Renal cell carcinoma (RCC) is the most common malignancy of the kidney and accounts for approximately 3% of adult cancers (1). RCC is more common in men than in women (ratio 2:1), and the median age at diagnosis is approximately 60 years. Although primarily a cancer of the proximal tubular epithelium (85%), RCC also includes nonepithelial kidney tumors, Wilms' tumor, and tumors of the renal pelvis (1, 2). During 2005, it is estimated that approximately 36,000 new cases of kidney cancer will be diagnosed and 16,000 people will die of the disease in the USA (3). There are wide regional differences in the incidence of RCC in the USA (4).
Annual estimates of the incidence for RCC indicate steady increases, with over a third of newly diagnosed patients presenting with advanced or metastatic disease (5–8). Surgical resection (including cytoreduction nephrectomy and/or metastasectomy) remains the most viable treatment option in patients regardless of the stage of disease at presentation (9–11). Despite recent advances in cancer therapy, the prognosis for patients with metastatic RCC remains dismal, with <5% overall 5-year survival. The role of systemic therapy in this setting has been studied; however, it has a limited effect on outcome and overall survival.
Based on morphologic, cytogenetic, and molecular criteria, there are five distinguishable types of RCC: clear cell (60%– 80%), papillary (7%–14%), chromophobe (4%–10%), oncocytic (2%–5%), and collecting duct carcinomas (1%–2%) (12, 13). A small percentage of patients have tumors of undefined cellular morphology. Several genetic mutations are associated with hereditary RCC (e.g., von Hippel-Lindau gene and c-met protooncogene). The sporadic forms of clear cell carcinoma likewise have a high frequency of VHL mutations or hypermethylation (6). The various tumor types have widely different disease courses; for example, genetic variants of papillary and collecting duct tumors are particularly aggressive cancers associated with short survival times (14), and eosinophilic variants of chromophobe tumors often have an indolent clinical course (15). Recent evidence suggests increased mortality (median survival of 9.4 months) in patients with non–clear-cell tumors, which tend to be resistant to chemotherapy and immunotherapy (2). Sarcomatoid tumors are high-grade transformants within each tumor type that carry a particularly poor prognosis for survival (16).
RCC is classified first according to disease stage and then according to disease grade. Stage of disease, defined using the TNM classification, differentiates size of primary tumor (T0–T4), lymph node involvement (N0–N2), and extent of metastasis into the vena cava and other tissues (M0–M1) (17). Tumor (nuclear) grade (G1–G4) reflects the differentiation of tumor cells as defined microscopically by increased nuclear size, irregularity, and nucleolar prominence (18). Nuclear grade is highly predictive of the development of metastatic disease.
Surgical resection (nephrectomy and partial nephrectomy) is the preferred treatment for localized primary tumors in patients with stage I through stage IV disease, but surgical cure of disease is strongly dependent on stage and grade of disease. For example, following radical nephrectomy, the overall 5-year survival of patients with stage IV disease is only 29.1%, with a corresponding 10-year survival of 0%. In contrast, patients with stage I disease have estimated 5-and 10-year survival rates of nearly 100% (19).
Patients with metastatic RCC have a poor prognosis, and relapse from therapy is common: those with larger renal or lymph node–positive tumors have postoperative metastatic recurrence rates of 59% by 12 months, 83% by 24 months, and 93% by 36 months (20). Radical nephrectomy combined with immunotherapy has been shown to significantly increase survival in patients with metastatic RCC beyond time periods achieved with immunotherapy alone (21, 22). However, nephrectomy may have limited clinical benefit in patients with unresectable or metastatic RCC. These patients, and those with recurrent disease following therapy, are candidates for additional chemotherapy or immunotherapy.
A variety of cytokines have been studied in patients with metastatic RCC. Two of these agents, interleukin 2 (rIL-2) and interferon-alpha (IFNα), have reproducible antitumor effects: objective response rates of 15% to 31%, which may be durable in some patients (23–33). Unfortunately, the majority of patients with metastatic RCC do not benefit from these therapies. At present, four novel agents with significantly greater antitumor activity are under evaluation in large, multinational phase III trials. It is expected that several will be approved by the Food and Drug Administration within the next 1 to 2 years.
BAYLOR SAMMONS CANCER CENTER TUMOR REGISTRY
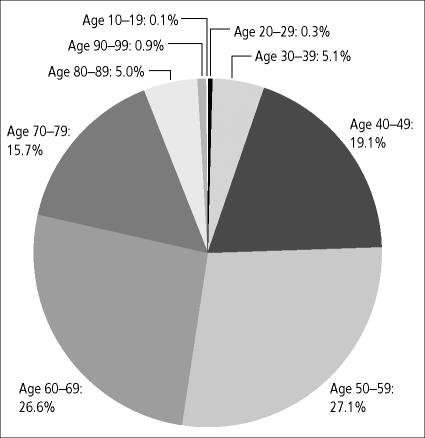
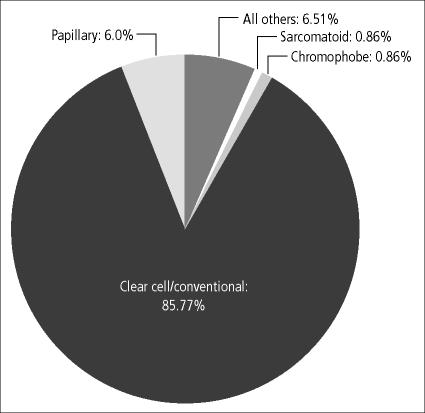
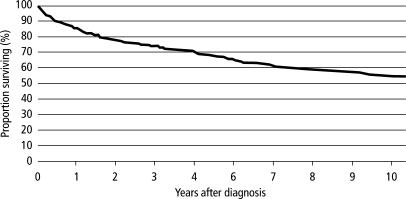
From 1994 to 2003, the tumor registry at Baylor University Medical Center at Dallas recorded 700 patients with RCC (424 men and 276 women); most patients were diagnosed in the fifth and sixth decades of life (Figure 1). The demographics and distribution of these cases compare with national data. Clear-cell carcinoma was the most common histologic type (Figure 2). Survival regardless of treatment type compares favorably with historical data of patients diagnosed with RCC nationally (Figure 3).
Figure 1.
Distribution of renal cell carcinoma cases by age at diagnosis at Baylor University Medical Center (n = 700).
Figure 2.
Distribution of renal cell carcinoma cases by histologic type at Baylor University Medical Center (n = 700).
Figure 3.
Overall survival for patients with renal cell carcinoma first seen at Baylor University Medical Center from 1994 to 2003 (n = 700).
The majority of patients (435) received surgical resection, nephrectomy or partial nephrectomy. Additional therapy—hormonal therapy, chemotherapy, immunotherapy, and radiation therapy alone or in combination—was administered in 48 cases and was most influenced by the stage of cancer at diagnosis and the time period in which the diagnosis occurred. Between 1994 and 1999, patients with stage IV RCC received various forms of systemic therapy (chemotherapy, radiation therapy, and hormonal therapy) irrespective of histologic type. In contrast, between 2000 and 2003, patients with non–clear-cell histologies were more likely not to be treated with systemic therapy. This shift parallels the global recognition that patients with non–clear-cell cancers do not benefit from traditional immunotherapy (interferon and/or interleukin-2) and chemotherapy (2).
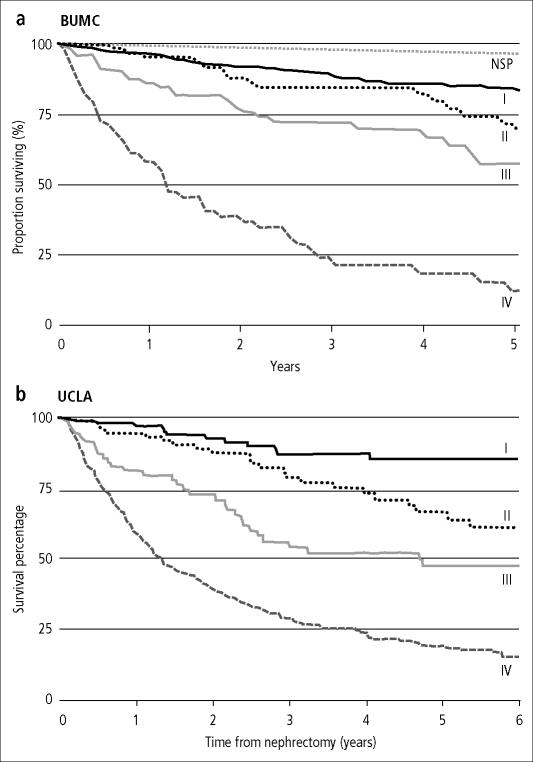
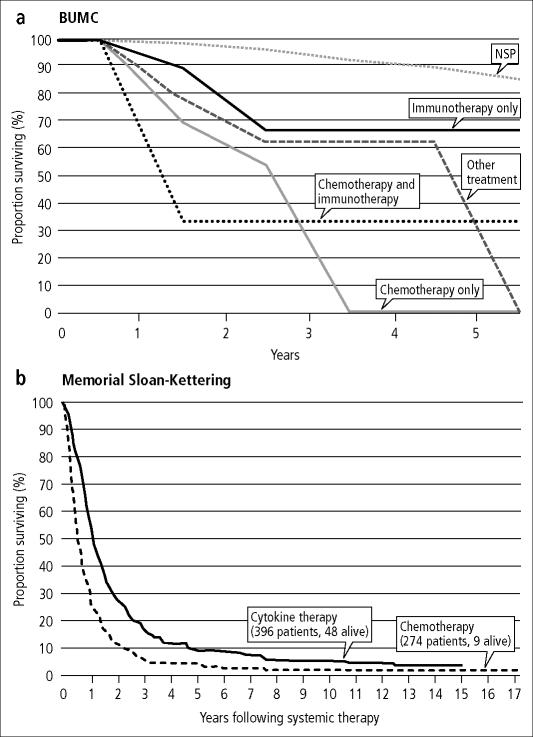
At present, there are three large, single-institution databases of RCC patients from which outcome comparisons can be made (University of California Los Angeles, Cleveland Clinic Foundation, and Memorial Sloan-Kettering Cancer Center). When separated by stage at diagnosis, the survival of cases registered at Baylor is similar to that reported by the University of California Los Angeles (34) (Figure 4). In addition, the survival by treatment (chemotherapy or immunotherapy) for advanced/metastatic RCC cases compares favorably with the survival from a 670 patient database at Memorial Sloan-Kettering Cancer Center (35) (Figure 5). In an attempt to facilitate the development of prognostic factors that may influence treatment and outcome, an international working group has formed a large database of more than 4000 patients with advanced RCC. This database is not yet accessible.
Figure 4.
Survival by stage of renal cell carcinoma: (a) for 700 patients first seen between 1994 and 2003 at Baylor University Medical Center and (b) for 661 patients who underwent nephrectomy between 1989 and 1999 at the University of California Los Angeles. NSP indicates normal survival probability. Part b reprinted from reference 34 with permission from the American Society of Clinical Oncology.
Figure 5.
Survival of patients with stage IV renal cell carcinoma: (a) for patients first seen between 1994 and 2003 at Baylor University Medical Center and (b) for 670 patients treated between 1975 and 1996 at Memorial Sloan-Kettering Cancer Center. NSP indicates normal survival probability. Part b reprinted from reference 35 with permission from American Urological Association/Lippincott Williams & Wilkins.
RENAL CELL CANCER CENTER
The Baylor Sammons Cancer Center has developed a multidisciplinary Renal Cell Cancer Center consisting of a dedicated genitourinary medical oncologist, urologic oncologist, and research staff. RCC patients at Baylor have access to the latest advancements in the treatment of all stages of this disease, including clinical trials. At present, the Baylor Sammons Renal Cell Cancer Center is a leading accruer to two major phase III trials evaluating novel agents for the treatment of metastatic disease, both of which will likely impact the treatment of this cancer. Through ongoing collaboration with physicians at other nationally recognized RCC centers, our patients are assured of access to the latest medical breakthroughs now and in the future.
Footnotes
Reprinted with permission from the 2004 Sammons Cancer Center Annual Report.
References
- 1.Linehan WM, Zbar B, Bates SE, Zelefsky MJ, Yang JC. Cancer of the kidney and ureter. In: DeVita VT Jr, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1362–1396. [Google Scholar]
- 2.Motzer RJ, Bacik J, Mariani T, Russo P, Mazumdar M, Reuter V. Treatment outcome and survival associated with metastatic renal cell carcinoma of non–clear-cell histology. J Clin Oncol. 2002;20:2376–2381. doi: 10.1200/JCO.2002.11.123. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Cancer Statistics Working Group . United States Cancer Statistics: 1999 Incidence. Atlanta, GA: Centers for Disease Control and Prevention; 2002. pp. 1–292. [Google Scholar]
- 5.Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma. J Urol. 2000;163:408–417. [PubMed] [Google Scholar]
- 6.Motzer RJ, Bander NH, Nanus DM. Renal cell carcinoma. N Engl J Med. 1996;335:865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 7.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 8.Tsui KH, Shvarts O, Smith RB, Figlin R, de Kernion JB, Belldegrun A. Renal cell carcinoma: prognostic significance of incidentally detected tumors. J Urol. 2000;163:426–430. doi: 10.1016/s0022-5347(05)67892-5. [DOI] [PubMed] [Google Scholar]
- 9.Staehler G, Brkovic D. The role of radical surgery for renal cell carcinoma with extension into the vena cava. J Urol. 2000;163:1671–1675. [PubMed] [Google Scholar]
- 10.Van Poppel H, Bamelis B, Oyen R, Baert L. Partial nephrectomy for renal cell carcinoma can achieve long-term tumor control. J Urol. 1998;160(3 Pt 1):674–678. doi: 10.1016/S0022-5347(01)62751-4. [DOI] [PubMed] [Google Scholar]
- 11.Flanigan RC, Blumenstein BA, Salmon S, Crawford E. Cytoreduction nephrectomy in metastatic renal cancer: The results of Southwest Oncology Group Trial 8949 [abstract] Proc Ann Mtg Am Soc Clin Oncol. 2000;19:2a. [PubMed] [Google Scholar]
- 12.Storkel S, Eble JN, Adlakha K, Amin M, Blute ML, Bostwick DG, Darson M, Delahunt B, Iczkowski K. Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) Cancer. 1997;80:987–989. doi: 10.1002/(sici)1097-0142(19970901)80:5<987::aid-cncr24>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 13.Delahunt B, Velickovic M, Grebe SK. Evolving classification of renal cell neoplasia. Expert Rev Anticancer Ther. 2001;1:576–584. doi: 10.1586/14737140.1.4.576. [DOI] [PubMed] [Google Scholar]
- 14.Leroy X, Zini L, Leteurtre E, Zerimech F, Porchet N, Aubert JP, Gosselin B, Copin MC. Morphologic subtyping of papillary renal cell carcinoma: correlation with prognosis and differential expression of MUC1 between the two subtypes. Mod Pathol. 2002;15:1126–1130. doi: 10.1097/01.MP.0000036346.88874.25. [DOI] [PubMed] [Google Scholar]
- 15.Onishi T, Oishi Y, Yanada S, Abe K, Hasegawa T, Maeda S. Prognostic implications of histological features in patients with chromophobe cell renal carcinoma. BJU Int. 2002;90:529–532. doi: 10.1046/j.1464-410x.2002.02977.x. [DOI] [PubMed] [Google Scholar]
- 16.Said JW, Thomas G, Zisman A. Kidney pathology: current classification of renal cell carcinoma. Curr Urol Rep. 2002;3:25–30. doi: 10.1007/s11934-002-0007-6. [DOI] [PubMed] [Google Scholar]
- 17.Guinan P, Sobin LH, Algaba F, Badellino F, Kameyama S, MacLennan G, Novick A. TNM staging of renal cell carcinoma: Workgroup No. 3. Union International Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) Cancer. 1997;80:992–993. doi: 10.1002/(sici)1097-0142(19970901)80:5<992::aid-cncr26>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 18.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Fiori E, De Cesare A, Galati G, Bononi M, D'Andrea N, Barbarosos A, Izzo L, Bolognese A. Prognostic significance of primary-tumor extension, stage and grade of nuclear differentiation in patients with renal cell carcinoma. J Exp Clin Cancer Res. 2002;21:229–232. [PubMed] [Google Scholar]
- 20.Saidi JA, Newhouse JH, Sawczuk IS. Radiologic follow-up of patients with T1–3a,b,c or T4N+M0 renal cell carcinoma after radical nephrectomy. Urology. 1998;52:1000–1003. doi: 10.1016/s0090-4295(98)00423-3. [DOI] [PubMed] [Google Scholar]
- 21.Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, Caton JR, Jr, Munshi N, Crawford ED. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–1659. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 22.Medical Research Council Renal Cancer Collaborators Interferon-alpha and survival in metastatic renal carcinoma: early results of a randomized controlled trial. Lancet. 1999;353:14–17. [PubMed] [Google Scholar]
- 23.Bukowski RM. Natural history and therapy of metastatic renal cell carcinoma: the role of interleukin-2. Cancer. 1997;80:1198–1220. doi: 10.1002/(sici)1097-0142(19971001)80:7<1198::aid-cncr3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 24.Negrier S, Escudier B, Lasset C, Douillard JY, Savary J, Chevreau C, Ravaud A, Mercatello A, Peny J, Mousseau M, Philip T, Tursz T, Negrier S, Escudier B, Lasset C, Douillard JY, Savary J, Chevreau C, Ravaud A, Mercatello A, Peny J, Mousseau M, Philip T, Tursz T, Groupe Francais d'Immunotherapie Recombinant human interleukin-2, recombinant human interferon alfa2a, or both in metastatic renal-cell carcinoma. N Engl J Med. 1998;338:1272–1278. doi: 10.1056/NEJM199804303381805. [DOI] [PubMed] [Google Scholar]
- 25.Ritchie AWS, Griffiths G, Cook P, Oliver RT, Hancock B, Parmer MK. Alpha interferon improves survival in patients with metastatic renal carci-noma—preliminary results of an MRC randomized controlled trial [abstract] Proc Ann Mtg Am Soc Clin Oncol. 1998;17:31. [Google Scholar]
- 26.Bower M, Roylance R, Waxman J. Immunotherapy for renal cell cancer. QJM. 1998;91:597–602. doi: 10.1093/qjmed/91.9.597. [DOI] [PubMed] [Google Scholar]
- 27.Boccardo F, Rubagotti A, Canobbio L, Galligioni E, Sorio R, Lucenti A, Cognetti F, Ruggeri E, Landonio G, Baiocchi C, Besana C, Citterio G, De Rosa M, Calabresi F. Interleukin-2, interferon-alpha and interleukin-2 plus interferon-alpha in renal cell carcinoma. A randomized phase II trial. Tumori. 1998;84:534–539. doi: 10.1177/030089169808400505. [DOI] [PubMed] [Google Scholar]
- 28.De Mulder PH, Oosterhof G, Bouffioux C, van Oosterom AT, Vermeylen K, Sylvester R, The EORTC Genitourinary Group EORTC (30885) randomised phase III study with recombinant interferon alpha and recombinant interferon alpha and gamma in patients with advanced renal cell carcinoma. Br J Cancer. 1995;71:371–375. doi: 10.1038/bjc.1995.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fossa SD, Martinelli G, Otto U, Schneider G, Wander H, Oberling F, Bauer HW, Achtnicht U, Holdener EE. Recombinant interferon alfa-2a with or without vinblastine in metastatic renal cell carcinoma: results of a European multi-center phase III study. Ann Oncol. 1992;3:301–305. doi: 10.1093/oxfordjournals.annonc.a058185. [DOI] [PubMed] [Google Scholar]
- 30.Kirkwood JM, Harris JE, Vera R, Sandler S, Fischer DS, Khandekar J, Ernstoff MS, Gordon L, Lutes R, Bonomi P, et al. A randomized study of low and high doses of leukocyte alpha-interferon in metastatic renal cell carcinoma: the American Cancer Society collaborative trial. Cancer Res. 1985;45:863–871. [PubMed] [Google Scholar]
- 31.Motzer RJ, Murphy BA, Bacik J, Schwartz LH, Nanus DM, Mariani T, Loehrer P, Wilding G, Fairclough DL, Cella D, Mazumdar M. Phase III trial of interferon alfa-2a with or without 13-cis-retinoic acid for patients with advanced renal cell carcinoma. J Clin Oncol. 2000;18:2972–2980. doi: 10.1200/JCO.2000.18.16.2972. [DOI] [PubMed] [Google Scholar]
- 32.Muss HB, Costanzi JJ, Leavitt R, Williams RD, Kempf RA, Pollard R, Ozer H, Zekan PJ, Grunberg SM, Mitchell MS, et al. Recombinant alfa interferon in renal cell carcinoma: a randomized trial of two routes of administration. J Clin Oncol. 1987;5:286–291. doi: 10.1200/JCO.1987.5.2.286. [DOI] [PubMed] [Google Scholar]
- 33.Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 34.Zisman A, Pantuck AJ, Chao D, Dorey F, Said JW, Gitlitz BJ, de Kernion JB, Figlin RA, Belldegrun AS. Reevaluation of the 1997 TNM classification for renal cell carcinoma: T1 and T2 cutoff point at 4.5 rather than 7 cm better correlates with clinical outcome. J Urol. 2001;166:54–58. [PubMed] [Google Scholar]
- 35.Motzer RJ, Mazumdar M, Bacik J, Russo P, Berg WJ, Metz EM. Effect of cytokine therapy on survival for patients with advanced renal cell carcinoma. J Clin Oncol. 2000;18:1928–1935. doi: 10.1200/JCO.2000.18.9.1928. [DOI] [PubMed] [Google Scholar]