical and urinary tract epithelia. After inoculation of genital strains of human papillomavirus or herpes simplex virus (HSV) in the genital region, infection is typically restricted to the epidermis or cervicovaginal epithelium. These cell and tissue tropisms, because of complex, organism-specific pathogenesis factors, require adaptations by the immune response. While humoral innate and acquired immune effector molecules such as complement and antibody can act at a distance, cellular effectors such as natural killer (NK) and T cells must localize to infected areas. This report, based on a presentation at a recent meeting, includes new data on the preferential expression of a functional E-selectin ligand by circulating HSV-2-specific CD8 T cells. A brief review of published and in press data on acquired, cellular immunity to HSV type 2 (HSV-2) is presented, emphasizing factors that modulate lymphocyte trafficking to infected skin and genital tract areas. Finally, T-cell homing literature relevant to STI and the genital tract are briefly reviewed.
The multi-step model of lymphocyte homing of Campbell et al.1 is useful for discussing local immunity to STI. The traditional initial step is the rolling adhesion of leukocytes to the lumenal surface of vascular endothelium, typically in post-capillary venules. In the skin, lymphocyte E-selectin ligand (ESL) interacts with E-selectin expressed on the lumenal surface of post-capillary venular endothelium. E-selectin, constitutively expressed at low levels in dermal venules, is assumed to be involved in baseline surveillance circulation of lymphocyte through normal skin.2 Tumor necrosis factor (TNF)-α and interleukin (IL)-1β, which are increased in vivo and in vitro in response to HSV, can increase E-selectin expression by endothelial cells through an NF-κB-mediated pathway.3,4 Chemokines, locally concentrated because of charge interactions with the endothelium, are assumed to trigger, via ‘inside out signaling’, the maturation of extracellular domains of integrins on the surface of loosely adherent lymphocytes. These structural changes promote tight adhesion. Finally, chemokines are again involved in diapedesis and further chemotaxis in the stroma towards the region of infection. Candidate chemokines and receptors involved in skin homing are discussed below.
The model above is relevant to memory T cells. Naïve T cells express the adhesion molecule l-selectin (CD62L) and chemokine receptor CCR7 to promote trafficking to lymph nodes, where priming with exogenous antigens can occur, and typically lack adhesion and chemotaxis molecules for migrating to peripheral tissues. There is increasing evidence that ‘programming for homing’ occurs, perhaps simultaneous with priming as traditionally understood. Immunologic priming may be the true ‘step zero’ for T-cell homing. For example, cutaneous sensitization, followed by priming in skin-draining lymph nodes, leads to ESL expression by resultant memory CD4 T cells.5 Priming in the gastrointestinal (GI) tract leads to the expression of the adhesion molecule A4B7 integrin and CCR9, and chemical factors in the Peyer’s patch microenvironment elaborated by gut dendritic cell (DC) that imprint this phenotype were recently described.6 Less data are available concerning programming for homing to skin. Some data suggest that plasmacytoid dendritic cells (pDC), which have a strong innate response to HSV perhaps mediated through TLR9,7 may influence programming for homing during T-cell priming to promote expression of skin-homing molecules.8
Many studies of skin homing have used ex vivo systems, recombinant mice, or non-infectious antigens. We have studied the classically HLA-restricted T-cell receptor (TCR) αβ cellular immune response to HSV, with the aims of understanding fine specificity, vaccine design, and disease correlation. While manipulation of the pathogen or host are not possible, markers of T-cell homing can be measured in a physiologically relevant human system. Tools developed in our HSV studies, and reagents and concepts from many other laboratories, have lead to data that reinforce findings from previous studies and call other models into question.
METHOD OF STUDY
Sampling of human genital herpes lesions by vesicle fluid harvest and biopsy, cervical and corneal T-cell recovery, and processing of blood to obtain peripheral blood mononuclear cells (PBMC) have been described.9–12 Interrogation of genomic HSV-2 DNA libraries to discover HSV-2 peptide epitopes recognized by CD4 and CD8 T cell clones has been detailed.12,13 To measure CLA and ESL on the surface of antigen-specific CD8 T cells, 5 × 106 PBMC in 70 μL T-cell medium9 were stained for 60 min at room temperature with allophycocyanin (APC)-labeled HLA B*0702 tetramers loaded with peptide HSV-2 VP22 amino acids 49–57 or HLA A*0201 tetramers loaded with peptide cytomegalovirus (CMV) pp65 amino acids 595–603 or Epstein–Barr virus (EBV) BMLF-1 amino acids 280–288.14 Tetramers were from the Immune Monitoring Core facility at Fred Hutchinson Cancer Research Center or the National Institutes of Health Tetramer Core at Emory University. Optimal dilutions determined in preliminary experiments. After two washes in PBS with 0.5% fetal bovine serum (FBS), cells were stained for 30 min, 37°C with 1 μg of human E-selectin-Fc (R&D Systems, Minneapolis, MN, USA) in the presence or absence of 10 mm ethylenediam-inetetraacetic acid (EDTA), washed twice as above, and stained with anti-CD8-PerCP (Caltag, Burlingame, CA, USA), anti-CLA-FITC (Pharmingen, Burlingame, CA, USA) and goat anti-human Fc-PE (Coulter, Fullerton, CA, USA) as described.15 Analyses used a FacsCalibur (Becton Dickenson, San Jose, CA, USA) flow cytometer and WinMDI 2.8 (http://facs.scripps.edu/software.html).
Studies of virus-reactive CD4 T cells were performed as described.15 Briefly, PBMC were stained with CFSE, a vital dye that is diluted in proportion to the number of cell divisions and is detectable by flow cytometry. CFSE-labeled PBMC were stimulated by the continual presence of whole Vero-cell derived, cell-associated UV-inactivated HSV-2 antigen, gradient-purified inactivated CMV virions or dialyzed influenza vaccine as control viral recall antigens, or phytohemagglutinin (PHA). After 5 days, virus-reactive CD4 T cells were identified by gating on large, granular lymphoblasts, and then on CD4+ cells with low CFSE fluorescence. Expression of CLA and ESL were detected as outlined above for CD8 cells. In some experiments, CLA+ cells were depleted prior to addition of antigen or mitogen by incubation of PBMC with biotinylated anti-CLA followed by negative selection with avidin-coated paramagnetic beads (Miltenyi, Auburn, CA, USA).
Subjects were studied after obtaining informed consent from the University of Washington Institutional Review Board. Skin biopsies were stained for E-selectin and CLA as previously described.14
RESULTS
HSV-2-specific CD4 and CD8 T Cells Localize to Sites of Infection
Genital HSV-2 is characterized by groups of small vesicles that evolve to pustules, erosions, and then crusts. Cells were recovered from vesicles and pustules by breaking them with a needle, and transferred with a cell scraper into a tube of cell media. When these cells were cloned immediately by limiting dilution, using PHA and IL-2 as mitogen and growth factor under standard conditions, respectively, HSV-2-specific CD4 T cells were readily obtained.9 These clones strongly proliferate with autologous γ-irradiated PBMC as antigen-presenting cells (APC) and UV-killed HSV-2 as antigen, compared with mock viral antigen, using a 3H-thymidine incorporation readout. The clones show classical HLA class II restriction. For many clones, the specific HSV-2-encoded proteins and constituent short peptides recognized by these clones were defined, confirming their reactivity with the virus.16 On a clonal frequency basis, HSV-2-specific CD4 T-cell clones comprised 6–10% of all clones recovered from HSV-2 vesicles.
To compare the abundance of HSV-specific T cells in lesions and blood, and therefore determine if specific trafficking occurs, it would be ideal to apply identical methods such as clonal frequency, ELISPOT, intracellular cytokine cytometry (ICC), or CD40L up-regulation to blood and local specimens. However, direct cloning of PBMC for clonal frequency is impractical given the expected rarity of HSV-specific cells, and direct ELISPOT, ICC, or CD40L tests on lesion-derived cells is limited by the small sample. We used limiting dilution assays (LDA), in which decreasing numbers of PBMC, with many replicates at each input number, were assayed for proliferative responses to HSV-2 (or mock control) antigen and APC. Typically, HSV-2-seropositive persons have LDA responder cell frequencies on the order of 1 in 1000 PBMC.17 ICC assays for interferon-γ and CD40L up-regulation assays, performed as described, both show somewhat higher net responder frequencies, in the order of 0.2–0.5% of CD4 T cells, are detected.15,18 Depending on the assay methodology, our data indicate that HSV-2-specific CD4 T cells are 10- to 100-fold enriched in genital lesions compared with blood.
We also analyzed the abundance of HSV-1-reactive CD4 T cells in corneas for patients with end-stage herpes stromal keratitis (HSK) requiring cornea transplant. Despite prolonged pre-operative antiviral therapy, HSV-specific CD4 cells are at least 100-fold enriched compared with blood in this disease state,10 which may be related to autoimmune or bystander damage to the cornea.19 Similar recovery of HSV-1-specific CD4 T cells from HSK corneas was reported by Verjans et al.20 Some evidence21 suggests that T cells that home to the eye express CD103 (AEB7 integrin, also known as human mucosal lymphocyte antigen or HML). CD103 and its ligand, E-cadherin, are of interest with regard to homing to the vagina and cervix, as lymphocytes freshly isolated from these epithelia preferentially express CD103.22,23
Local enrichment of HSV-2-specific CD8 T cells has been somewhat harder to measure. Direct cloning of vesicle fluid cells, or of biopsies of genital HSV-2 lesions that are immediately digested with collagenase, yield classically HLA class I-restricted CD8 CTL clones upon screening relatively small numbers of clones.12,24 Measure of the integrated abundance of virus-specific CD8 T cells in PBMC remains challenging: expansive peptide sets and large blood samples can cover the proteome in ICC surveys,25 but LDA with CTL readout, despite many shortcomings,26 remains the most practical tool for complex pathogens. Using LDA, we detect abundances on the order of 1 in 1000 PBMC for HSV-2-specific CD8 CTL.17 Direct tetramer analyses have yielded abundances, for T cells specific for a single HSV-2 epitope, of up to 0.6% of CD8 T cells (about 1 in 500 PBMC).14 The discrepancy between these two measures may be partially explained by the differential requirements for detection in the LDA assay (expansion in culture and cytotoxicity) and by tetramer (binding to peptide HLA). Regardless, the ease of recovery of HSV-2-specific CD8 CTL from lesions is consistent with some degree of local enrichment and therefore trafficking of these cells to sites of infection.
Perhaps the strongest evidence of local enrichment of HSV-specific T cells comes from biopsy studies including normal skin controls. Subjects with recurrent genital HSV-2 on skin surfaces acceptable for biopsy underwent two to three biopsies over several days, in the absence of antiviral therapy as well as at least one normal skin biopsy. Skin-infiltrating T cells were expanded in bulk with PHA and IL-2, conditions documented T and NK lymphocytes in a relatively unbiased fashion. After a single 2–3-week cycle of cell replication, the functional activities (HSV-specific CTL and proliferative responses, and NK activity represented by K562 killing) and cell-surface phenotypes of the bulk cultures were compared. There was always a markedly increased HSV-specific CTL and proliferative activity in the HSV biopsy-derived cultures. The CTL activity contained both CD4 and CD8 effector components.27 Cloning of these bulk cultures easily lead to large panels of HSV-2-reactive CD4;12,27 CD8 CTL clones were also readily derived after positive selection of bulk CD8-expressing cells. These results indicate that HSV-2-specific CD4 and CD8 T cells traffic to recurrent genital HSV-2 lesions in preference to normal skin. Interestingly, local NK cell activity and number was markedly higher in HSV lesions than in normal skin. A subset of NK cells express skin homing-related markers,28 but little is known about their homing to skin.
Circulating HSV-specific CD8 T Cells Express Skin-homing Markers
In 1990, Picker et al.29 described HECA-452, a monoclonal antibody (mAb)-reactive with an antigen selectively expressed on skin-infiltrating lymphocytes. The HECA-452-reactive epitope has been named CLA. The precise molecular structure of CLA has not been determined, although synthetic glycopeptides reactive with HECA-452 have been described.30 CLA is a fucose-containing carbohydrate structure structurally related to sialyl-lewis X.30 In this review, CLA is considered to be the set of HECA-452-reactive structures. ESL are functionally defined as structures that bind E-selectin. The precise structure(s) of ESL(s) are also unknown. Most data suggest that CLA and ESL are very tightly linked for T lymphocytes. HECA-452 can inhibit the binding of T cells to E-selectin-transfected cells.31 Other data, however suggest that some CLA and ESL are not identical. Stimulated naïve CD4 T cells acquire CLA and ESL with differing kinetics.32 Both CLA and ESL are thought to occur on memory T cells mainly as post-translational modifications of the transmembrane protein P-selectin glycoprotein ligand-1 (PSGL-1) (CD162).30 Transfection and other experiments have established a role for fucosyltransferases (FUT) VII and IV in the synthesis of CLA and ESL, focusing research on the regulation of skin homing onto the molecular control of FUTVII and FUTIV.33,34
Cloning of HSV-2-specific CD8 T cells from lesions enable the use of genomic libraries of HSV-2 DNA to determine their fine peptide specificity. HSV-2 has a large genome, encoding approximately 85 polypeptides. Expression cloning has defined many of these open-reading frames, encoding structural proteins in the viral scaffold, tegument, and envelope, as well as non-structural regulatory proteins, as CD8 antigens.12,35 Definition of minimal nine to 10 amino acid peptide epitopes in turn allowed the synthesis of fluorescent HLA class I-peptide tetramers to detect these cells directly in blood. CD8 T cells reactive with selected HSV-2 epitopes are abundant enough in the blood of HSV-2-infected, HLA-appropriate subjects to be detectable by flow cytometry of unmanipulated blood samples (Fig. 1).
Fig. 1.
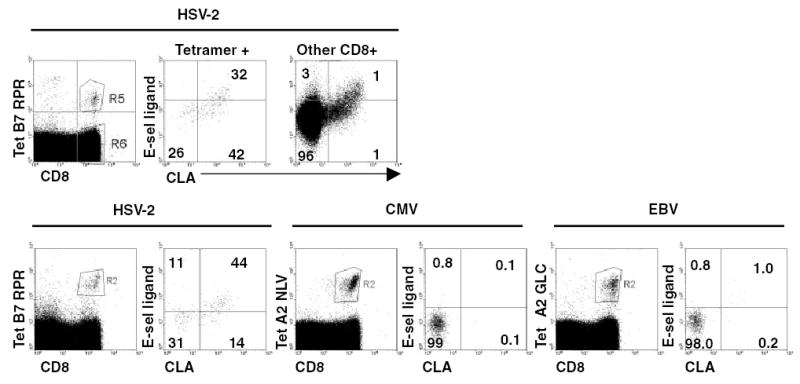
Expression of CLA and ESL by circulating HSV-2-specific CD8 T cells. Top: PBMC were stained with fluorescent tetramer of HLA B*0702 complexed with HSV-2 VP22 amino acids 49–57, followed by anti-CD8, anti-CLA, and soluble E-selectin as per Method of Study. Gating on lymphocytes (not shown) and either HSV-2-specific CD8 cells (middle) or bystander CD8 cells (right) was done and expression of CLA and ESL plotted. Numbers are percentages of cells in each quadrant. Bottom: similar analyses including PBMC stained for T-cells specific for cytomegalovirus pp65 amino acids 595–603 or Epstein–Barr virus BMLF-1 amino acids 280–288 in the context of HLA A*0201.
Expression of putative skin-homing markers was studied by multi-color flow cytometry. We found that 50–70% of circulating HSV-2-specific CD8 T cells expressed pre-formed CLA (Fig. 1). In contrast, only 5–10% of circulating CMV- or EBV-specific CD8 cells express CLA.14 While CMV is sometimes shed from the cervix and EBV from oral mucosa, neither agent is a typical STI pathogen.
When HSV-2-specific CD8+ lymphocytes in blood are stained for ESL, over-expression of ESL in comparison with bystander CD8 T cells or CD8 T cells specific for CMV (or EBV, not shown) is also detected. Inclusion of EDTA abrogates binding of E-selectin-chimeric protein to T cell (not shown) as expected, as selectin-ligand interactions require calcium ions. Comparing CLA and ESL, only the CLA-bright fraction express ESL. This is similar to the finding of Takahashi et al.32 for PBMC of undefined specificity. Functional binding assays also showed that HSV-specific CD8 T cells bind to E-selectin-expressing cell lines.14 CLA expression is also useful in a one-step enrichment of HSV-2-specific CD8 CTL directly from unmanipulated blood.35 We have no evidence to date that CD103, mentioned above in the context of the corneal and genital epithelia, is overexpressed by circulating. Overall, HSV-2-specific circulate ‘pre-armed’ with an adhesion molecule, ESL/CLA, involved in homing to skin. It remains to be determined how, when, and where this expression is programmed into these resting cells and how durable this phenotype might be in memory in the absence of periodic re-stimulation.
Expression of CLA by Circulating HSV-2-specific CD4 T Cells
Skin biopsy functional27 and immunohistologic36 studies show that CD4 T cells infiltrate recurrent genital HSV-2 lesions prior to CD8 CTL. We therefore predicted that circulating HSV-2-specific CD4 T cells would also highly express CLA and ESL. HLA class II tetramers can identify culture-enriched HSV-2-specific CD4 T cells, but not yet isolate these cells from PBMC, perhaps because of the low frequency of cells reactive with single epitopes.37 We identified circulating HSV-2-specific CD4 T cells by two ex vivo methods: accumulation of intracellular interferon-γ in response to HSV-2 antigen, or upregulation of surface CD40L after stimulation by HSV-2. In both assays, cells were stained on the surface with anti-CD4 and anti-CLA, and CMV stimulation was used as a control for a non-skin-tropic pathogen. These methods yielded similar results, with generally 15–25% of HSV-2-reactive T cells expressing CLA. This was in contrast to 5–10% for non-reactive ‘bystander’ CD4+ lymphocytes, and <2% for CMV-reactive lymphocytes.15 These data are consistent with some programming for HSV-2-specific CD4 T cells to express CLA, but do not explain their faster trafficking to herpetic lesions when compared with CD8 cells.
To further investigate this issue, we exposed PBMC to viral recall antigens in vivo and measured CLA and ESL expression after 5 days. The media conditions used had previously been found not to allow generalized, non-specific CLA expression.38 Many cytokines and small molecules have been found to promote [IL-12, IFN-α, transforming growth factor (TGF)-β], or decrease (IL-4, retinoids) CLA/ESL expression when T cells are stimulated through TCR or a surrogate.6,33 We observed that CD4+ lymphoblasts reactive with whole HSV-2 antigen were highly (40–60%) positive for CLA (Fig. 2), and also positive for ESL, while cells reactive with whole CMV antigen were not.15 These reactive cells also specifically bound to E-selectin-expressing cells. Depletion of all CLA+ cells from the starting population only slightly reduced the expression of CLA by CD4 T cells reacting to HSV-2 antigen, indicating that there is no strict precursor-product lineage relationship between circulating CLA+ cells and CLA+ progeny after exposure to recall antigen. At least two hypotheses can explain these data. In the first, HSV-2-specific memory T cells are somehow programmed to express CLA/ESL after re-stimulation through TCR, regardless of their CLA expression in the resting state. Re-stimulation might occur in draining lymph nodes during recurrent genital herpes, equipping cells to traffic to the skin. The second hypothesis is that innate immunity, stimulated by the presence of HSV, signals T cells reacting to cognate antigen to express adhesion molecules. These hypotheses are under investigation.
Fig. 2.
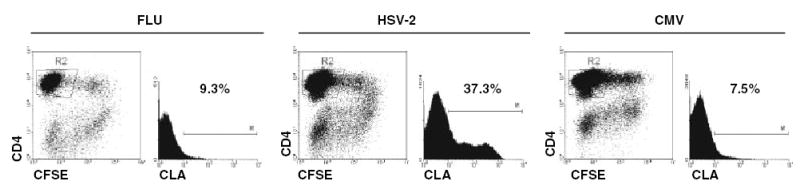
Expression of CLA by virus-reactive CD4 T lymphoblasts after 5 days of in vitro culture. PBMC were labeled with CFSE as described.15 They were cultured with UV-treated HSV-2 whole virus or purified CMV antigen (ABI, Bethesda, MD, USA), or dialyzed 2002–2003 influenza vaccine (Aventis Pasteur, Swiftwater, PA, USA) at 1:1000 for 5 days in human serum-based T-cell medium.27 The level of CLA expression was analyzed on gated CFSE-low (divided), CD4+ cells in the lymphoblast forward/side scatter gate (not shown). HSV-2-reactive cells expressed CLA to a greater extent than did influenza or CMV-reactive cells.
E-selectin is Overexpressed in HSV-2 Genital Herpes Lesions
As discussed in the ‘Introduction’, E-selectin (ELAM, CD62E) is a transmembrane glycoprotein specifically expressed in inflamed skin. It has also been reported to be expressed in the human vagina.39 We processed biopsies for cutaneous recurrent HSV-2 lesions and normal skin for E-selectin and CLA by immunohistochemistry (Fig. 3). Linear structures consistent with vasculature were stained with anti-E-selectin. The intensity of staining was lower in normal skin. Many cells stained with anti-CLA in the herpetic skin, while positive outlines were fewer in normal skin.
Fig. 3.
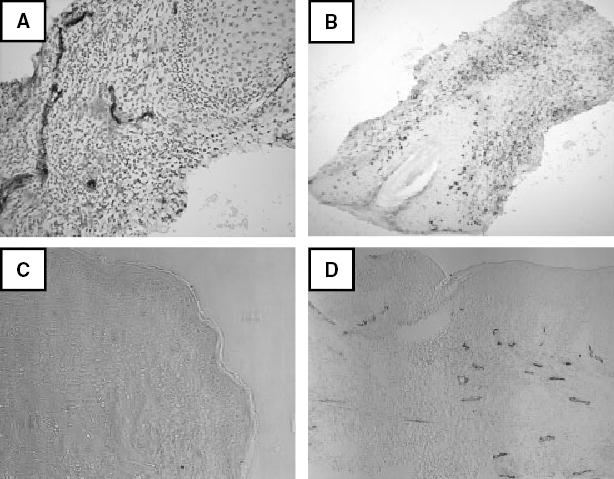
Expression of E-selectin and CLA in frozen sections of human skin. (A) HSV-2 skin lesion stained for E-selectin (brown) and counterstained with hematoxylin. Epidermis/dermis junction at upper right. 10× original magnification. (B) HSV-2 skin lesion stained for CLA. Epidermis at lower left with hair follicle (4× original magnification). Light counterstain with hematoxylin. (C) Normal skin lesion stained for E-selectin with no counterstain. Epidermis at right (4× original magnification). (D) HSV-2 skin lesion prepared similarly. Epidermis at top (43× original magnification).
DISCUSSION
This review has focused on the traditional initial stage of T-cell homing, rolling adhesion. Similar to T cells specific for melanoma antigens,40 CD8 T cells specific for HSV-2 are pre-armed with CLA and functional ESL while circulating in the blood. As forced expression of FUTVII is sufficient to cause CLA expression,33 our data imply that FUTVII and perhaps FUTIV are overexpressed in HSV-2-specific T cells. Experiments based on cell sorting and mRNA expression will hopefully determine if this is the case. CD4 cells may be different, more rarely expressing CLA while circulating in the blood. The regulation of FUT enzymes in these cells is clearly of interest.
The next stage in the multi-step model of T-cell trafficking is tight adhesion. This process is thought to be mediated by lymphocyte integrins that change conformation in response to stimuli received in the vasculature, such as endothelial-bound chemokines.1 Chemokines may also be involved in directed migration of cells through the vessel wall and into tissues. Based on co-expression by circulating CLA+ T cells, CCR4 and CCR10 are candidate skin homing-associated chemokine receptors.41,42 Ligands for these cytokines are variably specifically associated with the skin. These analyses are complicated by the association of some chemokine receptors with specific cytokine expression profiles. Detection of chemokine receptors on cells with a proven, physiologically appropriate ability to home to skin may resolve this issue. Using tetramers, we sought evidence for expression of these chemokine receptors on circulating HSV-2-specific CD8 T-cells. Our preliminary work is negative, but corroboration with another modality, such as mRNA expression, is required. CXCR3, CCR6, and CCR8 have also been implicated in homing to skin,43–46 and measurement of their expression is also underway.
In addition to tropism for skin and genital mucosal, HSV infects neuronal ganglia. In mice, a subtle but definite inflammatory state including T-cell cytokines persists in latently infected ganglia, with some evidence for low-grade HSV protein expression as reviewed by Hendricks et al.47 A striking finding by this group is that HSV-specific CD8 T cells persist long-term in latently infected ganglia.48 Little is known concerning homing mechanisms to neural ganglia or of the turnover and trafficking of these infiltrating T cells, but they seem to be functionally important in the maintenance of latency.49,50 Recent evidence suggests that latently infected human ganglia may also contain inflammatory cells.51
Studies of the GI tract have indicated that homing to epithelia and subepithelial layers may involve different molecules. In this skin, dermal infiltration of lymphocytes is common, while epidermal localization is rare and little is known about differential mechanisms of home. With regards to HSV, infection is strictly localized to the vaginal epithelium in mice after experimental inoculation of progesterone-prepared mice,52 and also limited to the epidermis after antero-grade transport down axons.53 Human immunohistologic data are generally similar, with viral antigen detection in the epidermis only.36 Interestingly, the lymphocyte infiltrate is largely dermal. While CD8 T cells recovered from the skin can kill HSV-infected keratinocytes in vitro,12 it is not clear if the relevant effector functions in vivo involve direct cytotoxicity or action at a distance mediated by cytokines.
Animal models of T cell homing have been very powerful. For example, deficiency of FUTIV and FUTVII eliminates delayed-type hypersensitivity reactions that involve lymphocyte homing to skin.33 It has recently been demonstrated that specific retinoids, preferentially made by gut-derived DC, may ‘imprint’-naïve CD4 T cells during priming to express a gut-homing phenotype (expression of A4B7 integrin), and also to downregulate ESL.6 Similar mechanisms for imprinting for skin homing are currently unknown.
Most models of HSV infection differ in important ways from human infection, restricting fusion of HSV infection and T-cell homing models. Mice do not have spontaneous recurrences, and heat- or stress-induced reactivations from latency are typically only detectable at the ganglionic level, failing to yield peripheral lesions.54 The guinea pig vaginal model has recurrent disease, but immunologic markers are limited. The zosteriform spread model, in which virus spreads acutely to epidermis, via the ganglia, after peripheral inoculation may be useful in future investigations.53
It is not yet clear if T-cell homing characteristics are important for vaccine efficacy, nor how to manipulate programming for homing in a clinically acceptable manner. Retrospective studies comparing intramuscular and oral typhoid vaccination detect differences in B-cell expression of candidate gastrointestinal tract homing markers.55 Mucosal vaccination with a retro-virus leads to expression of A4B7 integrin in resultant vaccine-induced T cells.56 A candidate HSV-2 vaccine, composed of recombinant gD2 protein in ASO4 adjuvant, is administered intramuscularly and would not be ‘expected’ to prime for skin- or genital tract-homing memory CD4 T cells. Despite this, the vaccine is partially effective in preventing HSV-2 infection and disease in HSV-uninfected women.57 Interestingly, a papillomavirus vaccine with clinical activity in preventing infection with genital strains was administered intramuscularly.58
CONCLUSIONS
Memory T lymphocytes may be most effective in response to local episodes of recurrent infection if they are equipped to travel efficiently to sites of antigen load. Evidence has been presented in this review that naturally occurring HSV-2-specific memory CD8 and CD4 T cells preferentially express CLA and functional E-selectin ligand. While these cells do not prevent all recurrences of genital herpes, their infiltration is temporally associated with clearance of infectious virus. HSV-specific lymphocytes are potentially frequently re-exposed to antigen,59 a distinction from some other STI that clear spontaneously or with therapy. It remains an open but important question whether administration of vaccines via specific routes, or with defined adjuvants, may influence the homing phenotype of vaccine-induced T cells and perhaps increase vaccine efficacy for STI and other infections.
Acknowledgments
This work was conducted while Dr González was a Pfizer Postdoctoral Fellow.
Footnotes
Based in part on a presentation at the 23rd Annual Meeting of the American Society for Reproductive Immunology, St Louis, MO, June 2004.
References
- 1.Campbell DJ, Kim CH, Butcher EC. Chemokines in the systemic organization of immunity. Immunol Rev. 2003;195:58–71. doi: 10.1034/j.1600-065x.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 2.Chong BF, Murphy JE, Kupper TS, Fuhlbrigge RC. E-selectin, thymus- and activation-regulated chemokine/CCL17, and intercellular adhesion molecule-1 are constitutively coexpressed in dermal microvessels: a foundation for a cutaneous immunosurveillance system. J Immunol. 2004;172:1575–1581. doi: 10.4049/jimmunol.172.3.1575. [DOI] [PubMed] [Google Scholar]
- 3.Cotran RS, Gimbrone MA, Bevilacqua MP, Mendrick DL, Pober JS. Induction and detection of a human endothelial activation antigen in vivo. J Exp Med. 1986;164:661–666. doi: 10.1084/jem.164.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montgomery KF, Osborn L, Hession C, Tizard R, Goff D, Vassallo C, Tarr PI, Bomsztyk K, Lobb R, Harlan M, Pohlman TH. Activation of endothelial-leukocyte adhesion molecule 1 (ELAM-1) gene transcription. Proc Natl Acad Sci USA. 1991;88:6523–6527. doi: 10.1073/pnas.88.15.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kate C, Song S-Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki H. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salio M, Cella M, Vermi W, Facchetti F, Palmowski MJ, Smith CL, Shepherd D, Colonna M, Cerundolo V. Plasmacytoid dendritic cells prime IFN-gamma-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions. Eur J Immunol. 2003;33:1052–1062. doi: 10.1002/eji.200323676. [DOI] [PubMed] [Google Scholar]
- 9.Koelle DM, Corey L, Burke RL, Eisenberg RJ, Cohen GH, Pichyangkura R, Triezenberg SJ. Antigenic specificity of human CD4+ T cell clones recovered from recurrent genital HSV-2 lesions. J Virol. 1994;68:2803–2810. doi: 10.1128/jvi.68.5.2803-2810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koelle DM, Reymond SN, Chen H, Kwok WW, McC-lurkan C, Gyaltsong T, Petersdorf EW, Rotkis W, Talley AR, Harrison DA. Tegument-specific, virus-reactive CD4 T-cells localize to the cornea in herpes simplex virus interstitial keratitis in humans. J Virol. 2000;74:10930–10938. doi: 10.1128/jvi.74.23.10930-10938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koelle DM, Schomogyi M, McClurkan C, Reymond SN, Chen HB. CD4 T-cell responses to herpes simplex virus type 2 major capsid protein VP5: comparison with responses to tegument and envelope glycoproteins. J Virol. 2000;74:11422–11425. doi: 10.1128/jvi.74.23.11422-11425.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koelle DM, Chen H, Gavin MA, Wald A, Kwok WW, Corey L. CD8 CTL from genital herpes simplex lesions: recognition of viral tegument and immediate early proteins and lysis of infected cutaneous cells. J Immunol. 2001;166:4049–4058. doi: 10.4049/jimmunol.166.6.4049. [DOI] [PubMed] [Google Scholar]
- 13.Koelle DM. Expression cloning for the discovery of viral antigens and epitopes recognized by T-cells. Methods. 2003;29:213–226. doi: 10.1016/s1046-2023(02)00344-4. [DOI] [PubMed] [Google Scholar]
- 14.Koelle DM, Liu Z, McClurkan CM, Topp M, Riddell SR, Pamer EG, Johnson AS, Wald A, Corey L. Expression of cutaneous lymphocyte-associated antigen by CD8+ T-cells specific for a skin-tropic virus. J Clin Invest. 2002;110:537–548. doi: 10.1172/JCI15537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez JC, Kwok WW, Wald A, McClurkan CL, Koelle DM. Programmed expression of cutaenous lymphocyte-associated antigen amongst circulating memory T-cells specific for HSV-2. J Infect Dis. 2004;191:243–254. doi: 10.1086/426944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koelle DM, Frank JM, Johnson ML, Kwok WW. Recognition of herpes simplex virus type 2 tegument proteins by CD4 T cells infiltrating human genital herpes lesions. J Virol. 1998;72:7476–7483. doi: 10.1128/jvi.72.9.7476-7483.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Posavad CM, Koelle DM, Shaughnessy MF, Corey L. Severe genital herpes infections in HIV-infected individuals with impaired HSV-specific CD8+ cytotoxic T lymphocyte responses. Proc Natl Acad Sci USA. 1997;94:10289–10294. doi: 10.1073/pnas.94.19.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Posavad CM, Wald A, Hosken N, Huang M-L, Koelle DM, Corey L. T cell immunity to herpes simplex virus in seronegative persons: silent infection or acquired immunity. J Immunol. 2003;170:4380–4388. doi: 10.4049/jimmunol.170.8.4380. [DOI] [PubMed] [Google Scholar]
- 19.Deshpande SP, Lee S, Zheng M, Song B, Knipe D, Kapp JA, Rouse BT. Herpes simplex virus-induced keratitis: evaluation of the role of molecular mimicry in lesion pathogenesis. J Virol. 2001;75:3077–3088. doi: 10.1128/JVI.75.7.3077-3088.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verjans GMGM, Remeijer L, Mooy CM, Osterhaus ADME. Herpes simplex virus specific T-cells infiltrate the cornea of patients with herpes stromal keratitis: no evidence for intra-corneal autoreactive T-cells in human HSK. Invest Ophthalmol Visual Sci. 2000;41:2607–2612. [PubMed] [Google Scholar]
- 21.Dua HS, Gomes JA, Donoso LA, Laibson PR. The ocular surface as part of the mucosal immune system: conjunctival mucosa-specific lymphocytes in ocular surface pathology. Eye. 1995;9 (Pt 3):261–267. doi: 10.1038/eye.1995.51. [DOI] [PubMed] [Google Scholar]
- 22.Kaul R, Thottingal P, Kimani J, Kiama P, Waigwa CW, Bwayo JJ, Plummer FA, Rowland-Jones SL. Quantitative ex vivo analysis of functional virus-specific CD8 T lymphocytes in the blood and genital tract of HIV-infected women. AIDS. 2003;17:1139–1144. doi: 10.1097/00002030-200305230-00004. [DOI] [PubMed] [Google Scholar]
- 23.Stevceva L, Kelsall B, Nacsa J, Moniuszko M, Hel Z, Tryniszewska E, Franchini G. Cervicovaginal lamina propria lymphocytes: phenotypic characterization and their importance in cytotoxic T-lymphocyte responses to simian immunodeficiency virus SIVmac251. J Virol. 2002;76:9–18. doi: 10.1128/JVI.76.1.9-18.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koelle DM, Abbo H, Peck A, Ziegweid K, Corey L. Direct recovery of HSV-specific T lymphocyte clones from human recurrent HSV-2 lesions. J Infect Dis. 1994;169:956–961. doi: 10.1093/infdis/169.5.956. [DOI] [PubMed] [Google Scholar]
- 25.Addo MM, Yu XG, Rathod A, Cohen D, Eldridge RL, Strick D, Johnston MN, Corcoran C, Wurcel AG, Fit-zpatrick CA, Feeney ME, Rodriguez WR, Basgoz N, Draenert R, Stone DR, Brander C, Goulder PJ, Rosenberg ES, Altfeld M, Walker BD. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J Virol. 2003;77:2081–2092. doi: 10.1128/JVI.77.3.2081-2092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheibenogen C, Romero P, Rivoltini L, Herr W, Schmittel A, Cerottini JC, Woelfel T, Eggermont AM, Keilholz U. Quantitation of antigen-reactive T cells in peripheral blood by IFN gamma ELISPOT assay and chromium release: a four-centre comparative trial. J Immunol Methods. 2000;244:81–89. doi: 10.1016/s0022-1759(00)00257-x. [DOI] [PubMed] [Google Scholar]
- 27.Koelle DM, Posavad CM, Barnum GR, Johnson ML, Frank JM, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest. 1998;101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuchiyama J, Yoshino T, Toba K, Harada N, Nishiuchi R, Akagi T, Furukawa T, Takahashi M, Fuse I, Aizawa Y, Harada M. Induction and characterization of cutaneous lymphocyte antigen on natural killer cells. Br J Haematol. 2002;118:654–662. doi: 10.1046/j.1365-2141.2002.03608.x. [DOI] [PubMed] [Google Scholar]
- 29.Picker LJ, Michie SA, Rott LS, Butcher EC. A unique phenotype of skin-associated lymphocytes in humans. Am J Pathol. 1990;136:1053–1068. [PMC free article] [PubMed] [Google Scholar]
- 30.Walcheck B, Leppanen A, Cummings RD, Knibbs RN, Stoolman LM, Alexander SR, Mattila PE, McEver RP. The monoclonal antibody CHO-131 binds to a core 2 O-glycan terminated with sialyl-Lewis X, which is a functional glycan ligand for P-selectin. Blood. 2002;99:4063–4069. doi: 10.1182/blood-2001-12-0265. [DOI] [PubMed] [Google Scholar]
- 31.Berg EL, Yoshino T, Rott LS, Robinson MK, Warnock RA, Kishimoto TK, Picker LJ, Butcher EC. The cutaneous lymphocyte antigen is a skin homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi R, Mizukawa Y, Yamazaki Y, Hayakawa K, Hayakawa J, Kudo A, Shiohara T. In vitro differentiation from naive to mature E-selectin binding CD4 T cells: acquisition of skin-homing properties occurs independently of cutaneous lymphocyte antigen expression. J Immunol. 2003;171:5769–5777. doi: 10.4049/jimmunol.171.11.5769. [DOI] [PubMed] [Google Scholar]
- 33.Kansas GS. Control of FucT-VII expression in CD4+ T cells. Ernst Schering Research Foundation Workshop, 2004, pp 95–107. [DOI] [PubMed]
- 34.Barry SM, Zisoulis DG, Neal JH, Clipstone NA, Kansas GS. Induction of FucT-VII by the Ras/MAP kinase cascade in Jurkat T cells. Blood 2003. [DOI] [PubMed]
- 35.Koelle DM, Liu Z, McClurkan CL, Cevallos RC, Vieira J, Hosken NA, Meseda CA, Snow DC, Wald A, Corey L. Immunodominance among herpes simplex virus-specific CD8 T-cells expressing a tissue-specific homing receptor. Proc Natl Acad Sci USA. 2003;100:12899–12904. doi: 10.1073/pnas.2131705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunningham AL, Turner RR, Miller AC, Para MF, Merigan TC. Evolution of recurrent herpes simplex lesions: an immunohistologic study. J Clin Invest. 1985;75:226–233. doi: 10.1172/JCI111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwok WW, Liu AW, Novak EJ, Gebe JA, Reymond SN, Ettinger RA, Nepom GT, Koelle DM. HLA-DQ tetramers identify epitope-specific T-cells in peripheral blood of herpes simplex virus-2-infected individuals: direct detection of immunodominant antigen responsive cells. J Immunol. 2000;164:4244–4249. doi: 10.4049/jimmunol.164.8.4244. [DOI] [PubMed] [Google Scholar]
- 38.Armerding D, Kupper TS. Functional cutaneous lymphocyte antigen can be induced in essentially all peripheral blood T lymphocytes. Int Arch Allergy Immunol. 1999;119:212–222. doi: 10.1159/000024197. [DOI] [PubMed] [Google Scholar]
- 39.Johansson EL, Rudin A, Wassen L, Holmgren J. Distribution of lymphocytes and adhesion molecules in human cervix and vagina. Immunology. 1999;96:272–277. doi: 10.1046/j.1365-2567.1999.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Gal FA, Avril MF, Bosq J, Lefebvre P, Deschemin JC, Andrieu M, Dore MX, Guillet JG. Direct evidence to support the role of antigen-specific CD8(+) T cells in melanoma-associated vitiligo. J Invest Dermatol. 2001;117:1464–1470. doi: 10.1046/j.0022-202x.2001.01605.x. [DOI] [PubMed] [Google Scholar]
- 41.Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, Andrew DP, Warnke R, Ruffing N, Kassam N, Wu L, Butcher EC. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 42.Homey B, Wang W, Soto H, Buchanan ME, Wiesenborn A, Catron D, Muller A, Dieu-Nosjean M-C, Orozco R, Ruzicka T, Lehmann P, Oldham E, Zlotnick A. Cutting edge: the orphan chemokine receptor G protein-coupled receptor-2 (GPR-2, CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC) J Immunol. 2000;164:3465–3470. doi: 10.4049/jimmunol.164.7.3465. [DOI] [PubMed] [Google Scholar]
- 43.Fitzhugh DJ, Naik S, Caughman SW, Hwang ST. Cutting edge: C-C chemokine receptor 6 is essential for arrest of a subset of memory T cells on activated dermal microvascular endothelial cells under physiologic flow conditions in vitro. J Immunol. 2000;165:6677–6681. doi: 10.4049/jimmunol.165.12.6677. [DOI] [PubMed] [Google Scholar]
- 44.Tensen VP, Flier J, Van Der Raaij-Helmer EM, Sampat-Sardjoepersad S, Van Der Schors RC, Leurs R, Scheper RJ, Boorsma DM, Willemze R. Human IP-9: A keratinocyte-derived high affinity CXC-chemokine ligand for the IP-10/Mig receptor (CXCR3) J Invest Dermatol. 1999;112:716–722. doi: 10.1046/j.1523-1747.1999.00581.x. [DOI] [PubMed] [Google Scholar]
- 45.Flier J, Boorsma DM, Bruynzeel DP, van Beek PJ, Stoof TJ, Scheper RJ, Willemze R, Tensen CP. The CXCR3 activating chemokines IP-10, Mig, and IP-9 are expressed in allergic but not irritant patch test reactions. J Invest Dermatol. 1999;113:574–578. doi: 10.1046/j.1523-1747.1999.00730.x. [DOI] [PubMed] [Google Scholar]
- 46.Schaerli P, Ebert L, Willimann K, Blaser A, Roos RS, Loetscher P, Moser B. A skin-selective homing mechanism for human immune surveillance T cells. J Exp Med. 2004;199:1265–1275. doi: 10.1084/jem.20032177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khanna KM, Lepisto AJ, Hendricks RL. Immunity to latent viral infection: many skirmishes but few fatalities. Trends Immunol. 2004;25:230–234. doi: 10.1016/j.it.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8(+) T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu T, Khanna KM, Carriere BN, Hendricks RL. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J Virol. 2001;75:11178–11184. doi: 10.1128/JVI.75.22.11178-11184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Theil D, Derfuss T, Paripovic I, Herberger S, Meinl E, Schueler O, Strupp M, Arbusow V, Brandt T. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am J Pathol. 2003;163:2179–2184. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 54.Sawtell NM. Quantitative analysis of herpes simplex virus reactivation in vivo demonstrates that reactivation in the nervous system is not inhibited at early times postinoculation. J Virol. 2003;77:4127–4138. doi: 10.1128/JVI.77.7.4127-4138.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kantele A, Kantele JM, Savilahti E, Westerholm M, Arvilommi H, Lazarovits A, Butcher EC, Makela PH. Homing potentials of circulating lymphocytes in humans depend on the site of activation: oral, but not parenteral, typhoid vaccination induces circulating antibody-secreting cells that all bear homing receptors directing them to the gut. J Immunol. 1997;158:574–579. [PubMed] [Google Scholar]
- 56.Evans DT, Chen LM, Gillis J, Lin KC, Harty B, Mazzara GP, Donis RO, Mansfield KG, Lifson JD, Desrosiers RC, Galan JE, Johnson RP. Mucosal priming of simian immunodeficiency virus-specific cytotoxic T-lymphocyte responses in rhesus macaques by the Salmonella type III secretion antigen delivery system. J Virol. 2003;77:2400–2409. doi: 10.1128/JVI.77.4.2400-2409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stanberry LR, Spruance S, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denes M, Vandepapeliere P, Dubin G. Prophylactic vaccination against genital herpes with adjuvanted recombinant glycoprotein D vaccine: two randomized controlled trials. N Engl J Med. 2002;347:1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 58.Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, Chiacchierini LM, Jansen KU. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 59.Wald A, Zeh J, Selke S, Warren T, Ryncarz A, Ashley R, Krieger JN, Corey L. Reactivation of genital herpes type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342:844–850. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]


