Abstract
Objective
Several studies have evaluated the relationship between physical activity and lung cancer. To summarize and review these studies, we conducted a meta-analysis of all relevant reports published from 1966 through October 2003.
Method
Adjusted odds ratios (ORs) from the original studies were pooled by the inverse of their variance, and all pooled estimates were accompanied by an assessment of heterogeneity across investigations. Test for linear trend across activity categories (low, moderate, high) were applied.
Results
The combinedORs were 0.87 (95%confidence interval = 0.79–0.95) for moderate leisure-time physical activity (LPA) and 0.70 (0.62–0.79) for high activity (p trend = 0.00). This inverse association occurred for both sexes, although it was somewhat stronger for women. No evidence of publication bias was found. Several studies were able to adjust for smoking, but none adjusted for possible confounding from previous malignant respiratory disease. Our simulations suggest that this condition is unlikely to entirely explain the inverse association.
Conclusion
The findings of this meta-analysis indicate that higher levels of LPAprotect against lung cancer. The inverse association is possible remains confounded by inadequately controlled smoking patterns. However on the whole, confounding seems an unlikely explanation for the findings of individual studies on non-smokers.
Keywords: leisure physical activity, lung cancer risk, meta-analysis
Introduction
Physical activity is a behavior defined as bodily movement produced by skeletal muscles resulting in a quantifiable level of energy expenditure. It is associated with daily living, work and leisure-time activities. Leisure-time physical activity (LPA) is often characterized by short-term, intensive energy expenditure, while occupational physical activity is more likely to occur over longer periods of time (e.g., hours) at lower rates of energy expenditure [1]. Recent reviews on the association between some types of physical activity and cancer [2, 3] have indicated the need for careful assessment of the possible preventive role of physical activity in the etiology of lung cancer across different assessment methods. The meta-analysis is a systematic identification, appraisal, synthesis, and statistical aggregation of all relevant prior studies using procedures that limit bias and random error.
Both occupational activity (OPA) and LPA have been evaluated vis-à-vis risk of lung cancer. The association of OPA and lung cancer has been developed in five studies [4–8]; one of them was later updated [4, 7]. Job title has typically served as a surrogate for occupational physical activity. Considerable variability exists in the type of activities and level of energy expenditure performed by employees within the same job title classification and similar job duties. Given the limited amount of data available the evidence for an association is inconclusive.
Studies that have evaluated the relationship between LPA and lung cancer have not proved entirely consistent. Lower lung cancer rates associated with high or moderate levels of LPA have been reported in five populations, including Norwegian men [8], US men and women [9, 10], Czech women [11] and Canadian men and women [12]. Other studies yielded inconsistent results [4, 13, 14] and one study failed to find an association [15].
Since occupational activity is tending to decrease for most people in developed societies, with leisure time and recreational activities becoming a greater component of overall activity, it is likely that occupational activity is becoming a less sensitive discriminator of risk [16]. Because LPA represents a powerful public health measure for reducing lung cancer, a clear understanding of the role of LPA in the etiology of lung cancer could have a major impact on public health. To address this issue we performed a systematic review of the literature and meta-analysis of published to assess the quantitative evidence that higher LPA might be associated with a reduced risk of lung cancer and to provide estimates of the proportion of lung cancer that might be preventable in sedentary people through enhanced LPA if the association appeared plausible.
Material and methods
Identification of study subjects
We conducted MEDLINE (1966 to October 2003) and EMBASE (1974 to October 2003) searches using the following terms: physical activity, exercise and lung cancer risk (PhALCR); and physical activity, exercise and cancer risk (PhACR). All cohort or case-control studies evaluating leisure-time physical activity as a risk factor for lung cancer incidence or mortality were initially selected. For inclusion, we required that relative risks be adjusted for the most important risk factor for lung cancer; i.e., smoking. Where several publications emanated from any given study, we selected the one with the longest follow-up and largest sample size. We contacted the authors of one study [10] for clarification of some data in the most recent report [17]. Our literature search was not language restricted. The PhALCR search yielded 27 and the PhACR search 648 potentially relevant papers: after reading the abstract of each, 27 and 67 papers were then respectively selected, based on inclusion criteria for closer examination. Reference lists cited in each of the selected papers were likewise examined. This additional search uncovered one ecological study [18], one cross-sectional study [19], three studies dealing only with occupational physical activity [5–7], and 13 reports of cohort or case-control studies analyzing LPA and lung cancer risk [4, 8–15, 20–23]. We excluded one case-control study because its relative risks were not adjusted for smoking [20], and three reports of cohort studies [21–23] because they preceded a more recent report [9].
After a full review of all eligible papers, nine met the inclusion criteria (LPA cohort or case-control studies adjusted for smoking), namely, two case-control and seven cohort studies (Table 1). Most studies measured three components of physical activity (i.e., type, duration, and/or intensity), and all used questionnaires to obtain information on LPA. Four questionnaires were self-administered [8–10, 12] and the remainder were based on in-person interviews [4, 11, 13–15]. Three of the LPA questionnaires had been evaluated for validity: these were the Norwegian Health Study [8], the Harvard Alumni Study [9], and the British Regional Heart Study [14]. Duration of LPA – amount of activity time per 24 hours – was classified into four strata according to “usual” activity [4, 10, 13, 14], activity during the last 10 years [11], or activity during the last year [8, 9, 12, 15]. Six studies [4, 10, 11, 13–15] classified LPA into three levels of intensity, i.e., low (sedentary, low, or inactive), moderate (moderate, medium, or moderately vigorous) and high (much exercise, high, vigorous, or heavy), while the remaining three [8, 9, 12] used four intensity categories, consisting of the three mentioned above plus an additional ‘very high’ category. Two of these latter studies published their results on three levels; the exception was the Harvard Alumni Study [9], and the Canadian cancer study [12], which also reported an analysis for the ‘very high’ group. For this meta-analysis we combined the high and very high categories, and thus classified LPA as low, moderate or high, with low activity used as the reference category. In our opinion, the intensities of the levels for classifying exposure were comparable across the studies.
Table 1.
Study summary of leisure time physical activity and lung cancer included in the meta-analysis
| Author, location | Design N | Type of measurement | Exposure level & RR (95% CI) | Adjustment |
|---|---|---|---|---|
| Prospective Cohort Studies | ||||
| Severson RK et al., 1989 (Honolulu heart study) | Cohort 8066
Men 194 cases |
Interview Questionnaire 65–68/86
Usual time per 24 h Basal, sleeping/lying down (34.4%) Sedentary, sitting/standing (39.06%) Moderate (gardening/carpentry)/Heavy (shoveling/digging) (26.56%) |
Reference Low 1st
2nd = 1.06(0.76–1.48) 3rd = 0.70(0.48–1.01) p = 0.039 |
Age
BMI Smoking |
| Albanes D et al., 1989 (NHANESa) | Cohort 5138
Men 114 cases |
Interview Questionnaire 71–75/82–84;
Much exercise (23.2%) Reference Moderate exercise (39.4%) Little or no exercise (37.4%) |
Much exercise RR = 1.00
Moderate exercise RR = 1.0(0.6–1.6) Little or no exercise RR = 0.9(0.6–1.5) p = 0.80 |
Age
BMI Smoking Race Energy intake |
| Sellers TA et al., 1991 | Cohort 41,837 | Mailing Questionnaires 86–89 | Reference Low/moderate | Age |
| Petersen et al., 2001 (IWHSb) | Women 565 cases | Random controls
Low Medium High |
OR = 1.00
Medium: 0.82(0.67–1.00) High: OR = 0.67(0.53–0.84) p < 0.001* |
BMI
Smoking Education Wais/circ |
| Thune and Lund 1997
Norway (Health survey cardiovascular diseases) |
Cohort 81,516
Men 413 cases Women 51 cases |
Mailing VALIDATED Questionnaires 72–78
Last year LPA. R1: reading, watching TV other sedentary R2: walking, bicycling/physical activities 4 h a week (M19.84%–F22.14%) R3: exercise to keep fit, 4 h a week (M54.61%–F66.73%) R4: regular hard training or participation in competitive sports several times a week (M25.22%–F9.85%) |
Men; Reference Low 1.00
Moderate 0.75(0.60–0.94) Regular training R3+R4 0.71(0.52–0.97) p = 0.01* Women Reference Low 1.00 Moderate 0.91(0.48–1.71) Regular training R3+R4 0.99(0.35–2.78) p = 0.88 |
Age
BMI Smoking Geog area |
| Lee IM et al., 1999
USA Harvard alumni |
Cohort 13,905
Men 245 cases |
Mailing VALIDATED questionnaires 1977–93
Last year LPA. Levels for kJ/wk. <4200 (32.2% men) (n = 4476) 4200–8399 (28.4% men) (n = 3946) 8400–12599 (18.1%) (n = 2513) ≥12600 Kj/week (21.4%) (n = 2970) |
Reference Low
−4200–8399− RR = 0.87 (0.64–1.18) −8400–12599 −RR = 0.76 (0.52–1.11) −≥ 12600 −RR = 0.61 (0.41–0.89) p-trend = 0.008* |
Age
BMI Smoking Current walking Stair climbing |
| Wannamethee SG et al., 2001
UK BRHSc |
Cohort 7735
Men 265 cases |
Nurse-administer VALIDATED Quest78/80/97
Life Usual Pattern of LPA Inactive/Moderate: cycling recreational activities, regular walking/sport 1 a week (78.57%) Moderately/vigorous: sporting activity 1 a week/frequent cycling, frequent activity/walking/frequent sport (14.67%) Vigorous: very frequent sporting/plus other (6.76%) |
Reference Low
RR = 1.00 Moderately-vigorous RR = 0.77(0.49–1.21) Vigorous RR = 0.76 (0.40–1.43) p = 0.19 |
Age
BMI Smoking Alcohol Social class |
| Colbert L et al., 2002
Finland ATBCd Study |
Cohort 27,082
Men 1441 cases |
Nurse administered questionnaire85/88/93
Last year LPA Sedentary: reading, watching TV (41.52%) Moderate: walking, hunting, gardening fairly regularly+Heavy: running, skiing, swimming fairly regularly (58.48%) |
Reference Low:
RR = 1.00 Moderate+Heavy: Active RR = 0.97 (0.87–1.07) |
Age
BMI Smoking Education Supplement Energy intake Veg intake |
| Hospital-based case-control study | ||||
| Kubik et al., 2002
Czechoslovakia |
Case-control
Women 269 cases |
In-person interviews
Last 10 years LPA Physical exercise (hours/week) 0 h (43.9%) 1–5 h: (26%) >5 h: (30%) |
Reference Low level:
R = 1.00 Moderate: 0.62(0.42–0.92) High active: 0.42 (0.29–0.62) p < 0.001* |
Age
Smoking Education Residence |
| Population-based case-control study | ||||
| Mao et al., 2003
Canada |
Case-control Men 1131 cases Women 997 cases |
Mailing questionnaire 94/97
Last year LPA Physical activity (Specific metabolic equivalent MET) Moderate MET ≥3 to ≤6: (%) Vigorous MET >6: (%) Total (Moderate plus Vigorous): (%) |
Reference Low level:
OR = 1.00 Men Moderate: 0.91 (0.71–1.17) High active: 0.79 (0, 61–1, 04) Women Moderate: 0.73 (0.55–0.98) High active: 0.69 (0.51–0.93) |
Age
BMI Alcohol Smoking ETS Occupation Education Residence Energy intake Veg intake |
National Health and Nutrition Examination Survey.
(IWHS) Iowa Women’s Health Study.
British Regional Heart Study.
Alpha-Tocopherol Beta-Carotene Cancer Prevention Study, ETS Exposure tobacco smoke.
p-trend significant.
Statistical analysis
Smoking-adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for lung cancer by LPA were extracted from the published studies. For the one study that took high LPA as the referent [4], we recalculated the OR – with low LPA as the reference category, using conventional procedures [24]. Due to a priori concerns regarding heterogeneity both in the study populations and in the methods used to assess physical activity, we decided that DerSimonian and Laird’s random-effects method for calculating summary statistics (1986) would be more appropriate than the fixed-effects model. The Q statistic, a test of homogeneity between studies, was calculated for the meta-analyses performed. Gender-based subgroup analyses were also performed. To test for linear trend we applied the Cuzick procedure [25]. Furthermore, we assessed potential publication bias by examining funnel plots [26], using Egger’s test [27]. All statistical analyses were performed using the Stata computer software program (Version 7.0.) Using meta-analysis OR, the LPA – preventable fraction of lung cancer cases was calculated as the number of cases in the “low” category that could be prevented if activity were increased to the level of the “moderate” or “high” categories [24]. The preventable fraction refers the proportion of cases that could theoretically be avoided if sedentary people had actually been more active.
Results
Of the studies that assessed the relationship between leisure-time physical activity and lung cancer risk, four were undertaken in the USA (National Health and Nutrition Examination Survey [4], Hawaii Honolulu Heart Study [13], Iowa Women’s Health Study (IWHS) [10] and the Harvard Alumni Study [9]), four in Europe (Norway Health Study of Cardiovascular Disease [8], British Regional Heart Study [14], a case-control study of Czech women [11], and the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study in Finland [15]) and one in Canada (Canadian National Enhanced Cancer Surveillance System (NECSS) [12]), (Table 1). Two studies reported results for men and women separately [8, 12], whereas two exclusively reported data for women [10, 11]. Risk of lung cancer was inversely associated with LPA in almost every study in Table 1, but most ORs were not statistically significant. Six studies [8–13] reported statistically significant ORs: three for women (the Norwegian Health Study, the IWHS and the Canadian NECS System) and two for men (the Norwegian Health Study and the Harvard Alumni Study).
Results from the meta-analysis (Figure 1) show and support an inverse relationship between LPA and lung cancer risk. The estimated combined risk for both sexes, was statistically significant, without heterogeneity (Table 2), and protective for both moderate LPA (OR = 0.87, 95% CI = 0.79–0.95; Q = 12.33, p = 0.16) and high LPA (OR = 0.70, 95% CI = 0.62–0.79; Q = 12.26, p = 0.61). The inverse dose-response relationship was statistically significant (p for trend = 0.00) and there was no evidence of publication bias (p = 0.50). This inverse pattern occurred for both men and women with significant reductions for the high LPA categories (OR for men = 0.75, 95% CI = 0.66–0.86; Q = 3.68, p = 0.39; and OR for women = 0.62, 95% CI = 0.48–0.79; Q = 5.73, p = 0.99). Somewhat greater protection, however, was suggested for women than for men, and non-significant results were obtained for moderate LPA among men.
Fig. 1.
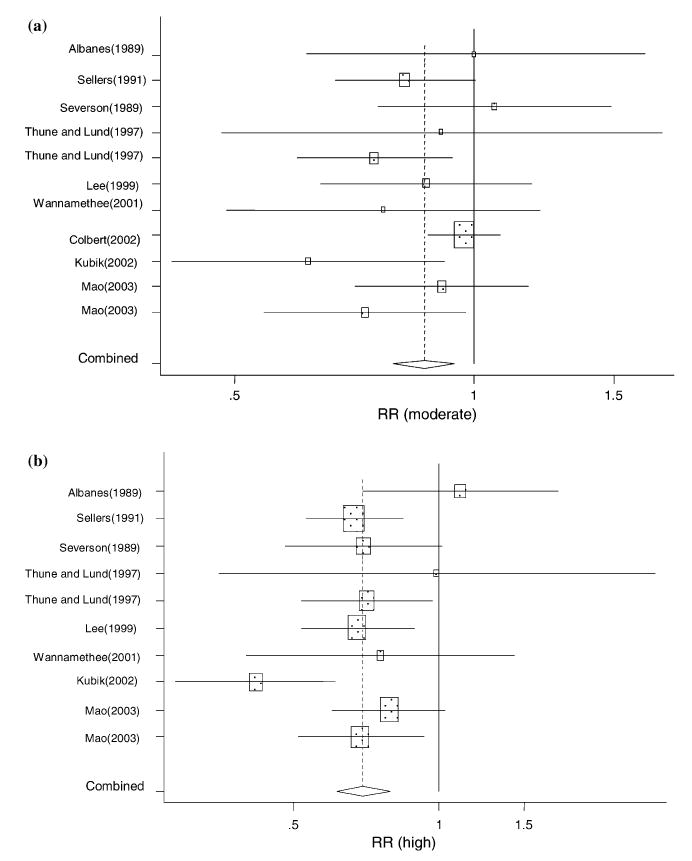
Relative risk of lung cancer for high LPA (versus low LPA) for individual cohort and case-control studies and on aggregate.
Table 2.
Results of meta-analysis of physical activity and lung cancer
| Activity level† | Total (11 studies)
|
Men (7 studies)
|
Women (4 studies)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | Q | Pub Bias* | OR Bias* | 95% CI | Q | Pub | OR | 95% CI | Q | Pub Bias* | |
| Moderate | 0.87 | 0.79–0.95 | 12.33 | 0.16 | 0.93 | 0.85–1.00 | 5.71 | 0.44 | 0.77 | 0.66–0.89 | 1.95 | 0.73 |
| High | 0.70 | 0.62–0.79 | 12.26 | 0.61 | 0.75 | 0.66–0.86 | 3.68 | 0.39 | 0.62 | 0.48–0.79 | 5.73 | 0.99 |
| Trend |
0.00 |
0.01 | 0.01 | |||||||||
| Results of meta-analysis of physical activity and lung cancer. Validated questionnaires studies | ||||||||||||
| Activity level† | Total (4 studies)
|
Men (3 studies)
|
||||||||||
| OR | 95% CI | Q | Pub Bias* | OR | 95% CI | Q | Pub Bias* | |||||
| Moderate | 0.79 | 0.68–0.93 | 0.78 | 0.44 | 0.79 | 0.66–0.93 | 0.60 | 0.78 | ||||
| High | 0.70 | 0.58–0.85 | 0.54 | 0.05 | 0.70 | 0.57–0.85 | 0.12 | 0.29 | ||||
p-value of test for publication bias (Egger et al., 1997).
Reference category: low level of physical activity.
Meta-analysis restricted to studies on men using previously published [8, 9, 14], validated questionnaires modified the results minimally, and showed a consistent inverse association – the pooled estimates were statistically significant – with very high homogeneity, and protective effects for both moderate (OR = 0.79, 95% CI = 0.66–0.93; Q = 0.598, p = 0.78) and high LPA (OR = 0.70, 95% CI = 0.57–0.85; Q = 0.116, p = 0.29) (Table 2).
The exposed estimated preventive fractions of lung cancer [24] (for women and men exposed combined) on the basis of the meta-analysis results were 0.13 and 0.30 for moderate and high LPA, respectively. The preventable fractions were somewhat larger for women than for men. Among exposed women, 38% of cases in the “low” category could be prevented by high LPA if those folk had actually been more active versus 25% among men. Stronger risk decreases were associated with total energy expended.
Discussion
This is the first meta-analysis performed on LPA and lung cancer at the literature. This meta-analysis of the association between lung cancer and leisure-time physical activity points to a reduced risk of lung cancer among more physically active individuals. The reduction affects men and women alike, is greater for high LPA (i.e., much, vigorous, or heavy exercise) than for moderate LPA (i.e., moderate or medium levels), and displays a significant dose-response relationship. The trend is somewhat stronger in women, but the gender difference is small and could be due to chance. Restricting the analysis to studies targeted men and used validated LPA questionnaires yielded a similar significant inverse association with very high homogeneity.
The use of meta-analysis as a tool for review and interpretation of epidemiological studies has grown in recent years. Nevertheless, use of this technique is not without controversy [28]. For example, by combining results from studies conducted with different methods, in different populations and at different times, such an approach may ignore the existence of true heterogeneity, which would require proper interpretation of detailed findings rather than summary estimates of the combined results. On the other hand, even conventional systematic reviews not employing some form of quantitative meta-analysis do combine, interpret, and summarize results from the different investigations, but in a manner that may be somewhat less clear and possibly less objective than a formal meta-analysis. Heterogeneity was not evident in the present analysis, as indicated by the Q statistics.
To determine the relationship between LPA and lung cancer, it is necessary to obtain valid and reproducible measurements. This can be difficult, since LPA is a very complex behavior that can be conducted and measured in many ways. Questionnaires and interviews used in surveys remain the most frequently used methods for assessment of LPA in epidemiological studies [16, 29]. In this meta-analysis, all assessments of LPA were self-administered or interview-based questionnaires, and three had been previously validated.
There is some concern that recall of LPA from earlier periods of time might be more accurate for strenuous than for light or moderately intense activity [16]. If so, some or the entire dose-response pattern could be due to differences in recall accuracy as regards time and level. This possible bias, however, would not explain the significant reduction in lung cancer risk for the high-activity group. Other methodological considerations include diagnosis-related recall bias and uncontrolled confounding. The inverse association observed between LPA and lung cancer in several prospective studies where data for LPA had been obtained several years prior to diagnosis of lung cancer, argues against disease-related recall bias as a likely explanation for the aggregate of the results.
All studies adjusted for important confounding factors such as age, education and tobacco smoking and all but one adjusted for body mass index [11]. One study adjusted for occupational exposure [12]. Some studies also adjusted for race, education level, energy intake, vegetable intake, residential exposure, cholesterol, triglyceride, alcohol and social class (Table 1). The single strongest predictor for lung cancer is cigarette smoking and the biggest concern regarding and observed relationship between LPA and lung cancer is the possible relationship that it could be effect modification or residual confounding from smoking specially the role of past smoking.
An apparent inverse lifestyle – lung cancer association was disappeared after smoking control [30]. To address this possibility, we carried out an in-depth examination into the way in which each study had controlled for tobacco use. All studies selected adjusted for smoking in terms of the amount of tobacco use (pack-years, or number of daily cigarettes and numbers of years smoked), and five studies [8, 9, 12, 15, 17] stratified current smokers by the number of cigarettes smoked. The Harvard University Alumni [9] study reported that highly physically active men, whether they were non-smokers (n = 83%), current smokers less than 20 cigarettes/day (n = 8%) or current smokers more than 20 cigarettes/day (n = 8%) had a lower risk of lung cancer than those who were less active. In a previous study [23] they examined only non smokers (n = 9457) and found a highly significant, inverse relation between activity level and lung cancer those who never smoked (OR = 0.47, 95% CI = 0.27–0.82). The Canadian study (12) found non-significant inverse associations for persons who never smoked (n = 1257; OR = 0.68, 95% CI = 0.39–1.19) and for ex-smokers (n = 2061; OR = 0.75, 95% CI = 0.62–1.13).
No one study adjusted for time since quitting, the depth of inhalation, or the choice of cigarette brand. One study adjusted for residential and occupational passive smoking. Residual confounding attributable to smoking could still exist and the inverse association remains confounded by inadequately measured smoking patterns, but on the whole, confounding seems an unlikely explanation for the findings of individual studies on non-smokers.
There is sometimes the perception that smokers cannot be physically active. This must occur if studies like these are to be informative. The Canadian study [12] reported that 16% of current smokers were in the highly physically active category (OR = 0.70, 95% CI = 0.51–0.96) and the Norwegian study [8] reported 11% of current smokers were highly physically active (OR = 0.59, 95% CI = 0.35–0.97) This study performed a stratified analysis on current smokers (n = 254) according to the number of cigarettes smoked, and found reduced risk of lung cancer from more physically active individuals among those who smoked fewer (n = 167) and those who smoked more than 15 cigarettes per day (n = 187) (OR = 0.79, 95% CI = 0.49–1.26 and OR = 0.59, 95% CI = 0.35–0.97). The Harvard University Alumni [9] reported a 7.5% of heavy smokers (>20 cigarettes/day) being highly physically active.
On the other hand, adenocarcinomas have always accounted for most lung cancers among non-smokers of both genders, and it has increased, as a proportion of all lung cancer, with increasing duration of smoking cessation [31]. Hence, if the inverse association was indeed attributable to residual confounding due to tobacco smoking, a stronger inverse association would be expected with squamous cell carcinoma (the histological subtype most strongly related to cigarette smoking) than with other histological subtypes. However, in the Norwegian study [8], inverse associations were noted for adeno-and small cell carcinoma, but not for squamous cell carcinoma. Mao et al. [12] found a greater protective effect for small cell carcinoma among men, but for squamous and small cell carcinoma among women. Protective effects observed for adeno-and small cell carcinoma might suggest that the lower risk of lung cancer among those more physically active was not simply a residual smoking-related effect, although some residual confounding is a possibility. Since most recent studies show quite similar smoking effects across all histologies the issue of confounding by smoking could be most effectively addressed in studies among never smokers, but low rates of lung cancer in this population makes such studies dificult [32].
Recently, studies have reported a possible elevated lung cancer risk associated with a history of previous lung disease (PLD), such as emphysema, with effect estimates ranging from 1.8–2.7 [33, 34]). Since individuals with lung disease may restrict their physical activity, this could confound the physical activity – lung cancer association. Unfortunately no study included in the meta-analysis controlled for PLD and could confound our lung cancer – LPA relationship. To assess this possibility we evaluated potential bias from PLD using the risk and prevalence information in earlier reports on lung cancer and PLD. We used the physical activity distribution seen in the studies in our meta-analysis, i.e., 35% low, 40% moderate and 25% high, in these calculations. We used ORs for lung cancer from PLD of between 1.8 and 2.7 and prevalence of PLD between 5 and 20% [32] in various hypothetical distributions of the study population. Estimated odds ratios for lung cancer did not change, except for situations with very high prevalence of PLD and low prevalence of physical activity. We believe PLD confounding is unlikely to entirely account for the observed inverse association between level of leisure time physical activity and risk of lung cancer because the US Health Interview Survey (HIS) estimates that approximately 6% of the population reports chronic obstructive pulmonary disease [35] and this has been relatively stable since 1980. Estimates of 4–7% have been made for the prevalence of asthma and chronic bronchitis have approximately [36] and these are considerably lower than the 20% prevalence by our calculations, which would be required to entirely account for our lung cancer – physical activity association.
Fruit and vegetable intake have consistently been associated with a protection of the lung cancer risk and a healthy diet maybe associated with physical activity. This has been seen in the Canadian study [12]. Two studies [12, 15] did adjust for possible confounding from diet, but there could still be residual confounding or effect modification from diet in our meta-analysis risk estimates.
Other possible uncontrolled confounding factors for this relationship are high exposure to outdoor and indoor air pollution, occupational exposures, family history of cancer, and history of radiotherapy [37] despite however that any of these factors would be strongly associated with LPA, which is an essential requirement for confounding to occur, there may still be residual confounding from these factors in our risk estimates.
There are several hypothesized underlying biological mechanisms for physical activity in cancer etiology. Exercise could affect cancer development through its impact on growth factors, such as IGFs and their binding proteins (IGFBPs) [2]. High levels of circulating IGF-I were associated with an increased risk of lung cancer and high levels of IGFBP-3 with a decreased risk [12]. Exercise significantly lowers insulin, glucose, triglycerides, and raises HDL cholesterol, which may also be associated with decreased cancer risk [2]. Another possibility is through the effect of exercise on the immune system. Immune function is enhanced with long-term exercise through increases in the number and activity of macrophages, natural killer cells and lymphokine-activated killer cells and their regulated cytokines, and increased mitogen-induced lymphocyte proliferation rates [2, 38–40]. It is also possible that the increased pulmonary ventilation and perfusion from physical activity is involved. Several studies have found that airway obstruction increases lung cancer risk, so that, even after taking cigarette smoking into account [39], increased pulmonary function following high levels of physical activity could result in decreased opportunity for airway exposure to inhaled carcinogens [8].
In conclusion, the epidemiological evidence showed that moderate and higher levels of LPA protect against lung cancer in men and women. The present data suggest that, through engaging in high levels of LPA, 25–38% of lung cancers in sedentary (exposed) men and women, respectively, could be prevented. It is possible that the inverse association remains confounded by inadequately controlled smoking patterns. However on the whole, confounding seems an unlikely explanation for the findings of individual studies on non-smokers. Leisure time physical activity come forward a useful and practical preventive measure and may well therefore represent a promising strategy for prevention of lung cancer for smokers and non-smokers. This is an important issue given the societal burden of lung cancer. However, further studies, particularly with carefully defined leisure time activity and biomarkers, such as IGFs and immune markers, are needed to clarify the mechanisms involved.
Acknowledgments
This work was partially funded by the Spanish Ministry of Education, Culture & Sport, (grant PR2001-0207).
The authors would like to express their appreciation for the helpful suggestions made by Dr. Paolo Boffetta of the International Agency for Research on Cancer.
References
- 1.Kohl HW, LaPorte RE, Blair SN. Physical activity and cancer. An epidemiological perspective. Sports Med. 1988;6:222–237. doi: 10.2165/00007256-198806040-00004. [DOI] [PubMed] [Google Scholar]
- 2.Friedenreich CM. Physical activity and cancer prevention: from observacional to intervention research. Cancer Epidemiol Biomarkers Prev. 2001;10(4):287–301. [PubMed] [Google Scholar]
- 3.Thune I, Furberg AS. Physical activity and cancer risk: doseresponse and cancer, all sites and site specific. Med Sci Sports Exerc. 2001;33(Suppl 6):S530–S550. doi: 10.1097/00005768-200106001-00025. [DOI] [PubMed] [Google Scholar]
- 4.Albanes D, Blair A, Taylor PR. Physical activity and risk of cancer in the NHANES I population. Am J Public Health. 1989;79(6):744–750. doi: 10.2105/ajph.79.6.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dosemeci M, Hayes RB, Vetter R, et al. Occupational physical activity, socio-economic status, and risks of 15 cancer sites in Turkey. Cancer Causes Control. 1993;4:313–321. doi: 10.1007/BF00051333. [DOI] [PubMed] [Google Scholar]
- 6.Browson RC, Chang JC, Davis JR, Smith CA. Physical activity on the job and cancer in Missouri. Am J Public Health. 1991;81(5):639–642. doi: 10.2105/ajph.81.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steenland K, Nowlin S, Palu S. Cancer incidence in the National Health and Nutrition Survey I. Follow-up data: diabetes, cholesterol, pulse, and physical activity. Cancer Epidemiol Biomarkers Prev. 1995;4(8):807–811. [PubMed] [Google Scholar]
- 8.Thune I, Lund E. The influence of physical activity on lungcancer risk. A prospective study of 81,516 men and women. Int J Cancer. 1997;70:57–62. doi: 10.1002/(sici)1097-0215(19970106)70:1<57::aid-ijc9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Lee IM, Sesso HD, Paffenbarger RS., Jr Physical activity and risk of lung cancer. Int J Epidemiol. 1999;28(4):620–625. doi: 10.1093/ije/28.4.620. [DOI] [PubMed] [Google Scholar]
- 10.Sellers TA, Potter JD, Folsom AR. Association of incident lung cancer with family history of female reproductive cancers: the Iowa Women’s Health Study. Genet Epidemiol. 1991;8(3):199–208. doi: 10.1002/gepi.1370080306. [DOI] [PubMed] [Google Scholar]
- 11.Kubik AK, Zatloukal P, Tomasek L, Petruzelka L. Lung cancer risk among Czech women: a case-control study. Prev Med. 2002;34(4):436–444. doi: 10.1006/pmed.2001.1002. [DOI] [PubMed] [Google Scholar]
- 12.Mao Y, Pan S, Wen SW, Johnson KC The Canadian Cancer. Physical activity and the risk of lung cancer in Canada. Am J Epidemiol. 2003;158(6):564–575. doi: 10.1093/aje/kwg186. [DOI] [PubMed] [Google Scholar]
- 13.Severson RK, Nomura AM, Grove JS, Stemmermann GN. A prospective analysis of physical activity and lung cancer. Am J Epidemiol. 1989;130(3):522–529. doi: 10.1093/oxfordjournals.aje.a115366. [DOI] [PubMed] [Google Scholar]
- 14.Wannamethee SG, Shaper AG, Walker M. Physical activity and risk of cancer in middle-aged men. Br J Cancer. 2001;85(9):1311–1316. doi: 10.1054/bjoc.2001.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colbert LH, Hartman TJ, Tangrea JA, et al. Physical activity and lung cancer risk in male smokers. Int J Cancer. 2002;98(5):770–773. doi: 10.1002/ijc.10156. [DOI] [PubMed] [Google Scholar]
- 16.IARC (2002) Weight Control and Physical Activity, Vol. 6. IARC Handbook of Cancer Prevention. IARC Press.
- 17.Petersen GM, Schmitz K, Cerhan J, Vierkant R, Yang P, Sellers T, Physical activity among smokers is inversely associated with lung cancer incidence in the Iowa women’s health study (IWHS). AACR 92nd Annual meeting. March 24–28, 2001. Ernest N. Morial Convention Center. New Orleans, LA, USA.
- 18.Garfinkel L, Stellman SD. Mortality by relative weight and exercise. Cancer. 1988;62:1844–1850. doi: 10.1002/1097-0142(19881015)62:1+<1844::aid-cncr2820621328>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.Frisch RE, Wyshak G, Albright NL, Albright TE, Schiff I. Lower prevalence of non-reproductive system cancers among female former college athletes. Med Sci Sports Exerc. 1989;21(3):250–253. [PubMed] [Google Scholar]
- 20.Pukkala E, Poskiparta M, Apter D, Vihko V. Life-long physical activity and cancer risk among Finnish female teachers. Eur J Cancer Prev. 1993;2(5):369–376. doi: 10.1097/00008469-199309000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Paffenbarger RS, Jr, Hyde RT, Wing AL. Physical activity and incidence of cancer in diverse populations: a preliminary report. Am J Clin Nutr. 1987;45(Suppl 1):312–317. doi: 10.1093/ajcn/45.1.312. [DOI] [PubMed] [Google Scholar]
- 22.Paffenbarger RS, Jr, Lee IM, Wing AL. The influence of physical activity on the incidence of site-specific cancers in college alumni. Adv Exp Med Biol. 1992;322:7–15. doi: 10.1007/978-1-4684-7953-9_2. [DOI] [PubMed] [Google Scholar]
- 23.Lee IM, Paffenbarger RS., Jr Physical activity and its relation to cancer risk: a prospective study of college alumni. Med Sci Sports Exerc. 1994;26(7):831–837. [PubMed] [Google Scholar]
- 24.Rothman KJ, Greenland S (1998) Modern Epidemiology, 2nd edn. USA: Lippincott-Raven Publishers.
- 25.Cuzick JA. Wilcoxon-type test for trend. Stat Med. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 26.Begg CB, Mazumbar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 27.Egger M, Smith GD, Scheneider M, Miner C. Bias in meta-analysis detected by a simple graphical test. Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999;354(9193):1896–1900. doi: 10.1016/s0140-6736(99)04149-5. (Review). [DOI] [PubMed] [Google Scholar]
- 29.Thune I. Assessments of physical activity and cancer risk. Eur J Cancer Prev. 2000;9(6):387–393. doi: 10.1097/00008469-200012000-00003. (Review). [DOI] [PubMed] [Google Scholar]
- 30.Feskanich D, Ziegler RG, Michaud DS, Giovannucci EL, Speizer FE, Willett WC, Colditz GA. Prospective study of fruit and vegetable consumption and risk of lung cancer among men and women. J Natl Cancer Inst. 2000;92(22):1812–1823. doi: 10.1093/jnci/92.22.1812. [DOI] [PubMed] [Google Scholar]
- 31.Franceschi S, Bidoli E. The epidemiology of lung cancer. Ann Oncol. 1999;10 (Suppl 5):S3–S6. doi: 10.1093/annonc/10.suppl_5.s3. [DOI] [PubMed] [Google Scholar]
- 32.Lee IM. Physical activity and cancer prevention – data from epidemiologic studies. Med Sci Sports Exerc. 2003;35(11):1823–1827. doi: 10.1249/01.MSS.0000093620.27893.23. [DOI] [PubMed] [Google Scholar]
- 33.Brownson RC, Alavanja M. Previous lung disease and lung cancer risk among women (United States) Cancer Causes Control. 2000;11:853–858. doi: 10.1023/a:1008999202040. [DOI] [PubMed] [Google Scholar]
- 34.Brenner AV, Wang Z, Kleinerman RA, et al. Previous pulmonary diseases and risk of lung cancer in Gansu Province, China. Int J Epidemiol. 2001;30:118–124. doi: 10.1093/ije/30.1.118. [DOI] [PubMed] [Google Scholar]
- 35.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance – United States, 1971–2000. MMWR. 2002;51:1–16. [PubMed] [Google Scholar]
- 36.Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States. Arch Intern Med. 2000;160(11):1683–1689. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- 37.Blot WJ, Fraumeni JF Jr (1996) Cancers of the lung and pleura. In: Shottenfeld D, Fraumeni JF Jr eds. Cancer Epidemiology and Prevention, 2nd edn. New York, NY: Oxford University Press, pp. 637–665.
- 38.Ballard-Barbash R, Blair A, Blair SN, et al. Physical activity across the cancer continuum: review of existing knowledge and innovative designs for future research. Cancer. 2002;95:1134–1143. doi: 10.1002/cncr.10771. [DOI] [PubMed] [Google Scholar]
- 39.Woods JA, Davis JM, Kohut ML, Ghaffar A, Mayer EP, Pate RR. Effects of the exercise on the immune response to cancer. Med Sci Sports Exerc. 1994;26(9):1109–1115. [PubMed] [Google Scholar]
- 40.Woods JA, Davis JM, Smith JA, Nieman DC. Exercise and cellular innate immune function. Med Sci Sports Exerc. 1999;31(1):57–66. doi: 10.1097/00005768-199901000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen BK, Ullum H. NK cell response to physical activity: possible mechanism of action. Med Sci Sports Exerc. 1994;26(2):140–146. doi: 10.1249/00005768-199402000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Nomura A, Stemmermann GN, Chyou PH, Marcus EB, Buist AS. Prospective study of pulmonary function and lung cancer. Am Rev Respir Dis. 1991;144(2):307–311. doi: 10.1164/ajrccm/144.2.307. [DOI] [PubMed] [Google Scholar]


