Abstract
Vascular endothelial growth factor receptor-3 (VEGFR-3/Flt4) binds two known members of the VEGF ligand family, VEGF-C and VEGF-D, and has a critical function in the remodelling of the primary capillary vasculature of midgestation embryos. Later during development, VEGFR-3 regulates the growth and maintenance of the lymphatic vessels. In the present study, we have isolated and cultured stable lineages of blood vascular and lymphatic endothelial cells from human primary microvascular endothelium by using antibodies against the extracellular domain of VEGFR-3. We show that VEGFR-3 stimulation alone protects the lymphatic endothelial cells from serum deprivation-induced apoptosis and induces their growth and migration. At least some of these signals are transduced via a protein kinase C-dependent activation of the p42/p44 MAPK signalling cascade and via a wortmannin-sensitive induction of Akt phosphorylation. These results define the critical role of VEGF-C/VEGFR-3 signalling in the growth and survival of lymphatic endothelial cells. The culture of isolated lymphatic endothelial cells should now allow further studies of the molecular properties of these cells.
Keywords: endothelium/Flt4/lymphatic/LYVE-1/podoplanin
Introduction
Vascular endothelial growth factor (VEGF) is a prime regulator of endothelial cell proliferation, angiogenesis, vasculogenesis and vascular permeability (Ferrara, 1999). Besides VEGF, the VEGF family of growth factors currently contains five other known members, namely placenta growth factor (PlGF), VEGF-B, VEGF-C, VEGF-D and orf viral VEGF homologs (Eriksson and Alitalo, 1999). Additional novel VEGF-like heparin-binding proteins were isolated recently from snake venom (Komori et al., 1999; Gasmi et al., 2000). Disruption of the genes encoding either VEGF or any of the three receptors of the VEGF family, VEGFR-1/Flt1, VEGFR-2/Flk1/KDR or VEGFR-3/Flt4, results in embryonic lethality because of failure of blood vessel development (Fong et al., 1995; Shalaby et al., 1995; Dumont et al., 1998).
VEGFR-2 is considered to be the main signal transducing VEGF receptor for angiogenesis and for mitogenesis of endothelial cells. VEGF induces endothelial cell proliferation, migration and survival via activation of VEGFR-2 and subsequent signal transduction pathways including the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and the phosphatidylinositol (PI) 3-kinase pathways (for reviews see Petrova et al., 1999; Shibuya et al., 1999). Activation of the p42/p44 MAPK (ERK1/ERK2) cascade is linked in many cells to a proliferation response. In addition, this pathway can lead to increased cell survival by stimulating the transcription of pro-survival genes and by post-translational modification and inactivation of components of the cell death machinery (Bonni et al., 1999; Gupta et al., 1999). The PI3-kinase pathway was also initially linked to mitogenesis, but several studies subsequently have shown that this pathway has an important function in regulating cell survival by activation of the serine-threonine kinase Akt (protein kinase B) (Datta et al., 1999). Recent studies have also indicated some cross-talk between the MAPK and PI3-kinase signalling pathways: phosphorylation of Raf by Akt resulted in inhibition of the Raf–MEK (MAP kinase kinase)–ERK pathway (Rommel et al., 1999; Zimmermann and Moelling, 1999).
The expression of the Vegfr-3 gene starts during mouse embryonic day 8.5 in developing blood vessels, and VEGFR-3-deficient embryos die at midgestation due to defects in the remodelling of primary vascular networks (Dumont et al., 1998). However, in adult tissues, VEGFR-3 expression occurs mainly in the lymphatic endothelia (Kaipainen et al., 1995; Dumont et al., 1998; Partanen et al., 2000). VEGFR-3 ligands VEGF-C and VEGF-D can induce the growth of both lymphatic and blood vessels (Jeltsch et al., 1997; Cao et al., 1998; Witzenbichler et al., 1998; Marconcini et al., 1999; Veikkola et al., 2001). In contrast, blocking of VEGFR-3 signalling by use of a soluble VEGFR-3 protein caused regression of developing lymphatic vessels by inducing endothelial cell apoptosis (Mäkinen et al., 2001). However, the biochemical signalling pathways activated via VEGFR-3 are less well characterized than those of VEGFR-2, mainly because isolated lymphatic endothelial cells have not been available for molecular studies. Also, little is known about the characteristic features of the lymphatic endothelial cells.
Here we show for the first time that primary cultures of human dermal microvascular endothelial cells consist of distinct lineages of blood vascular and lymphatic endothelial cells and that the latter can be isolated by using antibodies against VEGFR-3. We have used VEGF-C and its VEGFR-3-specific mutant to study VEGFR-3 signalling. Stimulation of VEGFR-3 alone induced a protein kinase C (PKC)-dependent activation of the p42/p44 MAPK and a wortmannin-sensitive pathway for the induction of Akt phosphorylation, as well as cell migration and survival in the lymphatic endothelial cultures. These results define the critical role of VEGFR-3 in the growth, migration and survival of lymphatic endothelial cells.
Results
Human dermal microvascular endothelial cells consist of distinct populations of blood vascular and lymphatic endothelial cells
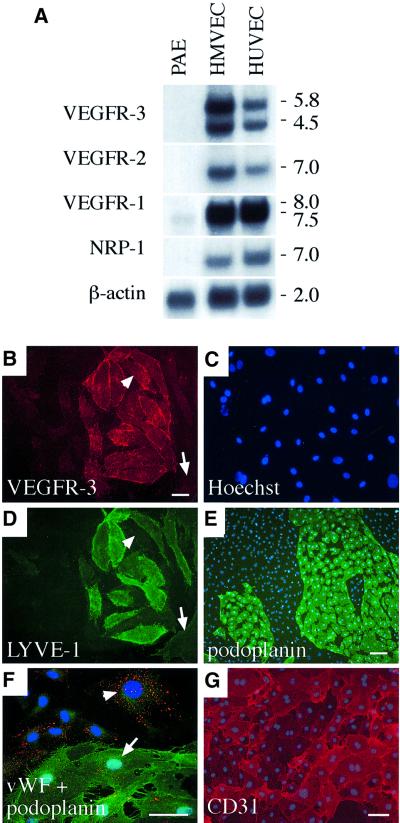
The functions of different VEGF receptors have been studied extensively in transfected cell lines, but the lack of an appropriate cellular background can compromise results obtained from such studies. In our present study, we therefore used primary endothelial cells, human dermal microvascular endothelial cells (HMVECs) and human umbilical vein endothelial cells (HUVECs) to elucidate VEGFR-3 signalling pathways promoting endothelial cell survival. All three VEGF tyrosine kinase receptors and the neuropilin-1 co-receptor were expressed in cultures of both HMVECs and HUVECs (Figure 1A). VEGFR-3 mRNA expression was stronger in microvascular endothelial cells and therefore these cells were used for the study of VEGFR-3 signalling in subsequent experiments.
Fig. 1. (A) Expression of VEGFR mRNAs in primary human dermal microvascular endothelial cells (HMVECs), human umbilical vein endothelial cells (HUVECs) and in the porcine aortic endothelial (PAE) cell line. A northern blot containing 8 µg of the mRNAs was probed with radiolabelled cDNA fragments of human VEGF receptors and with β-actin for the control of equal loading. Numbers to the right denote the sizes of the transcripts (kb). (B–G) HMVECs consist of two distinct populations of blood vascular and lymphatic endothelial cells. Immunofluorescence double staining using antibodies against VEGFR-3 (B) and LYVE-1 (D) with conterstaining of the nuclei by Hoechst fluorochrome (C). Note that LYVE-1 expression is not detected in all VEGFR-3-positive cells (arrowhead in B and D) while some VEGFR-3-negative cells are also stained weakly with LYVE-1 antibodies (arrow in B and D). Immunolabelling with antibodies against podoplanin (green in E and F), vWF (red, F) and CD31 (G). The nuclei were stained with the Hoechst fluorochrome (E–G). vWF expression occurs primarily in the podoplanin-negative cells (arrowhead in F), but weaker expression is also detected on podoplanin-positive cells (arrow in F). Stainings in (B–E) were carried out using live cells on ice, and in (F) and (G) after PFA fixation. Scale bars in (B–D), (F) and (G), 50 µm; in (E), 100 µm.
In immunofluorescence microscopy, only a subset of the HMVECs was positive for VEGFR-3 (Figure 1B and C). Antigen-blocking experiments and use of different monoclonal antibodies indicated that the VEGFR-3 staining was specific (data not shown). The VEGFR-3-expressing cells grew in distinct islands surrounded by VEGFR-3-negative cells (Figure 1B and C). Based on our previous immunostaining results from human tissues (Jussila et al., 1998; Lymboussaki et al., 1998), we assumed that the former represented lymphatic and the latter blood vascular endothelial cells. Most, but not all, of the VEGFR-3-expressing cells and a few of the VEGFR-3-negative cells were stained for the lymphatic endothelial cell marker LYVE-1 (Banerji et al., 1999) (Figure 1D). The VEGFR-3-positive cells were also stained specifically for podoplanin, another recently identified lymphatic endothelial marker (Breiteneder-Geleff et al., 1999) (Figure 1E, see also Figure 4J–L). Similar results were also obtained in fluorescence-activated cell sorting (FACS) analysis (data not shown). The von Willebrand factor (vWF) antigen (Figure 1F, red staining) was expressed more prominently in the blood vascular endothelial cells (arrowhead), which were negative for podoplanin (green staining in Figure 1F). The pan-endothelial cell marker CD31 was detected in all cells, confirming the absence of contaminating non-endothelial cells (Figure 1G). According to western and northern blot analyses, VEGFR-1 and VEGFR-2 were expressed in both endothelial cell populations, while in these analyses only small amounts of VEGFR-3 were detected in the blood vascular endothelial cells (data not shown, see Figure 3B, inset). Among the freshly isolated HMVECs, the proportion of VEGFR-3-positive cells was in general >50%, decreasing upon repeated subculture (data not shown).
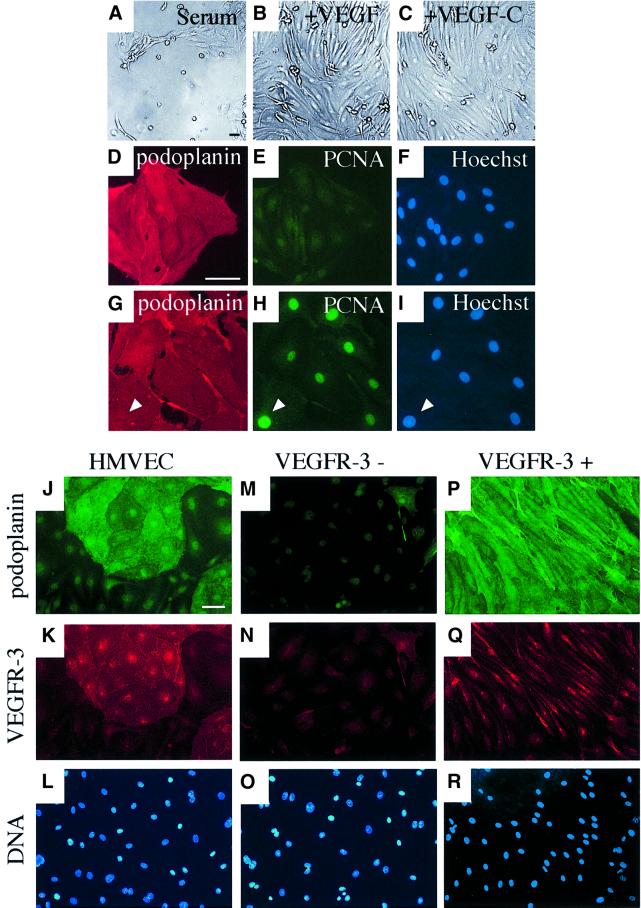
Fig. 4. Isolation of VEGFR-3/podoplanin-positive and -negative endothelial cells using magnetic microbeads. (A–I) Culture of VEGFR-3-expressing lymphatic endothelial cells in complete medium containing 5% serum (A) and supplemented with VEGF (10 ng/ml, B) or VEGF-C (100 ng/ml, C). Staining of the VEGFR-3-positive cells grown for 5 days after sorting in serum (D–F) or supplemented with VEGF-C (G–I) for podoplanin (red; D and G) or PCNA (green; E and H). The nuclei were stained with the Hoechst fluorochrome (F and I). Note that if supplemented with VEGF-C, the cells are stained for PCNA (arrowhead in G–I). Immunofluorescence double-staining of non-sorted cells (J–L) or VEGFR-3-negative (M–O) and VEGFR-3-positive (P–R) cell populations with antibodies against podoplanin (green; J, M and P) or VEGFR-3 (red; K, N and Q). The nuclei were stained with the Hoechst fluorochrome (L, O and R). The VEGFR-3+ cells were cultured in the presence of VEGF-C (100 ng/ml). Scale bars, 50 µm.
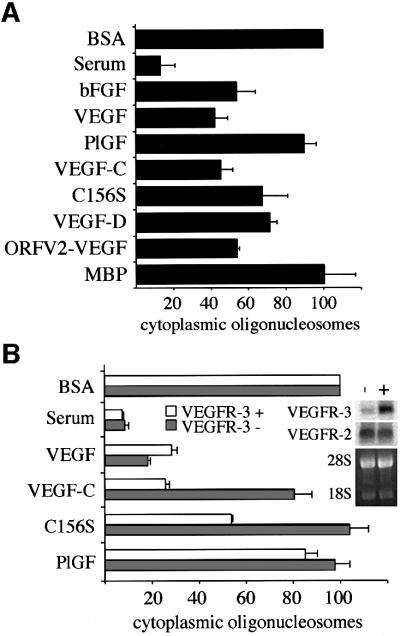
Fig. 3. VEGFR-2- and VEGFR-3-, but not VEGFR-1-activating ligands inhibit apoptosis of serum-deprived HMVECs. Measurement of the cytoplasmic histone-associated DNA fragments (mono- and oligo nucleosomes) in serum-starved HMVECs consisting of two cell populations of blood vascular and lymphatic endothelial cells (A) or in the isolated cell populations after magnetic cell sorting using VEGFR-3 antibodies (B). The enrichment factor of cytoplasmic oligonucleosomes in the apoptotic cells grown for 24 h in serum-free medium (BSA) was chosen as 100%. Data represent mean values from three independent experiments (mean ± SD). Grey bars in (B) represent blood vascular endothelial cells and white bars represent VEGFR-3-expressing lymphatic endothelial cells. The inset in (B) shows a northern blot containing 4 µg of total RNAs extracted from blood vascular (–) and lymphatic endothelial cells (+). The blot was probed with radiolabelled cDNA fragments of human VEGFR-2 or VEGFR-3, and the 28S and 18S rRNAs were visualized by ethidium bromide staining of the gel for the control of equal loading. The following concentrations of growth factors were used in the apoptosis assays: bFGF, 10 ng/ml; PlGF, 500 ng/ml; VEGF, 50 ng/ml; VEGF-C, 100 ng/ml; VEGF- C156S, 500 ng/ml; VEGF-D, 500 ng/ml; ORFV2-VEGF, 500 ng/ml; and myelin basic protein (MBP) as an irrelevant control protein, 500 ng/ml.
Analysis of VEGFR-specific ligands used for the cell survival experiments
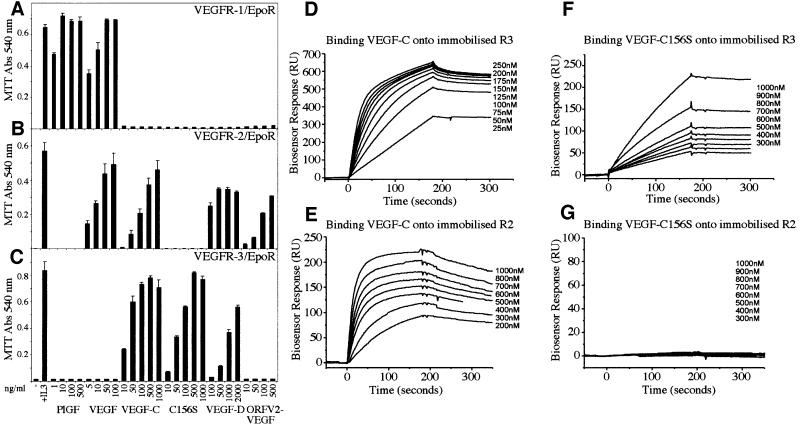
VEGF is an endothelial cell mitogen, which has also been shown to protect endothelial cells from starvation-induced apoptosis via activation of VEGFR-2 (Gerber et al., 1998). We wanted to compare the abilities of the different VEGFRs to promote endothelial cell survival by using VEGFR-specific VEGFs. The specificities of the growth factors used were determined using a cell survival bioassay. For the bioassay, Ba/F3 pre-B cells were stably transfected with a chimeric receptor containing the extracellular domain of human VEGFR-1, VEGFR-2 or VEGFR-3 fused with the transmembrane and cytoplasmic domains of the mouse erythropoietin receptor (EpoR). As expected, only VEGF and PlGF were able to induce the survival of VEGFR-1/EpoR cells (Figure 2A). VEGF, VEGF-C, VEGF-D and orf viral NZ2 (ORFV2-VEGF) were able to support the survival of the VEGFR-2/EpoR-expressing cells, whereas the mutant VEGF-C156S that binds to and activates only VEGFR-3 (Joukov et al., 1998) did not affect the survival of these cells (Figure 2B). Instead, VEGFR-3/EpoR-expressing cells survived in the presence of VEGF-C, VEGF-C156S and VEGF-D (Figure 2C). On the basis of these experiments, a VEGF-C concentration of 100 ng/ml and a VEGF- C156S concentration of 500 ng/ml, which gave maximal viability in VEGFR-3/EpoR cell survival assays, were chosen for the subsequent apoptosis and signalling experiments.
Fig. 2. (A–C) Analysis of the receptor specificities of different VEGFs using the Ba/F3 bioassay. Measurement of the viability of Ba/F3 cells expressing the chimeric receptors VEGFR-1/EpoR (A), VEGFR-2/EpoR (B) or VEGFR-3/EpoR (C) in the presence of different VEGFs at the indicated concentrations. Cell viability was determined using the MTT assay. Data represent the mean values from triplicate assays (mean ± SD). (D–G) Biosensor analysis of the interaction of VEGF-C (D and E) and VEGF-C156S (F and G) with VEGFR-3 (D and F) and VEGFR-2 (E and G). Chimeric receptor proteins were immobilized onto a carboxymethylated dextran surface. Growth factors were injected over the surface at a flow rate of 20 µl/min at the indicated concentrations. The sensorgrams shown have been subtracted with the corresponding signal obtained when the same sample was passed over a blank control channel. Kinetic data derived from the biosensor analysis are shown in Table I.
Biosensor analysis was used to investigate further the interactions of VEGF-C and VEGF-C156S with VEGFR-2 and VEGFR-3. Analysis of the biosensor-binding curves confirmed that VEGF-C156S binds only to the extracellular domain of VEGFR-3, whereas wild-type VEGF-C bound to both VEGFR-2 and VEGFR-3 receptors (Figure 2D–G). The analysis of the kinetics of the VEGF-C–VEGFR interactions (Table I) revealed lower KD values than reported previously using radioactive ligand-binding analysis in cultured receptor-expressing cells (Joukov et al., 1997). However, in both assays, the affinity of VEGF-C was higher towards VEGFR-3 than towards VEGFR-2. When compared with VEGF-C, the relative affinity of VEGF-C156S for VEGFR-3 was significantly lower, but of similar magnitude to that reported for the interaction of mouse VEGF-D with mouse VEGFR-3 (Baldwin et al., 2001).
Table I. Kinetic data derived from the biosensor analysis of the interaction of VEGF-C and VEGF-C156S with VEGFR-2 and VEGFR-3.
| Ligand | Receptor | ka (1/ms) | kd (1/s) | KD (M) | Reference |
|---|---|---|---|---|---|
| hVEGF-C | hVEGFR-2 | 5.5 × 104 | 12.3 × 10–4 | 2.2 × 10–8 | |
| hVEGF-C | hVEGFR-3 | 13.6 × 104 | 6.05 × 10–4 | 0.44 × 10–8 | |
| hVEGF-C156S | hVEGFR-2 | no binding | no binding | no binding | |
| hVEGF-C156S | hVEGFR-3 | 0.35 × 104 | 4.0 × 10–4 | 11.5 × 10–8 | |
| hVEGF-D | hVEGFR-2 | 1.3 × 104 | 6.3 × 10–4 | 4.8 × 10–8 | Baldwin et al. (2001) |
| hVEGF-D | hVEGFR-3 | 1.8 × 104 | 12 × 10–4 | 6.5 × 10–8 | Baldwin et al. (2001) |
| mVEGF-D | mVEGFR-2 | no binding | no binding | no binding | Baldwin et al. (2001) |
| mVEGF-D | mVEGFR-3 | 0.8 × 104 | 7.0 × 10–4 | 8.9 × 10–8 | Baldwin et al. (2001) |
The data were extracted by global fitting using BIAevaluation 3.0 assuming a 1:1 Langmuirian model with mass transfer.
h, human; m, mouse.
The ligands used in the study by Baldwin et al. are the mature forms of VEGF-D, as are VEGF-C and VEGF-C156S used in this study. All the other receptors used were bivalent immunoglobulin fusion proteins except mVEGFR-2, which was monovalent.
VEGFR-3 signalling protects endothelial cells from serum starvation-induced apoptosis
All VEGFs capable of stimulating VEGFR-2 or VEGFR-3, or both, including VEGF, VEGF-C, VEGF- C156S, VEGF-D and ORFV2-VEGF, were able to protect the HMVECs from starvation-induced DNA degradation, which was measured as the amount of cytoplasmic histone-associated DNA fragments (Figure 3A). In contrast, PlGF, which binds only to VEGFR-1, did not give significant protection. The lack of VEGFR-1-mediated survival signals was also suggested by the fact that the VEGFR-2-specific ligand, ORFV2-VEGF, gave protection comparable with that obtained with VEGF. VEGF-C and VEGF-C156S dose-dependently inhibited the accumulation of oligo- and mononucleosomes in the serum-deprived cells (data not shown). The maximum effect of VEGF-C was achieved at 100 ng/ml and that of VEGF-C156S at 500 ng/ml.
Isolation and growth of the lymphatic endothelial cells
In order to compare the effects of the VEGFs on the survival of lymphatic versus blood vascular endothelial cells, specific antibodies and magnetic microbeads were used to isolate and to culture the VEGFR-3/podoplanin-positive and -negative cells. The lymphatic endothelial cell cultures were >95% pure according to immunofluorescence staining (data not shown). The isolated VEGFR-3-positive cells did not adhere well on culture dishes and only a few cells proliferated in the complete culture medium containing 5% serum (Figure 4A and D–F). However, if supplemented with either VEGF (Figure 4B and data not shown) or VEGF-C (Figure 4C and G–I), most of the VEGFR-3- and podoplanin-positive cells proliferated readily, as observed by staining for proliferating cell nuclear antigen (PCNA). In contrast, the blood vascular endothelial cells grew well without the addition of these factors (data not shown). However, if the lymphatic endothelial cells were cultured on fibronectin, the cells adhered better and more proliferating nuclei were detected in comparison with the cells grown on the uncoated culture dishes (data not shown). This indicates that an appropriate extracellular matrix provides additional signals for the adhesion, survival and proliferation of these cells.
The morphology of the isolated lymphatic endothelial cells was more elongated and the cells displayed several protrusions when cultured in the presence of VEGF-C (Figure 4P and Q). Immunofluorescence for podoplanin (green) and VEGFR-3 (red) co-localized to the same cells in non-sorted, VEGFR-3-negative and VEGFR-3-positive cell populations (Figure 4). In VEGF-C supplemented cultures, only cytoplasmic staining for VEGFR-3 was observed (Figure 4Q), consistent with internalization of the ligand–receptor complexes. In contrast, in the presence of serum (Figure 4K) or VEGF (data not shown), VEGFR-3 was distributed on the cell surface.
VEGF-C promotes survival of mainly the VEGFR-3-expressing lymphatic endothelial cells
The accumulation of cytoplasmic mono- and oligonucleosomes was measured as a sign of apoptosis in the two endothelial cell populations during serum starvation. In the VEGFR-3-expressing cells, both VEGF-C and VEGF promoted cell survival (Figure 3B). However, for the VEGFR-3-negative cells, VEGF-C was a less efficient survival factor, requiring 5- to 10-fold higher concentrations for an equal effect as detected in VEGFR-3-positive cells (data not shown). As expected, VEGF-C156S induced the survival of only the VEGFR-3-positive cells (Figure 3B). Northern blot analysis revealed equally strong expression of VEGFR-2 in both blood vascular and lymphatic endothelial cell lineages (inset in Figure 3B), explaining the responsiveness of both of these cell types upon VEGF stimulation. However, only weak expression of VEGFR-3 was detected in blood vascular endothelial cells in comparison with the lymphatic endothelial cells (inset in Figure 3B). These results confirmed that VEGFR-3 alone can transduce endothelial cell survival signals and that VEGF-C differentially targets blood vascular and lymphatic endothelial cells.
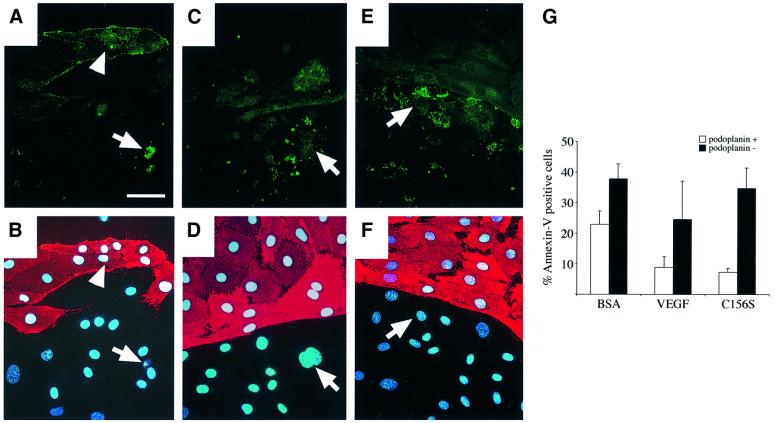
Serum deprivation-induced apoptosis was also monitored by analysing the exposure of phosphatidylserine at the cell surface using the fluorescence-conjugated phospholipid-binding protein, annexin-V. Annexin-V-stained cells were detected after 24 h of serum starvation and, by 72 h of starvation, ∼40% of the adherent cells were apoptotic, although the cells were more resistant to apoptosis in early passage and at confluence (data not shown).
Addition of VEGF to the starvation medium strongly decreased the number of cells displaying annexin-V positivity as well as cell detachment (Figure 5). The annexin-V-positive cells were not stained with propidium iodide and thus they represented apoptotic, not necrotic cells (data not shown). On the other hand, staining of the nuclei by the Hoechst fluorochrome revealed pyknotic nuclei typical for cells undergoing apoptosis (Figure 5B, arrow). These pyknotic nuclei were also positive for TUNEL staining (data not shown). Interestingly, stimulation with VEGF-C (data not shown) and especially with VEGF-C156S increased the survival of mainly the lymphatic endothelial cells (Figure 5E–G). The number of annexin-V-positive cells was also higher among the podoplanin-negative cells grown in the serum-free medium containing 0.2% bovine serum albumin (BSA) and in VEGF-treated cultures (Figure 5G). This may be partly an indirect effect, since blood vascular, but not lymphatic endothelial cells produce VEGF-C (data not shown) and therefore they can probably promote the survival of the lymphatic endothelial cells.
Fig. 5. (A–F) Annexin-V staining of HMVECs after 72 h of culture in serum-free medium alone (BSA, A) or with stimulation of VEGF (C) or VEGF-C (E). Simultaneous staining using antibodies against podoplanin (B, D and F) was used to distinguish lymphatic and blood vascular endothelial cells. Arrows indicate apoptotic, annexin-V-positive lymphatic endothelial cells, and arrowheads indicate apoptotic blood vascular endothelial cells. The nuclei were conterstained with Hoechst fluorochrome. (G) Quantitation of the annexin-V-positive cells (% of adherent cells) in the podoplanin-positive and -negative cell populations after 72 h of serum starvation. Note that detached cells were not included in the quantitation. Data represent mean values from five counted areas (×400) (mean ± SD). Scale bar in (A–F), 50 µm.
VEGFR-3 phosphorylation leads to PI3-kinase-dependent Akt activation
As discussed in the Introduction, a major signal transduction pathway by which growth factor receptors can promote cell survival employs the PI3-kinase and its downstream target, the serine-threonine kinase Akt. We analysed the effect of the different VEGFs on Akt by assessing Akt phosphorylation in Ser473 and Thr308 using phosphospecific antibodies. Akt was found to be phosphorylated at Ser473 in HMVECs stimulated by VEGF, VEGF-C, VEGF-C156S or VEGF-D, but not in PlGF- stimulated HMVECs (Figure 6). This indicated that Akt is activated by growth factor signals transduced via VEGFR-2 or VEGFR-3, but not via VEGFR-1. A similar increase in Akt Thr308 phosphorylation was also detected (data not shown). The PI3-kinase inhibitors wortmannin (30 nM) (Figure 6A) and LY294002 (20 µM) (data not shown) abolished the Akt phosphorylation in response to all the VEGFs studied, demonstrating that the VEGFR-3-mediated Akt activation is transduced via the PI3-kinase, as has been shown previously for VEGFR-2 (Gerber et al., 1998; Thakker et al., 1999).
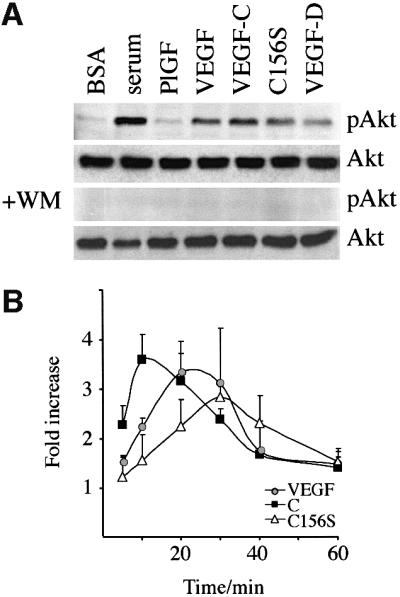
Fig. 6. VEGFR-2 or VEGFR-3 stimulation leads to PI3-kinase-dependent Akt phosphorylation. (A) The indicated ligands were used to stimulate HMVECs for 25 min, and Akt-Ser473 phosphorylation was analysed. Note that 30 nM wortmannin (WM) abolished the Akt activation in response to all VEGFs studied. (B) VEGF (grey circles), VEGF-C (black boxes) and VEGF-C156S (open triangles) induced phosphorylation of Akt with different kinetics. The data represent quantitations of optical densities of the signals from phosphorylated versus total Akt protein from three independent experiments (mean ± SD).
Akt was found to be phosphorylated maximally at 20–30 min after the exposure of HMVECs to VEGF, while the VEGF-C-induced Akt phosphorylation peaked at 10 min (Figure 6B). In striking contrast, VEGF-C156S stimulation resulted in slower Akt phosphorylation, peaking at 30–40 min. The differences in the activation of downstream targets suggested that Akt phosphorylation via VEGFR-2 or VEGFR-3 may be transduced via different routes. VEGFR-2 probably can transduce signals for Akt phosphorylation via the classical pathway as it constitutively associates with the regulatory p85 subunit of the PI3-kinase (Thakker et al., 1999). In contrast, we and others have not been able to detect association of p85 PI3-kinase with VEGFR-3 autophosphorylation (Pajusola et al., 1994; Borg et al., 1995; data not shown).
Simultaneous VEGFR-2 and VEGFR-3 stimulation by VEGF-C induces a sustained p42/p44 MAPK activation
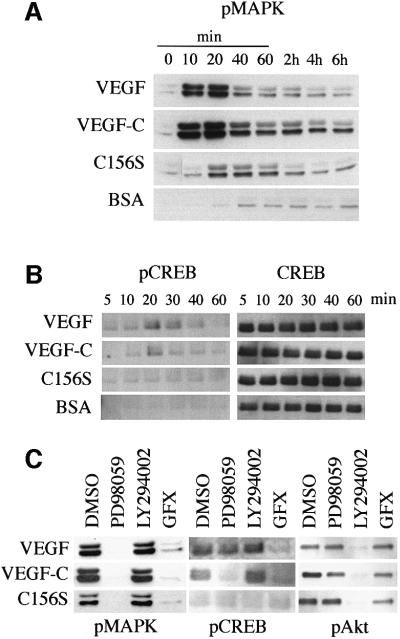
The MAPK signalling pathway is another mechanism implicated in growth factor-dependent cell survival. We detected MAPK activation in HMVECs after VEGFR-3 stimulation by VEGF-C156S (Figure 7A). However, MAPK phosphorylation induced by the VEGFR-2 ligands VEGF and VEGF-C was significantly stronger in these cells. Although MAPK activation after both VEGF-C and VEGF stimulation peaked at 10–20 min, the VEGF- induced activation was more transient than that induced by VEGF-C, which persisted for at least 6 h (Figure 7A).
Fig. 7. (A and B) Simultaneous signalling via VEGFR-2 and VEGFR-3 upon VEGF-C stimulation leads to sustained p42/p44 MAPK activation in HMVECs. The p42/p44 MAPK activation was detected by western blotting using phospho-Thr202/Tyr204-MAPK-specific antibodies (A) and CREB phosphorylation using phospho-Ser133-specific antibodies (B). The growth factor concentrations used are: VEGF, 10 ng/ml; VEGF-C, 100 ng/ml; and VEGF-C156S, 500 ng/ml. (C) The VEGFR- 3-induced p42/p44 MAPK activation is mediated via PKC in HMVECs. Effects of inhibition of PKC by GF109203X (GFX), MEK1 by PD98059, and PI-3 kinase by LY294002 on p42/p44 MAPK Thr202/Tyr204 phosphorylation, CREB Ser133 phosphorylation and Akt Ser473 phosphorylation in HMVECs. The growth factor concentrations used are: VEGF, 1 ng/ml; VEGF-C, 10 ng/ml; and VEGF-C156S, 500 ng/ml.
Downstream of the MAPKs, the MAPK-activated kinases, Rsks, have been shown to phosphorylate the transcription factor cAMP response element-binding protein (CREB) at Ser133, which promotes cell survival by increasing transcription of pro-survival genes (Bonni et al., 1999). CREB phosphorylation, which correlated with p42/p44 MAPK activation, was detected after stimulation of the HMVECs by VEGF or VEGF-C, but not by VEGF-C156S (Figure 7B). Although Akt has also been shown to phosphorylate CREB (Du and Montminy, 1998), inhibition of Akt with LY294002 did not affect CREB phosphorylation (Figure 7C). In contrast, inhibition of MEK1 (MAPK kinase) with PD98059 (Figure 7C) or U0126 (data not shown) inhibited VEGF-C-, but not VEGF-induced CREB phosphorylation.
The VEGFR-3-induced MAPK activation is mediated via PKC
VEGF-induced activation of the MAPK cascade has been shown to be mediated by PKC instead of the classical Ras pathway (Doanes et al., 1999; Takahashi et al., 1999; Yoshiji et al., 1999). In order to study the effect of PKC inhibition on VEGF-C- and VEGF-C156S-induced MAPK activation, the minimum concentrations of VEGF, VEGF-C and VEGF-C156S were titrated that gave maximal p42/p44 MAPK activation as measured by western blotting using phosphospecific antibodies. In these conditions, inhibition of PKC by GF109203X completely blocked p42/44 MAPK phosphorylation induced by VEGF, VEGF-C or VEGF-C156S and CREB phosphorylation induced by VEGF or VEGF-C (Figure 7C). Moreover, inhibition of MEK1 by PD98059 resulted in decreased phosphorylation of CREB upon VEGF-C stimulation. Suprisingly, this treatment did not inhibit VEGF-induced CREB phosphorylation (Figure 7C). In agreement, in a recent study, VEGF-induced CREB phosphorylation was shown to be mediated via PKC and p38 MAPK, not via p42/p44 MAPK (Mayo et al., 2001).
VEGFR-3 induces endothelial cell migration
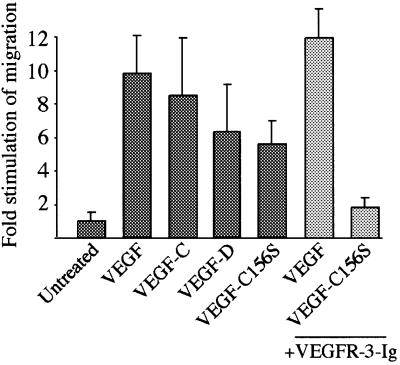
Migration of endothelial cells plays a critical role in angiogenesis, and at least some of the VEGF-induced migration signals are transduced via PI3-kinase (Gille et al., 2000, 2001; Qi and Claesson-Welsh, 2001). Since the VEGFR-3-deficient embryos die due to a failure of vascular remodelling (Dumont et al., 1998), we wanted to study whether VEGFR-3 signalling is also involved in the migration of endothelial cells. The HMVECs were incubated in the presence of different VEGFs in a Boyden chamber assay. VEGF induced a 10-fold stimulation of cell migration (Figure 8). In addition, both VEGF-C and VEGF-D as well as VEGF-C156S stimulated significant migration of HMVE cells. The VEGF-C156S-induced migration was blocked specifically by a 10-fold molar excess of soluble VEGFR-3 (Figure 8, light grey bars). When isolated blood vascular and lymphatic endothelial cells were tested in the same assay, both cell lineages migrated in response to VEGF and VEGF-C while only the VEGFR-3/podoplanin-positive cells migrated towards VEGF-C156S (data not shown). These results indicated that signalling via VEGFR-3 is sufficient for the induction of lymphatic endothelial cell migration.
Fig. 8. VEGFR-3 mediates endothelial cell migration. The migration of HMVECs in the presence of different VEGFs in a Boyden chamber assay. VEGF-C156S-, but not VEGF-stimulated migration was blocked by pre-incubating VEGF-C156S with a 10-fold molar excess of soluble VEGFR-3 (light grey bars). Data represent mean values from three independent experiments (mean ± SD). The growth factor concentrations used are: VEGF, 10 ng/ml; VEGF-C, 500 ng/ml; VEGF-D, 500 ng/ml; and VEGF-C156S, 3 µg/ml.
Discussion
Human dermal microvascular endothelial cells consist of two distinct populations of blood vascular and lymphatic endothelial cells. Here we have, for the first time, isolated and grown these two separate cell lineages in culture. We show that VEGFR-3 stimulation protects the lymphatic endothelial cells from serum deprivation-induced apoptosis and increases their growth and migration. We also show that VEGFR-3 can induce PKC-dependent p42/p44 MAPK activation and wortmannin-sensitive phosphorylation of Akt; two important signalling cascades that have been associated with cell proliferation and survival (Datta et al., 1999; Chang and Karin, 2001).
While stimulation of VEGFR-2 promotes cell viability, VEGF withdrawal results in endothelial cell apoptosis, inhibits angiogenesis and leads to blood vessel regression in vivo (Aiello et al., 1995; Ferrara et al., 1998; Gerber et al., 1999). Similarly, the inhibition of VEGFR-3 signalling causes regression of developing lymphatic vessels (Mäkinen et al., 2001). In agreement with previously published studies, VEGFR-2 stimulation strongly protected serum-deprived primary endothelial cells against apoptosis. This effect occurred via VEGFR-2 alone (stimulation by ORFV2-VEGF) as well as in combination with VEGFR-1 stimulation (by VEGF) or VEGFR-3 stimulation (by VEGF-C). However, VEGFR-1 (stimulation by PlGF) transmitted only very weak, if any, cell survival signals. Moreover, VEGFR-3 signalling alone was sufficient for inhibition of serum deprivation-induced apoptosis. Interestingly, while VEGF-C was a weaker survival factor than VEGF for blood vascular endothelial cells, it strongly promoted the survival of VEGFR-3-expressing lymphatic endothelial cells. The responsiveness of the lymphatic endothelial cells to VEGF is explained by the expression of VEGFR-2 in these cells in vitro (this study) and in vivo (Partanen et al., 1999).
The two markers, VEGFR-3 and podoplanin, which we used to distinguish blood vascular and lymphatic endothelial cells, appeared to be expressed quite homogenously on the lymphatic endothelial cells. However, heterogeneity was observed with regard to other markers. For example, only a subset of the lymphatic endothelial cells was stained for the hyaluronan receptor LYVE-1, which is relatively specific for the lymphatic endothelium in vivo (Banerji et al., 1999). In addition, vWF and CD31 were expressed heterogenously in both the blood vascular and lymphatic endothelial cell lineages. Such heterogeneity among the blood vascular endothelial cells is presumably due to the heterogenous mixture of arterial, venous and capillary endothelial cells in the HMVEC cultures. All these cells probably also differ in their responsivenesses to different VEGFs.
Although northern and western blot analysis of the isolated blood vascular endothelial cells revealed weak VEGFR-3 expression, the VEGFR-3 antibodies that we used for the isolation of the cells labelled exclusively the cell surfaces of the lymphatic, podoplanin-positive cells in both immunofluorescence and FACS analysis. In addition, no responsiveness of the blood vascular endothelial cells to the VEGFR-3-specific mutant form of VEGF-C was detected, while in the cultures of lymphatic endothelial cells it clearly induced cell survival. VEGFR-3 expression is detected in normal adult tissues almost exclusively in the lymphatic endothelium (Dumont et al., 1998; Jussila et al., 1998; Partanen et al., 2000). The detection of this receptor in the cultured blood vascular endothelial cells may result from up-regulation of VEGFR-3 expression in the culture conditions, for example due to lack of smooth muscle cells or appropriate extracellular matrix components in these cultures.
VEGFR-3 induced the phosphorylation of two important survival signalling molecules, p42/p44 MAPK and Akt. A PKC inhibitor severely reduced VEGFR-3-mediated p42/p44 MAPK phosphorylation, suggesting that this pathway is transmitted mainly via PKC, not via Ras, similarly to what has been shown previously for VEGFR-2 (Doanes et al., 1999; Takahashi et al., 1999; Yoshiji et al., 1999). Such a pathway is unique among receptor tyrosine kinases since classically PKC-dependent MAPK activation is thought to be employed mainly by certain seven-transmembrane, G-protein-coupled receptors. PKC regulates many endothelial cell processes involved in angiogenesis, including endothelial cell proliferation and migration (Harrington et al., 1997, 2000; Ilan et al., 1998), and inhibition of PKC was able to block tumour neovascularization (Yoshiji et al., 1999).
Although p42/p44 MAPK activation occurred with similar kinetics in HMVECs stimulated by VEGF or VEGF-C, the latter induced a more sustained response. Differences in the duration of activation and in the subcellular distribution of p42/p44 MAPK have been reported to lead to divergent cellular responses (Marshall, 1995; Pang et al., 1995; Kaiser et al., 1999). The differences may result from the fact that only VEGF-C can signal simultaneously via VEGFR-2 and VEGFR-3. However, although VEGF-induced homo- or heterodimeric complexes between VEGFR-1 and VEGFR-2 have been shown to regulate mitogenesis differentially (Rahimi et al., 2000), we could not detect heterodimer formation by VEGFR-2 and VEGFR-3 in the VEGF-C-stimulated cells (data not shown).
The VEGFR-3-specific mutant form of VEGF-C, VEGF-C156S, proved to be a valuable tool for studies of VEGFR-3-mediated signalling (Joukov et al., 1998; Veikkola et al., 2001). In the biosensor analysis, the affinity of VEGF-C156S for VEGFR-3 was reduced in comparison with the wild-type VEGF-C. Moreover, the VEGF-C156S-induced maximal VEGFR-3 phosphorylation (data not shown) or p42/p44 MAPK activation were not as strong as for VEGF-C. The reason for this is unclear, but VEGF-C156S may be more unstable than the wild-type VEGF-C because one of the eight conserved cysteine residues forming the cysteine knot growth factor domain has been changed into a serine residue. However, in a transgenic model, VEGF-C156S was as efficient as wild-type VEGF-C in promoting lymphangiogenesis (Veikkola et al., 2001). Furthermore, the concentrations used in our present assays should saturate VEGFR-3. Therefore, since even the highest VEGF-C156S concentrations were not as effective as VEGF-C in protecting cells from apoptosis, a simultaneous activation of both VEGFR-2 and VEGFR-3 may be required for the VEGF-C-induced maximal survival of the lymphatic endothelial cells.
In conclusion, we show for the first time that molecular markers such as VEGFR-3 allow the isolation of lymphatic endothelial cells and that the culture of these cells in the presence of specific growth factors is possible without losing their differentiated properties. We demonstrate, furthermore, that specific VEGFR-3 ligands can induce cell migration and protect serum-deprived lymphatic endothelial cells from apoptosis via the activation of two important signalling molecules associated with cell survival, Akt and p42/p44 MAPK. The ability to culture lymphatic endothelial cells should now allow the elucidation of the VEGFR-3 signalling pathways as well as the molecular characteristics and gene expression profiles of blood vascular versus lymphatic endothelial cells.
Materials and methods
Antibodies and growth factors
The primary antibodies used in immunofluorescence were mouse monoclonal antibodies against human CD31 (Dako), vWF (Dako) or VEGFR-3 (clones 9D9F9, 2E11D11 and 7B3F9; Jussila et al., 1998), rabbit antisera against human LYVE-1 (kindly provided by David G.Jackson, Oxford, and by Pirjo Laakkonen and Erkki Ruoslahti, La Jolla, CA; Banerji et al., 1999), affinity-purified rabbit anti-human podoplanin (Breiteneder-Geleff et al., 1999) or rabbit anti-human VEGF-C (882; Joukov et al., 1996). Monoclonal antibody against PCNA (clone PC10) was from Santa Cruz Biotechnology. Fluorescein isothiocyanate (FITC)- or tetramethylrhodamine isothiocyanate (TRITC)-conjugated goat anti-rabbit IgG, goat anti-mouse IgG and donkey anti-mouse IgG were obtained from Jackson Immunoresearch. The rabbit antiserum against human VEGFR-2 was a kind gift from Lena Claesson-Welsh (Uppsala, Sweden) and affinity-purified goat anti-human VEGFR-1 was from R&D Systems. Rabbit polyclonal antibodies against Akt, MAPK or CREB were from New England Biolabs. Basic fibroblast growth factor (bFGF), recombinant human VEGF165 and recombinant mature human VEGF-D (consisting of residues Phe93–Ser201) were from R&D. Recombinant human PlGF-1 was a kind gift from Graziella Persico (Naples, Italy). The recombinant human VEGF-C (Thr103–Leu215), VEGF-C156S (Thr103–Ile225), ORFV2-VEGF and human VEGFR-3-Ig were produced and purified as described earlier (Joukov et al., 1997; Wise et al., 1999; Mäkinen et al., 2001). Wortmannin, LY294002, PD98059 and bisindolylmaleimide I (GF109203X) were from Calbiochem, and U0126 was from Promega.
Cell culture
HMVECs and HUVECs were obtained from PromoCell (Heidelberg, Germany), cultured in endothelial cell medium provided by the supplier and used at passages 3–7. The murine Ba/F3 pre-B lymphocytes were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum, glutamine and 2 ng/ml interleukin-3 (Calbiochem).
Immunofluorescence staining
Cells on glass coverslips were fixed in 4% paraformaldehyde (PFA) or methanol:acetone (1:1) for 10 min. If required, the cells were permeabilized with 0.1% Triton X-100 in phosphate-buffered saline (PBS) for 5 min. After blocking in 5% goat serum, the cells were stained with the primary antibodies for 30 min at room temperature, followed by incubation with FITC- or TRITC-conjugated secondary antibodies (15 µg/ml) for 30 min. Hoechst 33258 fluorochrome (Sigma, 0.5 µg/ml in PBS) was used for the staining of the nuclei. If cells were stained alive, the procedure was carried out on ice, followed by fixation in PFA.
Isolation of lymphatic and blood vascular endothelial cells
Monoclonal VEGFR-3 antibodies (clone 2E11D11) or polyclonal affinity-purified podoplanin antibodies, MACS colloidal super-paramagnetic MicroBeads conjugated to rat anti-mouse IgG1 or to goat anti-rabbit IgG antibodies (Miltenyi Biotech, Bergisch Gladbach, Germany), MACS MS separation columns and MiniMACS separator (Miltenyi Biotech) were used for cell sorting according to the manufacturer’s instructions.
Bioassay for VEGFR stimulation
Viability assays using Ba/F3 pre-B cells expressing VEGFR-2/EpoR (Achen et al., 1998; Stacker et al., 1999) or VEGFR-3/EpoR (Achen et al., 2000) were carried out as described earlier (Mäkinen et al., 2001). For the generation of Ba/F3 VEGFR-1/EpoR cells, the chimeric receptor was constructed by introducing a BglII site into the human VEGFR-1 cDNA prior to the sequence encoding the transmembrane domain, followed by ligation of a BglII–NotI fragment consisting of the transmembrane and intracellular domains of mouse erythropoietin receptor (Achen et al., 2000). The VEGFR-1/EpoR cDNA was subcloned into the pEF-BOS expression vector (Mizushima and Nagata, 1990) and co-transfected into Ba/F3 cells with pCDNA3.1(+)Zeo vector (Invitrogen). Stable cell pools were generated by selection with 250 µg/ml zeocin.
Biosensor analysis
All protein preparations were analysed for homogeneity and buffer exchanged by micropreparative size exclusion HPLC using a Superose 12 (3.2/30) column installed in a SMART™ system (Amersham Pharmacia Biotech, Uppsala, Sweden) immediately prior to use (Nice and Catimel, 1999). The concentrations of VEGF-C and VEGF-D were determined by absorbance at 280 nm using an E280 1%1 cm of 0.65. Receptor domains were coupled to the carboxymethylated dextran layer of a CM5 sensor chip using standard amine coupling chemistry (Nice and Catimel, 1999) for analysis of ligand binding using a BIAcore 2000 optical biosensor (BIAcore, Uppsala, Sweden). The levels immobilized were 3000 and 7000 RU for VEGFR-2 and VEGFR-3, respectively. Following immobilization, residual activated ester groups were blocked by treatment with 1 M ethanolamine hydrochloride pH 8.5 followed by washing with 10 mM diethylamine to remove non-covalently bound material. Diethylamine (10 mM) or HCl (10 mM) was used to regenerate the sensor surface between analyses for VEGF-D or VEGF-C binding, respectively. Samples were diluted in running buffer (10 mM HEPES pH 7.4, 150 mM NaCl, 3.4 mM EDTA, 0.005% Tween-20). The apparent binding affinities of VEGF-C and VEGF-C156S for receptor domains were determined by analysis of the initial dissociation phase to obtain the kd, which was then used to constrain a global analysis of the association region of the curves, assuming a 1:1 Langmuirian model. Data were analysed using BIAevaluation 3.0 (BIAcore, Uppsala, Sweden) as described previously (Catimel et al., 1997).
Analysis of endothelial cell apoptosis
For the apoptosis assay, 70 000 cells/well were seeded into 24-well plates. Treatments were carried out in duplicate and apoptosis was detected by measuring cytoplasmic histone-associated DNA fragments using the death detection ELISAPLUS kit (Roche). The following ranges of growth factor concentrations were tested: bFGF, 10–20 ng/ml; PlGF-1, 50–1000 ng/ml; VEGF, 10–50 ng/ml; VEGF-C, 50–1000 ng/ml; VEGF-D, 50–1000 ng/ml; VEGF-C156S, 50–1000 ng/ml; and ORFV2-VEGF, 50–1000 ng/ml. Annexin-V-FLUOS (Roche) was used to detect phosphatidylserine on the apoptotic cells by fluorescence microscopy according to the manufacturer’s instructions. The simultaneous staining with propidium iodide (1 µg/ml) was used for discriminating possible necrotic cells.
Western blot analysis
Endothelial cells were cultured on 35 mm dishes to near confluence, starved for 24 h in serum-free medium and stimulated as indicated. Wortmannin (30 nM), LY294002 (10–20 µM), PD98059 (10–25 µM) or GF109203X (2.5–5 µM) were added 1–3 h before stimulation, where indicated. Dimethylsulfoxide (DMSO), in which the inhibitors were dissolved, was used as a control. After the stimulation, the cells were lysed in lysis buffer [50 mM HEPES pH 7.5, 150 mM NaCl, 10 mM EDTA, 100 mM NaF, 1% Triton X-100 supplemented with 2 mM Na3VO4, 0.5 mM phenylmethylsulfonyl fluoride (PMSF), 100 U/ml approtinin and 10 µg/ml leupeptin]. Clarified lysates were separated by SDS–PAGE, transferred to nitrocellulose and immunoblotted using the phosphospecific antibodies for Akt-Ser473, Akt-Thr308, p42/44 MAPK-Thr202/Tyr204 or CREB-Ser133. The bound antibodies were detected using horseradish peroxidase-conjugated secondary antibodies and an enhanced chemiluminescence detection system. The blots were stripped and reprobed with antibodies against Akt, MAPK or CREB for quantification by reading the optical densities of the signals with the Multi-Analyst 2.0.1 program (Bio-Rad).
Cell migration assay
Migration assays were performed in a 48-well chemotaxis Boyden chamber (Neuroprobe Inc.). Nucleopore polycarbonate filters (8 µm; Corning) were coated with 100 µg/ml of collagen type I (Upstate Biotechnology) overnight at +4°C and air dried. The filters were placed over the lower chamber wells containing the growth factors in serum-free growth medium supplemented with 0.2% BSA. For blocking experiments, VEGF and VEGF-C156S were pre-incubated with a 10-fold molar excess of soluble human VEGFR-3 for 30 min. HMVECs were suspended in the growth medium and 10 000 cells in 50 µl were added to each well in the upper chamber. The cells were allowed to migrate for 6 h at 37°C, after which the filter was fixed with cold methanol and stained with haematoxylin (Meyer). Non-migrated cells on the upper surface of the filter were removed by scraping with a cotton swab, and the number of migrated cells was counted. The assays were run in quadruplicate and repeated with three different batches of HMVECs.
Acknowledgments
Acknowledgements
We would like to thank Graziella Persico for providing PlGF-1, David G.Jackson and Pirjo Laakkonen for LYVE-1 antibodies, and Lena Claesson-Welsh for VEGFR-2 antibodies. Monica Schoulz is acknowledged for FACS analysis, and Tapio Tainola for expert technical assistance. This study was supported by grants from the Finnish Cancer Organization, Ida Montin Foundation and Emil Aaltonen Foundation. S.A.S. and M.G.A. were supported by the Anti-Cancer Council of Victoria and the National Health and Medical Research Council of Australia. D.K. was supported by a grant No. SFB05-Project07.
References
- Achen M.G., Jeltsch,M., Kukk,E., Makinen,T., Vitali,A., Wilks,A.F., Alitalo,K. and Stacker,S.A. (1998) Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl Acad. Sci. USA, 95, 548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achen M.G. et al. (2000) Monoclonal antibodies to vascular endothelial growth factor-D block its interactions with both VEGF receptor-2 and VEGF receptor-3. Eur. J. Biochem., 267, 2505–2515. [DOI] [PubMed] [Google Scholar]
- Aiello L.P., Pierce,E.A., Foley,E.D., Takagi,H., Chen,H., Riddle,L., Ferrara,N., King,G.L. and Smith,L.E. (1995) Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc. Natl Acad. Sci. USA, 92, 10457–10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin M.E. et al. (2001) The specificity of receptor binding by vascular endothelial growth factor-D is different in mouse and man. J. Biol. Chem., 276, 19166–19171. [DOI] [PubMed] [Google Scholar]
- Banerji S., Ni,J., Wang,S.X., Clasper,S., Su,J., Tammi,R., Jones,M. and Jackson,D.G. (1999) LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol., 144, 789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonni A., Brunet,A., West,A.E., Datta,S.R., Takasu,M.A. and Greenberg,M.E. (1999) Cell survival promoted by the Ras–MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science, 286, 1358–1362. [DOI] [PubMed] [Google Scholar]
- Borg J.-P., deLapeyrière,O., Noguchi,T., Rottapel,R., Dubreuil,P. and Birnbaum,D. (1995) Biochemical characterization of two isoforms of FLT4, a VEGF receptor-related tyrosine kinase. Oncogene, 10, 973–984. [PubMed] [Google Scholar]
- Breiteneder-Geleff S. et al. (1999) Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am. J. Pathol., 154, 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Linden,P., Farnebo,J., Cao,R., Eriksson,A., Kumar,V., Qi,J.-H., Claesson-Welsh,L. and Alitalo,K. (1998) Vascular endothelial growth factor C induces angiogenesis in vivo. Proc. Natl Acad. Sci. USA, 95, 14389–14392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catimel B., Nerrie,M., Lee,F.T., Scott,A.M., Ritter,G., Welt,S., Old,L.J., Burgess,A.W. and Nice,E.C. (1997) Kinetic analysis of the interaction between the monoclonal antibody A33 and its colonic epithelial antigen by the use of an optical biosensor. A comparison of immobilisation strategies. J. Chromatogr., 776, 15–30. [DOI] [PubMed] [Google Scholar]
- Chang L. and Karin,M. (2001) Mammalian MAP kinase signalling cascades. Nature, 410, 37–40. [DOI] [PubMed] [Google Scholar]
- Datta S.R., Brunet,A. and Greenberg,M.E. (1999) Cellular survival: a play in three Akts. Genes Dev., 13, 2905–2927. [DOI] [PubMed] [Google Scholar]
- Doanes A.M., Hegland,D.D., Sethi,R., Kovesdi,I., Bruder,J.T. and Finkel,T. (1999) VEGF stimulates MAPK through a pathway that is unique for receptor tyrosine kinases. Biochem. Biophys. Res. Commun., 255, 545–548. [DOI] [PubMed] [Google Scholar]
- Du K. and Montminy,M. (1998) CREB is a regulatory target for the protein kinase Akt/PKB. J. Biol. Chem., 273, 32377–32379. [DOI] [PubMed] [Google Scholar]
- Dumont D.J., Jussila,L., Taipale,J., Lymboussaki,A., Mustonen,T., Pajusola,K., Breitman,M. and Alitalo,K. (1998) Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science, 282, 946–949. [DOI] [PubMed] [Google Scholar]
- Eriksson U. and Alitalo,K. (1999) Structure, expression and receptor-binding properties of novel vascular endothelial growth factors. Curr. Top. Microbiol. Immunol., 237, 41–57. [DOI] [PubMed] [Google Scholar]
- Ferrara N. (1999) Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med., 77, 527–543. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Chen,H., Davis-Smyth,T., Gerber,H.P., Nguyen,T.N., Peers,D., Chisholm,V., Hillan,K.J. and Schwall,R.H. (1998) Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nature Med., 4, 336–340. [DOI] [PubMed] [Google Scholar]
- Fong G.H., Rossant,J., Gertsenstein,M. and Breitman,M.L. (1995) Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature, 376, 66–70. [DOI] [PubMed] [Google Scholar]
- Gasmi A., Abidi,F., Srairi,N., Oijatayer,A., Karoui,H. and Elayeb,M. (2000) Purification and characterization of a growth factor-like which increases capillary permeability from Vipera lebetina venom. Biochem. Biophys. Res. Commun., 268, 69–72. [DOI] [PubMed] [Google Scholar]
- Gerber H.P., McMurtrey,A., Kowalski,J., Yan,M., Keyt,B.A., Dixit,V. and Ferrara,N. (1998) Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J. Biol. Chem., 273, 30336–30343. [DOI] [PubMed] [Google Scholar]
- Gerber H.P. et al. (1999) VEGF is required for growth and survival in neonatal mice. Development, 126, 1149–1159. [DOI] [PubMed] [Google Scholar]
- Gille H., Kowalski,J., Yu,L.L., Chen,H., Pisabarro,M.T., Davis-Smyth,T. and Ferrara,N. (2000) A repressor sequence in the juxtamembrane domain of Flt-1 (VEGFR-1) constitutively inhibits vascular endothelial growth factor-dependent phosphatidylinositol 3′-kinase activation and endothelial cell migration. EMBO J., 19, 4064–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille H., Kowalski,J., Li,B., LeCouter,J., Moffat,B., Zioncheck,T.F., Pelletier,N. and Ferrara,N. (2001) Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2)—a reassessment using novel receptor-specific vascular endothelial growth factor mutants. J. Biol. Chem., 276, 3222–3230. [DOI] [PubMed] [Google Scholar]
- Gupta K., Kshirsagar,S., Li,W., Gui,L., Ramakrishnan,S., Gupta,P., Law,P.Y. and Hebbel,R.P. (1999) VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp. Cell Res., 247, 495–504. [DOI] [PubMed] [Google Scholar]
- Harrington E.O., Löffler,J., Nelson,P.R., Kent,K.C., Simons,M. and Ware,J.A. (1997) Enhancement of migration by protein kinase Cα and inhibition of proliferation and cell cycle progression by protein kinase Cδ in capillary endothelial cells. J. Biol. Chem., 272, 7390–7397. [DOI] [PubMed] [Google Scholar]
- Harrington E.O., Doyle,K.E., Brunelle,J.L. and Ware,J.A. (2000) Endothelial proliferation, migration and differentiation are blunted by conditionally expressed protein kinase C pseudosubstrate peptides. Biochem. Biophys. Res. Commun., 271, 499–508. [DOI] [PubMed] [Google Scholar]
- Ilan N., Mahooti,S. and Madri,J.A. (1998) Distinct signal transduction pathways are utilized during the tube formation and survival phases of in vitro angiogenesis. J. Cell Sci., 111, 3621–3631. [DOI] [PubMed] [Google Scholar]
- Jeltsch M. et al. (1997) Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science, 276, 1423–1425. [DOI] [PubMed] [Google Scholar]
- Joukov V., Pajusola,K., Kaipainen,A., Chilov,D., Lahtinen,I., Kukk,E., Saksela,O., Kalkkinen,N. and Alitalo,K. (1996) A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J., 15, 290–298. [PMC free article] [PubMed] [Google Scholar]
- Joukov V., Sorsa,T., Kumar,V., Jeltsch,M., Claesson-Welsh,L., Cao,Y., Saksela,O., Kalkkinen,N. and Alitalo,K. (1997) Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J., 16, 3898–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukov V., Kumar,V., Sorsa,T., Arighi,E., Weich,H., Saksela,O. and Alitalo,K. (1998) A recombinant mutant vascular endothelial growth factor-C that has lost vascular endothelial growth factor receptor-2 binding, activation and vascular permeability activities. J. Biol. Chem., 273, 6599–6602. [DOI] [PubMed] [Google Scholar]
- Jussila L. et al. (1998) Lymphatic endothelium and Kaposi’s sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res., 58, 1599–1604. [PubMed] [Google Scholar]
- Kaipainen A., Korhonen,J., Mustonen,T., van Hinsbergh,V.M., Fang,G.-H., Dumont,D., Breitman,M. and Alitalo,K. (1995) Expression of the fms-like tyrosine kinase FLT4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl Acad. Sci. USA, 92, 3566–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser G.C., Yan,F. and Polk,D.B. (1999) Conversion of TNFα from antiproliferative to proliferative ligand in mouse intestinal epithelial cells by regulating mitogen-activated protein kinase. Exp. Cell Res., 249, 349–358. [DOI] [PubMed] [Google Scholar]
- Komori Y., Nikai,T., Taniguchi,K., Masuda,K. and Sugihara,H. (1999) Vascular endothelial growth factor VEGF-like heparin-binding protein from the venom of Vipera aspis aspis (Aspic viper). Biochemistry, 38, 11796–11803. [DOI] [PubMed] [Google Scholar]
- Lymboussaki A., Partanen,T.A., Olofsson,B., Thomas-Crusells,J., Fletcher,C.D.M., de Waal,R.M.W., Kaipainen,A. and Alitalo,K. (1998) Expression of the vascular endothelial growth factor C receptor VEGFR-3 in lymphatic endothelium of the skin and in vascular tumors. Am. J. Pathol., 153, 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconcini L., Marchio,S., Morbidelli,L., Cartocci,E., Albini,A., Ziche,M., Bussolino,F. and Oliviero,S. (1999) c-fos-induced growth factor/vascular endothelial growth factor D induces angiogenesis in vivo and in vitro. Proc. Natl Acad. Sci. USA, 96, 9671–9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C.J. (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell, 80, 179–185. [DOI] [PubMed] [Google Scholar]
- Mayo L.D., Kessler,K.M., Pincheira,R., Warren,R.S. and Donner,D.B. (2001) Vascular endothelial growth factor activates CREB by signaling through the KDR receptor tyrosine kinase. J. Biol. Chem., 276, 25184–25189. [DOI] [PubMed] [Google Scholar]
- Mizushima S. and Nagata,S. (1990) pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res., 18, 5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkinen T. et al. (2001) Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nature Med., 7, 199–205. [DOI] [PubMed] [Google Scholar]
- Nice E.C. and Catimel,B. (1999) Instrumental biosensors: new perspectives for the analysis of biomolecular interactions. BioEssays, 21, 339–352. [DOI] [PubMed] [Google Scholar]
- Pajusola K., Aprelikova,O., Pelicci,G., Weich,H., Claesson-Welsh,L. and Alitalo,K. (1994) Signalling properties of FLT4, a proteolytically processed receptor tyrosine kinase related to two VEGF receptors. Oncogene, 9, 3545–3555. [PubMed] [Google Scholar]
- Pang L., Sawada,T., Decker,S.J. and Saltiel,A.R. (1995) Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J. Biol. Chem., 270, 13585–13588. [DOI] [PubMed] [Google Scholar]
- Partanen T.A., Makinen,T., Arola,J., Suda,T., Weich,H.A. and Alitalo,K. (1999) Endothelial growth factor receptors in human fetal heart. Circulation, 100, 583–586. [DOI] [PubMed] [Google Scholar]
- Partanen T.A., Arola,J., Saaristo,A., Jussila,L., Ora,A., Miettinen,M., Stacker,S.A., Achen,M.G. and Alitalo,K. (2000) VEGF-C and VEGF-D expression in neuroendocrine cells and their receptor, VEGFR-3, in fenestrated blood vessels in human tissues. FASEB J., 14, 2087–2096. [DOI] [PubMed] [Google Scholar]
- Petrova T.V., Makinen,T. and Alitalo,K. (1999) Signaling via vascular endothelial growth factor receptors. Exp. Cell Res., 253, 117–130. [DOI] [PubMed] [Google Scholar]
- Qi J.H. and Claesson-Welsh,L. (2001) VEGF-induced activation of phosphoinositide 3-kinase is dependent on focal adhesion kinase. Exp. Cell Res., 263, 173–182. [DOI] [PubMed] [Google Scholar]
- Rahimi N., Dayanir,V. and Lashkari,K. (2000) Receptor chimeras indicate that the vascular endothelial growth factor receptor-1 (VEGFR-1) modulates mitogenic activity of VEGFR-2 in endothelial cells. J. Biol. Chem., 275, 16986–16992. [DOI] [PubMed] [Google Scholar]
- Rommel C., Clarke,B.A., Zimmermann,S., Nunez,L., Rossman,R., Reid,K., Moelling,K., Yancopoulos,G.D. and Glass,D.J. (1999) Differentiation stage-specific inhibition of the Raf–MEK–ERK pathway by Akt. Science, 286, 1738–1741. [DOI] [PubMed] [Google Scholar]
- Shalaby F., Rossant,J., Yamaguchi,T.P., Gertsenstein,M., Wu,X.F., Breitman,M.L. and Schuh,A.C. (1995) Failure of blood island formation and vasculogenesis in Flk-1-deficient mice. Nature, 376, 62–66. [DOI] [PubMed] [Google Scholar]
- Shibuya M., Ito,N. and Claesson-Welsh,L. (1999) Structure and function of vascular endothelial growth factor receptor-1 and -2. In Claesson-Welsh,L. (ed.), Vascular Growth Factors and Angiogenesis. Vol. 237. Springer-Verlag, Heidelberg, Germany, pp. 59–83. [DOI] [PubMed]
- Stacker S.A., Vitali,A., Caesar,C., Domagala,T., Groenen,L.C., Nice,E., Achen,M.G. and Wilks,A.F. (1999) A mutant form of vascular endothelial growth factor (VEGF) that lacks VEGF receptor-2 activation retains the ability to induce vascular permeability. J. Biol. Chem., 274, 34884–34892. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Ueno,H. and Shibuya,M. (1999) VEGF activates protein kinase C-dependent, but Ras-independent Raf–MEK–MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene, 18, 2221–2230. [DOI] [PubMed] [Google Scholar]
- Thakker G.D., Hajjar,D.P., Muller,W.A. and Rosengart,T.K. (1999) The role of phosphatidylinositol 3-kinase in vascular endothelial growth factor signaling. J. Biol. Chem., 274, 10002–10007. [DOI] [PubMed] [Google Scholar]
- Veikkola T. et al. (2001) Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J., 20, 1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise L.M. et al. (1999) Vascular endothelial growth factor (VEGF)-like protein from orf virus NZ2 binds to VEGFR2 and neuropilin-1. Proc. Natl Acad. Sci. USA, 96, 3071–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzenbichler B. et al. (1998) Vascular endothelial growth factor-C (VEGF-C/VEGF-2) promotes angiogenesis in the setting of tissue ischemia. Am. J. Pathol., 153, 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiji H. et al. (1999) Protein kinase C lies on the signaling pathway for vascular endothelial growth factor-mediated tumor development and angiogenesis. Cancer Res., 59, 4413–4418. [PubMed] [Google Scholar]
- Zimmermann S. and Moelling,K. (1999) Phosphorylation and regulation of Raf by Akt (protein kinase B). Science, 286, 1741–1744. [DOI] [PubMed] [Google Scholar]