Abstract
In a significant number of patients, platelets do not respond to aspirin and/or clopidogrel. Furthermore, this lack of response has recently been shown to affect cardiovascular outcome. At the time of the CAPRIE, CURE, CREDO, and MATCH studies, no in vitro assessment was made to determine platelet response. In vitro platelet response should be considered as an important correctable risk factor for atherosclerotic events.
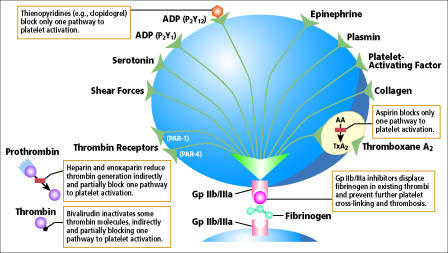
Four major studies have evaluated the antiplatelet effect of aspirin and clopidogrel on cardiovascular outcomes. The CAPRIE (1) trial in 1996 compared aspirin head to head with clopidogrel. CURE (2) in 2001 and CREDO (3) in 2002 compared clopidogrel versus placebo in addition to aspirin. MATCH (4) in 2004 compared aspirin versus placebo added onto clopidogrel. These trials were unable to consider the more recent finding of in vitro platelet resistance to both aspirin and clopidogrel within the general population, which can lead to clinical resistance to or failure of these drugs. This review focuses on our increasing knowledge of the antiplatelet response of aspirin and clopidogrel (Figure) and how this might affect the application of the results of these four major trials to the general population.
Figure.
Platelet activity is affected by multiple factors.
ASPIRIN RESISTANCE
Aspirin is the first-line antiplatelet therapy in cardiovascular medicine today. The Antithrombotic Trialists' Collaboration, a metaanalysis of now over 250 randomized trials, has documented the powerful effect of aspirin in reducing ischemic vascular events by 22% compared with control in a wide array of atherothrombotic conditions (5). Aspirin irreversibly inhibits cyclooxygenase (COX)-1 by acetylation of serine-530, which is close to the active site, and thereby affects platelet-dependent thromboxane A2 (TxA2) formation from arachidonic acid (AA) (6). TxA2 activates platelets through the thromboxane receptor. In anucleated platelets, COX-1 is rendered inactive. The half-life of aspirin is 20 minutes (7).
Numerous tests are available to assess in vitro platelet function (8). The degree of platelet inhibition within a population can vary from 5% to 40% depending on the method used; a standard test for clinical trials is clearly needed. The test should be easy to perform, inexpensive, and reproducible and best represent the in vivo physiology. Platelet aggregometry, either the turbidometric gold standard or impedance method, evaluates response to AA and adenosine diphosphate (ADP), is time consuming and expensive, and can be poorly reproducible. The VerifyNow rapid platelet functional assay offers point-of-care evaluation of platelet aggregation in response to AA and is highly reproducible, simple, and rapid. This assay has increasingly become the method of choice in recent trials. The PFA-100 analyzer determines cessation of high shear flow by platelet plug but is dependent on von Willebrand factor. The P-selectin test uses flow cytometry to detect activation-dependent changes in the platelet surface in response to AA. Although the method is expensive and complex, it is very accurate. The urinary 11-dihydro-thromboxane B2 test measures a metabolite of serum thromboxane B2. It is directly dependent on aspirin targeting COX-1, but it is an indirect measurement and is not platelet specific.
Many mechanisms have been proposed for aspirin nonresponse or resistance (9). Clinical factors include noncompliance and possible poor absorption of enteric-coated drug. Drug interaction has been demonstrated when ibuprofen is administered <4 hours before aspirin; the interaction is caused by steric interference at the COX-1 binding site (10). Possible cellular factors include inadequate suppression of COX-1, overexpression of COX-2 mRNA (11), erythrocyte-induced platelet activation (12), increased norepinephrine as found in acute coronary syndrome, and generation of 8-iso-prostagladin 2 alpha. Genetic polymorphisms have been speculated to affect COX-1 (13), platelet membrane glycoprotein P1(A1/A2) (14), collagen receptor, and von Willebrand factor receptor (15).
Monocytes/macrophages have been implicated in the mechanism of aspirin resistance. These cells are another rich source of TxA2, ranking behind platelets. However, unlike anucleated platelets, monocytes/macrophages can regenerate enzymes. The regenerated, uninhibited COX-1 in the macrophages produces prostaglandins that can then be shunted to the platelets, bypassing COX-1 to produce thromboxane. They also have thromboxane receptors. In macrophages, inducible COX-2 is also present and is the enzyme responsible for the major portion of the metabolism of AA, and this raises the possibility that low-dose aspirin may not be sufficient to block TxA2 production through this alternative pathway (16). Furthermore, COX-2 expression is augmented 10-fold to 20-fold by inflammatory stimuli, such as during acute coronary syndrome, and the TxA2 produced by these nucleated cells may in turn activate platelets (17).
In an interesting study, Zimmermann et al found that patients who responded to aspirin before coronary artery bypass surgery became transiently resistant to aspirin for up to 10 days after surgery, but terbogrel, a combined inhibitor of thromboxane synthetase and thromboxane receptor, equally prevented thromboxane formation by platelets before and after surgery (18). In the platelets from patients after coronary artery bypass surgery, aspirin significantly delayed the inhibition kinetics of COX-1, which might not allow sufficient time for enzyme inhibition before conversion to inactive salicylate. This result suggests that aspirin resistance might be overcome by prolonged administration, such as repeated doses during the day. Nitrosylation of platelet COX has been described and found to be associated with alteration of the enzyme's catalytic activity (19), but it is unknown whether this is related to the impairment of COX-1 acetylation by aspirin. In conclusion, it is clear that aspirin response can show temporal variation.
In 1994 Helgason et al reported the development of aspirin resistance in persons with previous ischemic stroke (20). They used platelet aggregation with the following four agonists: 500 μmol/L AA, 5 μmol/L ADP, 5 μmol/L epinephrine, and 0.8 μg/L collagen. Hyperaggregability was defined as increased sensitivity to more than one agent, the presence of spontaneous aggregation, or both. In contrast, the present methods of platelet aggregation use only AA and ADP as agonists. Helgason followed 306 patients for 33 months and found that the antiplatelet effect of a fixed dose of aspirin was not constant over time. Pulcinelli et al reported that inhibition of platelet aggregation by aspirin progressively decreased in patients for 24 months but showed no change with ticlopidine (7). However, it should be noted that this loss of aspirin platelet inhibition was found to be significant only when the agonist was 2 μg/mL of collagen and not when the agonist used with aggregometry was 1 mmol/L AA with 2 μmol/L ADP.
Over the past few years, there has been increasing evidence of a relationship between variability in response to aspirin and clinical events. The Heart Outcomes Prevention Evaluation (HOPE) study measured baseline urinary 11-dehydro-thromboxane B2 levels, which serve as a marker of thromboxane generation, in a subgroup of patients taking aspirin (21). The investigators found that those in the highest quartile of urinary thromboxane generation had twice the risk of myocardial infarction (MI) of those in the lowest quartile. The investigators concluded that incomplete suppression of thromboxane generation was the cause of increased events. Gum et al (22) performed a prospective, blinded analysis of 326 stable cardiovascular patients, 17 (5.2%) of whom were identified as aspirin resistant as determined by optical platelet aggregometry using 0.5 mg/mL AA and 10 μmol ADP. The aspirin-resistant patients were found to have an increased relative risk of 3.12 for death, MI, or stroke (CVA) over a mean follow-up of 679 ± 185 days (95% confidence interval [CI], 1.10 to 8.90; P = 0.03). It should be noted that these patients were also evaluated by PFA-100, which found aspirin resistance in 9.5% of the patients. However, there was no correlation between the two methods, and only 1.2% of the patients were found to be aspirin resistant by both methods (23). Chen et al (24) used the point-of-care rapid platelet functional assay to determine aspirin responsiveness in 151 patients scheduled for nonurgent percutaneous coronary intervention (PCI) with adequate clopidogrel pretreatment. They concluded that aspirin resistance was associated with a 2.9-fold increase of myonecrosis as evidenced by creatine kinase myocardial band elevation (95% CI, 1.2 to 6.9; P = 0.015).
CLOPIDOGREL RESISTANCE
Clopidogrel is a noncompetitive inhibitor of ADP. The effect of ADP on platelets is mediated by two P2Y receptors, designated P2Y1 and P2Y12. The latter is the target of the thienopyridine drugs, ticlopidine and clopidogrel (25). These drugs lead to inhibition of platelet activation, aggregation, and GpIIb/IIIa receptor activation.
The P2Y12 gene, which encodes the 342–amino acid receptor, was recently identified (26). Fontana et al (27) have identified a P2Y12 receptor haplotype that is strongly associated with an increase in ADP-induced platelet aggregation, and it is anticipated that other sequences will be found to explain interindividual variability. Clopidogrel is a prodrug activated by hepatic cytochrome P450 (CYP) 3A4. Lau et al (28) have demonstrated that interindividual variability of platelet inhibition by clopidogrel correlates with CYP3A4 activity. The next generation of P2Y12 inhibitors is expected to have shorter half-lives and adjustable dosing, which will allow flexibility in obtaining and maintaining a therapeutic effect.
The in vitro effect of clopidogrel on platelet function can be evaluated with aggregometry using the turbidometric or impedance method, or more recently the VerifyNow assay (8). As in aspirin evaluation, flow cytometry can be used for assessment of clopidogrel platelet effect but remains expensive and requires an experienced technician.
Response to clopidogrel has been shown to have an effect on clinical outcome. Gurbel et al (29) used platelet aggregation and flow cytometry to assess platelet inhibitory response to a standard loading dose of 300 mg of clopidogrel in 113 patients undergoing elective PCI at baseline and at 2 hours, 24 hours, 5 days, and 30 days after stenting. They found that platelet inhibitory response followed a normal distribution pattern and that patients with the highest pretreatment platelet reactivity remained the most reactive 24 hours after treatment.
Matetzky et al (30) used routine aggregometry as well as the Cone-and-Platelet analyzer to prospectively study 60 consecutive patients who underwent PCI with stenting for acute ST-elevation MI. They stratified patients into 4 quartiles according to the percentage reduction of ADP-induced platelet aggregation. Clopidogrel was administered on completion of the PCI. Forty percent of patients in the first quartile sustained a recurrent cardiovascular event during a 6-month follow-up, but only 1 patient (6.7%) in the second quartile and none in the third and fourth quartiles suffered an event. The authors concluded that up to 25% of ST-elevated MI patients undergoing PCI are resistant to clopidogrel and therefore may be at increased risk of recurrent cardiovascular events.
Serebruany et al (31) studied the variability in platelet responsiveness to clopidogrel among a group of 544 individuals, including volunteers, patients after PCI, patients with heart failure, and patients after CVA. The response of subjects followed a normal, bell-shaped distribution when aggregation was induced by 5 μmol/L ADP. When hyporesponsiveness and hyper-responsiveness were considered to be two standard deviations below and above the mean, the prevalence was 4.2% and 4.8%, respectively.
THE CAPRIE STUDY
The Clopidogrel versus Aspirin in Patients at Risk of Ischemic Event trial (CAPRIE) was a randomized, blinded trial designed to assess the relative efficacy of clopidogrel (75 mg once a day) and aspirin (325 mg once a day) in reducing a composite outcome of CVA, MI, or vascular death (1). The 19,185 patients—including subgroups of patients with atherosclerotic vascular disease manifested as either recent CVA, recent MI, or symptomatic peripheral vascular disease—were followed for 1 to 3 years, with a mean of 1.91 years. Clopidogrel-treated patients showed an annual 5.32% risk of CVA, MI, or vascular death compared with 5.83% in the aspirin group. These rates reflected a relative risk of 8.7% in favor of clopidogrel (95% CI, 0.3 to 16.5; P = 0.0043). Overall bleeding complications were similar in both groups, although there was a significantly increased risk of gastrointestinal bleeding in the aspirin group.
A subgroup analysis of the CAPRIE study involved 1480 patients with prior cardiac surgery (32). Clopidogrel had a marked benefit over aspirin, with a 31.2% relative risk (95% CI, 15.8 to 43.8; P = 0.003). ADP receptor blockage has been shown to inhibit shear stress-induced platelet aggregation more effectively than aspirin (33). This latter mechanism may be particularly important in surgical conduits, which are more likely to have perturbed flow. Histologically, there is also a difference between thrombus in venous grafts and thrombus in native coronary arteries. Dorsam et al have shown that antagonism of the P2Y12 receptor decreases both collagen- and thrombin-induced thrombin generation and thereby reduces platelet procoagulant activity (34). Exclusion of the cardiac surgery subgroup from the CAPRIE trial would bring the relative risk down from 8.7% to 7.7%.
In other subgroups of patients, such as those with diabetes, atherosclerotic peripheral vascular disease, or hypercholesterolemia, clopidogrel offered an additional incremental benefit over aspirin, further reducing the relative risk of the remaining patients. A multivariate model evaluation of diabetic patients in CAPRIE showed that, compared with aspirin therapy, clopidogrel was independently associated with a decrease in MI, CVA, and vascular death (relative risk, 13.1%; 95% CI, 1.2 to 23.7; P = 0.032) and also caused fewer bleeding complications (35). Osende et al found that in type 2 diabetes mellitus, when blood was perfused on a collagen surface under arterial shear, the extent of thrombus formation was proportional to the plasma hemoglobin A1C level (36), which might suggest that the incremental benefit of clopidogrel could be limited to those with poorly controlled diabetes. In this study, troglitazone, one of the thiazolidinedione drugs, was used to improve glycemic control. The thiazolidinediones have been shown through the platelet PPAR gamma receptor to blunt the release of thromboxanes and CD40 ligand (37). Alternative methods of inhibiting TxA2 have been studied. In the Drug Evaluation in Atherosclerotic Vascular Disease in Diabetics (DAVID) trial, 1000 high-risk diabetics were randomized to receive either aspirin or picotamide, a dual TxA2 synthetase and thromboxane receptor antagonist. For the outcome of vascular death, patients receiving picotamide had a relative risk reduction rate of 40% compared with those receiving aspirin (38).
It is also important to note that on entry into the CAPRIE trial, 80% of the patients were taking aspirin before randomization. The study does not mention how many of the patients were taking aspirin when they had the CVA or MI. It is certainly possible that a significant number of patients might have been on aspirin at the time of the qualifying event, and some of them might now be classified as aspirin resistant or nonresponders. The inclusion and randomization of unknown aspirin-resistant patients in the CAPRIE trial would lead to a greater-than-expected failure rate within the aspirin arm of the study. This would increase the reported relative risk advantage for clopidogrel. The problem of aspirin resistance suggests the possibility that the reported results of CAPRIE may not be valid for the general population.
THE CURE STUDY
The CURE study, which examined the effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation, enrolled 12,562 patients who had presented within 24 hours after the onset of symptoms and randomly assigned them to receive either clopidogrel with aspirin or placebo with aspirin for 3 to 12 months, with a mean of 6 months (2). The dose of aspirin varied from 75 to 325 mg, but this was not reported to affect the outcome. However, the higher doses increased the bleeding complication rate, a finding that is supported by the Antithrombotic Trialists' Collaboration. The first primary outcome—a composite of death from cardiovascular causes, nonfatal MI, or CVA—occurred in 9.3% of the patients in the clopidogrel group and 11.4% of the patients in the placebo group, giving a relative risk of clopidogrel with aspirin compared with aspirin plus placebo of 0.80 (95% CI, 0.72 to 0.90; P = 0.001). The benefit of clopidogrel was significant within the first 24 hours.
Within the CURE trial, 2658 patients underwent PCI and received open-label clopidogrel for 4 weeks, after which the study drug was restarted for a mean of 8 months. In the PCI-CURE study, 4.5% of the clopidogrel group reached the primary endpoint, compared with 6.4% of the placebo group (relative risk, 0.70; 95% CI, 0.50 to 0.97; P = 0.03). Sixty-five percent of the patients had been taking aspirin before being entered into the CURE trial, and it might be assumed that a certain number would be aspirin resistant and could be considered aspirin failures. It would be of interest to review the raw data of the study to see if this cohort of patients had different outcomes than the remaining patients who were naive to aspirin.
In the PCI-CURE study patients, clopidogrel appeared to show continued benefit at 12 months, whereas in the CURE study the benefit related to primary outcome seemed to wane at around 6 months (39). The extent to which clopidogrel benefits patients who do not require PCI, then, is unclear. In an acute ischemic event, the combination of aspirin and clopidogrel may be required initially, but perhaps a few months afterward patients with no complications could be managed with aspirin alone, if resistance is not noted.
THE CREDO STUDY
The Clopidogrel for the Reduction of Events During Observation (CREDO) (3) trial was designed to evaluate the efficacy and safety of clopidogrel therapy for 1 year and the efficacy and safety of a loading dose of clopidogrel prior to elective PCI.
Investigators randomly assigned 2116 patients to receive a 300-mg clopidogrel loading dose (n = 1053) or placebo (n = 1063) 3 to 24 hours before PCI. Thereafter, all patients received clopidogrel 75 mg/day through day 28. From day 29 through 12 months, patients in the loading-dose group received clopidogrel 75 mg/day, and those in the control group received placebo. Both groups received aspirin throughout the study, but the dose could vary from 81 to 325 mg/day. At 1 year, the combined risk of death, MI, or CVA in long-term clopidogrel therapy was associated with a relative risk reduction of 26.9% (P = 0.02). The combined endpoint occurrence rate in the clopidogrel group showed continued increasing advantage through the 12 months. Preloading with clopidogrel showed an advantage only in a subgroup who received the drug at least 6 hours before PCI.
Clopidogrel increased the risk of major bleeding at 1 year: 8.8% with clopidogrel versus 6.7% with placebo (P = 0.07), and approximately two thirds of major bleeding occurred in patients undergoing coronary artery bypass surgery.
Of the 2116 randomized patients, 831 permanently discontinued the study drug. Among these patients, those in the clopidogrel group reported 142 adverse events, and those in the placebo group reported 119 adverse events.
THE MATCH STUDY
The MATCH study compared aspirin 75 mg/day with placebo in 7599 high-risk patients with recent ischemic CVA or transient ischemic attack and at least one additional vascular risk factor; patients were already receiving clopidogrel 75 mg/day for up to 18 months (4). The primary endpoint was a composite of CVA, MI, vascular death, or acute ischemia, which was reached in 15.7% of the aspirin and clopidogrel group and in 16.7% of the placebo and clopidogrel group, for a relative risk reduction of 6.4% (95% CI, 4.6 to 16.3; P = 0.244). The endpoint of CVA, MI, or vascular death was attained in 11.7% of the aspirin and clopidogrel group and in 12.4% of the placebo and clopidogrel group, for a relative risk reduction of 5.9% (95% CI, 7.1 to 17.3; P = 0.360). The risk of life-threatening or major bleeding was increased by the addition of aspirin (P = 0.0001). The authors concluded that because of benefit-to-risk considerations, the trial did not show an additional value of adding aspirin to clopidogrel in high-risk patients with transient ischemic attack or CVA. A point of interest would be whether patients who are hyperresponders to clopidogrel might have an increased risk of major bleeding.
STUDIES IN PROGRESS
The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial includes more than 15,000 high-risk but stable patients. It compares clopidogrel and aspirin with aspirin alone in primary and secondary prevention, with a mean anticipated follow-up of 42 months.
Lev has evaluated aspirin and clopidogrel drug response in patients undergoing elective PCI. Preliminary data show that 50% of patients resistant to aspirin were also resistant to clopidogrel. Fifteen percent of the study patients were resistant to aspirin, 24% were resistant to clopidogrel, and 7.5% were resistant to both drugs. Several other important studies are in progress, one of which is the Research Evaluation to Study Individuals Who Show Thromboxane or P2Y12 Receptor Resistance (RESISTOR) trial, which will examine variability in response to antiplatelet therapies.
CONCLUSION
Our knowledge of platelet biology and pathology continues to expand rapidly. There is a need to determine which of the several in vitro assays available can be used as a common standard to assess in vitro platelet function. The VerifyNow assay for aspirin and clopidogrel is attractive because of its ease of use and reproducibility.
A significant number of patients are now reported to be nonresponsive to aspirin and clopidogrel, and some are nonresponsive to both. It remains to be seen whether further increases of the dose of the drug in these nonresponsive patients will lead to a response and how many will continue to be truly nonresponsive and may then be considered to be resistant to the in vitro assay. Responders exhibit a normal distribution of response to a fixed standard dose, and therefore it becomes important to determine, for each patient, the dose required to produce the in vitro level of response needed for the maximal clinical benefit-to-risk ratio.
Several recent studies now clearly show that the in vitro response to either aspirin or clopidogrel can affect the rate of atherosclerotic events. This important information was not available at the time of CAPRIE, CURE, CREDO, and MATCH, and so some of the conclusions of these trials cannot be extended to the general population. Nevertheless, the studies do indicate that the higher the patient's risk of a cardiovascular event, the greater the benefit of combined synergistic aspirin and clopidogrel action. In addition, in acute inflammatory states, such as acute coronary syndrome and PCI, the macrophage's increased TxA2 production may play an important role in overriding the effect that aspirin may have on platelet activity, and addition of a thromboxane receptor antagonist may be of benefit during these events.
The cost of clopidogrel is about 50 times that of aspirin. The combination of aspirin and clopidogrel clearly is known to increase the risk of bleeding morbidity and mortality. These are further reasons why it is important to carefully decide the choice as well as the dose of the prescribed drug.
Finally, it appears reasonable to consider including in vitro platelet response as an important correctable risk factor for atherosclerotic events.
References
- 1.CAPRIE Steering Committee A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK, Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial investigators Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. The CURE study. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 3.Steinhubl SR, Berger PB, Mann JT, III, Fry ET, DeLago A, Wilmer C, Topol EJ, CREDO investigators Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 4.Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias-Guiu J, Rupprecht HJ, MATCH investigators Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 5.Antithrombotic Trialists' Collaboration Collaboration meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schror K. Aspirin and platelets: the antiplatelet action of aspirin and its role in thrombosis treatment and prophylaxis. Semin Thromb Hemost. 1997;23:349–356. doi: 10.1055/s-2007-996108. [DOI] [PubMed] [Google Scholar]
- 7.Pulcinelli FM, Pignatelli P, Celestini A, Riondino S, Gazzaniga PP, Violi F. Inhibition of platelet aggregation by aspirin progressively decreases in long-term treated patients. J Am Coll Cardiol. 2004;43:979–984. doi: 10.1016/j.jacc.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 8.Michelson AD. Platelet function testing in cardiovascular diseases. Circulation. 2004;110:e489–e493. doi: 10.1161/01.CIR.0000147228.29325.F9. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt DL. Aspirin resistance: more than just a laboratory curiosity. J Am Coll Cardiol. 2004;43:1127–1129. doi: 10.1016/j.jacc.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Catella-Lawson F, Reilly MP, Kapoor SC, Cucchiara AJ, DeMarco S, Tournier B, Vyas SN, FitzGerald GA. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001;345:1809–1817. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 11.Weber AA, Zimmermann KC, Meyer-Kirchrath J, Schror K. Cyclooxygenase 2 in human platelets as a possible factor in aspirin resistance. Lancet. 1999;353:900. doi: 10.1016/S0140-6736(99)00498-5. [DOI] [PubMed] [Google Scholar]
- 12.Valles J, Santos MT, Aznar J, Osa A, Lago A, Cosin J, Sanchez E, Broekman MJ, Marcus AJ. Erythrocyte promotion of platelet reactivity decreases the effectiveness of aspirin as an antithrombotic therapeutic modality: the effect of low-dose aspirin is less than optimal in patients with vascular disease due to prothrombotic effects of erythrocytes on platelet reactivity. Circulation. 1998;97:350–355. doi: 10.1161/01.cir.97.4.350. [DOI] [PubMed] [Google Scholar]
- 13.Halushka MK, Walker LP, Halushka PV. Genetic variation in cyclooxygenase 1: effects on response to aspirin. Clin Pharmacol Ther. 2003;73:122–130. doi: 10.1067/mcp.2003.1. [DOI] [PubMed] [Google Scholar]
- 14.Macchi L, Christiaens L, Brabant S, Sorel N, Ragot S, Allal J, Mauco G, Brizard A. Resistance in vitro to low-dose aspirin is associated with platelet PlA1 (GP IIIa) polymorphism but not with C807T(GP Ia/IIa) and C-5T Kozak (GP Ibα) polymorphisms. J Am Coll Cardiol. 2003;42:1115–1119. doi: 10.1016/s0735-1097(03)00921-5. [DOI] [PubMed] [Google Scholar]
- 15.Quinn MJ, Topol EJ. Common variations in platelet glycoproteins: pharmacogenomic implications. Pharmacogenomics. 2001;2:341–352. doi: 10.1517/14622416.2.4.341. [DOI] [PubMed] [Google Scholar]
- 16.Cipollone F, Patrignani P, Greco A, Panara MR, Padovano R, Cuccurullo F, Patrono C, Rebuzzi AG, Liuzzo G, Quaranta G, Maseri A. Differential suppression of thromboxane biosynthesis by indobufen and aspirin in patients with unstable angina. Circulation. 1997;96:1109–1116. doi: 10.1161/01.cir.96.4.1109. [DOI] [PubMed] [Google Scholar]
- 17.Halushka MK, Halushka PV. Why are some individuals resistant to the cardioprotective effects of aspirin? Could it be thromboxane A2? Circulation. 2002;105:1620–1622. doi: 10.1161/01.cir.0000015422.86569.52. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann N, Wenk A, Kim U, Kienzle P, Weber AA, Gams E, Schror K, Hohlfeld T. Functional and biochemical evaluation of platelet aspirin resistance after coronary artery bypass surgery. Circulation. 2003;108:542–547. doi: 10.1161/01.CIR.0000081770.51929.5A. [DOI] [PubMed] [Google Scholar]
- 19.Boulos C, Jiang H, Balazy M. Diffusion of peroxynitrite into the human platelet inhibits cyclooxygenase via nitration of tyrosine residues. J Pharmacol Exp Ther. 2000;293:222–229. [PubMed] [Google Scholar]
- 20.Helgason CM, Bolin KM, Hoff JA, Winkler SR, Mangat A, Tortorice KL, Brace LD. Development of aspirin resistance in persons with previous ischemic stroke. Stroke. 1994;25:2331–2336. doi: 10.1161/01.str.25.12.2331. [DOI] [PubMed] [Google Scholar]
- 21.Eikelboom JW, Hirsh J, Weitz JI, Johnston M, Yi Q, Yusuf S. Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation. 2002;105:1650–1655. doi: 10.1161/01.cir.0000013777.21160.07. [DOI] [PubMed] [Google Scholar]
- 22.Gum PA, Kottke-Marchant K, Welsh PA, White J, Topol EJ. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J Am Coll Cardiol. 2003;41:961–965. doi: 10.1016/s0735-1097(02)03014-0. [DOI] [PubMed] [Google Scholar]
- 23.Campbell CL, Steinhubl SR. Variability in response to aspirin: do we understand the clinical relevance? J Thromb Haemost. 2005;3:665–669. doi: 10.1111/j.1538-7836.2005.01119.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen WH, Lee PY, Ng W, Tse HF, Lau CP. Aspirin resistance is associated with a high incidence of myonecrosis after non-urgent percutaneous coronary intervention despite clopidogrel pretreatment. J Am Coll Cardiol. 2004;43:1122–1126. doi: 10.1016/j.jacc.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 25.Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Conley PB. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- 26.Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, Lachowicz JE, Zhang FL, Gustafson E, Monsma FJ, Jr, Wiekowski MT, Abbondanzo SJ, Cook DN, Bayne ML, Lira SA, Chintala MS. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107:1591–1598. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fontana P, Dupont A, Gandrille S, Bachelot-Loza C, Reny JL, Aiach M, Gaussem P. Adenosine diphosphate–induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation. 2003;108:989–995. doi: 10.1161/01.CIR.0000085073.69189.88. [DOI] [PubMed] [Google Scholar]
- 28.Lau WC, Gurbel PA, Watkins PB, Neer CJ, Hopp AS, Carville DG, Guyer KE, Tait AR, Bates ER. Contribution of hepatic cytochrome P450 3A4 metabolic activity to the phenomenon of clopidogrel resistance. Circulation. 2004;109:166–171. doi: 10.1161/01.CIR.0000112378.09325.F9. [DOI] [PubMed] [Google Scholar]
- 29.Gurbel PA, Bliden KP, Hiatt BL, O'Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107:2908–2913. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 30.Matetzky S, Shenkman B, Guetta V, Shechter M, Bienart R, Goldenberg I, Novikov I, Pres H, Savion N, Varon D, Hod H. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004;109:3171–3175. doi: 10.1161/01.CIR.0000130846.46168.03. [DOI] [PubMed] [Google Scholar]
- 31.Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005;45:246–251. doi: 10.1016/j.jacc.2004.09.067. [DOI] [PubMed] [Google Scholar]
- 32.Bhatt DL, Chew DP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Superiority of clopidogrel versus aspirin in patients with prior cardiac surgery. Circulation. 2001;103:363–368. doi: 10.1161/01.cir.103.3.363. [DOI] [PubMed] [Google Scholar]
- 33.Makkar RR, Eigler NL, Kaul S, Frimerman A, Nakamura M, Shah PK, Forrester JS, Herbert JM, Litvack F. Effects of clopidogrel, aspirin and combined therapy in a porcine ex vivo model of high-shear induced stent thrombosis. Eur Heart J. 1998;19:1538–1546. doi: 10.1053/euhj.1998.1042. [DOI] [PubMed] [Google Scholar]
- 34.Dorsam RT, Tuluc M, Kunapuli SP. Role of protease-activated and ADP receptor subtypes in thrombin generation on human platelets. J Thromb Haemost. 2004;2:804–812. doi: 10.1111/j.1538-7836.2004.00692.x. [DOI] [PubMed] [Google Scholar]
- 35.Bhatt DL, Marso SP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol. 2002;90:625–628. doi: 10.1016/s0002-9149(02)02567-5. [DOI] [PubMed] [Google Scholar]
- 36.Osende JI, Badimon JJ, Fuster V, Herson P, Rabito P, Vidhun R, Zaman A, Rodriguez OJ, Lev EI, Rauch U, Heflt G, Fallon JT, Crandall JP. Blood thrombogenicity in type 2 diabetes mellitus patients is associated with glycemic control. J Am Coll Cardiol. 2001;38:1307–1312. doi: 10.1016/s0735-1097(01)01555-8. [DOI] [PubMed] [Google Scholar]
- 37.Akbiyik F, Ray DM, Gettings KF, Blumberg N, Francis CW, Phipps RP. Human bone marrow megakaryocytes and platelets express PPARγ, and PPARγ agonists blunt platelet release of CD40 ligand and thromboxanes. Blood. 2004;104:1361–1368. doi: 10.1182/blood-2004-03-0926. [DOI] [PubMed] [Google Scholar]
- 38.Neri Serneri GG, Coccheri S, Marubini E, Violi F, Drug Evaluation in Atherosclerotic Vascular Disease in Diabetics (DAVID) Study Group Picotamide, a combined inhibitor of thromboxane A2 synthase and receptor, reduces 2-year mortality in diabetics with peripheral arterial disease: the DAVID study. Eur Heart J. 2004;25:1845–1852. doi: 10.1016/j.ehj.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, Malmberg K, Rupprecht H, Zhao F, Chrolavicius S, Copland I, Fox KA, Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) investigators Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358:527–533. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]