Abstract
This overview of the oncologic applications of positron emission tomography (PET) focuses on the technical aspects and clinical applications of a newer technique: the combination of a PET scanner and a computed tomography (CT) scanner in a single (PET/CT) device. Examples illustrate how PET/CT contributes to patient care and improves upon the previous state-of-the-art method of comparing a PET scan with a separate CT scan. Finally, the author presents some of the results from studies of PET/CT imaging that are beginning to appear in the literature.
Positron emission tomography (PET), now almost 30 years after its initial development, has become an established nuclear imaging modality that has proved especially useful in oncology. PET was invented at the Mallinckrodt Institute of Radiology at Washington University in the mid 1970s and was soon adopted into neurology and cardiology as a valuable research tool. However, it took more than a decade for investigators to realize that PET also could be a powerful tool for oncology. PET is a very expensive modality, requiring not only a million-dollar-plus PET scanner but also expensive equipment and highly trained personnel to generate the radiopharmaceuticals used for PET imaging. Nevertheless, thousands of these scanners are in service around the world.
Unlike computed tomography (CT) or magnetic resonance imaging (MRI), which show anatomic detail, PET images biochemical or physiologic phenomena. Because of this, PET offers substantial advantages over anatomic imaging modalities in oncologic imaging. PET can often distinguish between benign and malignant lesions when CT and MRI cannot.
The basis of PET imaging is the labeling of small, biologically important molecules, such as sugars, amino acids, nucleic acids, receptor-binding ligands, or even water and molecular oxygen, with positron-emitting radionuclides. When these positron-emitting tracers undergo radioactive decay, their positions can be detected by the PET scanner. By imaging the temporal distribution of these labeled compounds, we can create “physiologic maps” of the functions or processes relevant to the labeled molecules.
Numerous different types of tracers have been developed for imaging with PET, but the vast majority of clinical oncologic PET studies performed at present utilize an analog of glucose, 18F-2-fluoro-2-deoxy-d-glucose (FDG). The use of FDG to image glucose metabolic rate takes advantage of the observation, first made 75 years ago, that malignant cells have higher rates of aerobic glycolysis than normal tissues (1). Thus, the malignant cell utilizes more glucose to meet its energy needs. Although malignant cells often differ from normal tissues in many other ways (e.g., levels of specific receptors, rate of nucleic acid uptake and incorporation, rate of amino acid uptake and incorporation, and a host of other biologic characteristics that could be measured with PET), FDG is currently the only agent approved by the Food and Drug Administration (FDA) for oncology studies. Fortunately, while FDG is not a perfect imaging agent (some tumors show poor FDG avidity and some benign processes show high FDG avidity), FDG does work very well in most malignant tumors of clinical importance, with the largest exception being prostate cancer.
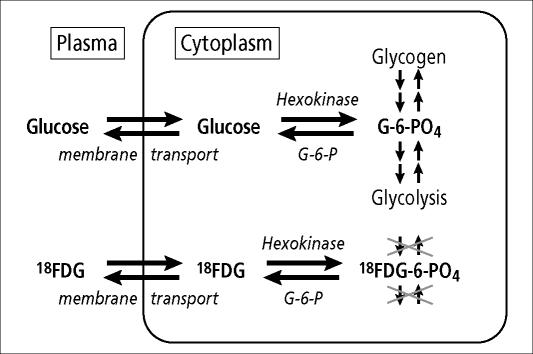
FDG is taken up by the same membrane transporters that take up glucose and is phosphorylated by the same hexokinases as is glucose (Figure 1). The difference is that when FDG is phosphorylated to become FDG-6-phosphate in the cell, it is metabolically trapped. It cannot go on to be stored as glycogen or go on to glycolysis the way glucose can; it is a polar molecule that cannot readily pass through the cell membrane to redistribute out of the cell. If high levels of glucose-6-phosphatase are present, then the phosphate group can be cleaved off to regenerate FDG, and the original molecule can then diffuse back out of the cell to be taken up in the bloodstream for uptake elsewhere. Studies have shown that, for most tumors, a major determinant of FDG uptake is an elevated level of the glucose membrane transporters. Fortunately for the application of FDG-PET in oncology, malignant tumors typically have elevated levels of the glucose transporters (especially one called Glut-1), often have elevated levels of hexokinases (especially HK-II), and typically have stable to decreased levels of glucose-6-phosphatase (2). For the typical clinical oncology study, FDG is administered intravenously in the quiet, resting state and is allowed to circulate through the body for 60 to 90 minutes before imaging is begun. In the case of most malignant neoplasms, sites of active tumor will show up as foci of hypermetabolism, or “hot spots” on the subsequent PET scan images.
Figure 1.
FDG is an analog of glucose in steps of membrane transport and phosphorylation but then becomes “metabolically trapped,” because it can be neither metabolized further nor stored as glycogen.
The Medicare-approved indications for PET are shown in Table 1. These indications encompass most patients presenting for cancer diagnosis and management. Some tumors are notably absent from the list, either because FDG-PET has proved less useful in those tumors or because the Centers for Medicare and Medicaid Services (CMS) considers the existing data insufficient to justify adding a relatively expensive test to the workup. In some tumors, there are specific limitations on the use of PET. For example, in the case of breast cancer, PET can be used for staging or restaging of suspected advanced disease but cannot be used for initial diagnosis. In patients with thyroid cancer, the CMS requirements for PET scanning are very specific: 1) the tumor must be of follicular origin, which excludes medullary tumors; 2) the patient must have had prior thyroidectomy and radioiodine ablation; 3) there must be suspicion of recurrence based on an elevated thyroglobulin level; and 4) the whole-body iodine study results must be negative. PET imaging has just recently been approved for use in the initial staging of cervical cancer; however, restaging with PET is not yet approved. Also, CMS has announced broader coverage for other tumors to be initiated in late 2005, but this will apply only to patients involved in clinical trials or entered into a to-be-developed PET tumor registry. Other payers have variable policies regarding the reimbursement for PET studies.
Table 1.
Medicare-approved oncologic indications for PET
| Non–small cell lung cancer | Colorectal cancer |
| Melanoma | Esophageal cancer |
| Lymphoma | Head and neck cancer |
| Solitary pulmonary nodule | Thyroid cancer* |
| Breast cancer* | Cervical cancer* |
*Special Medicare restrictions exist for these indications.
Table 2 is a partial list of other neoplasms for which FDG-PET has shown promise. For some of these tumors, such as hepatocellular carcinoma, cholangiocarcinoma, and some sarcomas, the results have been quite mixed, and use of PET should be approached with caution. Some tumors have either a relatively low overall glucose metabolic rate, relatively low levels of Glut-1 and other transporters, or high levels of glucose-6-phosphatase, yielding an overall poor FDG uptake. Of the major tumors for which FDG-PET has generally proved to be of little value, prostate cancer is the one with the highest incidence and population impact. Tracers other than FDG have shown better results with several of these non–FDG-avid tumors, but they await much further study.
Table 2.
Other oncologic indications for PET
| Brain tumor | Gastrointestinal stromal tumor |
| Ovarian cancer | Sarcoma* |
| Uterine cancer | Hepatocellular carcinoma* |
| Pancreatic cancer | Cholangiocarcinoma* |
| Testicular cancer | Tumors of unknown primary* |
| Small cell lung cancer |
*Published results have been variable with these tumors.
HARDWARE AND TECHNIQUES FOR PET
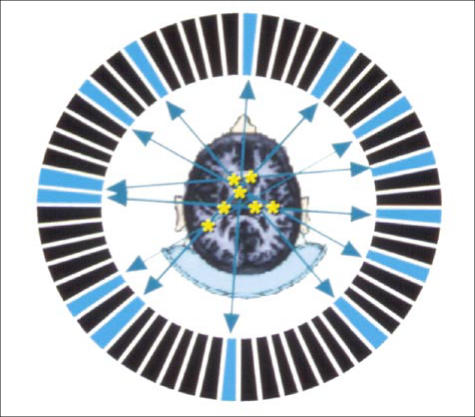
PET imaging is “coincidence” imaging, which is different than the other imaging techniques used in nuclear medicine. PET images are collected by surrounding the patient's body (or a part of the patient's body) with multiple rings of specialized detector crystals (Figure 2). Each decay event yields a positron, which is a positively charged electron. This positron typically travels only a few millimeters in tissue before undergoing an “annihilation reaction” with an electron, whereby both particles convert all of their mass into energy, which is predominantly released in the form of two 511-keV photons traveling in opposite directions. The PET scanner detects these photons simultaneously (or “in coincidence”), so the event is recorded and localized as a positron annihilation, or “event.” By collecting millions of these events, modern PET devices use sophisticated hardware and software to reconstruct images of the distribution of the PET tracer.
Figure 2.
A ring of multiple specialized crystals detect the “coincidence” 511-keV photons resulting from positron decay and the subsequent annihilation reaction. Detection of enough coincident events allows reconstruction of an image of the distribution of tracer. This image depicts a transaxial slice through the brain, with the metabolically active gray matter showing much greater intensity than the less active white matter.
Most PET scanners used since the early 1990s surround the patient with 18 to 24 rings of detectors, comprising thousands of individual detector crystal elements, capable of producing up to 47 transaxial “slices” through an approximate 14- to 15-cm swath through the body at once. Using a “step-and-shoot” technique, 7 or 8 bed-steps are required to scan the typical patient, covering the area from the base of the skull through the proximal femora (where most metastatic disease is likely to reside). The generated images include literally hundreds of coronal, sagittal, and transaxial images, similar to those seen with CT or MRI, as well as a rotating, 3-dimensional, maximum-intensity-pixel “projection” image that is often useful for a global perspective on this large amount of data.
The major drawback to standard PET is that the images are of substantially lower resolution than, for example, those of CT and MRI, and PET is generally poor at delineating anatomic detail. This lack of detail results in poor localization of lesions and poor demarcation of lesion borders. Moreover, lesions are often complex, with some portions more metabolically active than others. Consequently, better localization is often needed for biopsy or focal radiotherapy. The goal of integrating two modalities into a single PET/CT device is to bridge that gap, to facilitate interpretation of the images, and, therefore, to provide a more accurate diagnosis.
WHY PET/CT?
CT has been the cornerstone of oncologic imaging for over 20 years but lacks the ability to show crucial differences in physiology. PET has incomparable abilities to determine the metabolic activity of tissues but needs the assistance of higher-resolution, anatomic information that it cannot provide. CT is the easiest and highest-resolution tomographic modality to integrate into PET imaging. The combination of the two offers the best of both worlds in an integrated data set and thus improves diagnostic accuracy and localization of many lesions.
For years, the primary means of merging the metabolic information with the anatomic information was visual fusion, or having an expert review the separate PET and CT images and mentally synthesize the data. More recently, software fusion has been attempted by many, utilizing specialized software programs to realign and “fuse” the two separate sets of data. There are myriad problems with these software approaches, however, such as different positioning of the patient between the two studies, differences related to breath-hold at maximal inspiration for CT versus tidal breathing during PET imaging, or even simple differences in the contour of the tables on the two devices. The first major step toward solving this dilemma, introduced in 2000 by the group of Dr. David Townsend, an imaging physicist then working at the University of Pittsburgh, was to actually put the two units together in one gantry (3). This allows for the immediately sequential collection of both the PET and the CT data sets, with minimal potential for misregistration.
HARDWARE AND TECHNIQUES FOR PET/CT
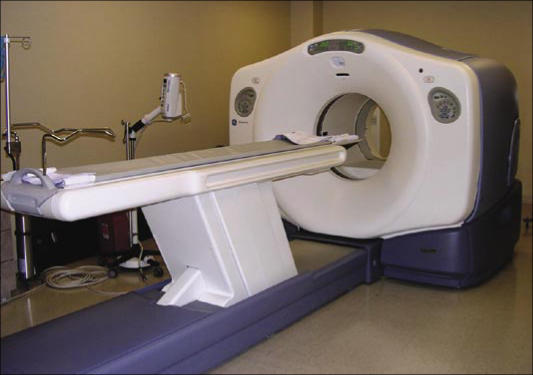
Several of the major imaging instrumentation vendors now manufacture PET/CT scanners. In fact, the market for PET devices has shifted so dramatically toward PET/CT in the past few years that very few PET-only scanners are now being sold in the USA. Figure 3 shows the current state-of-the-art PET/CT scanner that was installed at the North Texas Clinical PET Institute of the Baylor Sammons Cancer Center in early 2004. The “tunnel” of a PET/CT device is deeper than that of a CT scanner or a typical PET scanner but not quite as deep as that of an MRI scanner, and the diameter of the opening (the bore) is wider. In the short time that these scanners have been in production, the manufacturers have made substantial improvements in patient acceptance by making the gantry more compact and making the bore wider so that the patient is less claustrophobic. This does not completely eliminate the problem, however, and patients who are prone to claustrophobia may still need premedication.
Figure 3.
The General Electric Discovery ST PET/CT scanner in the North Texas Clinical PET Institute of the Baylor Sammons Cancer Center.
For the vast majority of devices sold today, the front of the gantry contains a state-of-the-art, multislice, spiral CT scanner. Behind this is a state-of-the-art PET scanner. Combining these two devices is a sophisticated engineering feat: a simple issue such as ensuring that the table position registration is exactly the same from one scanner to the other so that the images align appropriately can be a difficult proposition.
The PET/CT scanning process is as follows. Sixty to ninety minutes after the FDG has been injected, the patient is placed supine on the imaging table. Then, just as with a diagnostic CT, the CT tube and detector are used to obtain a topogram, which is essentially a digital x-ray of the entire field of interest. The topogram is used to map out the precise portion of the body to be scanned, and those coordinates are entered into the system. The scanner software then automatically realigns the table, and a spiral CT is performed of the area of interest (usually the base of the skull through the mid-thigh for most oncology studies), generating literally hundreds of transaxial images through the body.
Next, the table indexes back to the PET scanner part of the machine, which then begins detecting the radiation being emitted from the patient, performing the “emission” part of the PET scan. The patient is “stepped” through the scanner; each bed-step requires 2 to 5 minutes, images a swath of about 14 to 15 cm through the patient, and generates about 30 to 45 contiguous transaxial images. For the typical oncology study, the whole scanning process takes about 30 minutes. If it is necessary to scan the entire body, such as with melanoma patients, scan times are increased accordingly.
The volume of data generated is enormous. Hundreds of transaxial PET and CT images are first reconstructed. These are then reformatted into coronal and sagittal images to facilitate image interpretation. For each of these sets of PET and CT images, corresponding “fusion” images, combining the two types of data, also are generated. Thus, the average oncologic PET/CT scan can generate as many as 4000 to 8000 images. This places obvious burdens on the interpreting physicians, as well as on data archival and retrieval resources.
CHALLENGES IN INTERPRETING PET AND PET/CT STUDIES
Several factors can make the interpretation of PET studies challenging. Chief among these factors in daily practice are variable physiologic uptake of FDG by normal tissues, FDG uptake related to inflammation or infection, occasional malignant lesions with low avidity for FDG, unusual tumor sites, limited resolution of small lesions, altered biodistribution of FDG related to hyperglycemia or hyperinsulinemia, bone marrow activation commonly encountered in cancer patients, and motion artifacts. While these pitfalls are most important to the interpreting physician, it is also important for the referring physician to consider these factors when deciding whether to order a PET study and when interpreting the clinical significance of the PET findings.
FDG-PET measures only glucose uptake. Unfortunately, glucose uptake is prevalent in cells of the body other than malignant cells. Physiologic uptake in some normal tissues can be highly variable. Although many accumulate FDG to a predictable extent, there are others whose uptake cannot be predicted. For example, the brain typically shows intense uptake of FDG, because it metabolizes glucose exclusively, while myocardial uptake is intense in patients who have not fasted but highly variable in patients who have fasted. Adipose tissue typically shows minimal FDG uptake, but certain adipose deposits (so-called “brown fat”) that play a role in thermogenesis can be dramatically activated in a cold or nervous patient. This problem can often be minimized by premedication with benzodiazepines and/or beta-blockers. Sometimes, even relatively predictable activity can be confusing. For example, unlike glucose, FDG is not well reabsorbed by the proximal tubules of the kidney. Thus, it can be predicted that intense activity will be seen in the kidneys and bladder. However, focal pooling of excreted activity in a ureter could be confused with a hypermetabolic iliac lymph node metastasis.
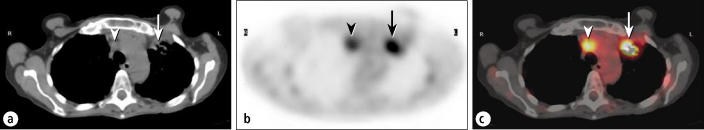
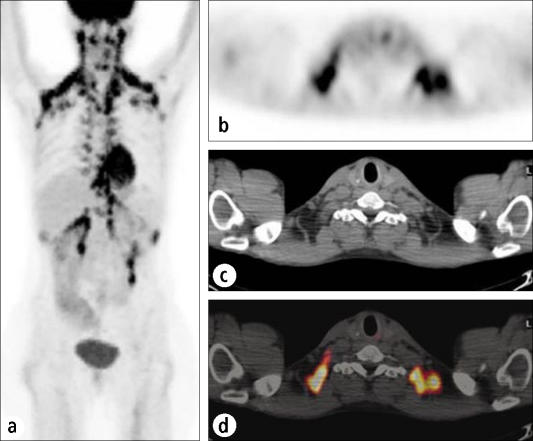
Inflammatory cells, especially macrophages, can sometimes accumulate FDG to a considerable extent, so inflammatory or infectious sites are sometimes visualized on PET. Granulomatous conditions, such as sarcoidosis, fungal infections, tuberculosis, and Mycobacterium avium-intracellulare infection, can cause particular problems in the PET evaluation of pulmonary lesions or lymph nodes (Figure 4). Even inflammation related to therapeutic procedures, such as surgery or radiotherapy, can cause significant uptake. When clinically possible, it is usually wise to wait at least 3 months after the completion of radiotherapy before performing a PET study to avoid confusion by inflammatory uptake of FDG. Other tracers, not yet FDA approved, may alleviate some of this confusion in the future by targeting processes less prevalent in inflammatory cells, such as nucleic acid uptake. These tracers also have drawbacks, however, because they are concentrated heavily in areas where FDG is not; for example, intense uptake of a nucleic acid analog will be seen not only in malignant cells but also in other rapidly dividing cells, such as within the bone marrow or gastrointestinal mucosa.
Figure 4.
A patient with an infiltrative mass lesion in the left upper lobe, being evaluated for malignancy. (a) The CT scan shows a left upper lobe mass (arrow) and an enlarged mediastinal node (arrowhead). (b) The corresponding transaxial PET scan. (c) The fusion image shows intense hypermetabolism, suspicious for a malignant tumor (arrow) and metastatic node (arrowhead). Subsequent biopsy showed Mycobacterium avium-intracellulare infection.
Some malignant lesions have low avidity for FDG. Prostate cancer was mentioned above; there are a few others—bronchoal veolar cell carcinoma, low-grade sarcomas, certain low-grade non-Hodgkin's lymphomas, and even a few well-differentiated adenocarcinomas of the lung—that may show poor concentration of FDG. Most neuroendocrine tumors are poorly seen on FDG-PET.
Small lesions or unusual presentations/locations always make the job of tumor detection and staging more difficult. PET alone is sometimes unable to localize small tumors or confirm whether FDG uptake in unusual sites reflects tumor or nontumor. PET/CT often helps overcome these limitations to a degree, but the inherent limitations of PET resolution, with or without CT, mean that tumors <8 to 10 mm typically must be very hypermetabolic to be visualized on PET.
Hyperglycemia and hyperinsulinemia are very important considerations when preparing a patient for a PET study with FDG. High blood levels of glucose will compete with the FDG for uptake by the tumor. High levels of insulin will push FDG, along with glucose, into skeletal muscle and myocardium, increasing the image background and decreasing the availability of the tracer for uptake by the tumor. Optimal imaging conditions can usually be achieved in euglycemic patients with a 6- to 8-hour fast. This fasting is essential for the quality of the test: altered tracer biodistribution caused by ingestion of even a small meal shortly before a FDG injection can impair the visualization of malignant lesions (Figure 5).
Figure 5.
Anterior projection images from a PET scan of a patient undergoing staging of lymphoma. (a) The initial study was performed after the patient had eaten a candy bar 30 minutes prior to FDG injection. Note the extensive myocardial and muscle uptake due to high insulin levels. Diminished activity is seen in the brain and in tumor sites in the neck and chest (arrows). (b) A repeat study after the patient complied with routine fasting preparation shows more normal biodistribution of tracer and better visualization of tumor deposits (arrows).
For diabetic patients, the situation is more complex. Several protocols are used by PET centers to optimize the chances of a good study. A non–insulin-dependent diabetic often can be studied early in the morning after an overnight fast if his or her early morning, fasting blood sugars routinely are below 150 mg/dL. For those with less optimal glucose control, as well as for insulin-dependent diabetics, our preferred approach is to have the patient ingest a small, low-carbohydrate breakfast and take his or her usual morning dose of insulin (or oral hypoglycemic). The FDG injection is scheduled for about 6 hours later, after the insulin level has decreased to an acceptable level but the glucose level has not yet risen above 150 mg/dL. A particular problem is posed by posttreatment cancer patients with previously unknown, therapy-induced glucose intolerance. At the North Texas Clinical PET Institute, the blood glucose level is checked before FDG administration in all patients.
Bone marrow activation is a major issue in the oncology population because many of the patients are receiving cytokine therapy or have rebounding bone marrow after a course of chemotherapy. PET usually has very high sensitivity for splenic or osseous metastases, but in a patient who has received cytokines, the marrow and spleen show greatly increased activity that can obscure such lesions. Similar, though usually less intense, uptake can be seen in patients with anemia or those recovering from chemotherapy. In general, PET should be delayed for at least 1 week after administration of short-acting cytokines and up to 3 weeks after administration of long-acting cytokines or chemotherapy.
Motion artifacts are a problem with almost any imaging modality but can be an even greater problem with PET/CT imaging than with standard PET. For both types of scanning, an “attenuation-correction” scan is collected to correct the emission data set for absorption of photons within the patient's body. Patient motion between the two types of data sets can seriously compromise the reconstructed images. A standard PET scanner obtains this attenuation scan by rotating a source of 511-keV photons around the body and measuring the attenuation through the body. At each body level being scanned, this scan occurs immediately before or immediately after the collection of the emission data, minimizing the chances for motion between the two. The PET/CT scanner, on the other hand, utilizes the CT images that are collected prior to the start of the emission data collection for attenuation correction. Since it takes some time for the PET scanner to “step” through the body, 20 or 30 minutes may pass between the attenuation scan and the final emission scan. There are substantial opportunities for motion in that time frame. On the other hand, the overall time required for a PET/CT scan is much shorter than for a standard PET scan, so motion due to patient discomfort may actually be less.
Because of these and other challenges, referring physicians should provide pertinent clinical information with the request for a PET scan to assist the interpreting physician in providing the most accurate information possible in oncology patients. Some of the most important factors include
Type of malignancy, date of diagnosis, and location of the lesion (even if it has been resected)
Recent chemotherapy or anemia
Recent cytokine therapy
Inflammatory or infectious processes
Radiation therapy, which can be a source of inflammation
Recent surgery, which can cause linear uptake along the incision
Granulomatous disease
Claustrophobia or anxiety
ADVANTAGES OF PET/CT
Aside from the overall decreased scan time, major clinical advantages of PET/CT include better localization of activity to normal vs abnormal structures, better identification of inflammatory lesions, CT visualization of PET-negative lesions (especially bone lesions), discovery of serendipitous abnormalities, confirmation of unusual or abnormal sites, and improved localization for biopsy or radiotherapy. Images from a variety of cases are presented to illustrate these advantages.
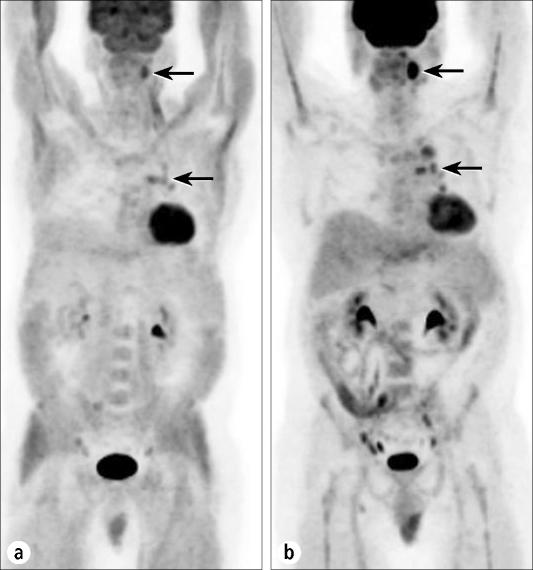
Figure 6 demonstrates how PET/CT can be useful in a problem often encountered in nervous patients. The intense activity seen in the supraclavicular region could be mistaken for metastatic adenopathy but actually represents brown fat. Patients who are anxious or cold may demonstrate activation of brown fat, which contains adrenergic receptors and generates heat by metabolizing glucose. If this is seen in a patient, a repeat study with diazepam premedication will often minimize this uptake, as brown fat also contains peripheral-type benzodiazepine receptors. Early reports suggest that beta-blockers also may minimize brown fat activity. In many of these cases, PET/CT can obviate a repeat study by localizing the activity to fatty tissue and not to lymph nodes.
Figure 6.
This case shows the effects of activation of brown fat in a nervous patient. (a) An anterior projection image shows abnormal activity extending from the neck through the supraclavicular and axillary regions, the anterior mediastinum, the paraspinal regions, and the retrocrural and perirenal regions. (b) Transaxial PET, (c) CT, and (d) fusion images localize the activity to fatty tissue rather than to lymph nodes or tumor masses.
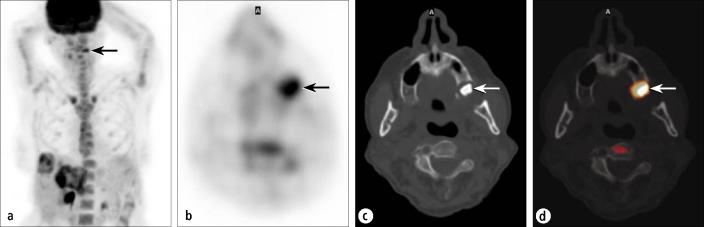
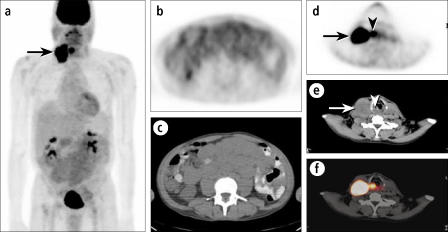
Localization and characterization of unusual lesions to confirm or exclude malignancy is a very important aspect of PET/CT. The patient shown in Figure 7 had recurrent colon cancer;clinical findings and a CT scan indicated that the disease was confined to the liver. The PET images showed that the cancer was confined to the liver except for one small focus in the facial region, which obviously would be a very atypical site for a colorectal metastasis. The PET/CT images showed that the intense activity was actually a residual periodontal abscess surrounding a previous root canal site rather than an atypical metastasis, thus eliminating the need for confirmatory testing.
Figure 7.
A patient being evaluated for metastatic colon cancer. (a) An anterior projection PET image shows known hepatic metastases, as well as an indeterminate focus in the left face (arrow). (b) Transaxial PET through the face shows a distinct hypermetabolic focus (arrow). The corresponding (c) CT and (d) fusion images show this focus to be a periodontal abscess in the maxillary alveolar ridge (arrows).
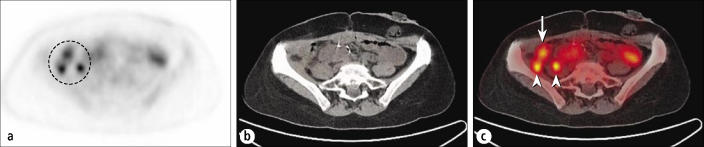
Even when PET clearly shows a malignant tumor, improved localization on PET/CT can assist in patient management. The patient in Figure 8 had a history of colon cancer. The PET images demonstrate the presence of recurrent lesions in the right abdomen, but it would be difficult to identify their exact location, and they likely would have been presumed to be serosal or nodal—possibly amenable to resection. PET/CT clearly localized these unresectable foci to the right psoas and iliacus muscles.
Figure 8.
A patient being restaged for colon cancer. (a) PET image shows focal uptake consistent with recurrence (dashed circle) but does not allow localization. (b) CT and (c) fusion images show intraabdominal recurrence (arrow), as well as lesions involving the psoas and iliacus muscles (arrowheads). Lesser activity elsewhere is physiologic bowel uptake.
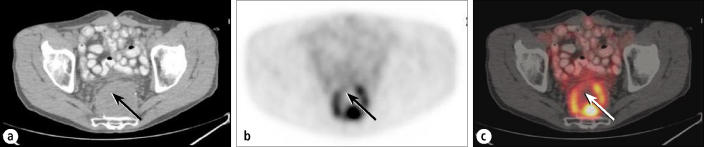
Biopsy localization also is often aided by PET/CT, especially for small lesions or for large, partially necrotic masses. The patient in Figure 9 had a history of rectal carcinoma and was seen 2 years after resection and radiotherapy. Diagnostic CT showed a presacral mass, but a CT-guided biopsy yielded only necrotic and inflammatory tissue. The fusion PET/CT images showed that the standard parasacral biopsy approach had passed through areas without significant amounts of viable tumor. A repeat biopsy based on the PET/CT images yielded recurrent tumor.
Figure 9.
Recurrent rectal carcinoma. (a) A CT scan shows a large presacral mass that had yielded a negative biopsy result. The arrow shows the course of the needle biopsy. (b) PET and (c) fusion images show the discontinuous rim of the active tumor surrounding a necrotic center. Prior biopsy (arrrows show biopsy track) had passed through the zone of tumor into predominant necrosis. Repeat biopsy based on these images revealed tumor recurrence.
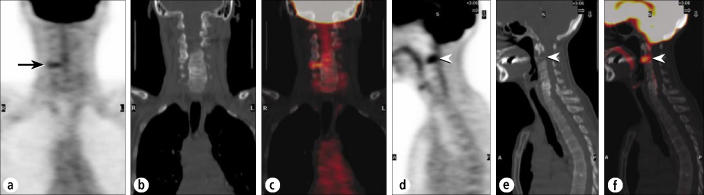
Frequently, suspicious foci on the PET images can be excluded or confirmed as tumor based on the corresponding CT appearance and localization. The patient in Figure 10 had recurrent breast cancer and was being restaged. The focal uptake in the spine on the coronal images might be considered suspicious for metastasis, but the CT images showed this to reflect inflammation at a site of facet joint arthritis. Unfortunately, in the same patient, there was a more intense focus that appeared to project within the anterior vertebral body or disk space; the fusion images showed that it projected within the body of C2. The CT images confirmed the metastasis, with nearly complete destruction of the lower half of the C2 vertebral body, and the patient was treated on an emergent basis, obviating the need for confirmatory examinations that might have delayed urgent treatment.
Figure 10.
A patient being restaged for breast cancer. (a) A coronal PET image through the neck shows a small, linear focus in the lower right cervical spine (arrow). (b) Coronal CT and (c) fusion images show this to reflect facet arthropathy. (d) A sagittal PET image shows a more suspicious focus anteriorly (arrowhead), worrisome for metastasis (or possibly severe degenerative disk disease). (e) Sagittal CT and (f) fusion images show this to reflect a destructive metastasis in C2 (arrowheads).
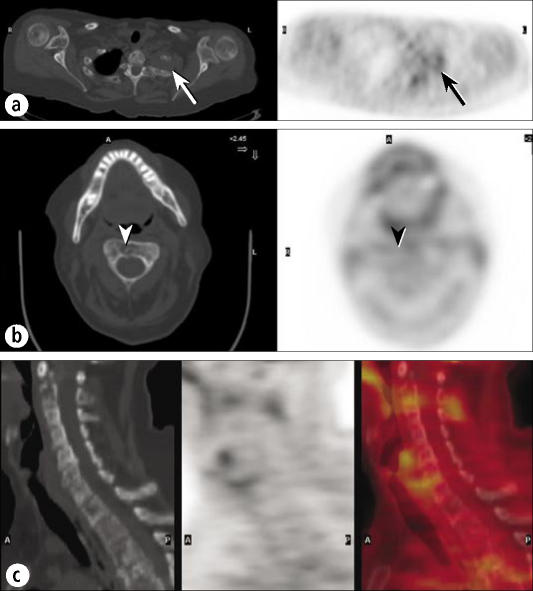
As mentioned previously, some tumors accumulate FDG poorly, and PET/CT can be more useful than PET in such lesions. Figure 11 shows very limited FDG uptake in a patient who had low-grade lymphoma. Most low-grade non-Hodgkin's lymphomas are well visualized on PET; however, some are not. While the PET study reveals the lesion in the left lung apex, it cannot show the destruction of the first and second ribs. In addition, several metastases in the cervical spine are impossible to identify on the PET scan but are readily visible on the CT scan as lytic metastases. This case is unusual; for bone lesions, PET typically visualizes lytic metastases well but blastic metastases less well, since visualization on PET depends on a significant volume of tumor tissue, and lytic lesions are more likely to contain such a volume of tumor.
Figure 11.
A patient with low-grade lymphoma. (a) PET and CT images through a left apical mass with rib involvement show only mild FDG uptake (arrows), very unusual for lymphoma. (b) A CT image through C2 clearly shows a destructive lesion (arrowhead), but a PET image shows minimal FDG uptake (arrowhead). (c) Sagittal CT, PET, and fusion images of the cervical spine show minimal or no FDG uptake in the extensive lytic disease seen on CT.
The “serendipity factor” of PET is very high, meaning that unsuspected malignancies are detected on PET studies performed for assessment of other malignancies. For example, incidental colon carcinomas can be discovered on PET studies performed to assess pulmonary nodules. PET/CT improves the characterization of these “incidentalomas.” The CT images of the PET/CT, while typically not diagnostically optimal, also afford the opportunity to detect other, clinically significant, non–FDG-avid abnormalities, such as aortic aneurysms or large renal masses. The combination modality also provides a great synergy for accurately characterizing unexpected or serendipitous tumor-related findings. The patient in Figure 12 had a low-grade non-Hodgkin's abdominal lymphoma that had only mild to moderate uptake of FDG. Massive adenopathy and small bowel involvement could be seen much more clearly on the CT than on PET. The patient also had cervical adenopathy that was presumed to represent lymphoma. The PET study showed that the cervical adenopathy was much more hypermetabolic than the primary lymphoma, suggesting a significant biological difference between the two. There also was a similarly hypermetabolic laryngeal lesion that could be localized only with the PET/CT and proved to be the primary tumor. In this patient, PET/CT showed that the cervical adenopathy was not lymphoma and made the serendipitous discovery of a head and neck carcinoma.
Figure 12.
A patient with recurrent low-grade lymphoma of the abdomen and cervical adenopathy, presumed lymphomatous. (a) An anterior projection PET image shows intense uptake in the cervical adenopathy (arrow) but only mild FDG uptake in the diffuse abdominal involvement, seen better on transaxial images (b, c). The intense uptake seen in the cervical adenopathy (arrows) proved to be metastatic from a primary laryngeal carcinoma (arrowheads) (d, e, f).
DISADVANTAGES OF PET/CT RELATIVE TO PET
PET/CT has many advantages over standard PET imaging but some disadvantages as well. First, the PET/CT scanner is more like a tunnel than a doughnut, so claustrophobia is a problem for some patients. Radiation dosimetry is another major issue. PET/CT involves doing a full CT scan, albeit usually performed at different settings than for a standard diagnostic CT to decrease the radiation dose. In general, the radiation from a typical PET scan is equivalent to about 3 to 5 times as much as a person would receive in 1 year from the naturally occurring “background” radiation exposure from our surroundings and from cosmic rays penetrating the atmosphere; depending on the technique used, radiation from a PET/CT often can be 5 to 10 times greater than annual background radiation. Although the addition of the CT adds a significant amount of radiation, the level is still similar to that of other diagnostic and interventional procedures, such as fluoroscopy. The radiation from a PET/CT, for example, is somewhat less than that from a typical gallium scan and can be much less than that from a complicated fluoroscopic procedure.
Technical difficulties also are more prevalent in PET/CT, because the scanner comprises two very complicated machines instead of one. A problem with either the PET or the CT may put the entire device out of commission. Also, the use of the CT-based attenuation correction of emission PET images limits some of the techniques that can be used. For example, the use of intravenous contrast for the CT scan can cause artifacts in the reconstruction of the PET images. This is because the iodine component of intravenous contrast absorbs the lower-energy CT x-rays much more efficiently than the high-energy, 511 keV photons emitted during PET imaging. This leads to an “overadjustment” for photon attenuation in the regions where dense contrast is present, resulting in an overestimation of the activity level at these sites when the PET images are reconstructed. With newer reconstruction techniques and adjustment of contrast administration and imaging protocols, this problem is likely to be substantially remedied in the near future.
As mentioned previously, motion artifacts can be amplified with PET/CT. A specific and important facet of this is respiratory motion. Because the patient is breathing during the PET study, it is important to perform the CT scan in a manner that best matches the positioning of the diaphragm and adjacent organs, in order to optimize registration of the two data sets. As a general rule, the CT images are best performed at end-tidal respiration, since that is close to the position that the diaphragm occupies for about 75% of the time during normal tidal breathing. This differs from diagnostic CT, which is typically performed at maximal inspiration.
Because misregistration does occur, the interpreters of these studies must not become overconfident in the ability of the PET/CT images to localize lesions. Overdependence on the CT information may explain some of the rare cases in which PET/CT may yield less accurate results than PET alone. For example, in one recent paper, the accuracy of staging lung cancer was actually lower with PET/CT than with PET alone (4), even though the confidence of the correct interpretations was higher. Since PET is generally more accurate than CT in such applications, discrepancies between the two data sets must be resolved carefully.
LIMITATIONS OF THE CT IN PET/CT
There are specific inherent limitations in the CT aspect of PET/CT. As discussed, in order to minimize radiation dosimetry, the CT scans are performed at lower energy settings, which produce lower-quality images than would a diagnostic-quality CT scan. Most studies do not employ intravenous contrast enhancement; many centers do not routinely use oral contrast. Dilute oral contrast is routinely used at the North Texas Clinical PET Institute because it improves the abdominal CT images and does not degrade the PET images. Intravenous contrast is not employed unless a separate, diagnostic CT exam is ordered, because of the increased cost and patient risk, as well as the possibility of imaging artifacts, as noted above.
In addition, there are minor limitations in using the PET/CT scanner as a diagnostic CT scanner. Probably the most clinically important is that, unlike the gantry of a diagnostic CT scanner, which can be tilted by several degrees (often performed for special studies of head and neck cases, in order to obtain direct coronal images or to minimize streak artifacts from dental fillings), the much larger gantry of the PET/CT scanner cannot be tilted.
CLINICAL DATA THUS FAR
Commercial PET/CT scanners were first introduced in 2002, so significant amounts of published data did not begin to appear until 2004. Some of these papers have examined a broad scope of patients to assess whether PET/CT imaging was better than PET alone, while others have evaluated specific disease categories. Antoch et al studied patients with a wide variety of solid tumors, heavily oriented toward lung, colon, and breast cancers, and found an incremental improvement in accuracy with PET/CT using integrated hardware. The addition of separately performed PET to diagnostic CT improved staging by 21%, while integrated PET was 34% more accurate than diagnostic CT alone (5).
Bar-Shalom et al looked at a larger number of patients with mixed types of cancer on a per-lesion basis (6) and found that having the integrated PET/CT improved image interpretation in 49% of the patients and 30% of the sites. PET/CT changed patient management in 14% of the cases. Importantly, when the PET/CT changed the interpretation of the study, 95% of the time that change was correct. It is always important, despite the allure of a newer technology, to ensure that the new technique is providing better information for patient care.
Several studies have looked at the incremental value of PET and PET/CT in non–small cell lung cancer, in terms of preoperative staging, restaging, and assessment of suspected recurrence. These studies confirmed the results of studies over the last decade in demonstrating that the accuracy of PET is substantially better than that of CT. Overall, the accuracy of CT alone ranged from 63% to 64%, and the accuracy of PET alone was 86% to 90%. The accuracy of integrated PET/CT was 84% to 93%, which is a very small incremental improvement over that of stand-alone PET;however, PET/CT increased the certainty of findings in 22% to 32% of patients—an important factor in terms of the direction and cost of further workup and therapy (4, 7, 8).
In studies of colorectal cancer patients, the accuracy of PET alone was 74% to 85%, and the accuracy of PET/CT was 89% to 97%. PET/CT increased the certainty of findings in 30% to 50% of lesions (9–11).
Freudenberg et al compared the results of various imaging modalities in 27 patients undergoing restaging of lymphoma (12). They found the following incremental improvement in accuracy: CT alone, 84%; PET alone, 95%; CT and separate PET, 98%; and hardware-integrated PET/CT, 99%. Again, given the high accuracy of PET (with or without CT) in this series, the primary contribution of PET/CT is in increasing the confidence of the diagnosis.
One particularly difficult area is the detection of the unknown primary tumor;no single imaging method is particularly successful at localizing such tumors that have eluded conventional evaluation. Gutzeit et al compared CT, PET, PET and CT, and hardware-integrated PET/CT in 45 such patients and found sensitivities of 19%, 28%, 31%, and 35%, respectively. Positive predictive values were 73%, 65%, 81%, and 83%, respectively (13).
Some of these early studies published in this field were performed with less-than-optimal technique, as the field was advancing. Clinical application of PET/CT is developing very quickly, the body of published literature is now growing rapidly, and ongoing studies are likely to show even better results, since they will be performed with more optimal techniques.
FUTURE DEVELOPMENTS
The field of PET/CT is, by its nature, driven by technology developments aimed at improving patient care. Only a few of those will be listed here. First, there will continue to be a push toward higher-resolution PET and CT components, which, of course, will be incorporated into newer PET/CT devices. The next major concern is the speed with which we can acquire acceptable-quality studies. Even though PET/CT shortens the overall time of the PET scan from 60 minutes to just over 30 minutes in most patients, faster PET scanning is needed to improve patient comfort and scanner throughput. In addition, organ-specific scanners are being developed; an organ-specific breast unit is already on the market, although its clinical utility is not yet certain and, in particular, its ability to stage the axilla is quite limited. There also is great interest in taking advantage of the additional information provided by MRI by developing a combination PET/MRI scanner, but this is a much higher hurdle than integrated PET/CT because of factors such as the effects of such a strong magnetic field on the currently available PET detectors. Finally, new tracers are needed to help improve the detection of poorly FDG-avid tumors and to better distinguish malignant tumor from nonmalignant, FDG-avid tissues.
CONCLUSION
In oncology, the combination of separate CT and FDG-PET has become, in the past decade, the standard of imaging care for many oncology patients. The recent integration of these two modalities into a single scanning device offers several major interpretive and clinical advantages. First, there is less confusion over nonmalignant sites of FDG accumulation, such as inflammatory foci, variable physiologic uptake in assorted tissues, and brown fat or muscle uptake, because these foci can be more precisely localized to nonmalignant structures. In addition, PET/CT offers improved localization of malignant lesions, including improved staging (especially for extranodal disease), better follow-up of sentinel lesions, improved targeting of biopsy and therapy, and greater confidence in interpretation. PET/CT also improves the detection of non–FDG-avid tumors that would not be evident on a PET study alone. Finally, studies to date typically have shown a 4% to 15% improvement in overall accuracy of staging/restaging and a 30% to 50% improvement in the confidence of lesion localization. These numbers may improve further with wider use and further technique development.
References
- 1.Warburg O. In: The Metabolism of Tumors. Dickens F, editor. London: Constable; 1930. pp. 129–169. [Google Scholar]
- 2.Mamede M, Higashi T, Kitaichi M, Ishizu K, Ishimori T, Nakamoto Y, Yanagihara K, Li M, Tanaka F, Wada H, Manabe T, Saga T. [18F]FDG uptake and PCNA, Glut-1, and Hexokinase-II expressions in cancers and inflammatory lesions of the lung. Neoplasia. 2005;7:369–379. doi: 10.1593/neo.04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, Jerin J, Young J, Byars L, Nutt R. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41:1369–1379. [PubMed] [Google Scholar]
- 4.Lardinois D, Weder W, Hany TF, Kamel EM, Korom S, Seifert B, von Schulthess GK, Steinert HC. Staging of non–small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500–2507. doi: 10.1056/NEJMoa022136. [DOI] [PubMed] [Google Scholar]
- 5.Antoch G, Saoudi N, Kuehl H, Dahmen G, Mueller SP, Beyer T, Bockisch A, Debatin JF, Freudenberg LS. Accuracy of whole-body dual-modality fluorine-18–2-fluoro-2-deoxy-d-glucose positron emission tomography and computed tomography (FDG-PET/CT) for tumor staging in solid tumors: comparison with CT and PET. J Clin Oncol. 2004;22:4357–4368. doi: 10.1200/JCO.2004.08.120. [DOI] [PubMed] [Google Scholar]
- 6.Bar-Shalom R, Yefremov N, Guralnik L, Gaitini D, Frenkel A, Kuten A, Altman H, Keidar Z, Israel O. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med. 2003;44:1200–1209. [PubMed] [Google Scholar]
- 7.Antoch G, Stattaus J, Nemat AT, Marnitz S, Beyer T, Kuehl H, Bockisch A, Debatin JF, Freudenberg LS. Non-small cell lung cancer: dual-modality PET/CT in preoperative staging. Radiology. 2003;229:526–533. doi: 10.1148/radiol.2292021598. [DOI] [PubMed] [Google Scholar]
- 8.Keidar Z, Haim N, Guralnik L, Wollner M, Bar-Shalom R, Ben-Nun A, Israel O. PET/CT using 18F-FDG in suspected lung cancer recurrence: diagnostic value and impact on patient management. J Nucl Med. 2004;45:1640–1646. [PubMed] [Google Scholar]
- 9.Cohade C, Osman M, Leal J, Wahl RL. Direct comparison of 18F-FDG PET and PET/CT in patients with colorectal carcinoma. J Nucl Med. 2003;44:1797–1803. [PubMed] [Google Scholar]
- 10.Israel O, Mor M, Guralnik L, Hermoni N, Gaitini D, Bar-Shalom R, Keidar Z, Epelbaum R. Is 18F-FDG PET/CT useful for imaging and management of patients with suspected occult recurrence of cancer? J Nucl Med. 2004;45:2045–2051. [PubMed] [Google Scholar]
- 11.Even-Sapir E, Parag Y, Lerman H, Gutman M, Levine C, Rabau M, Figer A, Metser U. Detection of recurrence in patients with rectal cancer: PET/CT after abdominoperineal or anterior resection. Radiology. 2004;232:815–822. doi: 10.1148/radiol.2323031065. [DOI] [PubMed] [Google Scholar]
- 12.Freudenberg LS, Antoch G, Schutt P, Beyer T, Jentzen W, Muller SP, Gorges R, Nowrousian MR, Bockisch A, Debatin JF. FDG-PET/CT in re-staging of patients with lymphoma. Eur J Nucl Med Mol Imaging. 2004;31:325–329. doi: 10.1007/s00259-003-1375-y. [DOI] [PubMed] [Google Scholar]
- 13.Gutzeit A, Antoch G, Kuhl H, Egelhof T, Fischer M, Hauth E, Goehde S, Bockisch A, Debatin J, Freudenberg L. Unknown primary tumors: detection with dual-modality PET/CT—initial experience. Radiology. 2005;234:227–234. doi: 10.1148/radiol.2341031554. [DOI] [PubMed] [Google Scholar]