Abstract
OBJECTIVES
We investigated the acute effect of orally administered high-dose folic acid on coronary dilator function in humans.
BACKGROUND
Folic acid and its active metabolite, 5-methyltetrahydrofolate, increase endothelium-dependent vasodilation in human peripheral circulation. However, the acute effect on coronary circulation is not known.
METHODS
Fourteen patients with ischemic heart disease, age 62 ± 12 years (mean ± SD), were enrolled in a double-blind, placebo-controlled crossover trial. Basal and adenosine-stimulated myocardial blood flow (MBF) were determined by positron emission tomography, and myocardial flow reserve was calculated. Each patient was studied after ingestion of placebo and after ingestion of 30 mg folic acid. Myocardial zones were prospectively defined physiologically as “normal” versus “abnormal” on the basis of MBF response to adenosine 140 μg/kg/min (normal = MBF > 1.65 ml/min/g). Abnormal and normal zones were analyzed separately in a patient-based analysis.
RESULTS
Folate was associated with a reduction in mean arterial pressure (100 ± 12 mm Hg vs. 96 ± 11 mm Hg, placebo vs. folate, p < 0.03). Despite the fall in mean arterial pressure, folic acid significantly increased the MBF dose response to adenosine (p < 0.001 using analysis of variance) in abnormal zones, whereas MBF in normal zones did not change. In abnormal segments, folic acid increased peak MBF by 49% (1.45 ± 0.59 ml/min/g vs. 2.16 ± 1.01 ml/min/g, p < 0.02). Furthermore, folate increased dilator reserve by 83% in abnormal segments (0.77 ± 0.59 vs. ml/min/g 1.41 ± 1.08 ml/min/g, placebo vs. folate, p < 0.05), whereas dilator reserve in normal segments remained unchanged (2.00 ± 0.61 ml/min/g vs. 2.12 ± 0.69 ml/min/g, placebo vs. folate, p = NS).
CONCLUSIONS
The data demonstrate that high-dose oral folate acutely lowers blood pressure and enhances coronary dilation in patients with coronary artery disease.
Abbreviations and Acronyms: Ado 140 = adenosine 140 μg/kg/min, MBF = myocardial blood flow, PET = positron emission tomography
Folic acid (folate) is a B vitamin that is required for transmethylation reactions, nucleic acid synthesis, homocysteine metabolism, and the enzymatic regeneration of tetra-hydrobiopterin (an essential co-factor of nitric oxide synthase) (1). Previous studies have shown that folate improves endothelial function in the peripheral circulation (2), an effect that may be independent of homocysteine lowering.
It is not known whether folic acid can acutely improve coronary dilation in patients with ischemic heart disease. Therefore, we conducted a double-blinded, placebo-controlled crossover study to test the hypothesis that folic acid improves coronary dilator function in patients with coronary artery disease. To assess the direct vascular effect of folic acid independently of its homocysteine-lowering effect, we studied patients with normal homocysteine concentrations and measured myocardial blood flow (MBF) acutely after ingestion of folate, before homocysteine lowering would occur.
METHODS
Patient population
Adult male and female patients with coronary artery disease and normal serum homocysteine concentration (<12 μM) were recruited from the greater Boston area. Coronary artery disease was defined as the presence of a >50% stenosis in at least one coronary artery identified by coronary arteriography within the last five years. Exclusion criteria included: inability to maintain a stable medical regimen during the study period, concurrent use of folic acid, or myocardial infarction or coronary intervention within the proceeding three months. The study protocol was approved by the local human research committee, and informed consent was obtained from each subject.
Study drug administration
Subjects were enrolled in a double-blinded, placebo-controlled, crossover study during which folate syrup (30 mg) or similar-tasting placebo syrup was administered orally in two divided doses, 10 to 12 h and 1 h before MBF measurements. The 1-h time point and the dose of 30 mg were chosen to achieve a similar peak plasma 5-methyltetrahydrofolate level (3) (the biologically active form of folic acid) that has previously been shown to improve peripheral nitric oxide-mediated vasodilation (1). Folate and placebo were administered at least one week apart, in random order, and in a double-blinded manner.
MBF measurements
Vasoactive medications were withheld for three to five half-lives, and subjects were asked to avoid caffeinated beverages for 24 h before blood flow measurements; MBF was assessed with positron emission tomography (PET) (GE Medical Systems Scanditronix PC4096, Chalfont St. Giles, United Kingdom) approximately 1 h after ingestion of the second dose of the study drug. Positron emission tomography measurements of MBF (13N-ammonia method) were performed at rest and during the infusion of two doses of adenosine (70 and 140 μg/kg/min), using a previously described method (4).
PET image analysis
Three short-axis rings corresponding to the proximal, middle, and distal thirds of the left ventricle were constructed for each K1 scan, and MBF was measured within 24 standard areas of interest as described previously (4). Data analysis was performed without knowledge of treatment order. To determine the variability of the MBF measurements, two readers independently assessed adenosine-stimulated MBF in 120 segments in five subjects, and the difference between and within readers was calculated. The mean (± SD) intra- and interobserver difference was 0.03 ± 0.10 ml/min/g and 0.03 ± 0.17 ml/min/g, respectively.
Prospective definition of myocardial zones
We prospectively sought to examine MBF in regions supplied by stenotic conduit vessels (which were expected to have abnormal dilator capacity) separately from regions with normal dilator capacity. Data obtained in our laboratory demonstrate that maximal MBF >1.65 ml/min/g with high-dose adenosine has very high negative predictive accuracy (91%) for exclusion of moderate-to-severe coronary artery stenosis and that 97% of moderate-to-severe stenoses had maximal MBF <1.65 ml/min/g (5). Accordingly, myocardial zones were physiologically as “normal” versus “abnormal” on the basis of MBF response to adenosine 140 μg/kg/min (Ado 140) during the placebo condition. Myocardial zones with MBF with Ado 140 of <1.65 ml/min/g (during placebo condition) were defined as abnormal. The corresponding myocardial regions were identified during the folate condition and were labeled abnormal regardless of their MBF during the folate condition. Values for abnormal MBF were combined and averaged to obtain a single value of abnormal blood flow for each patient at each condition (placebo and folic acid) for each scan acquisition (rest, adenosine 70 or adenosine 140). A patient-based analysis of MBF was performed.
Dilator reserve for both normal and abnormal zones was defined as the difference between peak MBF and rest MBF. Peak MBF was defined as the higher of the two average MBF values obtained during adenosine (i.e., the greater of the average MBF obtained during adenosine 70 vs. adenosine 140 dose). This was done, because in patients with ischemic heart disease, coronary “steal” may occur (6) and cause underestimation of dilator reserve in abnormal regions.
Coronary angiography
Coronary angiograms were reviewed in blinded fashion by an expert (G.H.) for the presence and grade of collaterals as previously described (6).
Statistical analysis
Data are expressed as mean values ± SD. A repeated-measures analysis of variance (ANOVA) was performed (Statview v 4.0, Abacus Concepts, Cary, North Carolina), with terms for the order of folate versus placebo administration, patient, and observations (blood pressure and MBF) for the three repeated measurements (adenosine 0, 70, and 140). To control for multiple testing, prospectively defined pairwise t tests comparing folate to placebo at each of the three adenosine doses were performed only if there was a significant main effect for folate or a significant interaction of folate with adenosine in the ANOVA analysis. Values of p < 0.05 were considered significant.
RESULTS
Subject characteristics
A total of 14 patients were studied. Subject characteristics are displayed in Table 1.
Table 1.
Subject Characteristics (Mean ± SD)
| Characteristic | Value |
|---|---|
| Gender (M/F) | 13/1 |
| Age (yrs) | 62 ± 12 |
| Diabetes (% subjects) | 20 |
| Smoking (% subjects) | 57 |
| Current | 7 |
| Former | 50 |
| SBP (mm Hg) | 125 ± 12 |
| DBP (mm Hg) | 74 ± 7 |
| Total cholesterol (mg/dl) | 155 ± 30 |
| LDL (mg/dl) | 88 ± 27 |
| HDL (mg/dl) | 41 ± 8 |
| Triglycerides (mg/dl) | 130 ± 79 |
| Statin use (% subjects) | 100 |
| Beta-blocker use (% subjects) | 80 |
| Calcium antagonist use (%) | 7 |
| Homocysteine (μmol/l) | 8.7 ± 1.5 |
| Coronary disease (% subjects) | |
| One-vessel | 29 |
| Two-vessel | 21 |
| Three-vessel | 50 |
| Prior CABG (% subjects) | 43 |
| Prior MI (% subjects) | 36 |
CABG = coronary artery bypass grafting; DBP = diastolic blood pressure; HDL = high-density lipoprotein; LDL = low-density lipoprotein; MI = myocardial infarction; SBP = systolic blood pressure.
Hemodynamics
Hemodynamic parameters are displayed in Table 2. There was no significant change in heart rate or rate-pressure product (folate vs. placebo) (Table 2, Fig. 1). However, folate caused a significant reduction in systolic, diastolic, and mean arterial blood pressures (p < 0.01 using ANOVA, Table 2). An order effect for placebo versus folate was never significant for any of the analyses of variance for the hemodynamic (or any other) measurements.
Table 2.
Effect of Folate on Hemodynamic Variables (Mean ± SD)
| Placebo | Folate | p Value | |
|---|---|---|---|
| HR (beats/min) | |||
| Rest | 61 ± 9 | 61 ± 8 | NS |
| Ado 70 | 68 ± 13 | 70 ± 14 | NS |
| Ado 140 | 82 ± 13 | 82 ± 15 | NS |
| RPP (mm Hg × beats/min) | |||
| Rest | 7,617 ± 1,717 | 7,483 ± 1,798 | NS |
| Ado 70 | 9,074 ± 2,639 | 8,604 ± 2,484 | NS |
| Ado 140 | 10,444 ± 2,156 | 9,998 ± 3,066 | NS |
| SBP (mm Hg)* | |||
| Rest | 125 ± 18 | 121 ± 15 | NS |
| Ado 70 | 131 ± 21 | 123 ± 18 | NS |
| Ado 140 | 127 ± 14 | 120 ± 21 | NS |
| DBP (mm Hg)* | |||
| Rest | 75 ± 9 | 70 ± 9 | 0.01 |
| Ado 70 | 74 ± 12 | 68 ± 11 | 0.04 |
| Ado 140 | 75 ± 12 | 69 ± 10 | 0.01 |
| MAP (mm Hg)* | |||
| Rest | 100 ± 12 | 96 ± 11 | 0.03 |
| Ado 70 | 103 ± 15 | 95 ± 13 | 0.01 |
| Ado 140 | 101 ± 14 | 95 ± 15 | 0.03 |
p < 0.05 analysis of variance for adenosine dose-response curve (placebo vs. folate conditions).
Ado 70 = adenosine 70 μg/kg/min; Ado 140 = adenosine 140 μg/kg/min; DAP = diastolic blood pressure; HR = heart rate; MAP = mean blood pressure; RPP = rate-pressure product (systolic blood pressure [SBP] × HR).
Figure 1.
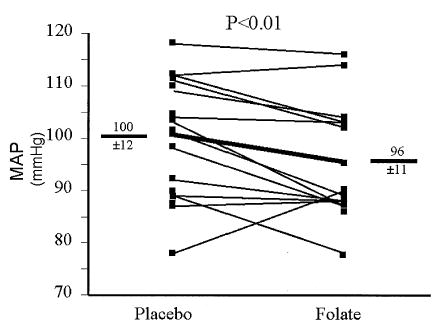
Effect of high-dose folate on mean arterial pressure (MAP). Resting MAP was measured. Individual responses are shown, and group mean data (± SD) are depicted by the thick line. Folate significantly reduced MAP (100 ± 3 mm Hg vs. 96 ± 2 mm Hg, placebo vs. folate, p < 0.03).
MBF
In normal regions, folate did not affect MBF (Table 3). However, in abnormal zones, despite the fall in mean arterial pressure, folic acid significantly increased the MBF response to adenosine (p < 0.001 using ANOVA, Table 3). This was accompanied by a 49% increase in peak MBF in abnormal zones (1.45 ± 0.59 ml/min/g vs. 2.16 ± 1.01 ml/min/g, placebo vs. folate p < 0.02; Table 3, Fig. 2). Furthermore, folate increased dilator reserve by 83% in abnormal segments (0.77 ± 0.59 ml/min/g vs. 1.41 ± 1.08 ml/min/g, placebo vs. folate, p = 0.04; Table 3, Fig. 3), whereas dilator reserve in normal segments remained unchanged (2.00 ± 0.61 ml/min/g vs. 2.12 ± 0.69 ml/min/g, placebo vs. folate, p = NS).
Table 3.
Effect of Folate on MBF (Mean ± SD)
| Placebo | Folate | p Value | |
|---|---|---|---|
|
MBF (ml/min/g) | |||
| Abnormal* | |||
| Rest | 0.68 ± 0.24 | 0.74 ± 0.27 | NS |
| Ado 70 | 1.30 ± 0.71 | 1.60 ± 1.15 | NS |
| Ado 140 | 1.18 ± 0.32 | 1.76 ± 0.75 | 0.03 |
| Peak | 1.45 ± 0.59 | 2.16 ± 1.01 | 0.02 |
| Normal | |||
| Rest | 0.84 ± 0.29 | 0.81 ± 0.19 | NS |
| Ado 70 | 2.10 ± 0.97 | 2.01 ± 0.88 | NS |
| Ado 140 | 2.78 ± 0.67 | 2.72 ± 0.74 | NS |
| Peak | 2.84 ± 0.61 | 2.86 ± 0.67 | NS |
| Ratio ABN:WNL* | |||
| Rest | 0.80 ± 0.15 | 0.95 ± 0.17 | 0.03 |
| Ado 70 | 0.67 ± 0.16 | 0.84 ± 0.37 | NS |
| Ado 140 | 0.47 ± 0.15 | 0.67 ± 0.21 | 0.02 |
| Peak | 0.54 ± 0.17 | 0.75 ± 0.24 | 0.01 |
|
Dilator Reserve (ml/min/g)
| |||
| Abnormal | 0.77 ± 0.59 | 1.41 ± 1.08 | 0.04 |
| Normal | 2.00 ± 0.61 | 2.12 ± 0.69 | NS |
| Ratio ABN:WNL | 0.43 ± 0.21 | 0.63 ± 0.36 | 0.01 |
p < 0.05 Analysis of variance for adenosine dose-response curve (placebo vs. folate conditions).
ABN = abnormal; Abnormal = myocardial segments with abnormal dilator capacity during placebo condition; Ado 70 = adenosine 70 μg/kg/min; Ado 140 = adenosine 140 μg/kg/min; Normal = myocardial segments with normal dilator capacity during placebo condition; Ratio = Abnormal/Normal zone myocardial blood flow (MBF); WNL = normal.
Figure 2.
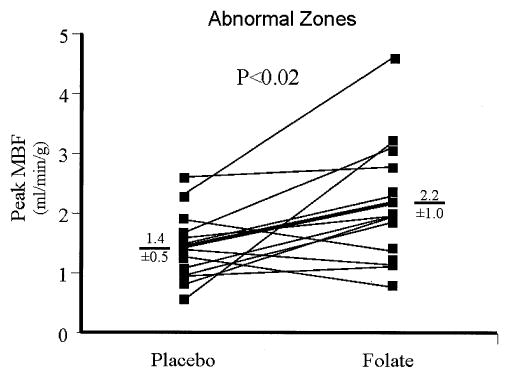
Effect of high-dose folate on peak (adenosine-stimulated) myocardial blood flow (MBF). Peak adenosine-stimulated MBF was measured in abnormal zones. Individual responses are shown, and group mean data are depicted by the thick line. Folate significantly increased peak MBF (1.45 ± 0.59 ml/min/g vs. 2.16 ± 1.01 ml/min/g, mean ± SD, placebo vs. folate, p < 0.02).
Figure 3.
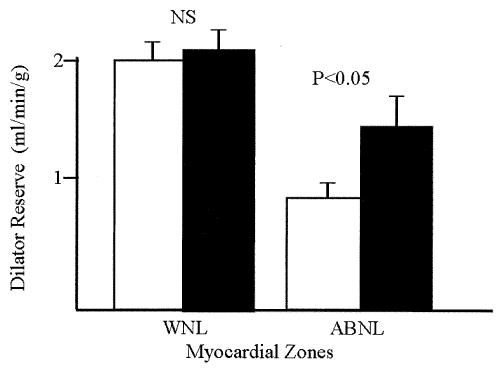
Effect of high-dose folate on coronary dilator reserve. Dilator reserve was measured in normal (WNL) and abnormal (ABNL) regions. Mean data and standard error bars are depicted. Folate increased dilator reserve by ~83% in abnormal segments (0.72 ± 0.60 ml/min/g vs. 1.31 ± 1.08 ml/min/g, mean ± SD, placebo vs. folate, p < 0.05), whereas dilator reserve in normal segments remained unchanged (2.00 ± 0.61 ml/min/g vs. 2.12 ± 0.69 ml/min/g, placebo vs. folate, p = NS). Open bars = placebo; solid bars = folate.
The ratio of MBF in abnormal versus normal zones was determined for each patient during each condition. There was a significant improvement in the abnormal/normal MBF ratio at rest and during adenosine after folate (Table 3). Notably, peak MBF increased in the abnormal zones relative to the normal zones in 85% of patients (Fig. 4). Similarly, dilator reserve increased in the abnormal zones relative to the normal zones in 83% of patients.
Figure 4.
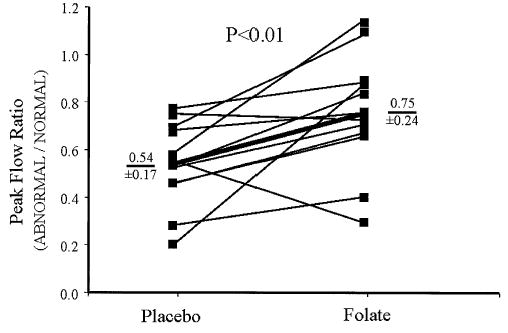
Effect of high-dose folate on peak flow ratio. The ratio for peak myocardial blood flow, in abnormal relative to normal segments, is shown for each patient. Group mean data are depicted by the thick line. Folate increased the ratio of flow in abnormal segments relative to normal segments (0.54 ± 0.17 vs. 0.75 ± 0.24, mean ± SD, placebo vs. folate, p < 0.01).
Coronary anatomy
A total of 94% of myocardial zones that were classified as abnormal by PET were found to be subserved by stenotic coronary arteries (>70% stenoses). Collaterals of varying grade (I to III) were observed supplying the abnormal zones in 9 of 13 patients. Of the 10 patients in whom abnormal zone MBF improved after folate, 8 had collaterals to the abnormal zone. In contrast, of three patients in whom abnormal zone MBF failed to improve after folate, only one of three exhibited collaterals to the abnormal zone. Sample size was insufficient to determine if peak MBF response correlated with collateral grade.
Biochemical data
Total plasma folate levels increased significantly after folic acid ingestion (20 ± 6 ng/ml vs. 473 ± 106 ng/ml, placebo vs. folate, p 3 0.01). Conversely, plasma homocysteine concentrations did not change after folic acid (7.1 ± 1.4 μmol/l vs. 7.8 ± 1.1 μmol/l, placebo vs. folate, p = NS).
DISCUSSION
The important new findings in this study are that high-dose folic acid: 1) increases both vasodilator-stimulated MBF as well as flow reserve in myocardial segments with impaired dilator function; and 2) acutely lowers arterial pressure independently of homocysteine lowering. As such, this study extends observations of folate’s effects made in the peripheral circulation to the coronary circulation, and highlights a potentially useful clinical role for folic acid in the therapy of ischemic heart disease.
Mechanism of the vasodilator effect of folic acid
Previous studies have demonstrated that folate enhances nitric oxide bioavailability (2,7). We and others (8) have previously shown that nitric oxide plays a significant role in adenosine-induced vasodilation in the coronary microcirculation. It is plausible, therefore, that in the current study, the increase in adenosine-induced blood flow that occurred after folate ingestion is also a result of increased nitric oxide bioavailability.
Vascular locus of folate’s effects
In the current study, both MBF (Fig. 2) and dilator reserve (Fig. 3) increased in regions with abnormal flow reserve after folate, a finding that is all the more noteworthy because diastolic blood pressure (coronary perfusion pressure) was significantly lower with folate (Table 2). The exact vascular locus of folate’s effects cannot be determined from the results of this study. Myocardial dilator capacity is impaired in collateral-dependent areas (9) in regions supplied by stenotic conduit vessels (5) and in resistance vessels distal to a stenosis (10,11). Thus, the potential for folate to improve vasomotion exists for any of these vessels.
Observation that folate reduces systemic blood pressure
Previous studies have demonstrated blood pressure lowering in the setting of long-term folate (four weeks to two years) in association with homocysteine lowering (12,13). In the current study, we observed a significant reduction in blood pressure after the ingestion of folate in 11 of 14 patients that occurred in the absence of changes in homocysteine concentration. As was postulated as the mechanism for folate’s effects on the coronary circulation, the reduction in systemic blood pressure observed after folate administration may result from enhanced nitric oxide bioavailability.
Study limitations
Two important limitations of this study should be noted. First, the study examined only acute effects of folate. Therefore, it is not known if long-term, high-dose folate therapy will have similar effects. Second, only one female patient was included in the study group. Accordingly, these results may not be generalizable to women.
Clinical implications
We observed a 5-mm Hg reduction in diastolic blood pressure with folate. Previous meta-analyses have demonstrated that a 5- to 6-mm Hg reduction in diastolic blood pressure is clinically significant and is associated with significant reductions in stroke (14,15) and heart disease (14). Should future studies demonstrate that folate’s antihypertensive effect is sustained with long-term use, then folic acid might prove to be a valuable addition to an antihypertensive therapy.
We also observed a significant increase in flow reserve. Because abnormalities in MBF and flow reserve are associated with manifestation of ischemia in patients with coronary artery disease (16), it follows logically that high-dose folate may reduce the occurrence of ischemia in patients with coronary disease. However, there are only limited data demonstrating that pharmacologic interventions that acutely improve MBF and flow reserve have a significant long-term effect on angina. Accordingly, the potential for folate-mediated cardiovascular benefits is theoretical but unproven.
The current study demonstrates that folic acid has acute vasodilating actions that occur in the absence of homocysteine lowering. Accordingly, the findings of the current study raise two possibilities that may impact the interpretation of ongoing trials: 1) doses of folate that are significantly higher than employed in previous trails may impart additional cardiovascular benefits; and 2) the vascular benefits of folic acid may extend beyond its homocysteine-lowering effect.
Conclusions
This study demonstrates that oral folate acutely enhances coronary dilation and modestly lowers arterial pressure in patients with coronary artery disease. These findings extend observations of effects of folate made in the peripheral circulation to the coronary circulation. Further, this study demonstrates an effect of folic acid on vascular function that is independent of its effect on homocysteine lowering and raises the possibility that administering doses of folic acid that are higher than previously employed may confer additional clinical benefits.
Footnotes
This study was supported, in part, by grants from the National Institutes of Health (RR16046) (to Dr. Tawakol) and the American Society of Nuclear Cardiology (to Dr. Tawakol).
References
- 1.Verhaar MC. 5-methyltetrahydrofolate, the active form of folic acid, restores endothelial function in familial hypercholesterolemia. Circulation. 1998;97:237–41. doi: 10.1161/01.cir.97.3.237. [DOI] [PubMed] [Google Scholar]
- 2.Chambers JC. Improved vascular endothelial function after oral B vitamins: an effect mediated through reduced concentrations of free plasma homocysteine. Circulation. 2000;102:2479–83. doi: 10.1161/01.cir.102.20.2479. [DOI] [PubMed] [Google Scholar]
- 3.Perry J, Chanarin I. Intestinal absorption of reduced folate compounds in man. Br J Haematol. 1970;18:329–39. doi: 10.1111/j.1365-2141.1970.tb01447.x. [DOI] [PubMed] [Google Scholar]
- 4.Huggins GS, Pasternak RC, Alpert NM, Fischman AJ, Gewirtz H. Effects of short-term treatment of hyperlipidemia on coronary vasodilator function and myocardial perfusion in regions having substantial impairment of baseline dilator reverse (see comments) Circulation. 1998;98:1291–6. doi: 10.1161/01.cir.98.13.1291. [DOI] [PubMed] [Google Scholar]
- 5.Gewirtz H. Quantitative PET measurements of regional myocardial blood flow: observations in humans with ischemic heart disease. Cardiology. 1997;88:62–70. doi: 10.1159/000177312. [DOI] [PubMed] [Google Scholar]
- 6.Holmvang G, Fry S, Skopicki HA, et al. Relation between coronary “steal” and contractile function at rest in collateral-dependent myocardium of humans with ischemic heart disease. Circulation. 1999;99:2510–6. doi: 10.1161/01.cir.99.19.2510. [DOI] [PubMed] [Google Scholar]
- 7.Doshi SN, McDowell IF, Moat SJ, et al. Folate improves endothelial function in coronary artery disease: an effect mediated by reduction of intracellular superoxide? Arterioscler Thromb Vasc Biol. 2001;21:1196–202. doi: 10.1161/hq0701.092000. [DOI] [PubMed] [Google Scholar]
- 8.Tawakol A, Forgione MA, Stuehlinger M, et al. Homocysteine impairs coronary microvascular dilator function in humans. J Am Coll Cardiol. 2002;40:1051–8. doi: 10.1016/s0735-1097(02)02069-7. [DOI] [PubMed] [Google Scholar]
- 9.Sellke FW, Quillen JE, Brooks LA, Harrison DG. Endothelial modulation of the coronary vasculature in vessels perfused via mature collaterals. Circulation. 1990;81:1938–47. doi: 10.1161/01.cir.81.6.1938. [DOI] [PubMed] [Google Scholar]
- 10.Merkus D, Vergroesen I, Hiramatsu O, et al. Stenosis differentially affects subendocardial and subepicardial arterioles in vivo. Am J Physiol Heart Circ Physiol. 2001;280:H1674–82. doi: 10.1152/ajpheart.2001.280.4.H1674. [DOI] [PubMed] [Google Scholar]
- 11.Fedele FA, Gewirtz H, Capone RJ, Sharaf B, Most AS. Metabolic response to prolonged reduction of myocardial blood flow distal to a severe coronary artery stenosis. Circulation. 1988;78:729–35. doi: 10.1161/01.cir.78.3.729. [DOI] [PubMed] [Google Scholar]
- 12.Mangoni AA, Sherwood RA, Swift CG, Jackson SHD. Folic acid enhances endothelial function and reduces blood pressure in smokers: a randomized controlled trial. J Intern Med. 2002;252:497–503. doi: 10.1046/j.1365-2796.2002.01059.x. [DOI] [PubMed] [Google Scholar]
- 13.van Dijk RAJM, Rauwerda JA, Steyn M, Twisk JWR, Stehouwer CDA. Long-term homocysteine-lowering treatment with folic acid plus pyridoxine is associated with decreased blood pressure but not with improved brachial artery endothelium-dependent vasodilation or carotid artery stiffness: a 2-year, randomized, placebo-controlled trial. Arterioscler Thromb Vasc Biol. 2001;21:2072–9. doi: 10.1161/hq1201.100223. [DOI] [PubMed] [Google Scholar]
- 14.Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2. Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335:827–38. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 15.Rodgers A, MacMahon S, Gamble G, Slattery J, Sandercock P, Warlow C. Blood pressure and risk of stroke in patients with cerebrovascular disease. BMJ. 1996;313:147. doi: 10.1136/bmj.313.7050.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe T. Relation between exercise-induced myocardial ischemia as assessed by nitrogen-13 ammonia positron emission tomography and QT interval behavior in patients with right bundle branch block. Am J Cardiol. 1998;81:816–21. doi: 10.1016/s0002-9149(98)00002-2. [DOI] [PubMed] [Google Scholar]


