Abstract
Physicians must make decisions about screening patients for abdominal aortic aneurysms (AAAs), monitoring or referring for surgery patients with AAAs of various sizes, and assessing patients with symptoms that may be related to AAAs. This review article analyzes the evidence for each scenario. The effectiveness and cost-effectiveness of screening for AAA is based on results from four randomized controlled trials. A cost-effectiveness analysis using a Markov model showed that ultrasound screening of white men beginning at age 65 is both effective and cost-effective in preventing AAA-related death. Such screening would have a small but real impact over a 20-year period in these men. For patients with a known AAA—which is often detected incidentally—the evidence clearly suggests periodic ultrasound surveillance for those with small AAAs (3.0–3.9 cm in diameter) and elective surgical repair for those with large AAAs (≥5.5 cm). Two recent randomized controlled trials have shown that early surgical repair confers no survival benefit compared with periodic surveillance for patients with intermediate-sized AAAs (4.0–5.5 cm in diameter), so those patients can also be monitored. Some centers choose to increase the frequency of monitoring to every 3 to 6 months when the AAA reaches 5.0 cm. Factors to consider in assessing symptomatic patients include the high risk of life-threatening conditions, the potential increased risk of death or poor outcome with delay in diagnosis, the limitations of ultrasound in identifying whether symptoms are due to known or suspected AAA, and the timely availability of computed tomography or other imaging tests. If available, computed tomography is preferred in patients with recent or severe symptoms, since it is better at detecting retroperitoneal hemorrhage and other complications and in providing preoperative definition of the anatomy.
DEFINITION OF THE DIAGNOSTIC PROBLEM
This review addresses the clinical use of common imaging tests for abdominal aortic aneurysms (AAA) in three clinical situations: screening, surveillance in a patient with an AAA, and evaluation of a symptomatic patient. AAA is a localized dilatation of the abdominal aorta: an aortic diameter >3.0 cm, or 1.5 times the normal diameter of 2.0 cm. The annual incidence of AAA is very small (<1/1000) in those younger than 60 years, peaks to approximately 7 per 1000 among those in their mid 60s, and then diminishes and remains at approximately 3 per 1000. Consequently, approximately 5% to 10% of older adult men have an AAA, but the majority of AAAs are small (1). A recent study found that the prevalence of AAA is approximately 6 times lower in women than in men (2).
A minority of AAAs will expand (3–5). Population-based studies indicate that AAAs that grow typically expand slowly, 0.2 cm/year. The rate of expansion increases with increasing AAA size. AAAs have an asymptomatic period of several years, during which detection is possible. When symptoms are present, they are due to pressure on adjacent structures or changes in the aneurysm wall. AAAs may rupture, resulting in hemorrhagic shock and death. Half or more of patients with a ruptured AAA die before reaching the hospital, and the operative mortality rate for emergency surgery for ruptured AAA is approximately 50% (6, 7). Overall, then, only 10% to 15% of patients with an acute ruptured AAA survive. Asymptomatic AAAs can be effectively repaired by aortic grafting via open surgery; the operative mortality rate is low (6). More recently, endovascular placement of an aortic stent has been used (8–10).
Two large randomized studies have shown lower operative mortality and fewer severe complications with endovascular repair compared with open surgery in patients suitable for both techniques (11, 12). Long-term results are less certain. While endovascular repair did result in a 3% higher aneurysm-related survival at 4 years in the EVAR trial 1, there was no difference in all-cause mortality or health-related quality of life. Moreover, endovascular repair was more expensive and led to a greater number of complications and reinterventions (13).
Tests for screening or case-finding for AAA
AAAs may be detected on physical examination by abdominal palpation and by ultrasonography or computed tomography (CT), as well as other imaging tests including radiography, magnetic resonance imaging (MRI), and angiography. Ultrasonography, a safe, inexpensive, noninvasive test for AAA, has been used to evaluate unselected patients in the community and patients at high risk of AAA in screening programs; it has also been used for case-finding in clinical practice settings. In these programs, patients with large AAAs are referred for elective repair with placement of an aortic graft or endovascular placement of a stent.
Four randomized clinical trials have provided evidence that ultrasound screening is effective in reducing AAA-related mortality but not all-cause mortality (2, 14–18). The first key issue in the use of diagnostic tests for AAA, therefore, concerns translation of the findings from the four randomized trials of ultrasound screening for AAA into clinical practice. Should AAA screening be included in routine preventive services for older men who are currently seeking care (case-finding)? Are community-based screening programs for AAA, modeled after the programs in the published randomized trials, effective and cost-effective?
Tests for surveillance for the patient with an AAA
The widespread clinical use of ultrasonography, CT, MRI, and other abdominal imaging tests often results in the incidental identification of AAAs. Thus, the second common clinical situation in which physicians must make decisions about imaging tests and management is the patient with a known AAA or newly discovered incidental AAA. The risk of rupture is related to the size of the AAA. In AAAs <4.0 cm, the risk of rupture is low, and observation with periodic ultrasonography is appropriate (1, 6). In AAAs >5.5 cm, the risk of rupture is high, and elective repair is indicated (19). Uncertainty about optimal management, especially for patients with intermediate-sized AAAs, led to the development of large multicenter randomized clinical trials of management of patients with AAAs (20). These trials compared immediate surgery with ultrasonography surveillance for patients with AAAs between 4.0 and 5.4 cm and reported no significant difference in outcomes between the two groups (21–23). Thus, the second key issue is the management of the patient with an intermediate-sized AAA. Should these patients be advised to have immediate surgery or periodic follow-up of aneurysm size? If surveillance is selected, what test should be ordered? How accurate are measurements of AAA size? What is the appropriate interval for testing?
Tests for the diagnostic evaluation of the symptomatic patient
The third clinical situation in which physicians must make decisions about ordering and interpreting tests for AAA is the evaluation of the symptomatic patient. For a patient with abdominal pain, flank pain, back pain, or other symptoms that may be due to an expanding or leaking AAA, or for a patient with signs of hemorrhage and shock that may be due to a ruptured AAA, the diagnostic task is to identify whether an AAA is the cause and, if so, then proceed with effective surgical treatment. A patient may be known to have an AAA, and consequently an AAA may be suspected as the cause of the symptoms or shock. However, even if the patient is not known to have an AAA, an AAA should be suspected, and physicians should be prepared to make decisions about ordering and interpreting tests for suspected AAA. The high risk of life-threatening conditions and the potentially increased risk of death or poor outcome if diagnosis and treatment are delayed should be considered when making decisions in this setting. Another key issue is the selection of tests. Ultrasound has limitations in identifying whether symptoms are due to known or suspected AAA, and CT and other imaging tests may not be readily available. Should an ultrasound, CT scan, or other test be ordered? Should hypotensive patients with suspected AAA be sent for testing or proceed immediately to surgery?
DIFFERENTIAL DIAGNOSIS AND PRETEST PROBABILITY
The approach to diagnosis in these three clinical settings differs considerably. In the first instance, the goal is to detect asymptomatic aneurysms efficiently and reduce eventual mortality and morbidity at a reasonable cost to society. Reasoning based on the prior probability of disease in an individual patient and the characteristics of the screening test can be applied to interpret test results. Community-based screening programs have provided information on the prevalence of AAA, the incidence of AAA, and the risk of rupture of AAA as a function of the AAA's diameter (24). In a community setting, information on the prevalence of AAA in the population can be used to estimate the pretest probability of AAA in unselected patients who have never been screened or tested for AAA, and information on the incidence of AAA can be used to estimate the probability of a new AAA in the interval since the last negative test for an AAA in a patient in a program of periodic screening. In addition, a systematic body of high-quality evidence has developed in the literature recently regarding the clinical course of AAA and outcomes of elective repair of asymptomatic AAA and emergency repair of symptomatic or ruptured AAA. Most importantly, recently completed randomized controlled trials of ultrasound screening provide evidence of risks and benefits of screening.
For a patient with an incidentally discovered AAA or with an AAA detected in a screening program, management decisions are based on initial AAA size and growth determined from later measurements. This information provides estimates of the probability of AAA rupture that are used by the clinician and patient to evaluate the risks and benefits of immediate elective repair or continued surveillance. Recently completed randomized controlled trials of immediate surgery, either by open surgery or endovascular placement of an aortic stent, compared with periodic surveillance with ultrasound provide evidence of the risks and benefits of surgery or surveillance that can be used by physicians and patients to make decisions about management.
A patient with a known AAA and abdominal, flank, or back pain due to suspected AAA leak or rupture, or a patient with no known AAA but with symptoms that may indicate a leak or rupture from an AAA, pose a more difficult diagnostic problem. AAAs can cause many symptoms, each with its own differential diagnosis and favored diagnostic tests (Table 1). The case literature is replete with unusual manifestations, but diagnostic predictors have not been evaluated rigorously. Thus, “causal reasoning,” inference from a knowledge of the pathologic anatomy and the time course of rupture, is a useful guide to the diagnostic strategy for a patient with symptoms due to a suspected AAA.
Table 1.
Potential clinical manifestations of AAAs
| Pathophysiology | Related clinical manifestations of AAA |
| Compression of adjacent structures | • Abdominal pain |
| • Chronic flank or back pain, vertebral effacement | |
| • Ureteral obstruction and hydronephrosis | |
| • Infection of the aneurysm wall | |
| Acute or subacute symptoms caused by change in the aneurysm | • Sudden expansion of the aneurysm |
| • Intramural hematoma | |
| • Aortoenteric fistula with intestinal bleeding | |
| • Contained retroperitoneal rupture | |
| • Distal arterial embolism of aneurismal thrombus | |
| • Hypotension | |
| • Lactic acidosis | |
| • Lethargy, coma | |
| Peritoneal free rupture | • Coagulopathy |
| • Multisystem organ failure |
AAA indicates abdominal aortic aneurysm.
Screening or case-finding for AAA
The pretest probability of AAA in the general population is very low but is increased when certain predictive factors are present. These include increasing age, male sex, white race, smoking, family history, history of other vascular aneurysms, hypertension, atherosclerotic diseases, and hypercholesterolemia (25–35). The largest US AAA screening program was conducted in asymptomatic veterans between 50 and 79 years old (33). The prevalence of AAA in the most important risk groups in this study is summarized in Table 2. In screening, fully two thirds of aneurysms detected by ultrasound were found to be <4.0 cm (33).
Table 2.
Association of gender, race, and smoking status with the prevalence of small and medium-sized AAAs among 73,451 US military veterans aged 50 to 79 years*
| Prevalence or pretest probability (%) | ||||
| Race | Sex | Smoking status | >3 cm | >4 cm |
| Smoker | 5.9 | 1.9 | ||
| White | Male | |||
| Nonsmoker | 1.9 | 0.4 | ||
| Smoker | 1.9 | 0.3 | ||
| White | Female | |||
| Nonsmoker | 0.6 | 0 | ||
| Smoker | 3.2 | 0.8 | ||
| Black | Male | |||
| Nonsmoker | 1.4 | 0.1 | ||
*The prevalence of AAAs among black females was not reported due to the low number of black females in the sample of veterans. Adapted from Table 2 in reference 33 with permission from the American College of Physicians.
AAA indicates abdominal aortic aneurysm.
The prevalence of AAA among patients in the health care setting is much higher than the prevalence of AAA in the general population. The risk factors for AAA include smoking, hypertension, hypercholesterolemia, coronary artery disease (myocardial infarction, angina pectoris), cerebrovascular disease (stroke, cerebrovascular ischemia), peripheral vascular disease, and intermittent claudication. Because of the morbidity associated with these conditions, patients with these factors are higher users of health services in the ambulatory and hospital setting. Thus, the pretest probability of AAA during physical examination and in case-finding when ultrasound or other imaging tests are ordered to identify an AAA in patients seeking care is increased. In one study, 9% of men aged 60 to 75 years with hypertension or coronary artery disease had an AAA >3.5 cm in diameter (36). Further, the high use of imaging procedures often results in the incidental identification of an AAA.Indeed,one study found that the majority of AAAs were incidentally identified (37).
Symptomatic patients with suspected AAA
The pretest probability of AAA in self-referred patients in emergency departments is very low: a diagnosis of AAA was made in only 4 of 21,902 patients included in a 1996 national probability sample of emergency department visits (38). About 1% of emergency department patients with abdominal pain were ultimately found to have an AAA in two studies, one based on 2-month follow-up of adult patients (39) and the other based on retrospective analysis of claims data (40). Another study of patients 65 years or older found a pretest probability of 1.5% (41). Among older patients, the main competing diagnoses, determined at 2-month follow-up, were nonspecific abdominal pain with a 30% prevalence and biliary colic, small bowel obstruction, and gastritis, each with about a 10% prevalence (41).
DIAGNOSTIC TESTS
Compared with studies of therapy, studies of diagnostic tests have been of variable quality (42). The diagnosis of AAA illustrates this problem well: asymptomatic aneurysms have been studied properly with large unbiased samples, but studies of tests for predicting aneurysm in symptomatic patients have been limited by small sample size and biased by failure to blind outcome status, by spectrum bias (failure to sample the whole spectrum of patients in whom AAA is ordinarily considered in the differential diagnosis), and by verification bias (limited use of the gold standard test among patients without the predictive finding of interest) (43).
Clinical examination
Asymptomatic patients. Among clinical findings potentially predictive of AAA in asymptomatic patients, only a palpably widened aorta above the umbilicus has been evaluated against an independent reference standard, usually ultrasound, by several different investigators (44). The technique involves placing one thumb along each side of the epigastric aortic pulsation. Since the umbilicus marks the aortic bifurcation, the presence of a pulsatile mass >3 cm wide in the epigastrium suggests an aneurysm and should be followed by a more accurate confirmatory test such as ultrasound or CT scan. An especially forceful aortic pulsation in the epigastrium is a common, sometimes startling finding that does not by itself imply aortic widening or call for confirmatory testing.
A single report examined the reproducibility of abdominal palpation. Two examiners agreed on the presence of an AAA 77% of the time, with agreement beyond chance rated “fair to good” (kappa = 0.55) (45). A systematic review of physical examination for AAA found that abdominal palpation was the only maneuver of value in detecting an AAA (44). The presence of a palpable mass has an overall sensitivity of approximately 39%, with a sensitivity of 29% for AAAs 3.0 to 3.9 cm, 50% for AAAs 4.0 to 4.9 cm, and 76% for AAAs ≥5.0 cm. The pooled likelihood ratio for abdominal palpation is 15.6 for AAAs >4 cm in diameter and 12.0 for AAAs >3 cm in diameter. Obesity substantially limits the effectiveness of abdominal palpation, and even large aneurysms are often not palpable in obese patients (abdominal girth >100 cm). A community-based AAA screening program that used ultrasound reported the sensitivity and specificity from abdominal palpation for an AAA >3.5 cm; data from this study indicate a lower likelihood ratio, 6.33 for abdominal palpation to detect an AAA >3.5 cm (46). The absence of a palpable aortic widening does not rule out AAA in any case: the negative likelihood ratio was 0.51 for AAAs >4.0 cm and 0.71 for AAAs >3.0 cm. Table 3 illustrates the hypothetical predictive values of abdominal palpation applied to the age and sex groups with AAA prevalence rates found in the VA study.
Table 3.
Posttest probability of AAA given the finding of a widened aorta on physical examination in various asymptomatic clinical subgroups*
| Posttest probability (%) | ||||
| Race | Sex | Smoking status | >3 cm | >4 cm |
| Smoker | 43 | 23 | ||
| White | Male | |||
| Nonsmoker | 19 | 6 | ||
| Smoker | 19 | 4 | ||
| White | Female | |||
| Nonsmoker | 7 | <1 | ||
| Smoker | 28 | 11 | ||
| Black | Male | |||
| Nonsmoker | 15 | 2 |
*Based on prevalence estimates from Table 2 and the pooled likelihood ratios of 12.0 and 15.6 for size thresholds of 3 and 4 cm, respectively (33, 44).
AAA indicates abdominal aortic aneurysm.
Analyses of the cost-effectiveness of AAA screening must account for the reduced effectiveness of physical examination and other tests in ordinary practice compared with the efficacy determined in the clinical research environment. Fewer than half of the patients in one study who had visited a physician in the previous 6 months had their aneurysm detected in the course of routine medical care. In a population-based AAA screening study that included abdominal palpation for AAA, only 20% of AAAs were detected by physical examination (46).
A large population-based study of ultrasound screening for AAA, defined as aorta diameter >3.5 cm or distal-to-proximal aortic diameter ratio >1.5, identified several items from the medical history and physical examination that were significant predictors for an AAA (46). Table 4 summarizes the individual characteristics of these factors. Abdominal palpation had the highest likelihood ratio for a positive test of 6.3, lower than the pooled value in the systematic review (44).
Table 4.
Risk factors for AAA identified in a population-based screening study*
| Factor | Sensitivity (%) | Specificity (%) | Likelihood ratio for positive | Likelihood ratio for negative |
| Male sex | 81 | 59 | 1.98 | 0.32 |
| Age 55–65 years | 22 | 43 | 0.39 | 1.81 |
| Age 66–75 years | 38 | 62 | 1.00 | 1.00 |
| Age >75 years | 39 | 81 | 2.05 | 0.75 |
| Current smoking | 38 | 79 | 1.81 | 0.78 |
| Antihypertensive drug use | 36 | 70 | 1.20 | 0.91 |
| Angina pectoris | 11 | 94 | 1.83 | 0.95 |
| Intermittent claudication | 5 | 99 | 5.00 | 0.96 |
| History of myocardial infarction | 17 | 94 | 2.83 | 0.88 |
| Cholesterol ≥6.5 mmol/L | 57 | 46 | 1.06 | 0.93 |
| Ankle-arm index ≤0.9 | 29 | 89 | 2.64 | 0.80 |
| Enlarged aorta on palpation | 19 | 97 | 6.33 | 0.84 |
| Bruit over abdominal aorta | 14 | 96 | 3.50 | 0.90 |
*AAA was defined as an aortic diameter >3.5 cm. Columns 1 to 3 from Table 2, reference 46; used with permission. Likelihood ratios determined based on study data.
AAA indicates abdominal aortic aneurysm.
Symptomatic patients. The first-contact physician fails to identify imminent aortic rupture about half the time (47, 48). Abdominal palpation alone was abnormal in roughly half of cases of rupture that presented to an internist (49). In two recent studies, the most common misdiagnoses were myocardial infarction, muscle pain, nonspecific abdominal pain, renal colic, and perforated ulcer. The sensitivities of various clinical findings for rupture in one case series are noted in Table 5 (47). Conversely, when the clinical examination did predict rupture, the clinician was in error 60% of the time (50, 51).
Table 5.
Sensitivity of clinical findings for AAA rupture in a series of 97 patients*
| Finding | Sensitivity (%) |
| Pulsatile mass | 69 |
| Abdominal pain | 53 |
| Back pain | 40 |
| Syncope | 23 |
| Groin pain | 13 |
| Flank pain | 10 |
| Ultrasound showing rupture | 51 |
*From reference 47.
AAA indicates abdominal aortic aneurysm.
Given the paucity of high-quality diagnostic test studies for symptomatic AAAs, the causal sequence leading to rupture may help in discriminating this entity from similar syndromes (6, 52). First, the aneurysm ruptures into a yielding retroperitoneal space, accompanied by severe abdominal and/or back pain and transient hypotension, tachycardia, weakness or syncope, but sometimes without complete intravascular collapse. In about half of cases, the posterior parietal peritoneum tamponades this hemorrhage, preventing immediate exsanguination. Patients with ruptured AAAs may even have normal vital signs due to homeostatic mechanisms. Retroperitoneal bleeding may provoke misleading symptoms such as back pain radiating into the groin, and these symptoms may dominate the clinical presentation. Later the patient suddenly collapses when the peritoneum yields to pressure and intraperitoneal rupture occurs. If a patient with an AAA rupture arrives in the emergency department after the initial rupture, the prior events may not be obvious if the patient has the nonspecific findings of hemorrhagic shock: stupor or coma, hypotension, lactic acidosis, disseminated intravascular coagulation, and multiorgan failure. Thus, ruptured AAA may not be suspected, and the diagnosis may be delayed or missed. Perioperative mortality after rupture is between 30% and 70% and often occurs after prolonged critical care (7, 53–55). Since about 50% of patients with ruptured AAA die without coming to medical attention, the overall community case-fatality rate of rupture is about 80% (48, 56, 57).
Abdominal ultrasound and computed tomography
Asymptomatic patients. Among asymptomatic patients, ultrasound detects the presence of AAA reproducibly, accurately, and inexpensively. Sensitivity and specificity are both close to 100% when compared with operative findings. In about 1% to 3% of the time, the aorta cannot be visualized because of bowel gas or obesity (58–60). In general, ultrasound is an ideal test for mass screening (61).
However, ultrasound is imprecise in measuring aneurysm size (62–65). Size is important for two reasons: as a single component of prognosis (66) and as a baseline to determine the aneurysm growth rate, another component of prognosis (67). A growth rate of >0.7 cm per 6 months or 1 cm per year has been suggested as a threshold for proceeding to surgery irrespective of aneurysm size (35, 68–70), though no population-based studies have demonstrated that the rate of growth influences rupture risk independent of aneurysm size. One study reported no AAA ruptures (0% risk; 95% confidence interval [CI], 0%–6%) in 32 patients with 50 patient-years of follow-up after rapid growth of >0.5 cm over 6 months but with an AAA diameter <5.5 cm (71).
CT is somewhat more reproducible than ultrasound, with fewer than 10% of remeasurements being >2 mm off from the initial reading when a magnifying glass and calipers are used rather than cursor placement (72). Ultrasound assessment of maximum diameter is on average slightly less reproducible and also 2.7 mm smaller than CT scan readings. Favoring ultrasound, however, is the observation that the cross-section of a tortuous aorta may not be in the transverse plane, causing axial CT images to exaggerate the aneurysm's maximum diameter. The advantages of portability, no need for intravenous contrast dye injection, and decreased expense make ultrasound the preferred diagnostic method for demonstrating the presence or absence of an aneurysm and for follow-up of asymptomatic aneurysms (61, 72–76).
The actual performance characteristics of ultrasonography as a screening test in the community setting have not been directly assessed against the standard of operative measurement of size of the aorta. Data from a community-based ultrasound screening program for AAA have been analyzed to provide indirect estimates of the characteristic of ultrasound screening for AAA.
In the Viborg County, Denmark, AAA screening study (74), men aged 65 to 73 were invited for a hospital-based ultrasound screening examination. Of the 5470 men invited, 4176 (76.3%) attended. The mean ± standard deviation of the proximal and distal aorta was 18.4 ± 2.45 mm and 17.9 ± 2.92 mm, respectively.
In 50 men, two observers measured the aortic diameter, and the difference in the measurements of the proximal aorta was –0.88 ± 1.76 cm and the difference in the distal aortic diameters was –0.10 ± 0.84 cm. The interobserver agreement was used to estimate the sensitivity and specificity of ultrasound examination for the presence of an aortic aneurysm, defined as an aortic diameter >3.0 cm. The sensitivity, specificity, and the likelihood ratios are summarized in Table 6. Ultrasound measurement of the distal aorta has a positive likelihood ratio of approximately 500 and a negative likelihood ratio of approximately 0.01, thus providing evidence that ultrasound measurement is appropriately considered the standard for the presence of an AAA.
Table 6.
Estimated characteristics of ultrasound screening for AAA in a community screening program*
| Measurement | Sensitivity (%) | Specificity (%) | Likelihood ratio for positive test | Likelihood ratio for negative test |
| Proximal aorta | 87.4 | 99.9 | 874 | 0.126 |
| Distal aorta | 98.9 | 99.8 | 494.5 | 0.011 |
*Data from reference 74.
AAA indicates abdominal aortic aneurysm.
Symptomatic patients. In symptomatic patients, ultrasound may be able to exclude AAA by identifying other causes of abdominal pain, including cholecystitis, renal colic, urinary retention, certain abdominal masses, pancreatitis, and a variety of other diagnoses. However, ultrasound is not very sensitive for detecting aortic rupture or other complications of AAA such as aneurysm inflammation or infection. In one case series, ultrasound examination failed to detect 49% of ruptures (47). However, ultrasound has the advantage of rapid availability in many institutions, especially when performed by the emergency physicians caring for the patient. In one large emergency department, the presence of AAA >3 cm (not ruptured) was determined with 100% sensitivity and 98% specificity among 125 patients with a 25% prevalence of AAA (77). However, attesting to the nonfasted nature of emergency patients, another study found that although emergency department physicians detected all aneurysms in this sample, they were unable to visualize a third or more of the abdominal aortas in 8% of 207 patients (78). Ultrasonography is a poor modality for imaging the aorta before surgery since it may not be able to detect such important features as accessory or anomalous renal arteries, the superior extent of the aneurysm, and the coexistence of iliac occlusive disease, each of which can modify the surgical approach.
In one study of patients in whom AAA rupture was clinically suspected, CT scan was found to be 90% sensitive and 91% specific (based on our reanalysis of tabulated data), as well as being a good modality for identifying other sources of symptoms, such as splenic infarction (51). However, in a British study of 653 patients with suspected rupture, 74 patients in whom the diagnosis was “in doubt on clinical grounds alone” had an urgent CT prior to surgery; CT scans in these patients with suspected rupture had a sensitivity of 79% and a specificity of 77% (79). In recent reports, multidetector CT without contrast demonstrates periaortic extravasation of blood and sometimes a “crescent sign” thought to predict impending rupture on the strength of case series and histological evidence (80, 81). Although these are preliminary studies, rapid high-resolution CT scan without contrast may have excellent test characteristics for AAA complications that require immediate surgery.
Other imaging methods
Plain films sometimes demonstrate an AAA unexpectedly when an aneurismal dilatation is outlined by aortic calcifications. This finding is reported as being 50% sensitive, and intuition suggests that it is close to 100% specific (82). The low sensitivity and concomitant low negative predictive value limit the value of this method, and it cannot be recommended for ruling out an AAA.
Aortography is an accurate and traditional way of providing a preoperative map of the vascular anatomy for elective surgical cases, but it is not sensitive in the detection of AAA because luminal thrombus may obscure the aortic dilatation. Many vascular surgeons will operate after only a CT scan, which can also image the iliac arteries. But like ultrasound, CT may also fail to image accessory renal arteries or to accurately localize the origins of the renal arteries or the proximal extent of the aneurysm. Nonetheless, a policy of selective angiography in one center led to a replacement of angiography by CT in 80% of elective aneurysm repairs (83).
CT angiography with three-dimensional reconstruction is a relatively new way of outlining the vascular anatomy but is more complex than traditional axial tomography. Contrast injection requires a secure peripheral intravenous line, breath-holding, and meticulous timing. Reconstructions may require prolonged computer processing on older machines to generate a satisfactory shaded-surface or other specialized display of the vascular anatomy. It is thus not usually suited for emergencies but is important for tailored stent-graft placement, a procedure sometimes performed by invasive radiologists and surgeons in collaboration (84).
Numerous imaging methods have been used for the preoperative evaluation of aortic anatomy, including MRI, magnetic resonance angiography, intravenous digital subtraction angiography, and duplex ultrasound (76, 85–88). The selection of imaging tests for preoperative evaluation of the patient with an AAA is less relevant to the common diagnostic problems faced by most practicing generalists but is important in deciding between stent-graft and traditional operative repair (89). Decisions about preoperative evaluation also depend on the local availability of imaging modalities and the experience and expertise of the radiologists and others involved in care of patients with AAA and should be made in consultation with the vascular surgeon.
MANAGEMENT STRATEGIES FOR ASYMPTOMATIC PATIENTS WITHOUT A KNOWN AAA
AAA screening programs using ultrasonography have been established in a community setting (26, 90, 91) as well as in medical practice settings (case-finding) (37, 92, 93). Ultrasound has been used in selected patients with family history (27, 29, 30, 34) or other risk factors for AAA (94). Ultrasound has also been used to detect AAA in patients with risk factors, including cardiovascular, cerebrovascular, and peripheral vascular disease (25, 28, 31, 95, 96). Published reports have documented the acceptability of ultrasonography screening for AAA, the prevalence of AAA by size in populations, adherence to recommendations for periodic surveillance for small or intermediate-sized AAAs, and adherence to recommendations for elective open surgical repair of large aneurysms at high risk of rupture. Most of these early reports were demonstration programs and lacked a comparison or control group.
Published analyses of the expected cost-effectiveness of ultrasound screening programs for AAA have concluded that they will be effective in reducing AAA-related mortality and, moreover, that ultrasound screening for an AAA would generally cost <$50,000 per quality-adjusted life-year (QALY) saved (27, 58, 97–102). Controlled trials of AAA screening have now convincingly demonstrated that AAA screening can reduce AAA-related mortality in men (15, 16, 18, 103, 104). Studies have also demonstrated the low yield of AAA on rescreening, suggesting that a single scan is sufficient (105, 106). These studies provide evidence for the benefits of ultrasound screening for AAA and identify many of the key factors that influence its effective implementation.
Selective screening for AAA
The identification of risk factors for AAA led to the evaluation of methods to improve the detection of AAA by selective screening of high-risk groups. One approach to identifying individuals with a high pretest probability is the use of a multivariate prediction score. A Canadian hospital-based case-control study of 78 men with AAA and 99 male controls identified risk of AAA using age, smoking, blood pressure, body mass index, history of heart disease, and serum high-density lipoprotein. The study found that if AAA risk was based on these clinical factors, 80% of AAA cases could be identified by studying 17% of patients, suggesting the feasibility of selective screening using predictors of AAA risk (107).
The Viborg County, Denmark, AAA screening program study used data from 4404 men, aged 65 to 73 years, to identify men at high risk of AAA. The six high-risk groups were men with hypertension, chronic obstructive airway disease, previous myocardial infarction, angina pectoris, lower limb atherosclerosis, previous stroke, or transient cerebral ischemia. Selective screening of these high-risk groups would have required screening only approximately 20% of men and would have identified approximately half of the AAA cases. The authors concluded that if AAA screening is desirable, mass screening is recommended because selective screening would miss approximately half of the AAA cases (108).
A study from the Western Australia AAA screening program of men aged 65 to 83 developed a multivariate AAA risk score from the first 8995 men and tested the AAA risk score on the remaining 2755 who were screened. The AAA risk score was based on age, height, place of birth, AAA in sister, smoking history, myocardial infarction, angina, hypertension, hypercholesterolemia, and regular vigorous daily exercise. Approximately half of the men had a high risk based on the score; screening these individuals identified 75% of the AAA cases. The authors concluded, however, that although selective screening is feasible, it is “not worthwhile” because approximately 25% of clinically significant AAAs would be missed (109).
Analysis of population-based ultrasound screening of AAA among men aged 55 or older from the Rotterdam study in the Netherlands was used to evaluate four strategies for selective AAA screening using multivariate prediction of AAA risk. The AAA prediction was based on age, sex, smoking, hypertension, hypercholesterolemia, previous myocardial infarction, angina pectoris, intermittent claudication, blood pressure, enlarged aorta by palpation, bruit over abdominal aorta, and reduced ankle-brachial index. The individual value of these predictors is summarized in Table 4. Screening approximately half of the men—those with at least a 1.5% risk of AAA based on the prediction score—could identity 94% of men with AAA (47). This approach would have, therefore, missed only 6% of the population who had an AAA. If these findings are validated in other settings, then selective screening of high-risk men may be an acceptable approach when resources for ultrasound screening are limited.
Screening programs for AAA: studies with nonrandomized controls
One of the first controlled evaluations of an ultrasound screening program for AAA compared outcomes as ultrasonography screening for AAA was implemented in a United Kingdom community practice over several years (110, 111). The study population consisted of all men older than 50 years registered with a general practitioner in the community. In this study, men in the community were initially in the control group and became eligible for the screening group when they were invited to participate in the screening program. The study subjects thus served as their own controls. The analyses compared the aggregate experience of the screened patient-years with the control patient-years. Although each study subject's time in the control group preceded his time in the screening group (individually nonconcurrent controls), ultrasound screening was implemented over several years, and groups of screened and control study subjects were followed concurrently in the community. In total, 18,059 men were invited and 13,466 men (75%) were screened during the study. The incidence of AAA rupture in the unscreened population was compared with the rates in the screened population.
There were 24 ruptured AAAs in the screening group with 92,939 person-years of follow-up, for a rate of 2.6 per 10,000 person-years (95% CI, 1.7–3.8), and 69 ruptured AAAs in those not invited for screening, representing 96,799 person-years of follow-up, for a rate of 7.1 per 10,000 person-years (95% CI, 5.6– 8.9). Thus, screening was associated with a 64% reduction in the incidence of ruptured AAAs. Ninety-eight elective AAA repairs were performed as a result of screening, with four perioperative deaths. Fifty-two deaths due to the aneurysms were expected in the screening group compared with the 17 observed. The investigators recorded screening and hospital costs and used estimates of background incidence of ruptured AAA in the population and life tables to estimate that 610 men would have to be invited for screening to save one life (“number needed to screen”), and that three elective operations on average would be needed to save one life. The study estimated that the total cost was $1173 per life-year gained in the first 5 years and $425 per life-year gained in the second round of screening (converting pounds to 1995 US dollars).
Screening programs for AAA: randomized controlled trials
Four randomized clinical trials of ultrasound screening for AAA with concurrent controls have been reported in the medical literature (Table 7).
Table 7.
Summary of four randomized controlled trials of ultrasound screening for AAA
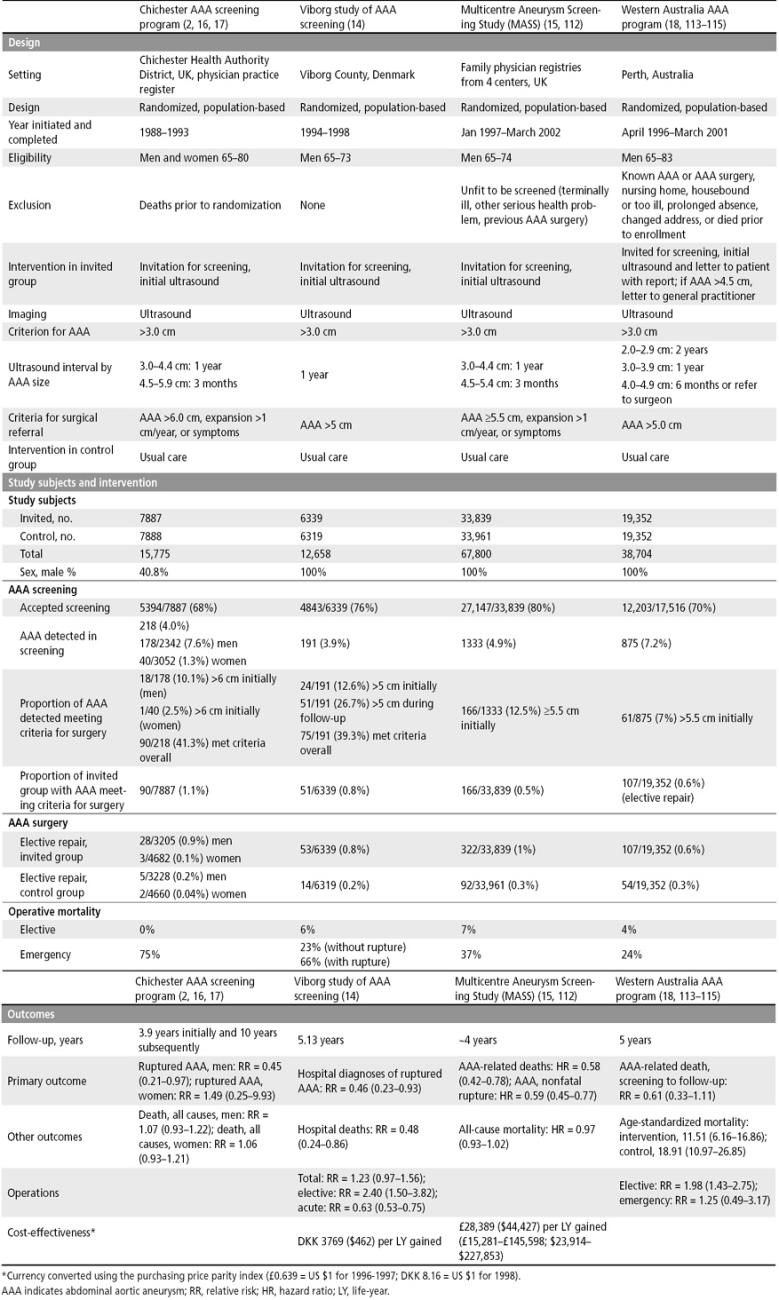
Chichester AAA screening program. The first published randomized controlled trial of ultrasound screening for AAA was conducted from 1988 through 1993 and first published in 1995 (2, 16, 17). In this study, 15,775 men and women, aged 65 to 80, were randomized to a group that was invited for ultrasonography screening for AAA or an age- and sex-matched control group. Patients with an aneurysm 3.0 to 4.4 cm in diameter were re-scanned annually, and those with an aneurysm 4.5 to 5.9 cm in diameter were rescanned every 3 months. Patients were referred for surgical repair if the diameter of their aneurysm measured >6 cm or increased >1 cm per year or if they developed symptoms. Of the 7887 invited for screening, 5394 (68.4%) accepted. An AAA was detected in 218 (4.0%) overall, including 178 of 2342 men (7.6%). Ninety of the 218 persons with AAA met criteria for surgical repair; 35 of the 90 (39%) had surgery.
During the 5-year follow-up, there were 9 ruptured AAAs in the 3205 men who were invited (0.28%) compared with 20 ruptured AAAs in the 3228 men in the control group (0.62%). This 55% reduction in the incidence of ruptured AAA in men was statistically significant. There was no significant reduction in the incidence of ruptured AAA in women. After 5 years, the mortality was 13% in the group invited for screening and 12.5% in the control group. The odds ratio for incidence of ruptured AAA recalculated using the data reported in the study is 0.45, with 95% confidence limits of 0.18 to 1.04 by the exact method, and 95% confidence limits of 0.21 to 0.97 by Cornfield's method, so the reduction in the incidence of ruptured AAA was at the border of statistical significance. After 10 years of follow-up, there was a 21% reduction in the mortality of AAA, relative risk 0.79 (95% CI, 0.53–1.40) (17). A separate report of ultrasound screening in women from this study found that the prevalence of AAA was 6 times lower in women than in men and that over 5- and 10-year follow-up the incidence of rupture was the same in the screened and control groups of women (2).
Among those invited for screening, the overall mortality rate was 10.4% in the 5394 men and women who accepted screening and 19% in the 2493 men and women who refused to participate in the screening program. This study found that those study subjects who were invited and accepted the invitation were healthier (they had a lower overall mortality rate) than the persons who refused the invitation to participate. If a study reported only outcomes in those who were invited and accepted screening compared with the outcomes in a control group, there would be a selection bias in favor of screening. Valid inferences can only be based on overall comparisons of the entire invited group with those not invited: an “intention to screen” comparison. In both men and women, AAA accounted for only 2% to 3% of all deaths over the 5-year study. Among men, ruptured AAA accounted for only 9 of the 532 deaths (1.5%) in those invited for screening and only 16 of the 508 deaths (3.1%) in the control group. This study provided a realistic estimate of the expected small difference in total mortality that may result from ultrasound screening for AAA; accordingly, subsequent studies of ultrasound screening for AAA have not been designed to demonstrate significant reductions in all-cause mortality.
Viborg study of AAA screening. The second randomized trial of ultrasound screening for AAA was conducted from 1994 through 1998 in Viborg, Denmark, and reported in 2002 (14). All 12,658 men in the county aged 65 to 73 years were randomly allocated to a group that was invited for abdominal ultrasound scanning at the regional hospital or to a control group. Ultrasound examinations were scheduled at 5-minute intervals. Men with aneurysms 3 cm or more in diameter were invited to consult with a doctor for information and were offered yearly follow-up examinations. Men with aneurysms 5.0 cm or more in diameter were referred to a vascular surgeon. There were 6339 men in the group invited for abdominal ultrasound screening and 6319 men in the control group. Of the 6339 men invited for screening, 4843 accepted (76%). Of these, 191 (4%) had an AAA, and 24 (0.5%) had an AAA >5 cm. During the 5-year follow-up, an additional 51 men were referred for surgery. Fifty of the 75 men (67%) who were referred for surgery had an elective AAA repair.
The hospital outcomes after 5 years in the entire group referred for screening were compared with the hospital outcomes for the control group. There was a 70% reduction in the number of ruptured AAAs, a 74% reduction in the number of emergency operations for AAAs, and a 68% reduction in hospital deaths for AAAs. Overall, there was a 51% increase in the total number of operations and a 278% increase in the number of elective operations for AAAs. A limitation of this study is that it was restricted to hospital mortality; nevertheless, all the outcomes were statistically significant.
The investigators used information on the costs of the screening, surveillance, elective surgery, and emergency operations, estimates of the proportion of AAAs occurring in the hospital, and estimates of remaining life expectancy (9 years) to calculate the cost per prevented hospital death and cost per life-year saved. The costs, converted to 1998 US dollars using the purchasing price parity index (DKK 8.16 = US $1 for 1998) were $8316 per prevented hospital death and $923 per life-year saved by the prevention of hospital AAA deaths. The investigators estimated that half of the AAA deaths prevented occurred in the hospital, so that overall the cost was $4158 per prevented death and $462 per life-year saved.
Multicentre Aneurysm Screening Study (MASS). The third randomized trial of ultrasound screening for AAA, the Multi-centre Aneurysm Screening Study (MASS), was conducted in four centers in the UK from January 1997 through May 1999 and reported in 2002 (15). Men aged 65 to 74 from family physician lists and health authority lists whose family doctors considered them fit for screening and gave permission for screening were randomly allocated to ultrasound screening or a control group. Men with aneurysms 3.0 to 4.4 cm in diameter were rescanned yearly, and men with aortic diameter of 4.5 to 5.4 cm were re-scanned at 3-month intervals. The patients with aneurysm >5.5 cm in diameter were referred for a vascular surgery consultation. The invited group consisted of 33,839 men, and the control group consisted of 33,961 men. Of the 33,839 men invited for screening, 27,147 (80%) accepted the invitation for screening. Of the 1333 AAAs detected, 166 (12%) were ≥5.5 cm.
The primary endpoints were total AAA-related deaths and nonfatal ruptured AAAs. There were 65 AAA-related deaths in the invited group, 0.49 deaths per 1000 person-years (95% CI, 0.39–0.63) compared with 113 AAA-related deaths in the control group, 0.85 per 1000 person-years (95% CI, 0.71–1.02). There was a significant reduction in AAA-related deaths, a hazard ratio of 0.58 (95% CI, 0.42–0.78). There were 17 nonfatal ruptured aneurysms in the invited group, 0.62 per 1000 person-years (95% CI, 0.50–0.77), compared with 27 nonfatal ruptured AAA in the control group, 1.06 per 1000 person-years (95% CI, 0.89–1.25). There was a significant reduction in nonfatal ruptured AAA: a hazard ratio of 0.59 (95% CI, 0.45–0.77). The all-cause mortality was 28.3 deaths per 1000 person-years in the invited group (95% CI, 27.5–29.3) compared with 29.1 per 1000 person-years in the control group (95% CI, 28.2–30.0). This was not statistically significant, with a hazard ratio of 0.97 (95% CI, 0.93–1.02). Overall, AAAs accounted for only 2% to 3% of deaths—65 of 3750 (2%) in the invited group and 113 of 3855 (3%) in the control group.
In the invited group, the 27,147 men who participated in the screening were generally healthier than the 6692 men who refused to participate: the men who participated had lower AAA-related deaths (0.40 vs 0.88 per 1000 person-years), a lower incidence of ruptured AAAs (0.53 vs 1.0 per 1000 person-years), and overall lower all-cause mortality (24.1 vs 46.4 per 10,000 person-years).
A separate report of the cost-effectiveness of screening for AAA was based on the 4-year results from the MASS randomized controlled trial (112). To facilitate comparison between published studies and the cost-effectiveness analysis conducted for this review, the costs reported in this study were converted to US dollars using the purchasing price parity index (£0.639 = US $1 for 1996–1997). Over 4 years, there were 47 fewer deaths related to AAA in the screening group than in the control group, but the additional costs incurred were £2.2 million. After adjustment for censoring and discounting the costs at 6% and the life-years at 1.5%, the mean additional cost of the screening program was $97.77 per patient, the mean incremental cost-effectiveness ratio of screening was £28,389 ($44,427) per life-year gained (95% CI, £15,281–£145,598; $23,914–$227,853). After 10 years, this figure was estimated to fall to approximately £8000 ($12,519) per life-year gained. In interpreting the cost-effectiveness ratio, however, it is important to note that it is customary to discount both costs and effectiveness (life-years) at the same rate (116), and with a 3% discount rate for both costs and effectiveness, the cost-effectiveness ratio was £30,784 ($48,175). Additionally, the MASS study measured quality of life using the EQ-5D instrument, and study subjects reported a quality of life of approximately 0.80 on this scale, so the cost-effectiveness of AAA screening was about £36,000 ($56,388) per QALY. The MASS study group concluded that although the cost-effectiveness of screening for AAA was at the margin of acceptability according to the current guidelines of the National Health Service, over a longer time the cost-effectiveness would improve substantially.
Western Australia AAA program. The fourth randomized trial of ultrasound screening for AAA was conducted in Perth, Australia. Men aged 65 to 83 residing in the Perth metropolitan area and not living in a nursing home were randomly allocated to a screening group or a comparison group. The men in the screening group were invited by mail to participate in an AAA screening program by attending a prearranged screening examination at one of five centers nearest to their home. After the screening, men were given a letter with the results and a letter for their general practitioners; a letter was also sent directly to the general practitioner for men with an AAA >4.5 cm. The men in the comparison group were not contacted and received their usual care. Screening occurred from April 1996 through March 2001.
There were 19,352 men randomly allocated to each group. In the screening group, 17,516 subjects were eligible for the study, and 12,203 (69.7%) participated. An AAA was detected in 875 men (7.2%), and 61 (7%) had an aneurysm >5.5 cm. Between screening and follow-up, 107 (0.6%) of the 19,352 men in the screening group and 54 (0.3%) of the 19,352 men in the control group had AAA repair.
The relative risk of death from AAA between screening and follow-up was 0.61 (95% CI, 0.33–1.11); however, the relative risk of death from AAA between randomization and follow-up was 0.85 (95% CI, 0.53–1.36). These relative risks were not statistically significant. An analysis restricted to men aged 65 to 74 years and comparable to the age group in the MASS study yielded a relative risk of AAA death of 0.19 (95% CI, 0.04–0.89) from scheduled screening to follow-up and 0.82 (95% CI, 0.37–1.84) from randomization to follow-up. The authors concluded that any benefit from AAA screening resulted from screening this subgroup. The authors further stated that the success of screening will depend on excluding ineligible men and minimizing the delay between becoming eligible for screening and actual screening and that it is necessary to assess the rate of elective surgery in the community before implementing a population-based screening program as, in some cases, this rate may approach a level that reduces the potential benefit of population-based screening (115).
Translating randomized controlled trials of AAA screening into clinical practice
In the randomized controlled trials of ultrasound screening for AAA, an AAA was identified in 4% to 7.6% of men screened, and 6.6% to 12.6% of AAAs met criteria for surgery. Ultrasound screening was shown to be effective in reducing AAA-related mortality but not in reducing overall mortality. AAA accounted for only 2% to 3% of deaths in men at risk of developing AAAs, and the studies were not designed to demonstrate a statistically significant reduction in overall mortality. The challenge is to translate findings from these randomized controlled trials into clinical practice. The factors that must be considered are the AAA incidence; the AAA prevalence; the clinical course of AAA; the risk of elective, urgent, and emergency surgery for AAA; the design of the AAA screening program; the costs of screening and treatment for AAA; the life expectancy and risk of non-AAA mortality; and the quality of life after elective, urgent, or emergency surgery.
AAAs have a high annual incidence in older men. At any one time, approximately 5% to 10% of older men have an AAA. Most AAAs, however, are small and are unlikely to become clinically symptomatic or rupture during the person's lifetime (66). Only a minority of AAAs will grow (at a rate of 0.3 to 0.4 cm/year), and the rate of growth increases with increasing AAA size. The costs of ultrasound screening for AAAs may be borne by hospitals, clinics, and other organizations that provide the personnel, equipment, and space for AAA screening. These costs may be covered in whole or in part by the payments for routine primary care for older adults.
The risk of AAA repair (risk of operative mortality and morbidity from complications arising from elective AAA surgery) are borne by those with an AAA that is detected and who agree to have surgical repair. The benefits of longer life accrue to those whose AAA is detected and repaired and whose AAA would have become symptomatic or ruptured during their lifetime. Many AAAs are detected incidentally during usual care as a result of physical examination or incidental abdominal imaging with radiography, ultrasonography, CT, MRI, or other imaging modalities. Furthermore, 20% to 25% of adults who are invited to have ultrasound screening for AAAs refuse to participate, and surprisingly less than half of men with a large AAA that meet criteria for elective repair based on AAA size or growth ultimately have elective AAA surgery. The low rate of elective AAA surgery reflects both patient preferences and the comorbid conditions that make patients unfit for surgery. Higher participation in screening and higher adherence to recommendations for elective repair may occur with endovascular repair of AAA, which has a lower operative mortality rate.
Thus, efforts to translate the findings from the randomized controlled trials of ultrasound screening for AAA, either in AAA screening as part of preventive services for adult men or in community-based AAA screening programs, must consider patient acceptance of screening, adherence to recommendations for elective surgery when criteria based on AAA size or growth are met, and incidental discovery of AAA and early surgery for incidental AAA that may not meet criteria for surgery based on AAA size or growth. Analyses must, of course, consider the clinical course of AAA, risks of AAA surgery, and the costs of screening, surveillance, and health services for surgery and follow-up care of patients with AAA. In these circumstances, decision analysis is an especially useful method for evaluating the effectiveness and cost-effectiveness of alternative methods of incorporating ultrasound screening for AAA into the primary care of ambulatory adults. We therefore developed a decision analysis model to evaluate the costs and effectiveness of ultrasound screening for AAA in a community setting.
Description of a decision analysis model and method of analysis
A Markov model of the clinical course of AAAs was used to analyze the implementation of ultrasound screening for AAA based on the findings of the randomized controlled trials of ultrasound screening for AAA. The cost-effectiveness of ultrasound screening was compared with usual care, and factors associated with the design of an AAA screening program were analyzed in detail. AAAs were classified as small (3.0–4.5 cm), intermediate (4.5–5.4 cm), or large (≥5.5 cm). The model was analyzed for white men, the demographic group with the highest prevalence of AAA.
Assumptions of the model. In the model, the clinical outcomes for a cohort of men invited to have ultrasound screening for AAA in a program similar to the MASS study, the largest of the four randomized trials of ultrasound screening for AAA, were compared with the outcomes of a cohort of men receiving usual care over a 20-year interval. Ultrasound screening was offered to men at age 65. Men could agree to have ultrasound screening or could refuse ultrasound screening. Men who refused screening were followed for the outcomes of their usual care. Men who agreed to have ultrasound screening were followed based on the results of the ultrasound: those with a negative ultrasound received usual care; those with a small AAA received an annual ultrasound; those with an intermediate-sized AAA received an ultrasound every 3 months; and those with a large AAA were advised to have elective AAA repair. Men with a large AAA could choose to follow the recommendation for elective AAA repair or decide not to have elective AAA repair and instead be followed with an ultrasound and follow-up physician visit every 3 months.
In the analysis, the proportion of men with an AAA was based on epidemiologic information on the age-specific prevalence rates of AAA, and the size distributions of the AAAs were based on population-based or community-based AAA screening programs. The model allowed for new AAAs to occur during follow-up, with age-specific rates derived from recent ultrasound screening programs for AAA (117). The model also allowed for some AAAs to grow. Information on the probability of a transition from one AAA size category to another was obtained from data from men followed in AAA screening programs (118). The risk of AAA symptoms or rupture, which varies with AAA size, was included in the model. The incidental rate of discovery of an AAA was calculated from information from the control groups in randomized controlled trials of screening (104).
AAA can be repaired by an open surgical procedure or by an endovascular procedure. The latter has become widespread and in many centers accounts for the majority of elective AAA surgery. Endovascular repair is also used for some AAA patients with AAA symptoms and for some patients with AAA rupture. This procedure has lower operative morbidity and mortality rates than open surgical repair but has a higher rate of endoleak, thrombosis, and other complications that require follow-up imaging and catheter-based interventional procedures (8, 11, 12). The cost of endovascular repair has been reported to be similar to that of open surgical repair, with the reduced costs of surgery and reduced length of stay offset by higher costs for imaging and supplies (119), but some studies have reported similar mortality rates and higher costs for endovascular repair compared with open surgical repair (89).
The model assumed that 60% of elective AAA surgery, 25% of urgent surgery for symptomatic AAA, and 25% of emergency surgery for AAA rupture would be endovascular repair. It assumed that after endovascular repair, 10% of patients would require a catheter-based interventional procedure for endoleak, thrombosis, or another complication. Emerging data suggest that the need for a catheter-based intervention following endovascular repair diminishes in subsequent years, and the rate was varied from 0% to 10% in a sensitivity analysis. The base case analysis assumed that there was no risk of rupture following AAA repair and that each year, 1% of patients with AAA repair would require a reoperation.
Although patients with a ruptured AAA have a 50% probability of out-of-hospital death, all AAA patients with symptomatic AAA were assumed to survive to have emergency department evaluation and hospital admission for urgent AAA surgery by open surgical repair. Elective AAA surgery, urgent surgery for symptomatic AAA, emergency department evaluation for symptomatic or ruptured AAA, and emergency surgery for ruptured AAA have associated costs, which were included in the analysis. AAA surgery also has a risk of major complications, including myocardial infarction, stroke, paraplegia, amputation, and renal failure requiring dialysis. These are associated with increased short-term morbidity, increased acute care costs, increased chronic care costs, and reduced quality of life. AAA patients who survived surgery and did not experience a complication were assumed to return to a normal quality of life. After endovascular repair, patients were assumed to have an annual follow-up examination, including CT scan. After open surgical repair, patients were assumed to have a follow-up evaluation, including CT scan, every 3 years. The model also considered the risk of long-term complications that may require a catheter-based procedure or reoperation, with the risk depending on whether the patient had endovascular or open surgical repair.
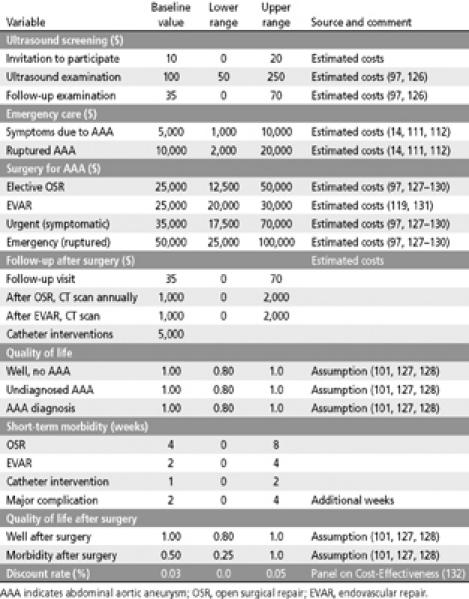
The clinical course of AAAs in men participating in screening or refusing to participate in the screening program was analyzed using the mortality rates from the US 2000 life tables. In the analysis, the all-cause mortality rates for men who participated and men who refused to participate in the screening program were assumed to be the same as the all-cause mortality rates in the general population. The risk of death for patients with AAA was greater than the risk in the general population, and the mortality rates were adjusted accordingly (120). Information on the AAA incidence, prevalence, size, growth, risk of rupture, AAA screening program, AAA surgery outcomes, and risk of death due to AAA and all-cause mortality—the probabilities used in the analysis—are summarized in Table 8. Information on the costs of health services for AAA screening, follow-up, and treatment and the quality of life assumptions—the utilities—are summarized in Table 9.
Table 8.
Probabilities used in a decision analysis of AAA screening: baseline values, upper and lower ranges for sensitivity analyses, and data sources
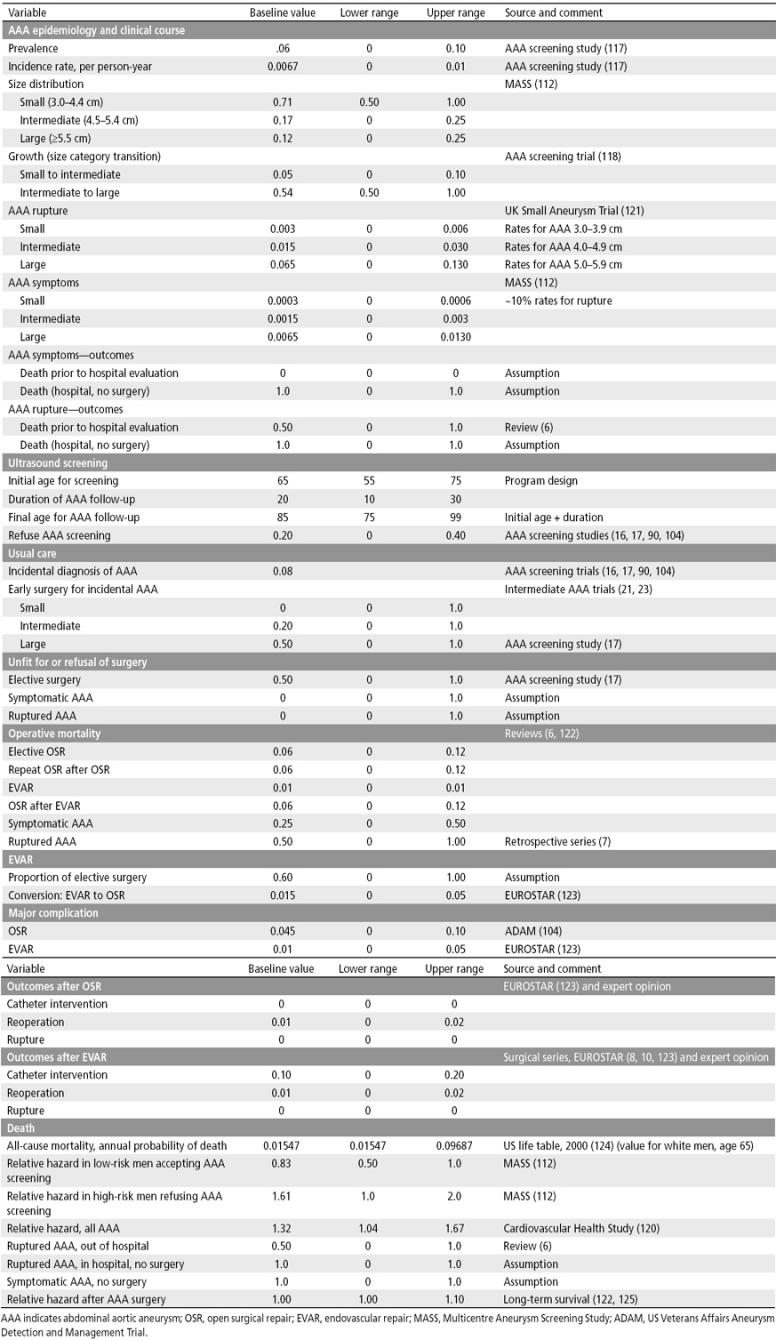
Table 9.
Costs and quality of life estimates used in decision analysis of AAA screening
Outcome measures. The decision to obtain ultrasound screening for AAAs was analyzed using three types of outcome measures. The first measure consisted of the distribution of outcomes for a hypothetical population of 100,000 white men who have ultrasound screening for AAA at age 65 and are followed for 20 years until age 85 compared with the outcomes in a hypothetical identical population not invited for screening. The second measure used in the analysis was the relative risk of fatal and nonfatal AAA rupture in men participating in the screening program compared with men not participating in the screening program. This measure is analogous to the relative risk or odds ratio that would be obtained from a randomized controlled trial of ultrasound screening. The third outcome measure summarized the cost-effectiveness analysis and included the incremental effectiveness of AAA screening compared with usual care, the incremental costs of AAA screening compared with usual care, and the incremental cost-effectiveness ratio, which is defined as the ratio of the incremental costs of screening and the incremental effectiveness of AAA screening. The incremental cost-effectiveness ratio is a measure of the AAA screening and treatment costs required to save an additional QALY. To account for the time value of resources, the cost-effectiveness measures were also reported using discount rates of 3% and 5% (132). A 17-state Markov model was developed to describe the clinical course of a cohort of adult men who were invited to have ultrasound screening for AAA or usual care. The final decision tree had over 1500 nodes. The analysis was performed using TreeAge Pro software (Williamstown, MA) and Microsoft Excel.
Outcomes, relative risk of AAA-related mortality, and cost-effectiveness of screening. The expected overall impact of screening 100,000 white men at age 65 and following their outcomes for 20 years is summarized in Table 10. In the screened cohort at age 85 after 20 years of observation, 32,469 men would still be alive with no AAA. After 20 years, 1592 men would have an undiagnosed AAA and 570 men would have a diagnosed AAA. After 20 years, 4098 men would have had AAA surgery without a complication and would still be alive, and 124 men would be alive with morbidity following AAA surgery. After 20 years, there would be 1413 deaths due to rupture or emergency surgery, 206 deaths due to elective AAA surgery by open surgical repair, and 54 deaths due to elective AAA surgery by endovascular repair. Most deaths that would occur, 59,474, would be due not to AAA but to other causes. Compared with usual care without ultrasound screening for AAA, ultrasound screening of 100,000 men for AAA would have prevented 764 AAA ruptures: 669 fatal and 95 nonfatal. Thus, ultrasound screening for AAA at age 65 would be expected to reduce the relative risk of fatal and nonfatal AAA to 0.69.
Table 10.
Outcomes and cost-effectiveness results of a decision analysis model of AAA screening*
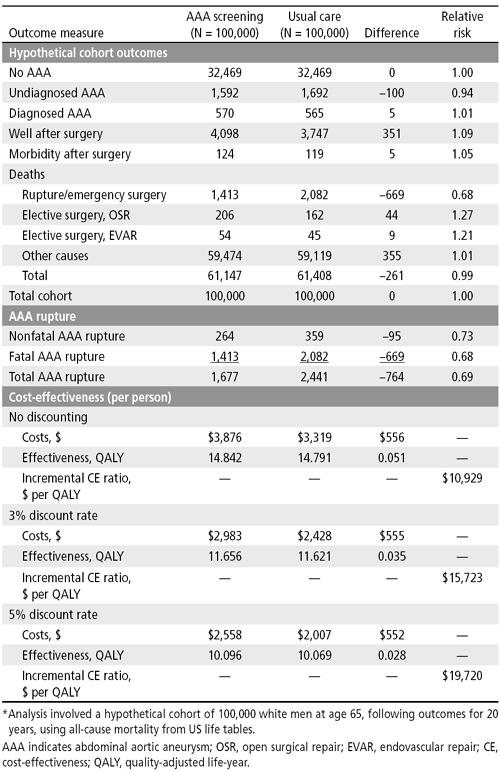
The average cost for AAA screening and management would be $3876 per person compared with $3319 per person in those receiving usual care, resulting in incremental costs of $556 per person. Ultrasound screening for AAA would result in a net gain of 0.050901 QALY, approximately 19 days. Ultrasound screening for AAA would therefore cost $10,929 per QALY saved. In an analysis that adjusts for the time value of money, the incremental costs of AAA screening would be $15,723 per QALY saved at a discount rate of 3% and $19,720 at a discount rate of 5%.
The results of the analysis can be expressed as the number needed to be invited or the number needed to be screened to prevent a ruptured AAA or a death due to a ruptured AAA. An AAA screening program would need to invite 131 men (100,000/764) and would need to screen 105 men (80,000/764) to prevent one ruptured AAA over the subsequent 20 years. An AAA screening program would have to invite 149 men (100,000/669) and screen 120 men (80,000/669) to prevent one death from a ruptured AAA over the subsequent 20 years. Ultrasound screening for AAA may cause harm by increasing the number of men who would die following elective repair for the AAA discovered by the screening program. We can express this as the number needed to be invited to cause harm or the number needed to be screened to cause harm. An AAA screening program would have to invite 1887 men (100,000/53) to cause an additional death and would have to screen 1509 men (80,000/53) to cause an additional death. An AAA screening program would have to invite 20,000 men (100,000/5) and screen 16,000 men (80,000/5) to cause an additional case of serious morbidity (myocardial infarction, stroke, paralysis, renal failure requiring dialysis or amputation).
Conclusions. The Markov model of ultrasound screening provided several important insights. First, the small net gain in QALY occurred over the 20-year time period; initially, there was a net loss of life-years in the screened group compared with usual care, and there was no net gain until after 4.2 years. The cost-effectiveness ratio was >$100,000 per QALY until approximately 7.7 years of follow-up and >$50,000 per QALY until approximately 9.8 years of follow-up. The findings are consistent with the observations from the early years of the MASS study (112).
Second, the cost-effectiveness of ultrasound screening for AAA was sensitive to the follow up interval for intermediate-sized AAAs. The follow-up interval in the randomized trials has ranged from 3 months to 12 months. Compared with a usual care strategy with 12-month follow-up of intermediate-sized AAA, ultrasound screening for AAA with 12-month follow-up of intermediate-sized AAA would have an incremental cost of $489 and incremental effectiveness of 0.0312 QALY (~11.4 days) for an incremental cost of $15,657 per additional QALY saved. Decreasing the follow-up interval would increase the costs due to more frequent visits, ultrasound examinations, and elective surgery (offset by some reduction in the cost of emergency surgery) and increase the QALY gained by reducing the number of ruptured AAAs. The absolute amounts of effectiveness in QALY and costs of more frequent follow-up were substantially smaller. The incremental cost of ultrasound screening of AAA with 6-month follow-up compared with ultrasound screening with 12-month follow-up of intermediate-sized AAA was $105; the incremental effectiveness was 0.0042 QALY (~1.5 days), for an incremental cost of $24,982 per additional QALY saved. The incremental cost of ultrasound screening with 3-month follow-up compared with ultrasound screening with 6-month follow-up of intermediate-sized AAA was $33,592 per additional QALY saved.
Third, the optimal age to initiate AAA screening as judged by the lowest cost per QALY gained was 61 years, but the cost per QALY gained was approximately $18,165 at age 55 and remained <$20,000 per QALY until age 70, suggesting that ultrasound screening for AAA could reasonably begin as early as age 55 and should certainly include adults as old as age 70. The effectiveness of ultrasound screening for AAA plateaued after 25 years of follow-up, suggesting little additional benefit in QALYs gained after 25 years of follow-up surveillance for patients with intermediate-sized AAAs and for patients with large AAAs who initially refused elective AAA repair.
Fourth, elective endovascular repair for AAA identified through ultrasound screening and the use of endovascular repair for some patients with symptomatic and ruptured AAA made ultrasound screening for AAA even more cost-effective than usual care. Our base case assumed that endovascular repair was used for 60% of elective AAA repair and 25% of surgery for symptomatic and ruptured AAA. If, for example, endovascular repair were to be used for 80% of elective AAA repair, the cost per additional QALY gained from ultrasound screening for AAA compared with usual care would be $14,874 in contrast to $15,723 with endovascular repair for 60% of elective AAA repair.
Fifth, the cost per additional QALY gained from ultrasound screening for AAA was <$20,000 even when the discount rate was 5%, suggesting that decisions to implement ultrasound screening for AAA should not be changed by uncertainty about the time value of resources (the discount rate).
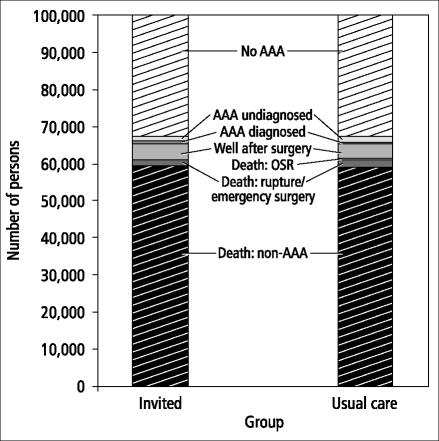
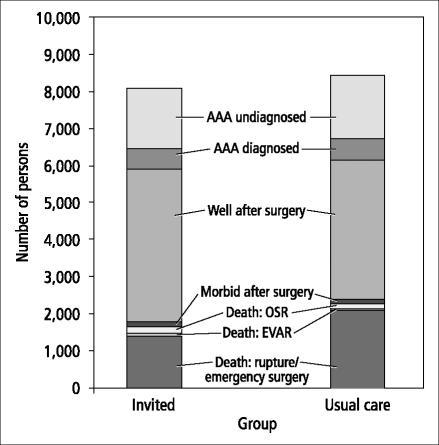
Sixth, although an AAA could potentially affect approximately 8% of adult men aged 65 and over during the subsequent 20 years (Table 10 and Figures 1 and 2), the overall impact of ultrasound screening for AAA in the population was rather small. In a cohort of 100,000 adult men aged 65 who were followed for 20 years, 31,469 (31.4%) would be alive and never develop an AAA and 61,147 (61.1%) would die from an unrelated cause. Of the entire cohort of 100,000 men, 8057 (8.1%) would have an AAA (Figure 2): 1592 (1.6%) would have an undiagnosed AAA (incident cases after the initial screening at age 65), 570 (0.6%) would have a known AAA, 4222 (4.2%) would have had AAA surgery, and 1673 (1.6%) would have died from a ruptured AAA or AAA surgery. In this cohort, only 764 men (0.7%) would have actually benefited by having a fatal or nonfatal ruptured AAA prevented.
Figure 1.
Outcomes of ultrasound screening for AAA vs usual care after 20 years for a cohort of 100,000 persons in a decision analysis model.
Figure 2.
AAA-related outcomes of ultrasound screening for AAA vs usual care after 20 years for a cohort of 100,000 persons in a decision model. Excludes persons without AAA and deaths due to non-AAA-related causes.
Discussion. The decision analysis has several strengths. First, it uses estimates of important factors that affect the costs and effectiveness of ultrasound screening from the four recently completed randomized controlled trials of ultrasound screening for AAA and the two randomized trials of immediate surgery compared with ultrasound surveillance for intermediate-sized AAAs. Second, it analyzes the cost-effectiveness of ultrasound screening with endovascular surgery, which is increasingly being used for elective AAA repair and also for urgent and emergency AAA surgery. Third, it includes patient and physician factors such as refusal of screening, incidental detection of AAA, early surgery for small and intermediate-sized AAAs, out-of-hospital death for ruptured AAAs, and patient refusal of or unfitness for surgery. Fourth, it includes a reasonable range of estimates for the epidemiology and clinical course of AAA, the morbidity and mortality of surgery, the increased mortality rate of AAA patients compared with the general population, and all-cause mortality rates from US life tables. Fifth, it includes a reasonable range of estimates for the cost of ultrasound screening, ultrasound follow-up of persons with AAA, AAA surgery, and CT follow-up of AAA patients after surgery. Sixth, it includes a range of health-related quality of life estimates. Seventh, it provides information to suggest an optimal initial age for AAA screening (age 61), but AAA screening would have a comparable low cost-effective ratio if AAA screening were to begin as early as age 55. Finally, the analysis uses several types of outcome measures—cost-effectiveness ratio ($15,723 per QALY gained), relative risk reduction of AAA rupture and death (0.69), impact on a population of men who would have AAA screening (669 AAA rupture deaths prevented over 20 years per 100,000 men screened), and number of men a physician would need to invite or screen (131 and 105, respectively) to prevent an AAA rupture over the subsequent 20 years.
In summary, ultrasound screening of white men beginning at age 65 is both effective and cost-effective in preventing AAA-related death. From a public health perspective, ultrasound screening for AAA would have a small but real impact over 20 years (749/100,000, or 0.7%) on white men aged 55 and older and on total mortality (669/61408, or 1%) in these white men. From an individual physician's or provider's perspective, ultrasound screening for AAA would have benefits for approximately 1% of older men who accepted ultrasound screening for AAA. An individual 55-year-old white man invited for ultrasound screening would have an 8% probability (8057/100,000) of having an AAA-related diagnosis, treatment, or outcome over the next 20 years; if he were to have an AAA-related condition, he would have a 9% probability (749/8057) that AAA rupture could be prevented. We therefore recommend ultrasound screening for AAA for white men.
MANAGEMENT STRATEGIES FOR PATIENTS WITH A KNOWN AAA
Small AAAs between 3.0 and 4.0 cm in diameter have a low annual risk of rupture, and periodic surveillance with abdominal ultrasonography is appropriate (1, 6). Large AAAs ≥5.5 cm in diameter have a high annual risk of rupture, and surgical repair is indicated (19). The optimal management of patients with intermediate-sized AAAs, a diameter between 4.0 and 5.5 cm, was uncertain until the recent publication of the eagerly awaited findings from two randomized controlled trials of immediate surgical repair compared with surveillance of small AAAs (Tables 11 and 12) (21, 23). The US Veterans Affairs Aneurysm Detection and Management Trial (ADAM) and the United Kingdom Small Aneurysm Trial (UKSAT) demonstrated that long-term survival was not improved by immediate elective repair of intermediate-sized AAAs compared with periodic ultrasound surveillance.
Table 11.
Randomized controlled trials of immediate surgery vs surveillance for intermediate-sized AAAs
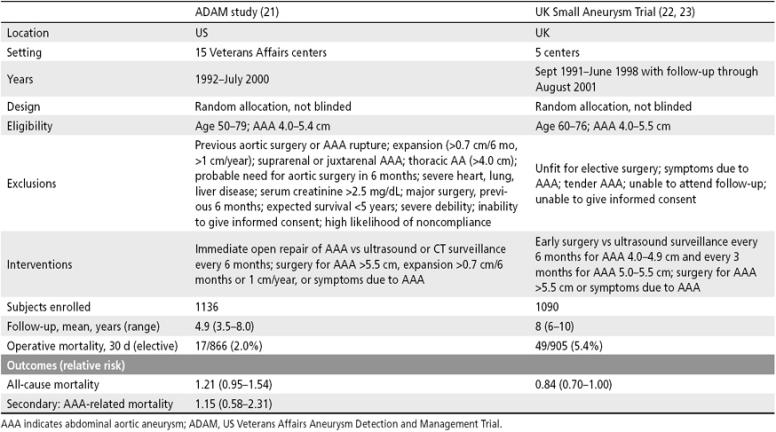
Table 12.
Study subjects, interventions, and primary outcomes of randomized controlled trials of early surgery vs surveillance for intermediate-sized AAAs
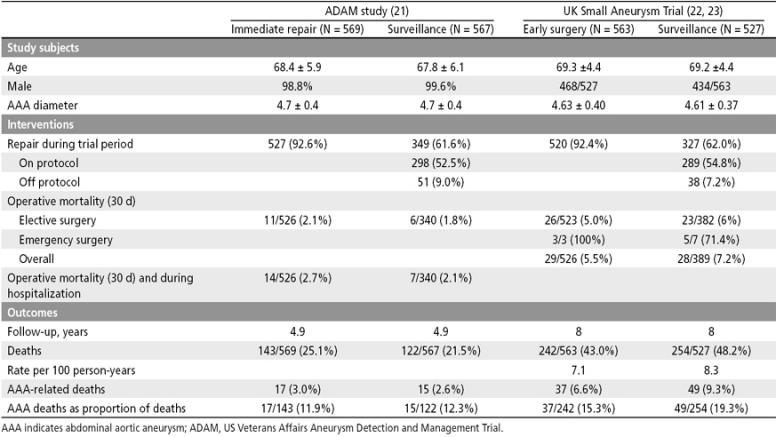
The US ADAM trial, initiated in 1992 and completed in 2000, was conducted in 15 Veterans Affairs centers (21). Men aged 50 to 79 with AAAs 4.0 to 5.4 cm in diameter were randomly allocated to immediate open repair of the AAA or to a program of ultrasound or CT scan surveillance every 6 months. Surgery was recommended for AAAs >5.5 cm in diameter, for AAAs with expansion >0.7 cm over 6 months or >1.0 cm over 1 year, or symptoms due to AAA. The study enrolled 1136 men who were followed for a mean of 4.9 years. The overall operative mortality was 2.0%. There was no significant difference in all-cause mortality (relative risk, 1.21; 95% CI, 0.95–1.54) or in AAA-related mortality (relative risk, 1.15; 95% CI, 0.58–2.31). There was no significant difference in cumulative survival, which was higher in the surveillance group throughout the course of the study (Figure 3).
Figure 3.
Cumulative survival according to treatment group in the Aneurysm Detection and Management (ADAM) study. Reprinted with permission from reference 21. Copyright © 2002 Massachusetts Medical Society. All rights reserved.
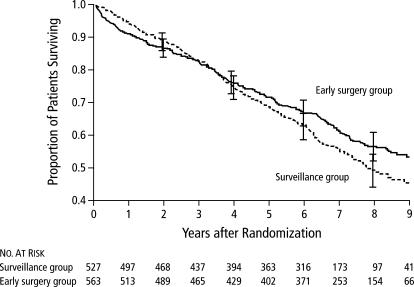
UKSAT was initiated in 1991 and completed in June 1998 (22); subsequent follow-up through August 2001 was recently reported (23). The study was conducted in five centers in the United Kingdom. Men aged 60 to 76 with AAAs 4.0 to 5.5 cm in diameter were randomly allocated to early surgery or a program of ultrasound surveillance every 6 months for patients with AAAs 4.0 to 4.9 cm or every 3 months for patients with AAAs 5.0 to 5.5 cm in diameter. Surgery was recommended for AAAs >5.5 cm in diameter or for patients with symptoms due to an AAA. The study enrolled 1090 men, who were followed for a mean of 8 years. Operative mortality was 5.4%. There is no significant difference in all-cause mortality (relative risk, 0.84; 95% CI, 0.70–1.0). There was no significant difference in overall survival; the early surgery group had lower initial survival but higher long-term survival, a difference that was attributed to lifestyle changes in the surgery group (Figure 4).
Figure 4.
Kaplan-Meier estimates of overall survival according to treatment-group assignment in the UK Small Aneurysm Trial. P = 0.05 by the log-rank test. I represent the 95% confidence intervals for the point estimates. Reprinted with permission from reference 23. Copyright © 2002 Massachusetts Medical Society. All rights reserved.
Translating research into practice
The important differences between the two studies, which are summarized in Table 11, relate to eligibility criteria, the interval for ultrasound surveillance, and operative mortality. The ADAM study age eligibility criteria were wider, although the actual ages of enrolled subjects were similar. The ADAM study had more explicit criteria for excluding study subjects with serious comorbidity, whereas the UKSAT excluded patients “unfit” for elective surgery. The ADAM study used expansion as one of the criteria for surgery, but no patients in the surveillance group underwent repair solely because of a rapid rate of expansion of the aneurysm. The ADAM study surveillance group had ultrasound or CT scan examination every 6 months, whereas the UKSAT study surveillance group had ultrasound every 3 months. Operative mortality was lower in the ADAM study (2.0%) compared with the UKSAT study (5.4%). At the end of approximately 5 years of follow-up, 21.5% of patients in the surveillance group and 25.1% of patients in the immediate repair group in the ADAM study had died. AAA-related deaths accounted for 3% of the deaths in the immediate repair group and 2.6% of deaths in the surveillance group. After approximately 8 years of follow-up in the UKSAT study, 45.9% of patients in the early surgery group and 45.1% of patients in the surveillance group had died.
Both studies were designed to look at the endpoint of total mortality. Long-term survival was not significantly different in the two studies (Figures 3 and 4). In the ADAM study, long-term survival was lower in the immediate repair group as a consequence of early operative mortality. In the UKSAT study, long-term survival was initially lower in the early surgery group, but after 8 years long-term survival was higher in the early surgery group, a difference that was attributed in part to the beneficial changes in lifestyle adopted by members of the early surgery group.
The ADAM study reported the complications of surgery (Table 13). In the ADAM study, 52.3% of patients in the immediate repair group had a complication, 20.5% were hospitalized for a complication, and 4.7% had a major complication defined as reoperation, myocardial infarction, amputation, paraplegia, stroke, pulmonary embolism, or need for dialysis. In contrast, 56.8% of patients in the surveillance group had a complication, 16.5% required hospitalization for the complication, and 7.7% had a major complication. The higher proportion of complications in the surveillance group was predominantly due to a larger number of myocardial infarctions.
Table 13.
Complications of repair of unruptured AAAs in a randomized controlled trial of immediate surgery vs surveillance for intermediate-sized AAAs*
| Variable | Immediate Repair group (N = 526) | Surveillance group (N = 340) |
| no. (%) | ||
| Major complications with no | ||
| operative death | ||
| Reoperation required | 9 (1.7) | 4 (1.2) |
| Myocardial infarction† | 5 (1.0) | 13 (3.8) |
| Amputation | 2 (0.4) | 2 (0.6) |
| Paraplegia | 0 | 2 (0.6) |
| Stroke | 3 (0.6) | 2 (0.6) |
| Pulmonary embolism | 4 (0.8) | 1 (0.3) |
| Dialysis | 1 (0.2) | 2 (0.6) |
| Any complication | 275 (52.3) | 193 (56.8) |
| Rehospitalization for complications | 108 (20.5) | 56 (16.5) |
*Reprinted with permission from reference 21. Copyright © 2002 Massachusetts Medical Society. All rights reserved.
†P = 0.004 for the comparison between groups.
AAA indicates abdominal aortic aneurysm.
Another metric that can be used in interpreting the studies is the proportion of all deaths due to AAA rupture or repair. In the ADAM study, 11.9% of the deaths were AAA related in the immediate repair group compared with 12.3% in the surveillance group. In the UKSAT, 15.3% of deaths were AAA related in the early surgery group compared with 19.3% in the surveillance group. AAA-related deaths accounted for 7.0% of patients in the early surgery group compared with 8.7% of patients in the surveillance group. The UKSAT trial reported the causes of the 242 deaths in the early surgery group and the 254 in the surveillance group (Table 14). Cardiovascular disease accounted for 60% of deaths in the early surgery group and 68% of deaths in the surveillance group. A primary or secondary ruptured AAA accounted for 4% of deaths in the early surgery group compared with 9% of deaths in the surveillance group. Death attributed to AAA repair, defined as death within 14 days of surgery, accounted for 11% of deaths in the early surgery group compared with 10% in the surveillance group. Thus, only a minority of the deaths that occurred in the AAA patients with intermediate-sized AAAs were due to AAA-related causes.
Table 14.
Causes of death according to treatment group in the UK Small Aneurysm Trial*
| Cause of death | Surveillance group | Early surgery group |
| no. (%) | ||
| Any | 254 | 242 |
| Cardiovascular causes | 172 (68) | 146 (60) |
| Myocardial infarction | 48 (19) | 42 (17) |
| Stroke | 12 (5) | 9 (4) |
| Ruptured thoracic aortic aneurysm | 11 (4) | 5 (2) |
| Ruptured AAA | 21 (8) | 10 (4) |
| Secondary AAA rupture | 3 (1) | 0 |
| AAA repair | 25 (10) | 27 (11) |
| Other | 52 (20) | 53 (22) |
| Cancer | 44 (17) | 56 (23) |
| Other | 37 (15) | 38 (16) |
| Unknown | 1 (<1) | 2 (1) |
*Reprinted with permission from reference 23. Copyright © 2002 Massachusetts Medical Society. All rights reserved.
AAA indicates abdominal aortic aneurysm.
While there were some differences in the design of the two studies, there was remarkable agreement in the important findings that early surgery confers no survival benefit compared with periodic surveillance for patients with intermediate-sized AAAs. Operative repair during the trial period was undertaken in 92% of patients in the early surgery group of both studies compared with approximately 62% of patients in the surveillance group of both studies. In both the ADAM and the UKSAT studies, 7% to 9% of patients in the surveillance groups underwent surgical repair of the AAA for indications that did not meet study protocol. Most of the surgical repairs were open repair in both studies. The recent development of endovascular repair with its potentially lower risk of operative mortality raises consideration of potential benefits of immediate endovascular repair for patients with intermediate-sized AAAs. However, there was no survival benefit from immediate repair compared with surveillance in the ADAM trial, which had an overall operative mortality of 2%. In the UKSAT, there was no significant difference in operative mortality after open repair and endovascular repair (22, 23).
Patients with small AAAs (3.0–3.9 cm) can be followed with yearly ultrasound examination. Patients with intermediate-sized AAAs (4.0–5.4 cm) can be advised that immediate elective surgery and periodic ultrasound surveillance every 6 months have essentially similar outcomes and that decisions about AAA management can be based on patient preferences. Patients with large AAAs (≥5.5 cm) should be advised to have elective AAA surgery. Studies suggest that the outcomes of a number of surgical procedures, including elective AAA surgery, are best when surgeons and hospitals with high-volume practices are used (133–135).
MANAGEMENT STRATEGIES FOR PATIENTS WITH ABDOMINAL PAIN, BACK PAIN, OR OTHER SYMPTOMS REFERABLE TO AAA
Patients with known aneurysms may develop increasing aneurysm tenderness, acute back pain, or abdominal pain, suggesting a change in the aneurysm's status but without clinical evidence of hypovolemia. Such aneurysms have been called “active,” “expanding,” or suspected of “leaking” (52, 136, 137). Given the lack of empirical predictors of imminent rupture in this clinical scenario and the surprisingly poor surgical outcomes reported for emergency surgery in this group even when the aneurysm has not ruptured (136–138), the clinician should focus on confidently and rapidly ruling in other causes of these symptoms. If this cannot be done, then preparation should be made for prompt surgery, keeping in mind that cardiovascular risk will have a great impact on surgical mortality if the aneurysm has not ruptured. Simultaneous coronary revascularization in symptomatic individuals with coincidental severe coronary heart disease has been advocated by some in this relatively uncommon circumstance, though the evidence for this maneuver is anecdotal (139). One detailed analysis of the variables relevant to preoperative coronary revascularization demonstrated that it is expected to save lives only when surgical risk is high, as with symptomatic AAAs (140).
No management strategies have been evaluated that specifically address the issue of new abdominal pain in patients with epidemiological risk factors for AAA. Unlike the management of asymptomatic aneurysms, this area lacks valid empirical evidence of diagnostic and therapeutic effectiveness. In an elderly patient with moderate abdominal or back pain for more than a day, abdominal ultrasound can determine if the patient has an aneurysm. If an aneurysm is present, it can be considered symptomatic. An axial CT scan with contrast can now discriminate between contained retroperitoneal hemorrhage and other complications of aneurysm growth, thus reducing the urgency of the intervention while providing a preoperative assessment of other relevant vascular anatomy.
The few case series of rupture are necessarily biased samples of the actually relevant population, namely a representative sample of patients in whom impending AAA rupture is a diagnostic consideration. There is much disagreement about whether a CT scan should be obtained in an unstable patient. The presence of instability is easily biased by hindsight, and a competing alternative diagnosis may strongly influence the decision to obtain a CT scan (141, 142). The decision is sensitive to the pretest probability of rupture and, to a lesser extent, to the risks of decreased vigilance, delay of surgery, and intravenous contrast exposure posed by CT scan. The risk of contrast dye–induced renal failure can be reduced by periprocedural hydration, use of a low-osmolality contrast, and a reduced amount of contrast agent (143).
When AAA rupture is a consideration, CT scan with intravenous contrast is a more accurate imaging test than abdominal ultrasound, as long as the patient is not already hypotensive and is carefully monitored, the surgical team is explicitly and fully involved, and the scan is interpreted immediately. But in many community hospitals after hours, when support personnel and radiography interpretation are most scarce and ruptured AAAs are most likely to present, immediate surgery may be preferred. Although not perfectly sensitive, CT scan is more accurate than ultrasound because it can demonstrate retroperitoneal blood and more accurately identify other sources of the patient's severe symptoms.
Among nonhypotensive patients with a suspected AAA rupture, one study found CT scan to be safe, leading probably to improved overall surgical management (144). Additional prospective studies are needed to evaluate the safety and effectiveness of CT without contrast in the evaluation of the patient with a high probability of having an AAA or with a known AAA that is suspected of having ruptured.
If the patient has ongoing hypotension or other evidence of hemorrhagic shock, the main priority is mobilizing the surgical team for immediate AAA repair. While this is being arranged, venous access with large-diameter catheters, cross-matching for blood, and a critical care environment are mandatory. Euvolemic resuscitation with massive fluid loading before the repair should probably be avoided because it may lead to increased hemorrhage (6, 145). Prognostic stratification to avoid futile operations has been proposed, but studies to date have included only small samples with imprecise rates (7, 146–148).
RECOMMENDATIONS
Screening or case-finding for AAA
Population screening for large AAAs (≥ 5.5 cm) in elderly men has recently been shown to reduce the risk of AAA rupture but not overall mortality in several randomized trials. Population or community screening for AAA with ultrasonography is both effective and cost-effective in preventing AAA rupture and death and most likely would have a small but real impact in reducing overall mortality. One-time ultrasound screening for AAA in older men at age 65, or possibly as early as age 55, can be recommended as cost-effective in reducing the risk of AAA-related mortality. This is in contrast to the current US Preventive Services Task Force recommendation for one-time screening by ultrasonography for men aged 65 to 75 who have ever smoked (149).
Management of the patient with a known AAA
Patients with asymptomatic aneurysms <5.5 cm in maximum diameter should be followed with ultrasound imaging at least every year. A conventional surveillance strategy is to increase this frequency to every 3 to 6 months after the AAA reaches 5.0 cm in diameter. Aneurysms ≥5.5 cm should be repaired if individual surgical risk is acceptable. The repair should be performed at centers with an operative mortality of <6% for elective open surgical AAA repair. Patients with small aneurysms may benefit from smoking cessation.
Symptoms in the patient with a known AAA or risk factors for AAA
In patients with abdominal or back pain (especially those with a known AAA or risk factors for AAA such as age >50, white race, male gender, or a smoking history), ultrasound imaging is recommended to determine if an AAA is present and to identify other causes of abdominal pain or back pain. If an aneurysm is detected, the patient should have a CT scan to rule out contained rupture and should be referred to a surgeon who frequently operates on AAAs. In patients with recent or severe symptoms or a palatable pulsatile epigastric mass, CT scan would be the preferred initial test because it can identify an aneurysm, detect retroperitoneal hemorrhage and other complications, and provide preoperative definition of the anatomy, without the delay required for confirmatory testing posed by choosing ultrasound initially. Patients with suspected AAA rupture should be intensively monitored. Blood should be sent for cross-matching and the surgical team mobilized emergently. Among those patients who are not hypotensive, an emergency CT scan with contrast would enable a more accurate surgical approach and probably save lives. Surgery should be performed immediately without further imaging for hypotensive patients.
References
- 1.Powell JT, Greenhalgh RM. Clinical practice. Small abdominal aortic aneurysms. N Engl J Med. 2003;348:1895–1901. doi: 10.1056/NEJMcp012641. [DOI] [PubMed] [Google Scholar]
- 2.Scott RA, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg. 2002;89:283–285. doi: 10.1046/j.0007-1323.2001.02014.x. [DOI] [PubMed] [Google Scholar]
- 3.Grimshaw GM, Thompson JM. The abnormal aorta: a statistical definition and strategy for monitoring change. Eur J Vasc Endovasc Surg. 1995;10:95–100. doi: 10.1016/s1078-5884(05)80204-7. [DOI] [PubMed] [Google Scholar]
- 4.Wilmink AB, Pleumeekers HJ, Hoes AW, Hubbard CS, Grobbee DE, Quick CR. The infrarenal aortic diameter in relation to age: only part of the population in older age groups shows an increase. Eur J Vasc Endovasc Surg. 1998;16:431–437. doi: 10.1016/s1078-5884(98)80013-0. [DOI] [PubMed] [Google Scholar]
- 5.Wilmink AB, Hubbard CS, Day NE, Quick CR. The incidence of small abdominal aortic aneurysms and the change in normal infrarenal aortic diameter: implications for screening. Eur J Vasc Endovasc Surg. 2001;21:165–170. doi: 10.1053/ejvs.2000.1285. [DOI] [PubMed] [Google Scholar]
- 6.Ernst CB. Abdominal aortic aneurysm. N Engl J Med. 1993;328:1167–1172. doi: 10.1056/NEJM199304223281607. [DOI] [PubMed] [Google Scholar]
- 7.Gloviczki P, Pairolero PC, Mucha P, Jr, Farnell MB, Hallett JW, Jr, Ilstrup DM, Toomey BJ, Weaver AL, Bower TC, Bourchier RG, et al. Ruptured abdominal aortic aneurysms: repair should not be denied. J Vasc Surg. 1992;15:851–857. discussion 857–859. [PubMed] [Google Scholar]
- 8.Elkouri S, Gloviczki P, McKusick MA, Panneton JM, Andrews JC, Bower TC, Noel AA, Sullivan TM, Canton LG, Harmsen WS, Hoskin TL, Cherry KJ. Endovascular repair of abdominal aortic aneurysms: initial experience with 100 consecutive patients. Mayo Clin Proc. 2003;78:1234–1242. doi: 10.4065/78.10.1234. [DOI] [PubMed] [Google Scholar]
- 9.Moore WS, Brewster DC, Bernhard VM. Aorto-uni-iliac endograft for complex aortoiliac aneurysms compared with tube/bifurcation endografts: results of the EVT/Guidant trials. J Vasc Surg. 2001;33:S11–S20. doi: 10.1067/mva.2001.111681. [DOI] [PubMed] [Google Scholar]
- 10.Zarins CK, White RA, Moll FL, Crabtree T, Bloch DA, Hodgson KJ, Fillinger MF, Fogarty TJ. The AneuRx stent graft: four-year results and worldwide experience 2000. J Vasc Surg. 2001;33:S135–S145. doi: 10.1067/mva.2001.111676. [DOI] [PubMed] [Google Scholar]
- 11.Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, Buskens E, Grobbee DE, Blankensteijn JD, Dutch Randomized Endovascular Aneurysm Management (DREAM) Trial Group A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351:1607–1618. doi: 10.1056/NEJMoa042002. [DOI] [PubMed] [Google Scholar]
- 12.Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG, EVAR trial participants Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet. 2004;364:843–848. doi: 10.1016/S0140-6736(04)16979-1. [DOI] [PubMed] [Google Scholar]
- 13.EVAR trial participants Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005;365:2179–2186. doi: 10.1016/S0140-6736(05)66627-5. [DOI] [PubMed] [Google Scholar]
- 14.Lindholt JS, Juul S, Fasting H, Henneberg EW. Hospital costs and benefits of screening for abdominal aortic aneurysms. Results from a randomised population screening trial. Eur J Vasc Endovasc Surg. 2002;23:55–60. doi: 10.1053/ejvs.2001.1534. [DOI] [PubMed] [Google Scholar]
- 15.Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RA, Thompson SG, Walker NM, Multicentre Aneurysm Screening Study Group The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet. 2002;360:1531–1539. doi: 10.1016/s0140-6736(02)11522-4. [DOI] [PubMed] [Google Scholar]
- 16.Scott RA, Wilson NM, Ashton HA, Kay DN. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br J Surg. 1995;82:1066–1070. doi: 10.1002/bjs.1800820821. [DOI] [PubMed] [Google Scholar]
- 17.Vardulaki KA, Walker NM, Couto E, Day NE, Thompson SG, Ashton HA, Scott RA. Late results concerning feasibility and compliance from a randomized trial of ultrasonographic screening for abdominal aortic aneurysm. Br J Surg. 2002;89:861–864. doi: 10.1046/j.1365-2168.2002.02133.x. [DOI] [PubMed] [Google Scholar]
- 18.Norman PE, Jamrozik K, Lawrence-Brown M, Dickinson J. Western Australian randomized controlled trial of screening for abdominal aortic aneurysm [abstract] Br J Surg. 2003;90:492. [Google Scholar]
- 19.Lederle FA, Johnson GR, Wilson SE, Ballard DJ, Jordan WD, Jr, Blebea J, Littooy FN, Freischlag JA, Bandyk D, Rapp JH, Salam AA, Veterans Affairs Cooperative Study #417 investigators Rupture rate of large abdominal aortic aneurysms in patients refusing or unfit for elective repair. JAMA. 2002;287:2968–2972. doi: 10.1001/jama.287.22.2968. [DOI] [PubMed] [Google Scholar]
- 20.Lederle FA. Risk of rupture of large abdominal aortic aneurysms. Disagreement among vascular surgeons. Arch Intern Med. 1996;156:1007–1009. [PubMed] [Google Scholar]
- 21.Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW, Ballard DJ, Messina LM, Gordon IL, Chute EP, Krupski WC, Busuttil SJ, Barone GW, Sparks S, Graham LM, Rapp JH, Makaroun MS, Moneta GL, Cambria RA, Makhoul RG, Eton D, Ansel HJ, Freischlag JA, Bandyk D, Aneurysm Detection and Management Veterans Affairs Cooperative Study Group Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437–1444. doi: 10.1056/NEJMoa012573. [DOI] [PubMed] [Google Scholar]
- 22.The UK Small Aneurysm Trial participants Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet. 1998;352:1649–1655. [PubMed] [Google Scholar]
- 23.United Kingdom Small Aneurysm Trial participants Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1445–1452. doi: 10.1056/NEJMoa013527. [DOI] [PubMed] [Google Scholar]
- 24.Bengtsson H, Sonesson B, Bergqvist D. Incidence and prevalence of abdominal aortic aneurysms, estimated by necropsy studies and population screening by ultrasound. Ann N Y Acad Sci. 1996;800:1–24. doi: 10.1111/j.1749-6632.1996.tb33294.x. [DOI] [PubMed] [Google Scholar]
- 25.Allardice JT, Allwright GJ, Wafula JM, Wyatt AP. High prevalence of abdominal aortic aneurysm in men with peripheral vascular disease: screening by ultrasonography. Br J Surg. 1988;75:240–242. doi: 10.1002/bjs.1800750318. [DOI] [PubMed] [Google Scholar]
- 26.O'Kelly TJ, Heather BP. General practice-based population screening for abdominal aortic aneurysms: a pilot study. Br J Surg. 1989;76:479–480. doi: 10.1002/bjs.1800760517. [DOI] [PubMed] [Google Scholar]
- 27.Bengtsson H, Norrgard O, Angquist KA, Ekberg O, Oberg L, Bergqvist D. Ultrasonographic screening of the abdominal aorta among siblings of patients with abdominal aortic aneurysms. Br J Surg. 1989;76:589–591. doi: 10.1002/bjs.1800760620. [DOI] [PubMed] [Google Scholar]
- 28.Shapira OM, Pasik S, Wassermann JP, Barzilai N, Mashiah A. Ultrasound screening for abdominal aortic aneurysms in patients with atherosclerotic peripheral vascular disease. J Cardiovasc Surg (Torino) 1990;31:170–172. [PubMed] [Google Scholar]
- 29.Webster MW, Ferrell RE, St Jean PL, Majumder PP, Fogel SR, Steed DL. Ultrasound screening of first-degree relatives of patients with an abdominal aortic aneurysm. J Vasc Surg. 1991;13:9–13. doi: 10.1067/mva.1991.25382. discussion 13–14. [DOI] [PubMed] [Google Scholar]
- 30.Bengtsson H, Sonesson B, Lanne T, Nilsson P, Solvig J, Loren I, Bergqvist D. Prevalence of abdominal aortic aneurysm in the offspring of patients dying from aneurysm rupture. Br J Surg. 1992;79:1142–1143. doi: 10.1002/bjs.1800791108. [DOI] [PubMed] [Google Scholar]
- 31.MacSweeney ST, O'Meara M, Alexander C, O'Malley MK, Powell JT, Greenhalgh RM. High prevalence of unsuspected abdominal aortic aneurysm in patients with confirmed symptomatic peripheral or cerebral arterial disease. Br J Surg. 1993;80:582–584. doi: 10.1002/bjs.1800800510. [DOI] [PubMed] [Google Scholar]
- 32.Lindholt JS, Juul S, Henneberg EW, Fasting H. Is screening for abdominal aortic aneurysm acceptable to the population? Selection and recruitment to hospital-based mass screening for abdominal aortic aneurysm. J Public Health Med. 1998;20:211–217. doi: 10.1093/oxfordjournals.pubmed.a024745. [DOI] [PubMed] [Google Scholar]
- 33.Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, Krupski WC, Barone GW, Acher CW, Ballard DJ. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med. 1997;126:441–449. doi: 10.7326/0003-4819-126-6-199703150-00004. [DOI] [PubMed] [Google Scholar]
- 34.van der Graaf Y, Akkersdijk GJ, Hak E, Godaert GL, Eikelboom BC. Results of aortic screening in the brothers of patients who had elective aortic aneurysm repair. Br J Surg. 1998;85:778–780. doi: 10.1046/j.1365-2168.1998.00652.x. [DOI] [PubMed] [Google Scholar]
- 35.Cronenwett JL, Sargent SK, Wall MH, Hawkes ML, Freeman DH, Dain BJ, Cure JK, Walsh DB, Zwolak RM, McDaniel MD, et al. Variables that affect the expansion rate and outcome of small abdominal aortic aneurysms. J Vasc Surg. 1990;11:260–268. discussion 268–269. [PubMed] [Google Scholar]
- 36.Lederle FA, Walker JM, Reinke DB. Selective screening for abdominal aortic aneurysms with physical examination and ultrasound. Arch Intern Med. 1988;148:1753–1756. [PubMed] [Google Scholar]
- 37.Karkos CD, Mukhopadhyay U, Papakostas I, Ghosh J, Thomson GJ, Hughes R. Abdominal aortic aneurysm: the role of clinical examination and opportunistic detection. Eur J Vasc Endovasc Surg. 2000;19:299–303. doi: 10.1053/ejvs.1999.1002. [DOI] [PubMed] [Google Scholar]
- 38.McCaig LF. National Hospital Ambulatory Medical Care Survey: 1992 emergency department summary. Adv Data. 1994;245:1–12. [PubMed] [Google Scholar]
- 39.Brewer BJ, Golden GT, Hitch DC, Rudolf LE, Wangensteen SL. Abdominal pain. An analysis of 1,000 consecutive cases in a university hospital emergency room. Am J Surg. 1976;131:219–223. doi: 10.1016/0002-9610(76)90101-x. [DOI] [PubMed] [Google Scholar]
- 40.Kizer KW, Vassar MJ. Emergency department diagnosis of abdominal disorders in the elderly. Am J Emerg Med. 1998;16:357–362. doi: 10.1016/s0735-6757(98)90127-9. [DOI] [PubMed] [Google Scholar]
- 41.Bugliosi TF, Meloy TD, Vukov LF. Acute abdominal pain in the elderly. Ann Emerg Med. 1990;19:1383–1386. doi: 10.1016/s0196-0644(05)82602-2. [DOI] [PubMed] [Google Scholar]
- 42.Reid MC, Lachs MS, Feinstein AR. Use of methodological standards in diagnostic test research. Getting better but still not good. JAMA. 1995;274:645–651. [PubMed] [Google Scholar]
- 43.Jaeschke R, Guyatt G, Sackett DL, Evidence-Based Medicine Working Group Users' guides to the medical literature. III. How to use an article about a diagnostic test. A. Are the results of the study valid? JAMA. 1994;271:389–391. doi: 10.1001/jama.271.5.389. [DOI] [PubMed] [Google Scholar]
- 44.Lederle FA, Simel DL. Does this patient have abdominal aortic aneurysm? JAMA. 1999;281:77–82. doi: 10.1001/jama.281.1.77. [DOI] [PubMed] [Google Scholar]
- 45.Fink HA, Lederle FA, Roth CS, Bowles CA, Nelson DB, Haas MA. Accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med. 2000;160:833–836. doi: 10.1001/archinte.160.6.833. [DOI] [PubMed] [Google Scholar]
- 46.Pleumeekers HJ, Hoes AW, Hofman A, van Urk H, van der Does E, Grobbee DE. Selecting subjects for ultrasonographic screening for aneurysms of the abdominal aorta: four different strategies. Int J Epidemiol. 1999;28:682–686. doi: 10.1093/ije/28.4.682. [DOI] [PubMed] [Google Scholar]
- 47.Akkersdijk GJ, van Bockel JH. Ruptured abdominal aortic aneurysm: initial misdiagnosis and the effect on treatment. Eur J Surg. 1998;164:29–34. doi: 10.1080/110241598750004922. [DOI] [PubMed] [Google Scholar]
- 48.Johansson G, Swedenborg J. Ruptured abdominal aortic aneurysms: a study of incidence and mortality. Br J Surg. 1986;73:101–103. doi: 10.1002/bjs.1800730205. [DOI] [PubMed] [Google Scholar]
- 49.Lederle FA, Parenti CM, Chute EP. Ruptured abdominal aneurysm: the internist as diagnostician. Am J Med. 1994;96:163–167. doi: 10.1016/0002-9343(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 50.Beede SD, Ballard DJ, James EM, Ilstrup DM, Hallet JW., Jr Positive predictive value of clinical suspicion of abdominal aortic aneurysm. Implications for efficient use of abdominal ultrasonography. Arch Intern Med. 1990;150:549–551. [PubMed] [Google Scholar]
- 51.Zarnke MD, Gould HR, Goldman MH. Computed tomography in the evaluation of the patient with symptomatic abdominal aortic aneurysm. Surgery. 1988;103:638–642. [PubMed] [Google Scholar]
- 52.Mannick JA, Whittemore AD. Management of ruptured or symptomatic abdominal aortic aneurysms. Surg Clin North Am. 1988;68:377–384. doi: 10.1016/s0039-6109(16)44483-x. [DOI] [PubMed] [Google Scholar]
- 53.Johansen K, Kohler TR, Nicholls SC, Zierler RE, Clowes AW, Kazmers A. Ruptured abdominal aortic aneurysm: the Harborview experience. J Vasc Surg. 1991;13:240–245. discussion 245–247. [PubMed] [Google Scholar]
- 54.Ouriel K, Geary K, Green RM, Fiore W, Geary JE, DeWeese JA. Factors determining survival after ruptured aortic aneurysm: the hospital, the surgeon, and the patient. J Vasc Surg. 1990;11:493–496. doi: 10.1067/mva.1990.18639. [DOI] [PubMed] [Google Scholar]
- 55.Stonebridge PA, Callam MJ, Bradbury AW, Murie JA, Jenkins AM, Ruckley CV. Comparison of long-term survival after successful repair of ruptured and non-ruptured abdominal aortic aneurysm. Br J Surg. 1993;80:585–586. doi: 10.1002/bjs.1800800511. [DOI] [PubMed] [Google Scholar]
- 56.Kantonen I, Lepantalo M, Brommels M, Luther M, Salenius JP, Ylonen K. Mortality in ruptured abdominal aortic aneurysms. The Finnvasc Study Group. Eur J Vasc Endovasc Surg. 1999;17:208–212. doi: 10.1053/ejvs.1998.0708. [DOI] [PubMed] [Google Scholar]
- 57.Ingoldby CJ, Wujanto R, Mitchell JE. Impact of vascular surgery on community mortality from ruptured aortic aneurysms. Br J Surg. 1986;73:551–553. doi: 10.1002/bjs.1800730711. [DOI] [PubMed] [Google Scholar]
- 58.Quill DS, Colgan MP, Sumner DS. Ultrasonic screening for the detection of abdominal aortic aneurysms. Surg Clin North Am. 1989;69:713–720. doi: 10.1016/s0039-6109(16)44878-4. [DOI] [PubMed] [Google Scholar]
- 59.Graeve AH, Carpenter CM, Wicks JD, Edwards WS. Discordance in the sizing of abdominal aortic aneurysm and its significance. Am J Surg. 1982;144:627–634. doi: 10.1016/0002-9610(82)90539-6. [DOI] [PubMed] [Google Scholar]
- 60.Gomes MN, Schellinger D, Hufnagel CA. Abdominal aortic aneurysms: diagnostic review and new technique. Ann Thorac Surg. 1979;27:479–488. doi: 10.1016/s0003-4975(10)63352-8. [DOI] [PubMed] [Google Scholar]
- 61.Wilmink AB, Forshaw M, Quick CR, Hubbard CS, Day NE. Accuracy of serial screening for abdominal aortic aneurysms by ultrasound. J Med Screen. 2002;9:125–127. doi: 10.1136/jms.9.3.125. [DOI] [PubMed] [Google Scholar]
- 62.Comstock CE, Bluth EI, Peattie RA, Schrader T, Leslie BR. Inter-observer variability in ultrasonic evaluation of abdominal aortic aneurysms. J La State Med Soc. 1994;146:526–530. [PubMed] [Google Scholar]
- 63.Jaakkola P, Hippelainen M, Farin P, Rytkonen H, Kainulainen S, Partanen K. Interobserver variability in measuring the dimensions of the abdominal aorta: comparison of ultrasound and computed tomography. Eur J Vasc Endovasc Surg. 1996;12:230–237. doi: 10.1016/s1078-5884(96)80112-2. [DOI] [PubMed] [Google Scholar]
- 64.Singh K, Jacobsen BK, Solberg S, Bonaa KH, Kumar S, Bajic R, Arnesen E. Intra- and interobserver variability in the measurements of abdominal aortic and common iliac artery diameter with computed tomography. The Tromso study. Eur J Vasc Endovasc Surg. 2003;25:399–407. doi: 10.1053/ejvs.2002.1856. [DOI] [PubMed] [Google Scholar]
- 65.Wanhainen A, Bergqvist D, Bjorck M. Measuring the abdominal aorta with ultrasonography and computed tomography—difference and variability. Eur J Vasc Endovasc Surg. 2002;24:428–434. doi: 10.1053/ejvs.2002.1748. [DOI] [PubMed] [Google Scholar]
- 66.Nevitt MP, Ballard DJ, Hallett JW., Jr Prognosis of abdominal aortic aneurysms. A population-based study. N Engl J Med. 1989;321:1009–1014. doi: 10.1056/NEJM198910123211504. [DOI] [PubMed] [Google Scholar]
- 67.Reed WW, Hallett JW, Jr, Damiano MA, Ballard DJ. Learning from the last ultrasound. A population-based study of patients with abdominal aortic aneurysm. Arch Intern Med. 1997;157:2064–2068. doi: 10.1001/archinte.157.18.2064. [DOI] [PubMed] [Google Scholar]
- 68.Lederle FA, Wilson SE, Johnson GR, Littooy FN, Acher C, Messina LM, Reinke DB, Ballard DJ, ADAM VA Cooperative Study Group Design of the abdominal aortic Aneurysm Detection and Management Study. J Vasc Surg. 1994;20:296–303. doi: 10.1016/0741-5214(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 69.Limet R, Sakalihassan N, Albert A. Determination of the expansion rate and incidence of rupture of abdominal aortic aneurysms. J Vasc Surg. 1991;14:540–548. doi: 10.1067/mva.1991.30047. [DOI] [PubMed] [Google Scholar]
- 70.Sterpetti AV, Schultz RD, Feldhaus RJ, Cheng SE, Peetz DJ., Jr Factors influencing enlargement rate of small abdominal aortic aneurysms. J Surg Res. 1987;43:211–219. doi: 10.1016/0022-4804(87)90073-4. [DOI] [PubMed] [Google Scholar]
- 71.Sharp MA, Collin J. A myth exposed: fast growth in diameter does not justify precocious abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2003;25:408–411. doi: 10.1053/ejvs.2002.1850. [DOI] [PubMed] [Google Scholar]
- 72.Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW, Messina LM, Ballard DJ, Ansel HJ, Abdominal Aortic Aneurysm Detection and Management Veterans Administration Cooperative Study Group Variability in measurement of abdominal aortic aneurysms. J Vasc Surg. 1995;21:945–952. doi: 10.1016/s0741-5214(95)70222-9. [DOI] [PubMed] [Google Scholar]
- 73.Lederle FA, Johnson GR, Wilson SE, Gordon IL, Chute EP, Littooy FN, Krupski WC, Bandyk D, Barone GW, Graham LM, Hye RJ, Reinke DB, the Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study investigators Relationship of age, gender, race, and body size to infrarenal aortic diameter. J Vasc Surg. 1997;26:595–601. doi: 10.1016/s0741-5214(97)70057-0. [DOI] [PubMed] [Google Scholar]
- 74.Lindholt JS, Vammen S, Juul S, Henneberg EW, Fasting H. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472–475. doi: 10.1053/ejvs.1999.0835. [DOI] [PubMed] [Google Scholar]
- 75.Baker SR. Abdominal CT screening: inflated promises, serious concerns. AJR Am J Roentgenol. 2003;180:27–30. doi: 10.2214/ajr.180.1.1800027. [DOI] [PubMed] [Google Scholar]
- 76.Sparks AR, Johnson PL, Meyer MC. Imaging of abdominal aortic aneurysms. Am Fam Physician. 2002;65:1565–1570. [PubMed] [Google Scholar]
- 77.Tayal VS, Graf CD, Gibbs MA. Prospective study of accuracy and outcome of emergency ultrasound for abdominal aortic aneurysm over two years. Acad Emerg Med. 2003;10:867–871. doi: 10.1111/j.1553-2712.2003.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 78.Blaivas M, Theodoro D. Frequency of incomplete abdominal aorta visualization by emergency department bedside ultrasound. Acad Emerg Med. 2004;11:103–105. doi: 10.1197/j.aem.2003.05.012. [DOI] [PubMed] [Google Scholar]
- 79.Adam DJ, Bradbury AW, Stuart WP, Woodburn KR, Murie JA, Jenkins AM, Ruckley CV. The value of computed tomography in the assessment of suspected ruptured abdominal aortic aneurysm. J Vasc Surg. 1998;27:431–437. doi: 10.1016/s0741-5214(98)70317-9. [DOI] [PubMed] [Google Scholar]
- 80.Mehard WB, Heiken JP, Sicard GA. High-attenuating crescent in abdominal aortic aneurysm wall at CT: a sign of acute or impending rupture. Radiology. 1994;192:359–362. doi: 10.1148/radiology.192.2.8029397. [DOI] [PubMed] [Google Scholar]
- 81.Arita T, Matsunaga N, Takano K, Nagaoka S, Nakamura H, Katayama S, Zempo N, Esato K. Abdominal aortic aneurysm: rupture associated with the high-attenuating crescent sign. Radiology. 1997;204:765–768. doi: 10.1148/radiology.204.3.9280256. comment in Radiology 1998;206:843–844. [DOI] [PubMed] [Google Scholar]
- 82.Gomes MN. Diagnosis of abdominal aortic aneurysms. Am Fam Physician. 1982;25:167–176. [PubMed] [Google Scholar]
- 83.Todd GJ, Nowygrod R, Benvenisty A, Buda J, Reemtsma K. The accuracy of CT scanning in the diagnosis of abdominal and thoracoabdominal aortic aneurysms. J Vasc Surg. 1991;13:302–310. [PubMed] [Google Scholar]
- 84.Rubin GD, Walker PJ, Dake MD, Napel S, Jeffrey RB, McDonnell CH, Mitchell RS, Miller DC. Three-dimensional spiral computed tomographic angiography: an alternative imaging modality for the abdominal aorta and its branches. J Vasc Surg. 1993;18:656–664. discussion 665. [PubMed] [Google Scholar]
- 85.Beebe HG, Kritpracha B. Imaging of abdominal aortic aneurysm: current status. Ann Vasc Surg. 2003;17:111–118. doi: 10.1007/s10016-001-0345-8. [DOI] [PubMed] [Google Scholar]
- 86.Vowden P, Wilkinson D, Ausobsky JR, Kester RC. A comparison of three imaging techniques in the assessment of an abdominal aortic aneurysm. J Cardiovasc Surg (Torino) 1989;30:891–896. [PubMed] [Google Scholar]
- 87.Nasim A, Thompson MM, Sayers RD, Boyle JR, Hartshorne T, Moody AR, Bell PR. Role of magnetic resonance angiography for assessment of abdominal aortic aneurysm before endoluminal repair. Br J Surg. 1998;85:641–644. doi: 10.1046/j.1365-2168.1998.00675.x. [DOI] [PubMed] [Google Scholar]
- 88.Lamah M, Darke S. Value of routine computed tomography in the preoperative assessment of abdominal aneurysm replacement. World J Surg. 1999;23:1076–1080. doi: 10.1007/s002689900626. discussion 1080–1081. [DOI] [PubMed] [Google Scholar]
- 89.Maher MM, McNamara AM, MacEneaney PM, Sheehan SJ, Malone DE. Abdominal aortic aneurysms: elective endovascular repair versus conventional surgery—evaluation with evidence-based medicine techniques. Radiology. 2003;228:647–658. doi: 10.1148/radiol.2283012185. [DOI] [PubMed] [Google Scholar]
- 90.Scott RA, Ashton HA, Kay DN. Abdominal aortic aneurysm in 4237 screened patients: prevalence, development and management over 6 years. Br J Surg. 1991;78:1122–1125. doi: 10.1002/bjs.1800780929. [DOI] [PubMed] [Google Scholar]
- 91.Smith FC, Grimshaw GM, Paterson IS, Shearman CP, Hamer JD. Ultrasonographic screening for abdominal aortic aneurysm in an urban community. Br J Surg. 1993;80:1406–1409. doi: 10.1002/bjs.1800801117. comments in Br J Surg. 1994:81, 472–623. [DOI] [PubMed] [Google Scholar]
- 92.Zuhrie SR, Brennan PJ, Meade TW, Vickers M. Clinical examination for abdominal aortic aneurysm in general practice: report from the Medical Research Council's General Practice Research Framework. Br J Gen Pract. 1999;49:731–732. [PMC free article] [PubMed] [Google Scholar]
- 93.Thurmond AS, Semler HJ. Abdominal aortic aneurysm: incidence in a population at risk. J Cardiovasc Surg (Torino) 1986;27:457–460. [PubMed] [Google Scholar]
- 94.Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, Makaroun MS, Barone GW, Bandyk D, Moneta GL, Makhoul RG, Aneurysm Detection and Management Veterans Affairs Cooperative Study investigators The Aneurysm Detection and Management Study screening program: validation cohort and final results. Arch Intern Med. 2000;160:1425–1430. doi: 10.1001/archinte.160.10.1425. [DOI] [PubMed] [Google Scholar]
- 95.Carty GA, Nachtigal T, Magyar R, Herzler G, Bays R. Abdominal duplex ultrasound screening for occult aortic aneurysm during carotid arterial evaluation. J Vasc Surg. 1993;17:696–702. [PubMed] [Google Scholar]
- 96.Berridge DC, Griffith CD, Amar SS, Hopkinson BR, Makin GS. Screening for clinically unsuspected abdominal aortic aneurysms in patients with peripheral vascular disease. Eur J Vasc Surg. 1989;3:421–422. doi: 10.1016/s0950-821x(89)80049-0. [DOI] [PubMed] [Google Scholar]
- 97.Frame PS, Fryback DG, Patterson C. Screening for abdominal aortic aneurysm in men ages 60 to 80 years. A cost-effectiveness analysis. Ann Intern Med. 1993;119:411–416. doi: 10.7326/0003-4819-119-5-199309010-00010. [DOI] [PubMed] [Google Scholar]
- 98.St Leger AS, Spencely M, McCollum CN, Mossa M. Screening for abdominal aortic aneurysm: a computer assisted cost-utility analysis. Eur J Vasc Endovasc Surg. 1996;11:183–190. doi: 10.1016/s1078-5884(96)80049-9. [DOI] [PubMed] [Google Scholar]
- 99.Pentikainen TJ, Sipila T, Rissanen P, Soisalon-Soininen S, Salo J. Cost-effectiveness of targeted screening for abdominal aortic aneurysm. Monte Carlo–based estimates. Int J Technol Assess Health Care. 2000;16:22–34. doi: 10.1017/s0266462300016135. [DOI] [PubMed] [Google Scholar]
- 100.Connelly JB, Hill GB, Millar WJ. The detection and management of abdominal aortic aneurysm: a cost-effectiveness analysis. Clin Invest Med. 2002;25:127–133. [PubMed] [Google Scholar]
- 101.Lee TY, Korn P, Heller JA, Kilaru S, Beavers FP, Bush HL, Kent KC. The cost-effectiveness of a “quick-screen” program for abdominal aortic aneurysms. Surgery. 2002;132:399–407. doi: 10.1067/msy.2002.126510. [DOI] [PubMed] [Google Scholar]
- 102.Boll AP, Severens JL, Verbeek AL, van der Vliet JA. Mass screening on abdominal aortic aneurysm in men aged 60 to 65 years in The Netherlands. Impact on life expectancy and cost-effectiveness using a Markov model. Eur J Vasc Endovasc Surg. 2003;26:74–80. doi: 10.1053/ejvs.2002.1773. [DOI] [PubMed] [Google Scholar]
- 103.Lindholt JS, Henneberg EW, Fasting H, Juul S. Hospital based screening of 65-73 year old men for abdominal aortic aneurysms in the county of Viborg, Denmark. J Med Screen. 1996;3:43–46. doi: 10.1177/096914139600300111. [DOI] [PubMed] [Google Scholar]
- 104.Lederle FA. Ultrasonographic screening for abdominal aortic aneurysms. Ann Intern Med. 2003;139:516–522. doi: 10.7326/0003-4819-139-6-200309160-00016. [DOI] [PubMed] [Google Scholar]
- 105.Crow P, Shaw E, Earnshaw JJ, Poskitt KR, Whyman MR, Heather BP. A single normal ultrasonographic scan at age 65 years rules out significant aneurysm disease for life in men. Br J Surg. 2001;88:941–944. doi: 10.1046/j.0007-1323.2001.01822.x. [DOI] [PubMed] [Google Scholar]
- 106.Scott RA, Vardulaki KA, Walker NM, Day NE, Duffy SW, Ashton HA. The long-term benefits of a single scan for abdominal aortic aneurysm (AAA) at age 65. Eur J Vasc Endovasc Surg. 2001;21:535–540. doi: 10.1053/ejvs.2001.1368. [DOI] [PubMed] [Google Scholar]
- 107.Cole CW, Hill GB, Millar WJ, Laupacis A, Johnston KW. Selective screening for abdominal aortic aneurysm. Chronic Dis Can. 1996;17:51–55. [PubMed] [Google Scholar]
- 108.Lindholt JS, Henneberg EW, Fasting H, Juul S. Mass or high-risk screening for abdominal aortic aneurysm. Br J Surg. 1997;84:40–42. [PubMed] [Google Scholar]
- 109.Spencer CA, Jamrozik K, Norman PE, Lawrence-Brown MM. The potential for a selective screening strategy for abdominal aortic aneurysm. J Med Screen. 2000;7:209–211. doi: 10.1136/jms.7.4.209. [DOI] [PubMed] [Google Scholar]
- 110.Wilmink TB, Quick CR, Hubbard CS, Day NE. The influence of screening on the incidence of ruptured abdominal aortic aneurysms. J Vasc Surg. 1999;30:203–208. doi: 10.1016/s0741-5214(99)70129-1. [DOI] [PubMed] [Google Scholar]
- 111.Wilmink AB, Quick CR, Hubbard CS, Day NE. Effectiveness and cost of screening for abdominal aortic aneurysm: results of a population screening program. J Vasc Surg. 2003;38:72–77. doi: 10.1016/s0741-5214(03)00135-6. [DOI] [PubMed] [Google Scholar]
- 112.Multicentre Aneurysm Screening Study Group Multicentre Aneurysm Screening Study (MASS): cost effectiveness analysis of screening for abdominal aortic aneurysms based on four year results from randomised controlled trial. BMJ. 2002;325:1135. doi: 10.1136/bmj.325.7373.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jamrozik K, Norman PE, Spencer CA, Parsons RW, Tuohy R, Lawrence-Brown MM, Dickinson JA. Screening for abdominal aortic aneurysm: lessons from a population-based study. Med J Aust. 2000;173:345–350. doi: 10.5694/j.1326-5377.2000.tb125684.x. [DOI] [PubMed] [Google Scholar]
- 114.Lawrence-Brown MM, Norman PE, Jamrozik K, Semmens JB, Donnelly NJ, Spencer C, Tuohy R. Initial results of ultrasound screening for aneurysm of the abdominal aorta in Western Australia: relevance for endoluminal treatment of aneurysm disease. Cardiovasc Surg. 2001;9:234–240. doi: 10.1016/s0967-2109(00)00143-5. [DOI] [PubMed] [Google Scholar]
- 115.Norman PE, Jamrozik K, Lawrence-Brown MM, Le MTQ, Spencer CA, Tuohy RJ, Parsons RW, Dickinson JA. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ. 2004;329:1259–1262. doi: 10.1136/bmj.38272.478438.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 117.Vardulaki KA, Prevost TC, Walker NM, Day NE, Wilmink AB, Quick CR, Ashton HA, Scott RA. Incidence among men of asymptomatic abdominal aortic aneurysms: estimates from 500 screen detected cases. J Med Screen. 1999;6:50–54. doi: 10.1136/jms.6.1.50. [DOI] [PubMed] [Google Scholar]
- 118.Couto E, Duffy SW, Ashton HA, Walker NM, Myles JP, Scott RA, Thompson SG. Probabilities of progression of aortic aneurysms: estimates and implications for screening policy. J Med Screen. 2002;9:40–42. doi: 10.1136/jms.9.1.40. [DOI] [PubMed] [Google Scholar]
- 119.Quinones-Baldrich WJ, Garner C, Caswell D, Ahn SS, Gelabert HA, Machleder HI, Moore WS. Endovascular, transperitoneal, and retroperitoneal abdominal aortic aneurysm repair: results and costs. J Vasc Surg. 1999;30:59–67. doi: 10.1016/s0741-5214(99)70176-x. [DOI] [PubMed] [Google Scholar]
- 120.Newman AB, Arnold AM, Burke GL, O'Leary DH, Manolio TA. Cardiovascular disease and mortality in older adults with small abdominal aortic aneurysms detected by ultrasonography: the Cardiovascular Health Study. Ann Intern Med. 2001;134:182–190. doi: 10.7326/0003-4819-134-3-200102060-00008. [DOI] [PubMed] [Google Scholar]
- 121.Brown LC, Powell JT, UK Small Aneurysm Trial participants Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. Ann Surg. 1999;230:289–296. doi: 10.1097/00000658-199909000-00002. discussion 296–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Norman PE, Semmens JB, Lawrence-Brown MM. Long-term relative survival following surgery for abdominal aortic aneurysm: a review. Cardiovasc Surg. 2001;9:219–224. doi: 10.1016/s0967-2109(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 123.Schermerhorn ML, Finlayson SR, Fillinger MF, Buth J, van Marrewijk C, Cronenwett JL. Life expectancy after endovascular versus open abdominal aortic aneurysm repair: results of a decision analysis model on the basis of data from EUROSTAR. J Vasc Surg. 2002;36:1112–1120. doi: 10.1067/mva.2002.129646. [DOI] [PubMed] [Google Scholar]
- 124.Arias E. National Vital Statistics Reports, Vol. 51 (No. 3) Hyattsville, MD: National Center for Health Statistics; 2002. United States Life Tables, 2000. [PubMed] [Google Scholar]
- 125.Norman PE, Semmens JB, Lawrence-Brown MM, Holman CD. Long term relative survival after surgery for abdominal aortic aneurysm in western Australia: population based study. BMJ. 1998;317:852–856. doi: 10.1136/bmj.317.7162.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Katz DJ, Stanley JC, Zelenock GB. Operative mortality rates for intact and ruptured abdominal aortic aneurysms in Michigan: an eleven-year statewide experience. J Vasc Surg. 1994;19:804–815. doi: 10.1016/s0741-5214(94)70005-2. discussion 816–817. [DOI] [PubMed] [Google Scholar]
- 127.Katz DA, Cronenwett JL. The cost-effectiveness of early surgery versus watchful waiting in the management of small abdominal aortic aneurysms. J Vasc Surg. 1994;19:980–990. doi: 10.1016/s0741-5214(94)70209-8. discussion 990–991. [DOI] [PubMed] [Google Scholar]
- 128.Patel ST, Korn P, Haser PB, Bush HL, Jr, Kent KC. The cost-effectiveness of repairing ruptured abdominal aortic aneurysms. J Vasc Surg. 2000;32:247–257. doi: 10.1067/mva.2000.105959. [DOI] [PubMed] [Google Scholar]
- 129.Benzaquen BS, Eisenberg MJ, Challapalli R, Nguyen T, Brown KJ, Topol EJ. Correlates of in-hospital cost among patients undergoing abdominal aortic aneurysm repair. Am Heart J. 1998;136:696–702. doi: 10.1016/s0002-8703(98)70018-3. [DOI] [PubMed] [Google Scholar]
- 130.Huber TS, Wang JG, Derrow AE, Dame DA, Ozaki CK, Zelenock GB, Flynn TC, Seeger JM. Experience in the United States with intact abdominal aortic aneurysm repair. J Vasc Surg. 2001;33:304–10. doi: 10.1067/mva.2001.112703. discussion 310–311. [DOI] [PubMed] [Google Scholar]
- 131.Patel ST, Haser PB, Bush HL, Jr, Kent KC. The cost-effectiveness of endovascular repair versus open surgical repair of abdominal aortic aneurysms: A decision analysis model. J Vasc Surg. 1999;29:958–972. doi: 10.1016/s0741-5214(99)70237-5. [DOI] [PubMed] [Google Scholar]
- 132.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 133.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, Welch HG, Wennberg DE. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 134.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 135.Pearce WH, Parker MA, Feinglass J, Ujiki M, Manheim LM. The importance of surgeon volume and training in outcomes for vascular surgical procedures. J Vasc Surg. 1999;29:768–776. doi: 10.1016/s0741-5214(99)70202-8. discussion 777–778. [DOI] [PubMed] [Google Scholar]
- 136.Galland RB, Magee TR. Acute (non-ruptured) abdominal aortic aneurysms [editorial] Eur J Vasc Endovasc Surg. 1997;14:421–422. doi: 10.1016/s1078-5884(97)80116-5. [DOI] [PubMed] [Google Scholar]
- 137.Sullivan CA, Rohrer MJ, Cutler BS. Clinical management of the symptomatic but unruptured abdominal aortic aneurysm. J Vasc Surg. 1990;11:799–803. [PubMed] [Google Scholar]
- 138.Cambria RA, Gloviczki P, Stanson AW, Cherry KJ, Jr, Hallett JW, Jr, Bower TC, Pairolero PC. Symptomatic, nonruptured abdominal aortic aneurysms: are emergent operations necessary? Ann Vasc Surg. 1994;8:121–126. doi: 10.1007/BF02018859. [DOI] [PubMed] [Google Scholar]
- 139.Ruby ST, Whittemore AD, Couch NP, Collins JJ, Cohn L, Shemin R, Mannick JA. Coronary artery disease in patients requiring abdominal aortic aneurysm repair. Selective use of a combined operation. Ann Surg. 1985;201:758–764. doi: 10.1097/00000658-198506000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mason JJ, Owens DK, Harris RA, Cooke JP, Hlatky MA. The role of coronary angiography and coronary revascularization before noncardiac vascular surgery. JAMA. 1995;273:1919–1925. [PubMed] [Google Scholar]
- 141.Eskind SJ. Computed tomography for ruptured abdominal aortic aneurysm? N Engl J Med. 1996;334:1272–1273. doi: 10.1056/nejm199605093341917. [DOI] [PubMed] [Google Scholar]
- 142.Gervais DA, Whitman GJ. Images in clinical medicine. Ruptured abdominal aortic aneurysm. N Engl J Med. 1996;334:27. doi: 10.1056/NEJM199601043340107. [DOI] [PubMed] [Google Scholar]
- 143.Briguori C, Tavano D, Colombo A. Contrast agent–associated nephrotoxicity. Prog Cardiovasc Dis. 2003;45:493–503. doi: 10.1053/pcad.2003.YPCAD16. [DOI] [PubMed] [Google Scholar]
- 144.Kvilekval KH, Best IM, Mason RA, Newton GB, Giron F. The value of computed tomography in the management of symptomatic abdominal aortic aneurysms. J Vasc Surg. 1990;12:28–33. comment in J Vasc Surg 1991;13: 351. [PubMed] [Google Scholar]
- 145.Bickell WH, Wall MJ, Jr, Pepe PE, Martin RR, Ginger VF, Allen MK, Mattox KL. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105–1109. doi: 10.1056/NEJM199410273311701. [DOI] [PubMed] [Google Scholar]
- 146.Bradbury AW, Bulstrode NW, Gilling-Smith G, Stansby G, Mansfield AO, Wolfe JH. Repair of ruptured thoracoabdominal aortic aneurysm is worthwhile in selected cases. Eur J Vasc Endovasc Surg. 1999;17:160–165. doi: 10.1053/ejvs.1998.0753. [DOI] [PubMed] [Google Scholar]
- 147.Akkersdijk GJ, van der Graaf Y, Moll FL, de Vries AC, Kitslaar PJ, van Bockel JH, Hak E, Eikelboom BC. Complications of standard elective abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 1998;15:505–510. doi: 10.1016/s1078-5884(98)80110-x. [DOI] [PubMed] [Google Scholar]
- 148.Prance SE, Wilson YG, Cosgrove CM, Walker AJ, Wilkins DC, Ashley S. Ruptured abdominal aortic aneurysms: selecting patients for surgery. Eur J Vasc Endovasc Surg. 1999;17:129–132. doi: 10.1053/ejvs.1998.0718. [DOI] [PubMed] [Google Scholar]
- 149.US Preventive Services Task Force Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med. 2005;142:198–202. doi: 10.7326/0003-4819-142-3-200502010-00011. [DOI] [PubMed] [Google Scholar]