Abstract
Age-related macular degeneration (AMD), affecting the retina, afflicts one out of ten people aged 80 years or older in the United States. AMD often results in vision loss to the central 15–20 deg of the visual field (i.e. central scotoma), and frequently afflicts both eyes. In most cases, when the central scotoma includes the fovea, patients will adopt an eccentric preferred retinal locus (PRL) for fixation. The onset of a central scotoma results in the absence of retinal inputs to corresponding regions of retinotopically mapped visual cortex. Animal studies have shown evidence for reorganization in adult mammals for such cortical areas following experimentally induced central scotomata. However, it is still unknown whether reorganization occurs in primary visual cortex (V1) of AMD patients. Nor is it known whether the adoption of a PRL corresponds to changes to the retinotopic mapping of V1. Two recent advances hold out the promise for addressing these issues and for contributing to the rehabilitation of AMD patients: improved methods for assessing visual function across the fields of AMD patients using the scanning laser ophthalmoscope, and the advent of brain-imaging methods for studying retinotopic mapping in humans. For the most part, specialists in these two areas come from different disciplines and communities, with few opportunities to interact. The purpose of this review is to summarize key findings on both the clinical and neuroscience issues related to questions about visual adaptation in AMD patients.
Keywords: Age-related macular degeneration, Preferred retinal locus, Primary visual cortex, Retinotopic reorganization, Brain plasticity
Introduction
Contrary to the long-held dogma that adult sensory systems are structurally invariant following early perceptual development, numerous recent studies have shown that experience can influence the organization of sensory cortex. This paper is about the functional and cortical adaptations associated with macular degeneration, one of the most prevalent forms of vision loss.
The Eye Disease Prevalence Research Group (2004b) has shown that approximately 12% of people aged 80 years and older in the United States have some form of age-related macular degeneration (AMD). In total, about 1.75 million Americans had AMD in 2000, and this number is expected to rise to nearly 3 million by the year 2020. There are important racial and ethnic differences: AMD accounts for 54%, 4%, and 14% of blindness among Caucasian, African, and Hispanic Americans, respectively (aEye Disease Prevalence Research Group, 2004a), and about 10% to 12% of blindness and visual impairment among Chinese (Dunzhu et al., 2003; Hsu et al., 2004). AMD is an especially serious health problem among Caucasians, who are about twice as likely to have AMD as Chinese (Chang et al., 1999).
Studies have shown that AMD is associated with decreased quality of life (Brown et al., 2002) and depression (Rovner & Casten, 2002). At present, there is no prevention or cure for AMD. Insights into factors that govern the possibilities for rehabilitation are of fundamental importance for the well-being of AMD patients and their families. This paper will begin with a review of the retinal characteristics of AMD, followed by a review of the retinotopy of the visual cortex. Possible linkages between neurophysiological findings of cortical reorganization and behavioral observations of functional adaptation among AMD patients will be discussed. Given the existing research findings on brain plasticity, we will present a cortical adaptation hypothesis for AMD patients.
Age-related macular degeneration (AMD)
Physiology of AMD
AMD can be classified into two general forms: dry AMD and wet AMD (Ambati et al., 2003; Chopdar et al., 2003). Dry AMD is often caused by geographic atrophy. Geographic atrophy is characterized by confined areas of cell death in the retinal pigment epithelium (RPE) layer. Dysfunction of the overlying photoreceptors follows and results in vision loss. Wet AMD is the more serious form of the two. Wet AMD is caused by choroidal neovascularization. Choroidal neovascularization refers to the growth of new blood vessels in the choroid. The new blood vessels can grow through the RPE into the retina and can cause damage to visual function due to blood and lipid leakage. Retinal damage, either from dry or wet AMD, often results in scotomata (singular: scotoma). Scotoma is an area of reduced light sensitivity on the retina. Relative scotoma refers to an area of some residual light sensitivity. Absolute scotoma describes the absence of any light perception.
AMD, as suggested by the name, affects mainly the macula of the retina. The macula refers to an area of approximately 5 mm in diameter centered on the fovea, and can be identified by its intense yellow pigmentation. Since 1 deg of visual angle spans about 0.3 mm on the retina, the macula corresponds to the central 15–20 deg in the visual field. AMD often results in central (or macular) scotomata in both eyes of the patient. Schuchard et al. (1999) found that 92% of their 255 AMD patients, who were evaluated for low-vision rehabilitation, had bilateral central scotoma. Bilateral scotoma occurs when the scotomata in both eyes affect the same region in the visual field. While the overall prevalence of bilateral central scotoma may be lower than in the patient sample of Schuchard et al.’s study, advanced AMD, especially when it affects both eyes, often leads to serious visual impairment due to central vision loss.
Characteristics of central scotoma
Measurements of scotomata (“scotometry”) can be done by conventional perimetry. A grid spanning 30 deg both vertically and horizontally with test points 6 deg apart is frequently used in conventional perimetry. In some macular perimetry, only the central 10 deg is mapped with test points 2 deg apart (see Anderson, 2003 for a brief review of conventional perimetry). Conventional perimetry has been found to be useful in identifying scotomata among patients with relatively healthy maculae and reasonably stable fixation (e.g. Westcott et al., 2002). However, conventional perimetry assumes stable foveal fixation, which is often a false assumption in patients with macular diseases like AMD. Patients with macular scotoma often use an eccentric retinal location for fixation, which is often less stable than foveal fixation (see the section on PRL below). Thus, conventional perimetry, falsely assuming foveal fixation, will show an eccentric displacement of the macular scotoma in such patients (Markowitz & Muller, 2004). As a result, macular scotometry based on conventional perimetry often has limited accuracy due to unstable eccentric fixation.
Use of a scanning laser ophthalmoscope (SLO) provides a more accurate alternative for scotometry (Webb et al., 1980; Webb & Hughes, 1981; Timberlake et al., 1982 Timberlake et al., 1986; Sunness et al., 1995). A SLO uses a laser of low power to illuminate a retinal area of 20-μm diameter. Light reflected from that spot is then collected for image formation. The laser is swept over the retina in a raster pattern to compile a full two-dimensional (2-D) image. The resulting images of the retina are usually displayed on a TV monitor and videotaped. Anatomical structures of the retina can be identified from these images. Further development of the SLO as described by Timberlake et al. (1986) allows not only viewing of the retina, but also presentation of stimuli to the retina at the same time using a single yellow laser beam. In the SLO used by Timberlake et al., the raster scan of the laser beam is controlled by a computer. Target stimuli can be presented to designated retinal locations by turning the scanning laser on and off. Upon the patient’s report of seeing or not seeing the targets, visual function at these locations can be noted. The retinal areas of no percept indicate the locations of scotomata. More recent advances in SLO technology involve a nearly invisible (near infrared) laser to illuminate the retina and a visible red laser, with 256 adjustable luminance levels, to present the stimuli on the retina (Fletcher et al., 1994; Sunness et al., 1995). A general discussion of the current SLO technology can be found in the reviews by Sharp and Manivannan (1997) and Sharp et al. (2004). Detailed descriptions of SLO microperimetry and scotometry can be found in the studies by Fletcher et al. (1994), Sunness et al. (1995), and Fujii et al. (2003). The widely used SLO produced by Rodenstock, Germany is no longer commercially available. However, SLO models with comparable capabilities are described by Kelly et al. (2003) and Sharp et al. (2004).
Previous research with conventional perimetry has shown that target size significantly influences the results of scotometry (Bek & Lund-Andersen, 1989). Large bright targets that are presented within an absolute scotoma but near the boundary may be detectable by patients due to light scatter. Therefore, researchers typically use a small mapping target ranging from 3 min to 12 min of arc in size (equivalent to Goldmann 0 to II target) to map the boundaries of the scotomata with a SLO (Timberlake et al., 1986; Fletcher et al., 1994; Schuchard et al., 1999).
In SLO mapping studies, central scotomata have been found to have a variety of shapes—circular, ring-shaped (i.e. scotoma surrounding a functioning fovea), and many irregular shapes (Fletcher et al., 1994). Table 1 shows summary findings on scotoma size from five SLO studies. As summarized in Table 1, most central scotomata in patients with advanced AMD have a diameter of 10–20 deg. However, near the onset of the disease, the scotomata in AMD patients are usually smaller with a diameter of 10 deg or less (Sunness et al., 1999). In a longitudinal study of AMD patients with geographic atrophy, GA (i.e. dry AMD), Sunness et al. showed a progression of increasing scotoma size at different stages of AMD. Sunness et al. collected data from 123 patients with GA at an initial visit and at a one-year follow-up visit. Additional data were collected from 81 of the 123 patients at a two-year follow-up visit. At the initial visit, the total atrophic area within the central 12 deg of the retina ranged from 0.25 mm2 to 10.16 mm2, with a median of 4.83 mm2. Sunness et al. did not provide corresponding angular measures for the atrophic area, but assuming circular shape, the areal measures convert to diameters ranging from 1.90 deg to 11.99 deg, with a median of 8.26 deg. Given that only 54% of the patients had a single solid scotoma (others had multiple, horseshoe, or ring scotomata), these diameters provide only a rough comparison with the other studies summarized in Table 1. At the two-year follow-up, among those with a baseline central atrophic area of 7.62 mm2 or smaller, the median increase in the central atrophic area was 2.29 mm2, with a range between 0–6.35 mm2. Thus, the increase in approximate diameter ranged from 0 deg to 9.48 deg, with a median of 5.69 deg. At the two-year follow-up, the scotoma size among these GA patients matched the findings from other studies in general. Compared with the physiological blind spot, which is about 6 deg wide and 8 deg high (Armaly, 1969), in most cases, especially for more advanced stages of AMD, central scotomata have a much larger size. Also, according to Schuchard et al. (1999), the scotoma width is usually larger than the scotoma height and thus the scotoma looks like a horizontally oriented ellipse. By comparison, the physiological blind spot looks like a vertically oriented ellipse.
Table 1.
Central scotoma size from SLO studies
| Study | Sample size | Summary findings |
|---|---|---|
| Déruaz et al. (2002) | 5 patients | Mean diameter = 11 deg |
| Guez et al. (1993) | 24 patients (40 eyes) | Mean diameter = 10.3 deg |
| Hassan et al. (2002) | 21 patients | Mean diameter = 14.8 deg |
| Schuchard et al. (1999) | 255 patients | Median width = 21.8 deg; median height = 17.9 deg |
Impact of central scotoma
The real-world impact of central scotomata is often characterized by the loss of daily activities such as reading and driving. Rovner and Casten (2002) found that 87.5% of 51 AMD patients gave up reading due to vision loss and 33.3% gave up driving. The deleterious impact of central scotomata on reading performance has been demonstrated clearly by a number of studies (Legge et al., 1985, 1992; Fine & Peli, 1995; Fletcher et al., 1999). Legge et al.(1985) tested 16 low-vision participants in a psychophysical study of reading. Seven of these low-vision participants had absolute scotomata covering part or all of the central 5 deg of the visual field. The highest reading speed among these participants with central vision loss was 70 words/min (wpm) over a range of print size from 12 deg to 24 deg. On the other hand, the participants with other forms of low vision but residual central vision read at least 90 wpm, given appropriate magnification. In their regression model for predicting peak reading speed, the central vision status (intact vs. loss) was the best predictor and accounted for 59% of the variance in the data. Fletcher et al. (1999) also found that the patients with central scotomata read a factor of 2 slower than the patients with other forms of low vision but without central scotomata. Ninety-nine patients with different kinds of low vision were included in their study. The no-scotoma group had an average reading speed of 232 wpm, while the scotoma group had an average reading speed of 110 wpm. Moreover, reading speed has been found to decrease as the central scotoma size increases (Cummings et al., 1985; Sunness et al., 1996; Ergun et al., 2003).
Vision loss from macular degeneration is also associated with termination or reduction of driving. In a study of 126 AMD patients by Decarlo et al. (2003), only 24% of the patients continued to drive, and these drivers severely reduced the number of miles driven. The consequence is a reduction of mobility and sometimes increasing social isolation.
Central scotomata can result from retinal diseases other than AMD, for example, juvenile forms of macular degeneration (JMD). Among the different causes of central vision loss, AMD results in a more serious reading problem (Legge et al., 1992). Legge et al. conducted a reading study that included 141 low-vision participants. Ninety-seven participants suffered from central vision loss. Forty-one participants had AMD, 11 had JMD, and 45 had central vision loss due to other eye conditions. Overall, the reading speeds of the AMD group were about a factor of 2 slower than the reading speeds of acuity-matched groups with JMD and other causes of central-field loss.
Can slower reading in AMD compared with JMD participants be explained by a general age-related decline in reading performance? Some studies have shown that reading speed among normally sighted adults is slower among the elderly (e.g. Bowers, 2000). Akutsu et al. (1991) showed that their older normally sighted group read more slowly than their younger group. However, when they used the results of a careful screening for subclinical age-related eye problems to identify a “visually healthy” older group (aged 63–75 years, mean = 68), they found that this group read as fast as the younger group (aged 19–30 years, mean = 22) at print sizes yielding fastest reading. Akutsu et al.’s findings suggest that age-related decline in normal reading speed, when it occurs, may be mediated by other age-related factors. Lott et al. (2001) reported findings that are consistent with this idea. Among a group of 544 participants aged 58–102 years (mean = 73), Lott et al. found that the youngest group (58–64 years) read 48% faster than the oldest group (85–102 years). Reading speeds were about the same for the 58–64-year group and the 65–69-year group. Reading speed dropped about 10% between the 65–69-year group and the 70–74-year group. Their findings suggest that reading speed begins decreasing around age 70 years. Nonetheless, in a multiple-regression model of reading speed with different vision and motor-related measures as predictors, age did not add extra predictive power. In other words, after taking other vision and motor-related measures into account, age no longer had a significant effect on reading speed. From these findings, it seems unlikely that age differences per se can explain the slower reading in AMD compared with JMD (Legge et al., 1992).
Preferred retinal locus (PRL)
Central scotomata often result in a dysfunctional fovea. In such cases, patients often adopt a peripheral retinal location for fixation and other visual functions without explicit training or instruction. Fuchs’s (1922/1938) work on hemianopia contained one of the earliest documented observations about such an adaptation phenomenon. Fuchs used the term “pseudo-fovea” to denote a peripheral retinal location that was used for fixation. In the last two decades, researchers have adopted the term “preferred retinal locus” (PRL) to describe the shift of fixation to a peripheral retinal location (Cummings et al., 1985; Schuchard & Fletcher, 1994).
Fixation stability at the PRL has been of interest to researchers (Fletcher & Schuchard, 1997; Schuchard et al., 1999). Crossland and Rubin (2002) reported SLO measurements of fixation stability for a group of six normally sighted participants. Participants were asked to fixate on a cross of height 2.5 deg for 10 s, while SLO images were taken at 25 Hz. Eye movements were determined by tracking the positions of retinal landmarks. Crossland and Rubin defined fixation stability as an elliptical area containing the eye’s position 68% of the time during fixational drift. Across the six normally sighted participants, fixation stability ranged from about 100 min to 650 min arc squared (i.e. equivalent to circular areas of about 0.2–0.5 deg in diameter). Fletcher and Schuchard (1997) used a similar method to measure fixation stability in 1339 eyes of patients with different forms of low vision. They used the diameter of the fixation area to characterize fixation stability. The range of fixation stability was 1–9 deg. About 65% of these eyes had a fixation stability of 2.5 deg or smaller. From a group of 255 AMD patients, Schuchard et al. (1999) found that fixation stability ranged from 1 deg to 8 deg with a median of 3 deg. Therefore, PRL fixation in patients with central scotomata is substantially less stable than normal fixation; the fixation areas are 2–15 times larger than normal for the AMD patients. Furthermore, Whittaker et al. (1988) showed that fixation stability decreased as scotoma size increased.
Decreased fixation stability at a PRL can be linked to the decreased fixation stability in normal peripheral vision. It has been shown that participants with normal vision who endeavor to fixate on a peripheral target exhibit a substantial decrease in fixation stability. Sansbury et al. (1973) developed a paradigm for comparing stability of central fixation and peripheral fixation at about 10 deg. Fixation was 3–4 times less stable with peripheral fixation.
It seems likely that lower fixation stability at the PRL would be counterproductive for perception. Recently, however, Déruaz et al. (2004) have proposed that fixation instability at the PRL has the functional benefit of overcoming the perceptual fading of peripheral targets during stable fixation (Troxler’s Effect). This proposal is reminiscent of the view that fixational drift and microsaccades during normal fixation are important in sustaining perception of fixated targets.
Given the importance of high-resolution central vision for reading and many other visual activities in daily life, what kind of cortical adaptation processes may happen after central vision loss? We will discuss research findings on retinotopic organization in the visual cortex and cortical plasticity in the following sections. Discussion of hypotheses for PRL development and its possible link with cortical reorganization will then follow.
Retinotopic organization in the visual cortex
Although the primary visual cortex (V1) receives feedback connections from higher visual areas (Girard et al., 2001; Lyon & Kaas, 2002), its spatial organization is closely tie to the afferent inputs from the retino-geniculate pathway. Brain-lesion and brain-imaging studies have demonstrated a retinotopic organization in V1 (e.g. Tootell et al., 1988; Horton & Hoyt, 1991; Engel et al., 1997). The key findings on retinotopic organization in V1 will be reviewed here. A more detailed historical review on the representation of the visual field in V1 can also be found in McFadzean et al. (2002).
Early brain-lesion and animal studies
Early evidence for retinotopic organization in human visual cortex came from case studies of individuals with gunshot, brain injury, or stroke (Holmes & Lister, 1916; Holmes, 1918 Holmes, 1919 Holmes, 1945; Bender & Furlow, 1945; Horton & Hoyt, 1991). During World War I, Holmes conducted a series of studies on cases of gunshots or other war-related brain injuries (Holmes & Lister, 1916; Holmes, 1918 Holmes, 1919). In some cases, a very confined area of the brain was damaged due to gunshot. Holmes estimated the anatomical locations of the brain lesions and measured the resulting visual-field defects. Holmes (1918/1945) drew a retinotopic map of the visual cortex by putting drawings of the occipital lobe and a visual field side by side and indicated the different retinotopic regions by different symbols on both drawings (see Fig. 1). As shown in Holmes’s map, as retinal stimulation goes from the central visual field to the periphery, the corresponding V1 representation moves from the most posterior part to more anterior parts of the calcarine sulcus in the occipital lobe. As retinal stimulation goes from the upper visual field to the lower visual field, the V1 representation moves from the lower bank to the upper bank of the calcarine sulcus, with the horizontal meridian projecting to the deepest part of the calcarine sulcus. In addition to the results shown in Fig. 1, Holmes’s studies also demonstrated that the left visual field projects to the right hemisphere and vice versa for the right visual field, with the vertical meridian marking the border between V1 and secondary visual cortex (V2). These essential facts have all been confirmed in recent brain-imaging studies of healthy human subjects (DeYoe et al., 1996).
Fig. 1.
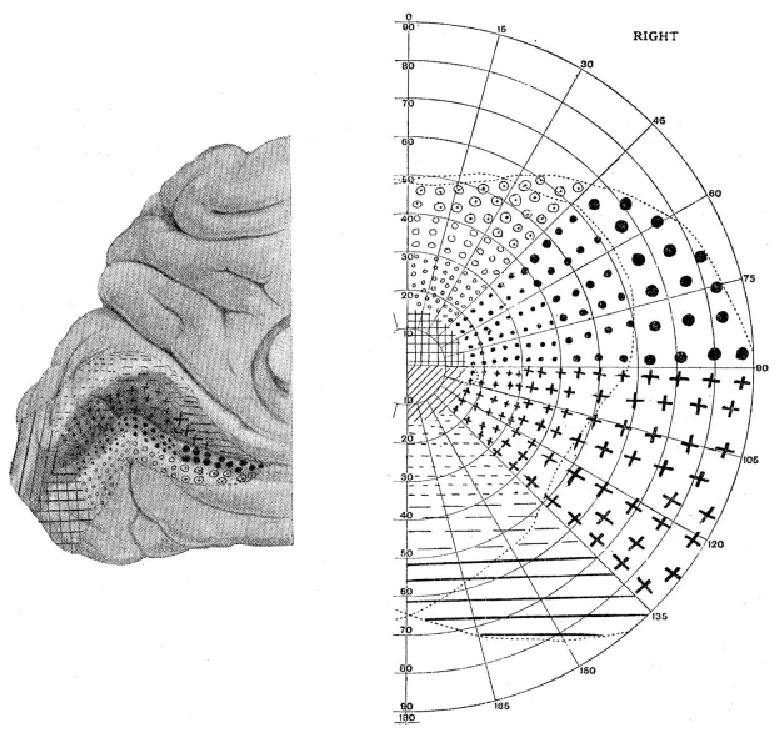
Holmes’s (1918/1945) retinotopic map of the visual cortex. Different locations of the right visual field (right panel) and the corresponding cortical representation in the left hemisphere (medial view, left panel) are marked by different symbols. (Reproduced by permission from Holmes G. “The organization of the visual cortex in man” in the Proceedings of the Royal Society B (London) 132 (869) (April 10, 1945), p. 352 (Fig. 5); © 1945 by the Royal Society).
Brindley and Lewin (1968) conducted a cortical stimulation study of a 52-year-old patient who became blind from bilateral glaucoma and right retinal detachment late in life. They implanted an array of 80 electrodes onto the calcarine sulcus and surrounding areas of the patient’s right hemisphere. Cortical stimulation through different electrodes generated localized light perception (phosphenes) at different locations. The patient was asked to point to the phosphenes. Brindley and Lewin mapped the locations of these phosphenes onto the “visual field”, thereby linking locations of cortical stimulation to locations in the visual field. Their findings were qualitatively consistent with Holmes’s (1918/1945) map. However, Brindley and Lewin’s study was limited in the precision with which they could designate the anatomical positions of cortical stimulation. Accuracy of the visual-field estimation from their patient’s pointing response posed another limitation of their study.
Cortical magnification was one of the important details in Holmes’s (1918/1945) map. Holmes (1945) noted that “the cortical representation of the more highly evolved and specialized maculae, which subserve central vision, occupies a relatively very much greater portion of the striate area than peripheral parts.” The notion of cortical magnification has been further confirmed and demonstrated anatomically in animal studies (e.g. Azzopardi & Cowey, 1993), and in human brain-imaging studies (e.g. Sereno et al., 1995; Engel et al., 1997; Duncan & Boynton, 2003). A more detailed review of cortical magnification can be found in Wilson et al. (1990).
Daniel and Whitteridge (1961) used the term “linear magnification factor”, which is now commonly known as cortical magnification factor, to describe the amount of cortex in millimeters devoted to 1 deg of the visual field. From electrophysiology in macaque monkeys, Daniel and Whitteridge found that the cortical magnification factor at the fovea was about 40 times larger than that at 60-deg eccentricity. From Daniel and Whitteridge’s study, the central 10 deg of the visual field was represented by about 55% to 60% of V1. However, according to Horton and Hoyt’s (1991) estimates based on Holmes’s (1945) map, only 25% of V1 was linked to the central 15 deg of the visual field. Horton and Hoyt did a study to quantitatively check the accuracy of the representation of cortical magnification in Holmes’ map. They studied three patients with peripheral visual field loss due to brain lesions. All three patients still had 20/20 visual acuity. Visual field testing, using tangent screen and conventional perimetry, was done on the three patients to map the visual field defects. Then structural MRI was used to precisely locate the brain lesions in the three patients. Horton and Hoyt predicted the size and location of the visual field defects using the magnetic resonance imaging (MRI) results and Holmes’s map. They found a mismatch between the predictions that were based on Holmes’s map and the actual measurements of the visual field defects. From these three cases, Horton and Hoyt concluded that Holmes’s map underrepresented the cortical magnification factor in central vision. Horton and Hoyt used the cortical magnification data from previous monkey studies, taking into account the areal difference between the monkey V1 and human V1, to revise Holmes’s map. Horton and Hoyt found a very good match between the predictions from their revised map and the actual measurements of the visual field defects and of the brain lesions. Horton and Hoyt estimated a cortical magnification factor of 9.9 mm/deg at 1 deg and 1.6 mm/deg at 10 deg.
The major limitation of Holmes’s (1918/1945) and Horton and Hoyt’s (1991) retinotopic maps is the inferential nature of their studies. Their maps were not derived from direct empirical coupling of retinal stimulation and neuronal response in the visual cortex. One of the first studies with such direct coupling was done by Tootell et al. (1988) on monkeys. Tootell et al. used infusion with 14C-2-deoxy-d-glucose (2-DG) to study the retinotopic organization in V1 of macaque monkeys. Radial lines and concentric rings were presented as stimuli in the visual fields of monkeys after 2-DG was injected. The radial lines and concentric rings were composed of high-contrast flickering checks yielding continuous strong retinal stimulation. The monkeys were then sacrificed for histological processing. The primary visual cortex was flattened and placed against a film to show the 2-DG markings (local concentration of 2-DG marks the level of neuronal activity) as a result of the retinal stimulation, and thus to show the retinotopic map of the monkeys (see Fig. 2). Tootell et al.’s map confirmed the topography of Holmes’s map. Tootell et al. reported a cortical magnification factor of about 15 mm/deg at the fovea for their monkeys. They also found that the cortical magnification factor was larger along the vertical meridian compared to the horizontal meridian given the same eccentricity. For example, at 10 deg, the cortical magnification factor would be 1.3 mm/deg for the horizontal meridian and 1.7 mm/deg for the vertical meridian. Fig. 3 shows plots of cortical magnification factor against eccentricity based on Tootell et al.’s estimated functions for both horizontal and vertical meridians.
Fig. 2.
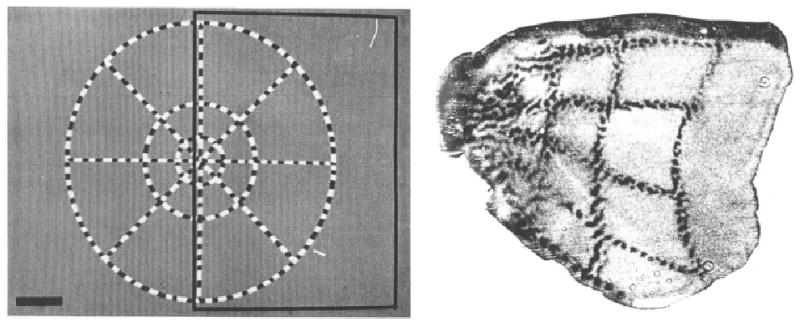
Retinotopic map of visual cortex in a macaque monkey. Left panel shows the visual stimulus with check-filled radial lines and concentric rings. Right panel shows the flattened visual cortex with representation of the radial lines and concentric rings marked by 2-DG. (Reproduced by permission from Tootell R.B.H., Switkes E., Silverman M.S. & Hamilton S.L. “Functional anatomy of macaque striate cortex. II. Retinotopic organization” in the Journal of Neuroscience 8(5), p. 1534 (Fig. 1) and p. 1535 (Fig. 2B); © 1988 by the Society for Neuroscience)
Fig. 3.
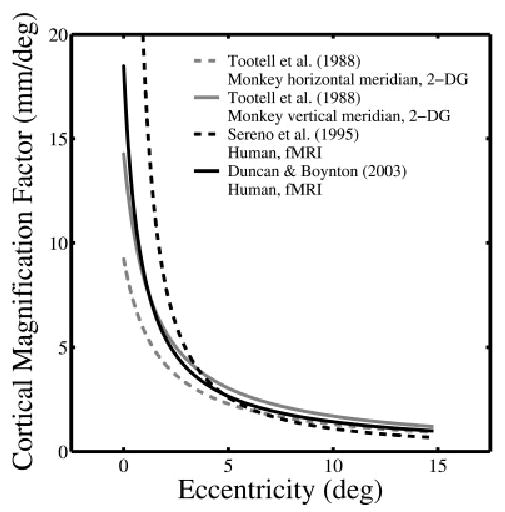
Plots of cortical magnification factor (M) vs. eccentricity. The gray curves are estimated functions from Tootell et al.’s (1988) 2-DG study on monkeys. The corresponding equations are: horizontal meridian (dashed line) M = (0.108 + 0.066 * E)−1, and vertical meridian (solid line) M = (0.070 + 0.052 * E)−1. The black curves are estimated functions from Sereno et al.’s (1995) human fMRI study: M = 20.05 * (0.08 + E)−1.26, and Duncan and Boynton’s (2003) human fMRI study: M = (0.054 + 0.065 * E)−1.
Functional brain-imaging studies
The recent development of brain-imaging methods, such as positron-emission tomography (PET) and functional magnetic resonance imaging (fMRI), allows researchers to study cortical activity in humans (Fox et al., 1984, 1986; Bandettini et al, 1992; Kwong et al., 1992; Ogawa et al., 1992). In PET, concentration of a preinjected radioactive substance inside the body is revealed by the scanner. One of the often-used radioactive substances is water labeled with radioactive oxygen (15O) (Fox et al., 1984). Change in blood flow is marked by the preinjected radioactive water and can be imaged by a PET scanner. Local increase of blood flow in the brain is used as an indication of increased neuronal activity. Fox et al (1987) used PET to study the retinotopic organization of human visual cortex. They presented three annuli of different sizes sequentially to stimulate different regions of the participants’ visual field. Cortical activity corresponding to the three annuli was recorded. The annuli were filled with red and black checks, which alternated colors at 10 Hz to maximize the cortical response. The inner and outer radii were 0.1 and 1.5 deg for the macular annulus, 1.5 and 5.5 deg for the perimacular annulus, and 5.5 and 15.5 deg for the peripheral annulus. All three annuli were centered on a fixation mark. Fox et al. found that as the stimulation stepped from the macular region to the perimacular region, then to the peripheral region, the corresponding cortical activity in the occipital lobe progressed in a posterior-to-anterior direction. They also estimated the cortical magnification factor as 3.4, 1.6, and 0.9 mm/deg at 0.7, 3.5, and 10.5 deg, respectively. However, due to the limitations of resolution, cortical infolding and curvature of the cortex, Fox et al. acknowledged that their estimates only marked the lower bounds. This is the first functional brain-imaging study on human retinotopic organization. Findings from this study confirmed the observations of Holmes (1918).
Blood-oxygenation-level-dependent (BOLD) fMRI has been used widely to study human cortical activity in recent years. The exact linkage between neural activity and the BOLD signal is still a topic of on-going research. The BOLD signal originates from a mismatch in the demand for more oxygen and the increase in oxygenated blood flow (Fox & Raichle, 1986; Fox et al., 1988). An increase in cortical activity results in an increased oxygen consumption to support the metabolism in the neurons. The increased oxygen consumption results in a local decrease of oxygen level, which in turn, increases the flow of oxygenated blood to that local cortical region. The increased supply of oxygenated blood overcompensates the demand of oxygen and leads to an increase in oxygen level as a response to increased cortical activity. However, the reason for such a cortical-activity-driven mismatch is still unknown. The changes in oxygen level can be detected by fMRI and produce a BOLD contrast. Logothetis et al. (2001) found that the BOLD signal was more closely associated with the synaptic inputs to the neurons and local intracortical processes (termed “local field potentials”) than with neuron spike rates. For a comprehensive review on the interpretation of the BOLD signal, see Logothetis and Wandell (2004).z
During the mid 1990s, several research groups used fMRI to study retinotopic organization in human visual cortex (Engel et al., 1994, 1997; DeYoe et al., 1994 , 1996; Sereno et al, 1995). Engel et al. (1994) used check-filled annuli to stimulate the participants’ visual field during fMRI measurements. Instead of using stationary annuli of different sizes as Fox et al. (1987) had done, Engel et al. used an expanding annulus to continuously stimulate retinal regions of different eccentricities. Both the inner and outer radii of the annulus increased in time until the outer radius reached 12 deg. When the outer radius reached 12 deg, the annulus started from the center again. Fig. 4 illustrates this stimulus. With multiple cycles of the expanding annulus, retinal regions of different eccentricities were stimulated periodically. The periodic retinal stimulation led to a periodic activation pattern of the V1 neurons. Fig. 5 is a schematic time course of retinal stimulation (black solid line) and the resulting V1 activity (gray dashed line) from 4 cycles of the expanding annulus for one particular eccentricity. Different eccentricities would produce similar plots except for a horizontal shift of the curves. Engel et al. fitted the V1 activity as measured by fMRI with sinusoid functions of different phase shifts (peak offsets). The amount of phase shift in the periodic activation pattern indicates the eccentricity because both the amount of phase shift and eccentricity increased together with time. This procedure was called the phase-encoding method for retinotopic mapping.
Fig. 4.
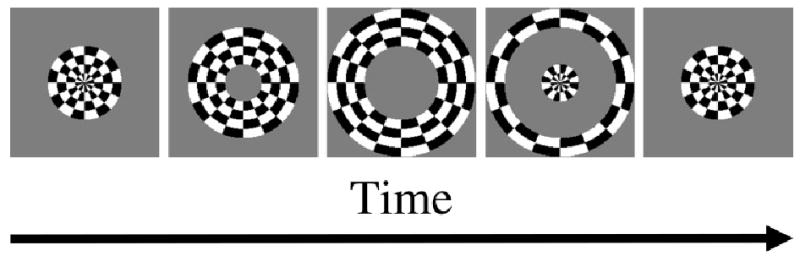
The expanding-annulus stimulus. The annulus expands until the outer annulus reaches the boundary of the display (third panel) and then starts from the center again (fourth and fifth panels). A complete cycle is shown in panels 1–4. The fifth panel shows the beginning of the next cycle. The annulus is filled with flickering checks to optimize V1 response.
Fig. 5.
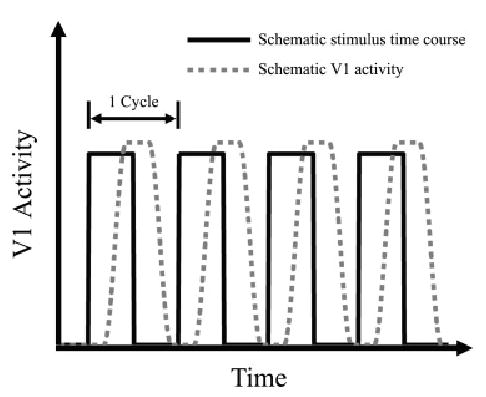
Schematic V1 activity in response to periodic retinal stimulation. The black curve represents the periodic retinal stimulation. The gray dashed curve represents the corresponding schematic V1 activity. Four cycles of activity are depicted.
DeYoe et al. (1994, 1996) introduced another stimulus for mapping the retinotopy of the visual cortex. DeYoe et al.’s idea was based on the fact that each point on the visual field can be described by referring to a polar-coordinate system. Engel et al.’s (1994) expanding annulus could be used to map the radius-coordinate (i.e. the eccentricity) of the visual field onto the visual cortex. A stimulus for mapping the angle-coordinate was needed. DoYoe et al. used a rotating check-filled half circle to map the angle-coordinate of the visual field. A schematic illustration of the rotating half circle is shown in Fig. 6. The half circle rotated about the fixation point in steps of 18 deg every 2 s and thus, one complete rotation took 40 s. DeYoe et al. used 5 cycles of the rotating half circle to generate a periodic activation pattern in the visual cortex. Similar to the phase encoding of eccentricities as used in Engel et al., the angle-coordinate was also phase encoded. In 1997, Engel et al. used both the expanding (and contracting) annulus and a set of rotating wedges to study the retinotopy of the visual cortex with high precision.
Fig. 6.
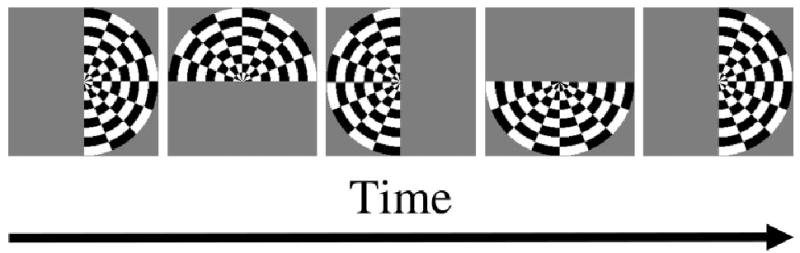
Rotating-half-circle stimulus. The half circle rotates about the fixation point periodically. The first and fifth panels represent the beginning of the first and second cycle. The half circle is filled with flickering checks to optimize V1 response.
In tandem with the development of the phase-encoding method for retinotopic mapping, algorithms were developed to produce a flattened representation of the human cortex for both analysis and visualization (Dale & Sereno, 1993; Carman et al., 1995; Drury et al., 1996; Wandell et al., 1996, 2000). The use of the phase-encoding stimuli for testing, followed by software-based visualization of activation patterns on a flattened cortical surface, has subsequently become the standard retinotopic mapping method in fMRI.
While the retinotopy studies reviewed above provided evidence for a point-to-point correspondence between retinal stimulation and neuronal response in V1, they did not address the question of whether such correspondence would hold at a very fine grain. Adams and Horton (2002, 2003) showed evidence for a fine-grain point-to-point correspondence between the retina and V1. Adams and Horton’s studies were based on the properties of the ocular-dominance columns (Hubel & Wiesel, 1969). Studies in nonhuman mammals have shown that visual experience early in life influences the formation of the ocular-dominance columns (e.g. Hubel & Wiesel, 1970; Issa et al., 1999). For example, Hubel and Wiesel (1970) showed in their studies on cats that when visual input from one eye was deprived for less than a week during the fourth and fifth weeks after birth, the ocular-dominance columns for the other eye would expand. Adams and Horton pointed out that such monocular visual deprivation actually happens naturally in normally sighted animals. Retinal blood vessels cast shadows on the retina, preventing light from stimulating the underlying photoreceptors. There is an imbalance of signals from the corresponding retinae because one is shadowed by the blood vessel and the other receives normal stimulation. The result is a representation of the retinal blood vessels in V1 as revealed by the ocular-dominance columns. Adams and Horton enucleated one of the two eyes in adult squirrel monkeys, so that the activity in V1 would be due to only one eye. Then, they used cytochrome oxidase (CO) staining to mark the metabolic activity in the visual cortex. Lighter colored CO staining indicated the reduced activity for the deprived columns and thus revealed the column structure. V1 of the monkeys was flattened showing a map of the blood vessel representation and was compared with fundus images of the retina. Adams and Horton were able to create a retinotopic map by referencing to the blood vessel representation in V1. Their study provided evidence for a fine-grain retinotopy at V1 on the scale of ±1 deg in the visual field, which was the estimated error in locating retinal landmarks.
The relationship between the cortical magnification factor and eccentricity has been revisited in fMRI retinotopic mapping data. Sereno et al. (1995) did an fMRI retinotopic mapping study using both the expanding and contracting annulus and the rotating half circle. From the resulting maps, Sereno et al. estimated the equation for the cortical magnification factor (M) against eccentricity (E) as M = 20.05 * (E + 0.08)−1.26. From this formula, the cortical magnification factor at the fovea would be 483 mm/deg, an unreasonably large value.* The relationship between M and E can also be described in terms of a linear relationship between the inverse of M (M−1) and E, similar to the approach taken by Tootell et al. (1988) (see Fig. 3). Duncan and Boynton (2003) have recently estimated the relationship between M−1 and E based on human fMRI data, M−1 = 0.054 + 0.065 * E. Curve fits based on Sereno et al.’s and Duncan and Boynton’s functions are shown with the estimated functions from Tootell et al.’s study on monkeys in Fig. 3. According to Duncan and Boynton’s equation, the cortical magnification factor would be 18.5, 8.4, and 1.4 mm/deg at 0, 1, and 10 deg eccentricities, respectively. These are in close agreement with Horton and Hoyt’s (1991) estimates of 9.9 and 1.6 mm/deg at 1 and 10 deg, respectively. The enlarged cortical representation of central vision has also been linked to better performance in psychophysical tasks in central vision compared with peripheral vision (Rovamo et al., 1978; Rovamo & Virsu, 1979; Virsu & Rovamo, 1979; Levi et al., 1985).
Cortical adaptation due to central scotoma
Baseler et al. (2002) estimated that the foveola, which is the rod-free zone and spans 1.25 deg, was represented by almost 20% of the surface area of V1 based on Horton and Hoyt’s (1991) map. As mentioned above, most central scotomata from macular degeneration have a diameter in the range of about 10–20 deg at the advanced stage. According to Horton and Hoyt’s map, which has been confirmed by different functional brain-imaging studies on humans (Engel et al., 1997; Duncan & Boynton, 2003), a macular region of 15 deg in diameter would correspond to about 50% of the surface area of V1. What happens to this area of visual cortex in patients with central scotomata? In the absence of any cortical adaptation or reassignment of function, this large area of visual cortex would be unused and unresponsive to incoming sensory signals. As yet, no conclusive answer has been given to this question. As early as 1945, Holmes pointed out that the adoption of a PRL in the presence of central scotomata could be a result of reorganization in the visual cortex. Results from animal studies may shed some light on changes of cortical organization in the presence of retinal scotomata.
Animal studies
Cat and monkey studies have shown that reorganization in adult mammalian visual cortex occurs following visual field loss (Kaas et al., 1990; Heinen & Skavenski, 1991; Gilbert & Wiesel, 1992; Chino et al., 1992, 1995; Darian-Smith & Gilbert, 1994, 1995; Calford et al., 2000). Prior to these studies, prevailing wisdom held that connections along the retinocortical visual pathways are very rigid following a developmental critical period (weeks old) (see Boothe et al., 1985 for a review). Contrary to this view, Kaas et al. (1990) found evidence for retinotopic reorganization in the visual cortex of adult cats. Kaas et al. created experimental retinal lesions just above the area centralis (area centralis in cats is analogous to fovea in humans) in one of the two retinae in adult cats. Then they eliminated all inputs from the other eye by enucleation. This left a strictly monocular retinal input with a “hole” in the center. Kaas et al. monitored cortical activity through single-cell recording. At 2–6 months after the removal of the nonlesioned eye, Kaas et al. found that the deafferented neurons became responsive to the retinal areas next to the lesion. In four of the studied cats, the size of the retinal lesion was about 5–10 deg in diameter. All deafferented neurons became reactivated again in these cats. In two other cats, the size of the retinal lesion was about 10–15 deg in diameter. A cortical region corresponding to the center of the retinal lesion remained unresponsive, indicating reorganization might not be complete.
Gilbert and Wiesel (1992) further showed that retinotopic reorganization could begin as soon as minutes after inducing the retinal lesions. Unlike Kaas et al.’s (1990) procedure of monocular lesion and removal of the nonlesioned eye, Gilbert and Wiesel induced corresponding retinal lesions in the parafoveal region of both eyes of six adult monkeys and four adult cats. The resulting visual field defect was analogous to the visual experience faced by patients with bilateral central scotoma. However, the induced retinal lesions (in the monkeys) were of relatively small size with 3–5 deg diameter centered 3–5 deg below the fovea. The neurons that were originally responsive to retinal areas just inside the border of the lesion started to respond to an expanded retinal area (i.e. increased receptive-field size) immediately after the lesion. On the other hand, the neurons that were originally responsive to the more central area of the lesion did not respond to any retinal stimulation immediately after the lesion. Two months following lesion induction, these originally unresponsive neurons were activated by retinal stimulation outside the lesion. The rapid expansion of the receptive fields of the neurons near the border of the retinal lesion suggested that the early establishment of reorganization was due to reweighting or unmasking of existing neural connections because sprouting of new axons and dendrites could not happen in minutes. However, rapid reorganization did not rule out the possibility of later axonal and dendtritic sprouting. Indeed, Darian-Smith and Gilbert (1994) showed evidence of axonal sprouting in retinotopic reorganization in adult cats. Additional evidence for axonal sprouting as a mechanism in retinotopic reorganization comes from a study by Obata et al. (1999). They found an increase in the concentration of molecules associated with the sprouting of axons.
In summary, both cat and monkey studies provide evidence for retinotopic reorganization as a result of retinal deafferentation (Kaas et al., 1990; Heinen & Skavenski, 1991; Gilbert & Wiesel, 1992; Chino et al., 1992, 1995; Darian-Smith & Gilbert, 1994, 1995; Calford et al., 2000). Extensive reviews on cortical plasticity of sensory and motor organization can be found in Chino (1995), Gilbert (1998), and Kaas and Collins (2003).
Retinotopic maps in humans with abnormal retinal inputs
Baseler, Morland, and colleagues reported on retinotopic maps in three people with abnormal retinal inputs (Morland et al., 2001; Baseler et al., 2002). Baseler et al. measured retinotopic organization in three rod monochromats and two participants with normal vision. Baseler et al. used fMRI in conjunction with the phase-encoding stimuli that Engel et al. (1997) used. Rod monochromats suffer from a condition in which none (or very few) of the cones function due to a genetic deficiency. The malfunction is related to the phototransduction of the cones. A rod-free region exists in the fovea in which the photoreceptors are exclusively cones and is called the foveola. Thus, the maculae of rod monochromats are packed with nonfunctional cones. As a result, the rod monochromats are congenitally color-blind and have small, central scotomata. Baseler et al. did retinotopic mapping on normally sighted controls under both normal (photopic) and low-luminance (scotopic) viewing conditions. The low-luminance viewing condition was intended to isolate the rod input to the retinocortical pathway. These rod-driven retinotopic maps were created on the normally sighted controls for comparisons with the retinotopic maps from the rod monochromats. Baseler et al. found that the cortical region that would otherwise have responded to the foveola was activated by retinal stimulation next to the foveola in the rod monochromats. In other words, there was no region in the cortical representation corresponding to the small central scotoma. Fixation stability was ± 1 to 2 deg across the three rod monochromats, measured with either a scleral search coil or a standard ophthalmoscope. Although the fixation was less stable in the rod monochromats, fixation instability could not account for the activation of the “foveolar region” in V1. Visual-field-eccentricity plots showing eccentricity in visual angles against cortical distance in millimeters (referencing to a cortical point that responded to stimulation at 10 deg) were also created for each participant. The scotopic eccentricity plots from the normally sighted controls showed no representation of the foveola, which spanned about 2 cm in V1. Comparison between the scotopic eccentricity plots from the normally sighted controls and the eccentricity plots from the rod monochromats showed that the parafoveal region spanned a larger cortical distance in rod monochromats than in normally sighted controls. These findings show that retinotopic organization in these rod monochromats was different from that of people with normal visual experience. The activation of the foveolar region and the expansion of the parafoveal representation in V1 of the rod monochromats provided evidence for an experience-driven retinotopic organization. However, since rod monochromatism is an inherited disorder present from birth, the “reorganization” presumably could have occurred during early visual development. Thus, these findings provided evidence for abnormal retinotopic organization due to congenital vision loss, but not necessarily evidence for reorganization of adult human visual cortex.
Hypotheses for the development of PRL
One of the key, yet unresolved, questions related to central scotomata is what determines the location of the PRL. Ideas for the answer to this question can be categorized into (1) function-driven, (2) performance-driven, or (3) retinotopy-driven explanations.
Function-driven explanation of PRL
Intuition suggests that certain PRL placements may be more appropriate for specific visual activities. For example, a visual field loss to the right of the point of fixation (i.e. a left-field PRL) would occlude part of the upcoming text on the fixated line in English reading. Thus, a PRL above or below the retinal lesion, which corresponds to a lower- or upper-field PRL respectively, should be more advantageous for English reading. Rehabilitation specialists favor the view that a lower-field PRL is best for reading. Nilsson et al. (1998, 2003) showed that their patients could read faster with a trained lower- or upper-field PRL, compared with their patients’ naturally occurring “undesirable” PRL locations. Nilsson et al. termed the newly trained eccentric retinal location the trained retinal locus (TRL). In one of their studies, 11 of 20 patients initially had a left-field PRL. Among the 20 patients, 18 established a TRL. Ten of the patients with initial left-field PRL established a TRL above or below the central scotomata after the training. Reading speed for the 18 patients who established a TRL improved from an average of 9.0 (±5.8) wpm before training to an average of 68.3 (±19.4) wpm after training.†
It is possible that the impressive improvement in reading speed in Nilsson et al.’s study might have been due, at least in part, to training in peripheral reading per se, rather than to the advantage of the TRL location. Chung et al. (2004) found a significant but smaller increase in peripheral reading speed among normally sighted participants after training on a letter-recognition task in peripheral vision. Their participants were trained 2 h per day for 4 days, one group at 10 deg above fixation and a second group 10 deg below fixation. Pre-training and post-training reading speeds at 10 deg above and below were measured. Chung et al. found a 41% increase in peripheral reading speed after the training. They attributed the improvement to perceptual learning, that is, to plastic changes in perceptual processing. It therefore remains to be determined how the impressive and encouraging improvements in AMD reading speed due to training (Nilsson et al., 2003) depend on PRL placement, perceptual learning, and strategic factors related to the use of magnifiers.
A simulated scotoma study by Fine and Rubin (1999) also showed that reading performance was best when the simulated scotoma forced their normally sighted participants to read with the lower visual field. Petre et al. (2000) also found that peripheral reading speed was faster in the lower visual field and left visual field among normally sighted participants. However, Fletcher et al. (1999) did not find a statistically significant difference in maximum reading speed between the lower-/upper-field PRL group and other PRL groups in a study of 99 patients with low vision. The average maximum reading speeds were 124 wpm for the lower-/upper-field PRL group and 105 wpm for the other PRL groups. The reason for the discrepancy between Fletcher et al.’s study on patients with low vision, and the TRL (Nilsson et al., 1998, 2003) and normal peripheral reading studies (Fine & Rubin, 1999; Petre et al., 2000) is yet to be determined. While either a lower- or upper-field PRL may be advantageous in English reading, a visual field loss below the PRL (i.e. an upper-field PRL) would likely pose a serious problem in walking, because visual information from beneath the fixation is important for obstacle avoidance. Thus, a lower-field PRL seems to be more advantageous for mobility (Turano et al., 2004).
Since having a lower-field PRL benefits visual function, the function-driven explanation of PRL predicts a larger proportion of lower-field PRLs among patients with central scotomata. Surprisingly, this is not the case. Instead, several studies have shown a comparable or even higher prevalence of left-field PRL among patients with central scotomata. Fletcher, Schuchard and colleagues found that the majority of patients with central scotomata, due to different kinds of pathology, had either a lower-field or a left-field PRL (Fletcher et al., 1994; Fletcher & Schuchard, 1997). In one of their studies, 39.0% of 825 patients with central scotomata had a lower-field PRL and 33.7% had a left-field PRL. Sunness et al. (1996) found that 63% of 27 eyes with geographic atrophy (dry AMD) and an established eccentric PRL had a left-field PRL. The high prevalence of left-field PRL is particularly puzzling in the context of English reading as discussed above. The seemingly inappropriate site selection for the PRL might help explain reading difficulty among people with central scotomata, especially from AMD, as discussed earlier in this paper (Legge et al., 1985, 1992; Fletcher et al., 1999). The high frequency of PRL to the left of central scotomata argues against the function-driven hypothesis for the spontaneous site selection of PRL.
Performance-driven explanation of PRL
When the macula becomes dysfunctional, the visual system may select a peripheral retinal location to maximize visual performance. For example, the visual system may place the PRL at a peripheral retinal location that has the best visual acuity outside of the dysfunctional macula. More than a century ago, Wertheim (1891/1980) documented differences in visual acuity at different parts of the visual field with the same eccentricity. Wertheim’s report was based on observations on his left eye. Within 15 deg of the fovea, he found that the left and right visual fields had about the same visual acuity, and the upper visual field had a higher visual acuity than the lower visual field. Beyond 15 deg, the left (temporal) visual field had higher visual acuity than the right (nasal) visual field, and the lower visual field had higher visual acuity than the upper visual field. Expressed in terms of gradients, the upper meridian had the highest rate of decline in visual acuity, followed by the lower meridian, and then by the right (nasal) meridian. The left (temporal) meridian had the lowest rate of decline in visual acuity. Wertheim also showed that the horizontal meridian had a higher visual acuity than the vertical meridian given the same eccentricity. Wertheim’s findings have been confirmed by many psychophysical studies (e.g. Fahle & Schmid, 1988; Carrasco et al., 2001). Since the width and height of a typical central scotoma are always smaller than 30 deg, we need to consider the relevance of acuity anisotropy only within 15 deg of the fovea. Adopting the simplifying assumptions that scotomata are circular, centered on the fovea, and that the highest acuity peripheral location is selected for the PRL, we would expect (1) a preference for PRL on the horizontal meridian, (2) no preference between PRLs to the left and right, and (3) a preference for upper-field PRL over lower-field PRL. But, since scotomata tend to be horizontally elongated (see above), the advantage of higher visual acuity along the horizontal meridian compared with the vertical meridian may be lost. If so, acuity considerations might lead us to expect a preference for upper-field PRL. As mentioned in the previous paragraph, the skewed distribution of PRL with high frequencies of left- and lower-field PRL argues against this kind of performance (acuity)-driven explanation for the placement of PRL.
Another performance-driven explanation of PRL is based on the idea of visual attention. Performance in different visual tasks (e.g. detection or identification) often increases with attention. He et al. (1996) found that attentional resolution, the finest separation of objects that allows isolated visual attention, was better in the lower visual field compared to the upper visual field. Altpeter et al. (2000) asked 22 patients with macular diseases and 15 normally sighted participants to report the orientation (among 4 choices) of the character E at cued and attended locations in different parts of the visual field. Overall, the horizontal meridians yielded better performance than the vertical meridians. They also found that 64% of the patient group and 47% of the control group showed poor performance in the upper visual field compared to the lower visual field. Given the relatively high prevalence of lower-field PRL, Altpeter et al. concluded that this was evidence for a link between visual attention ability and the placement of PRL. However, Alt-peter et al. found no difference between the left and right visual fields. Therefore, the high-prevalence of left-field PRL was left unexplained.
Retinotopy-driven explanation of PRL
The retinotopy-driven hypothesis and the two hypotheses mentioned above are not mutually exclusive. However, the retinotopy-driven hypothesis comes from a cortical mechanism point of view. As shown from the discussion on animal studies on retinotopic reorganization, the deafferented V1 neurons spontaneously re-mapped to the inputs from retinal locations near the scotoma. PRL selection might be a result of such spontaneous reorganization. The retinotopy-driven hypothesis predicts a PRL at the border of the central scotoma. Among 883 eyes with different forms of maculopathy in Fletcher and Schuchard’s (1997) study, 88.7% of the PRL were within 2.5 deg of the borders of the scotomata. Sunness et al. (1996) also found that the 27 eyes with dry AMD and eccentric PRL always had a PRL within 2 deg of the scotoma border. These findings on the proximity of the PRL placement and the scotoma borders seem consistent with the retinotopy-driven hypothesis.
Research has shown that 72%–84% of the eyes with central scotomata due to AMD or JMD adopt a PRL (Cummings et al., 1985; Fletcher & Schuchard, 1997). While spontaneous retinotopic reorganization provides a hypothesis for the initial development of PRL, it still leaves room for possible retraining of the PRL location. As discussed earlier, Nilsson et al. (1998, 2003) showed that an eccentric retinal location other than the initial PRL can be trained (i.e. the TRL) to replace the initial PRL. Nilsson et al. (1998) also reported that five of their six patients (83%) used the TRL as their stable PRL. A question arising from the TRL studies is whether the training-induced TRL produces a plastic change in visual cortex. Brain-imaging studies of humans with extensive sensory or motor training show evidence of experience-/training-dependent cortical reorganization. Pantev et al. (1998) has shown that musicians have enlarged auditory cortical representations. Elbert et al. (1995) has also shown that string players have enlarged representations in their somatosensory cortex for fingers on their left hands, used for playing string instruments. In both musician studies, an alternative explanation for the findings is that the musicians were born with larger auditory or somatosensory cortex. However, both studies showed that the amount of enlargement was correlated with the age at which the participants started practicing. Such correlations support the interpretation of training-induced cortical reorganization.
Another piece of evidence that suggests an increased extent of reorganization with experience or training comes from observations on patients with JMD. Sunness et al. (1996) found that 70% of the eyes with JMD in their study had a PRL at least 2 deg from a scotoma border. They also found that 90% of these JMD eyes had a lower-field PRL, likely to be the most functionally adaptive position. The differences between AMD and JMD suggest that the PRL placement in AMD may be influenced more by spontaneous retinotopic reorganization, while JMD may be linked to the experience-/training-based cortical reorganization. As mentioned above, the studies by Gilbert and Wiesel (1992) and Darian-Smith and Gilbert (1994) have demonstrated two cortical mechanisms underlying retinotopic reorganization in adult mammals: (1) an initial immediate unmasking of existing cortical connections, and (2) axonal sprouting that results in long-range reorganization. If only the initial unmasking happens in AMD, but not the axonal sprouting, as indicated by Gilbert and Wiesel’s study, we should expect an area in V1 to remain unresponsive to visual stimulation. Recently, Sunness et al. (2004) reported a retinotopic mapping study on a patient with GA (dry AMD). A horseshoe macula scotoma developed during the patient’s late 50s. Sunness et al. found an unresponsive area in V1, which corresponded to the scotoma.
If extensive retinotopic reorganization does not happen in V1, what underlies the adoption of a peripheral PRL as a new fixation reference among AMD patients? It is possible that the PRL serves primarily as a new oculomotor reference point, so the reorganization might be found in brain areas dedicated to eye-movement control. Eye movement control can be roughly divided into two categories: saccadic control and smooth pursuit control. Different cortical and subcortical areas have been linked to eye movement control (see Munoz, 2002 and Krauzlis, 2004 for a review). Bergeron et al. (2003) has shown that neurons in the superior colliculus encode the distance between the current gaze position and the goal of a saccadic eye movement. Goldberg and Wurtz studied the activity in monkey superior colliculus and its role in eye movements (Goldberg & Wurtz, 1972a,b; Wurtz & Goldberg, 1972a,b). They found a retinotopic organization in the monkey superior colliculus. Recent studies have also shown evidence for retinotopy in human superior colliculus (DuBois & Cohen, 2000; Schneider et al., 2004). It remains to be determined if superior colliculus demonstrates reorganization in the presence of central scotoma, and whether the retinotopic organization adopts the PRL as its origin.
While more extensive retinotopic reorganization in V1 by axonal sprouting may be absent in AMD, JMD may follow a different scenario. Casco et al. (2003) recently reported on a comparison in parafoveal and peripheral visual function between a 21-year-old JMD patient and a group of normally sighted control participants. Casco et al. found comparable or even better performance from the JMD patient in some of the parafoveal and peripheral visual tasks including lexical decision, recognition of crowded letters and visual search. Casco et al. suggested that such findings supported the idea of cortical reorganization. While such observations could be due to remapping of cortical resources to the functional retinal areas outside the central scotoma, an alternative explanation would be better performance due to training similar to the improved reading performance in peripheral vision in Chung et al.’s (2004)perceptual learning study (see above), and perhaps unrelated to remapping. More research is needed to distinguish between cortical remapping and perceptual learning as explanations for improved performance in peripheral vision.
The high prevalence of left-field PRL is still not explained by the retinotopy-driven hypothesis. Sunness et al. (1996) reported on the relationship between the estimated proximity of PRL to fovea and PRL placement among 24 eyes with dry AMD. The proximity of PRL to fovea was estimated by determining the position of the fovea within the scotoma. The position of the fovea was categorized into left-third, central-third, or right-third of the scotoma. For cases in which the fovea is at the right-third of the scotoma in the visual field, a right-field PRL should be chosen for a closer proximity to the fovea. However, among the five eyes with the fovea in the right-third of the scotoma, three eyes had a left-field PRL instead. Although 16 of 24 eyes had the fovea in the central third horizontally and 23 of 24 eyes had the fovea in the central third vertically, 16 of the 24 eyes had a left-field PRL. Thus, these findings, with the knowledge of retinotopy in V1, suggest that the final placement of PRL is not strictly governed by the cortical distance between the V1 neurons that correspond to the PRL and the deafferented cortical region in V1.
Multiple PRLs
SLO studies have shown that patients with central scotomata may have more than one PRL. Whittaker et al. (1988) suggested that patients with a central scotoma larger than 20 deg were more likely to have multiple PRLs. There is evidence that patients with multiple PRLs use different PRLs for different tasks (Guez et al., 1993; Sunness et al., 1996; Lei & Schuchard, 1997; Déruaz et al., 2002). Lei and Schuchard studied the effects of stimulus luminance on the locations of PRLs. They found that patients used two different PRLs for fixating targets of different levels of luminance. They had 28 patients with central scotomata. The borders for both the absolute and relative scotomata were mapped by a SLO. Given targets with high luminance, the patients would use an area within the relative scotomata, either at fovea or just outside the absolute scotomata, to perform the visual tasks. When the targets had low luminance, the patients used a different and relatively more healthy retinal location for the visual tasks. Lei and Schuchard concluded that these patients had two stable PRLs for different luminance levels. The switch between the two PRLs occurred at 106 to 3437 trolands across different patients.
Although most dry AMD patients in Sunness et al.’s (1996) study used a single PRL for fixation with a cross, or reading of a letter or word, five of the 35 patients used different PRLs for the different tasks. Two patients used a left-field PRL to fixate on a cross, but a lower-field PRL for reading words. Two patients used a left-field PRL together with a right-field PRL to read words. One patient used a left-field PRL to fixate on a letter, but an upper-field to fixate on a cross. Guez et al. also found that among their 24 patients, four had different PRLs depending on the size of the fixation target. Déruaz et al. (2002) noted that patients could use multiple PRLs during reading for better performance. Déruaz et al. made SLO recordings on five patients with central scotomata while the patients were reading words of different lengths and different character sizes. All of their five patients used multiple PRLs during the reading task. Two of them used different PRLs depending on the character size of the words. The other three used different PRLs depending on word length, but not the character size. Déruaz et al. suggested that patients with central scotoma could be trained to use multiple PRLs for more efficient reading. On the other hand, Nilsson et al. (1998, 2003 recommended that patients with multiple PRLs should be trained to use a single PRL for reading. The question of whether multiple PRLs are adaptive or maladaptive has not yet been resolved.
Multiple PRLs could also be a transitional stage. Patients with dry AMD often have a relatively functional fovea surrounded by absolute scotomata until late in the pathology. Such patients may use the functional fovea with a ring scotoma for high-acuity tasks and use an eccentric location outside of the scotoma for other tasks. Finally, as mentioned earlier, PRL placement may relate to visual attention (Altpeter et al., 2000). The multiple PRL phenomenon may have mechanisms in common with switching attention among different peripheral areas in the visual field.
Conclusion
In this review, we have discussed (1) the deleterious effects of age-related macular degeneration (AMD) and the preferred retinal locus (PRL) phenomenon, (2) the retinotopy in visual cortex, (3) the limitations of the function- or performance-driven explanations for PRL, and (4) the possible link between retinotopic reorganization findings and the development of PRL. Different cortical adaptation mechanisms may be active in AMD and juvenile forms of macular degeneration (JMD). Studies on retinotopic reorganization in patients with AMD and a comparison between the AMD and JMD patients are needed. These will lead to a better understanding of the degree of plasticity of the adult visual brain and also the cortical adaptation that is related to AMD. A better understanding of the adaptive cortical changes associated with AMD will help in designing better intervention and rehabilitation methods.
Acknowledgments
The preparation of this paper was supported by a University of Minnesota Doctoral Dissertation Fellowship to S.-H. Cheung and NIH Grant EY02934 to G.E. Legge. We thank Susana Chung and Sheng He for reviewing an earlier draft of this paper. We thank Sheng He for detailed discussions on the topic, and Daniel Kersten and Paul Schrater for their comments on a presentation of an earlier version of this paper.
Footnotes
The relationship between M and E has often been described by a power function, which often misrepresented the cortical magnification factor at 0 deg eccentricity.
It should be noted that the untrained reading speeds of about 10 wpm in the Nilsson et al. (2003) study were lower than those reported in other AMD studies (e.g. Legge et al., 1985e.g. , 1992; Fletcher et al., 1999). The discrepancy may be due to the different methods used to measure reading speed. Nilsson et al. had their patients read printed text with high-power magnifiers (10–15×) and very short viewing distances (1.7–2.5 cm), which may have contributed to slower reading. Legge et al. (1985) used a drifting-text method in which speed was not limited by the manual demands of a hand-held magnifier.
References
- Adams DL, Horton JC. Shadows cast by retinal blood vessels mapped in primary visual cortex. Science. 2002;298:572–576. doi: 10.1126/science.1074887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DL, Horton JC. A precise retinotopic map of primate striate cortex generated from the representation of angioscotomas. Journal of Neuroscience. 2003;23:3771–3789. doi: 10.1523/JNEUROSCI.23-09-03771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akutsu H, Legge GE, Ross JA, Schuebel K. Psychophysics of reading. X. Effects of age-related changes in vision. Journal of Gerontology: Psychological Sciences. 1991;46:325–331. doi: 10.1093/geronj/46.6.p325. [DOI] [PubMed] [Google Scholar]
- Altpeter E, Mackeben M, Trauzettel-Klosinski S. The importance of sustained attention for patients with maculopathies. Vision Research. 2000;40:1539–1547. doi: 10.1016/s0042-6989(00)00059-6. [DOI] [PubMed] [Google Scholar]
- Ambati J, Ambati BK, Yoo SH, anchulev S, Adamis AP. Age-related macular degeneration: Etiology, pathogenesis, and therapeutic strategies. Survey of Ophthalmology. 2003;48:257–293. doi: 10.1016/s0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- Anderson DR. Standard perimetry. Ophthalmology Clinics of North America. 2003;16:205–212. doi: 10.1016/s0896-1549(03)00005-1. [DOI] [PubMed] [Google Scholar]
- Armaly MF. The size and location of the normal blind spot. Archives of Ophthalmology. 1969;81:192–201. doi: 10.1001/archopht.1969.00990010194009. [DOI] [PubMed] [Google Scholar]
- Azzopardi P, Cowey A. Preferential representation of the fovea in the primary visual cortex. Nature. 1993;361:719–721. doi: 10.1038/361719a0. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magnetic Resonance in Medicine. 1992;25:390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- Baseler HA, Brewer AA, Sharpe LT, Morland AB, Jägle H, Wandell BA. Reorganization of human cortical maps caused by inherited photoreceptor abnormalities. Nature Neuroscience. 2002;5:364–370. doi: 10.1038/nn817. [DOI] [PubMed] [Google Scholar]
- Bek T, Lund-Andersen H. The influence of stimulus size on perimetric detection of small scotomata. Graefe’s Archive for Clinical and Experimental Ophthalmology. 1989;227:531–534. doi: 10.1007/BF02169446. [DOI] [PubMed] [Google Scholar]
- Bender MB, Furlow LT. Visual disturbances produced by bilateral lesions of the occipital lobes with central scotomas. Archives of Neurology and Psychiatry. 1945;53:165–170. [Google Scholar]
- Bergeron A, Matsuo S, Guitton D. Superior colliculus encodes distance to target, not saccade amplitude, in multi-step gaze shifts. Nature Neuroscience. 2003;6:404–413. doi: 10.1038/nn1027. [DOI] [PubMed] [Google Scholar]
- Boothe RG, Dobson V, Teller DY. Postnatal development of vision in human and nonhuman primates. Annual Review of Neuroscience. 1985;8:495–545. doi: 10.1146/annurev.ne.08.030185.002431. [DOI] [PubMed] [Google Scholar]
- Bowers AR. Eye movements and reading with plus-lens magnifiers. Optometry and Vision Science. 2000;77:25–33. doi: 10.1097/00006324-200001000-00010. [DOI] [PubMed] [Google Scholar]
- Brindley GS, Lewin WS. The sensations produced by electrical stimulation of the visual cortex. Journal of Physiology. 1968;196:479–493. doi: 10.1113/jphysiol.1968.sp008519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MM, Brown GC, Sharma S, Landy J, Bakal J. Quality of life with visual acuity loss from diabetic retinopathy and age-related macular degeneration. Archives of Ophthalmology. 2002;120:481–484. doi: 10.1001/archopht.120.4.481. [DOI] [PubMed] [Google Scholar]
- Calford MB, Wang C, Taglianetti V, Waleszczyk WJ, Burke W, Dreher B. Plasticity in adult cat visual cortex (area 17) following circumscribed monocular lesions of all retinal layers. Journal of Physiology. 2000;524.2:587–602. doi: 10.1111/j.1469-7793.2000.t01-1-00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman GJ, Drury HA, Van Essen DC. Computational methods for reconstructing and unfolding the cerebral cortex. Cerebral Cortex. 1995;5:506–517. doi: 10.1093/cercor/5.6.506. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Talgar CP, Cameron EL. Characterizing visual performance fields: Effects of transient covert attention, spatial frequency, eccentricity, task and set size. Spatial Vision. 2001;15:61–75. doi: 10.1163/15685680152692015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casco C, Campana G, Grieco A, Musetti S, Perrone S. Hyper-vision in a patient with central and paracentral vision loss reflects cortical reorganization. Visual Neuroscience. 2003;20:501–510. doi: 10.1017/s0952523803205046. [DOI] [PubMed] [Google Scholar]
- Chang TS, Hay D, Courtright P. Age-related macular degeneration in Chinese-Canadians. Canadian Journal of Ophthalmology. 1999;34:266–271. [PubMed] [Google Scholar]
- Chino YM. Adult plasticity in the visual system. Canadian Journal of Physiology and Pharmacology. 1995;73:1323–1338. doi: 10.1139/y95-187. [DOI] [PubMed] [Google Scholar]
- Chino YM, Kaas JH, Smith ELIII, Langston AL, Cheng H. Rapid reorganization of cortical maps in adult cats following restricted deafferentation in retina. Vision Research. 1992;32:789–796. doi: 10.1016/0042-6989(92)90021-a. [DOI] [PubMed] [Google Scholar]
- Chino YM, Smith ELIII, Kaas JH, Sasaki Y, Cheng H. Receptive-field properties of deafferentated visual cortical neurons after topographic map reorganization in adult cats. Journal of Neuroscience. 1995;15:2417–2433. doi: 10.1523/JNEUROSCI.15-03-02417.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopdar A, Chakravarthy U, Verma D. Age related macular degeneration. British Medical Journal. 2003;326:485–488. doi: 10.1136/bmj.326.7387.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung STL, Legge GE, Cheung SH. Letter-recognition and reading speed in peripheral vision benefit from perceptual learning. Vision Research. 2004;44:695–709. doi: 10.1016/j.visres.2003.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossland MD, Rubin GS. The use of an infrared eye-tracker to measure fixation stability. Optometry and Vision Science. 2002;79:735–739. doi: 10.1097/00006324-200211000-00011. [DOI] [PubMed] [Google Scholar]
- Cummings RW, Whittaker SG, Watson GR, Budd JM. Scanning characters and reading with a central scotoma. American Journal of Optometry and Physiological Optics. 1985;62:833–843. doi: 10.1097/00006324-198512000-00004. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. Journal of Cognitive Neuroscience. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Daniel PM, Whitteridge D. The representation of the visual field on the cerebral cortex in monkeys. Journal of Physiology (Paris) 1961;159:203–221. doi: 10.1113/jphysiol.1961.sp006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith C, Gilbert CD. Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature. 1994;368:737–740. doi: 10.1038/368737a0. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Gilbert CD. Topographic reorganization in the striate cortex of the adult cat and monkey is cortically mediated. Journal of Neuroscience. 1995;15:1631–1647. doi: 10.1523/JNEUROSCI.15-03-01631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarlo DK, Scilley K, Wells J, Owsley C. Driving habits and health-related quality of life in patients with age-related maculopathy. Optometry and Vision Science. 2003;80:207–213. doi: 10.1097/00006324-200303000-00010. [DOI] [PubMed] [Google Scholar]
- Déruaz A, Matter M, Whatham AR, Goldschmidt M, Duret F, ssenhuth M, Safran AB. Can fixation instability improve text perception during eccentric fixation in patients with central scotomas? British Journal of Ophthalmology. 2004;88:461–463. doi: 10.1136/bjo.2003.025601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Déruaz A, Whatham AR, Mermoud C, Safran AB. Reading with multiple preferred retinal loci: Implications for training a more efficient reading strategy. Vision Research. 2002;42:2947–2957. doi: 10.1016/s0042-6989(02)00354-1. [DOI] [PubMed] [Google Scholar]
- DeYoe EA, Bandettini P, Neitz J, Miller D, Winans P. Functional magnetic resonance imaging (FMRI) of the human brain. Journal of Neuroscience Methods. 1994;54:171–187. doi: 10.1016/0165-0270(94)90191-0. [DOI] [PubMed] [Google Scholar]
- DeYoe EA, Carman GJ, Bandettini P, Glickman S, Wieser J, Cox R, Miller D, Neitz J. Mapping striate and extra-striate visual areas in human cerebral cortex. Proceedings of the National Academy of Sciences of the U.S.A. 1996;93:2382–2386. doi: 10.1073/pnas.93.6.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury HA, Van Essen DC, Anderson CH, Lee CW, Coogan TA, Lewis JW. Computerized mappings of the cerebral cortex: A multiresolution flattening method and a surface-based coordinate system. Journal of Cognitive Neuroscience. 1996;8:1–28. doi: 10.1162/jocn.1996.8.1.1. [DOI] [PubMed] [Google Scholar]
- DuBois RM, Cohen MS. Spatiotopic organization in human superior colliculus observed with fMRI. Neuroimage. 2000;12:63–70. doi: 10.1006/nimg.2000.0590. [DOI] [PubMed] [Google Scholar]
- Duncan RO, Boynton GM. Cortical magnification within human primary visual cortex correlates with acuity thresholds. Neuron. 2003;38:659–671. doi: 10.1016/s0896-6273(03)00265-4. [DOI] [PubMed] [Google Scholar]
- Dunzhu S, Wang FS, Courtright P, Liu L, Tenzing C, Noer tjojo K, Wilkie A, Santangelo M, Bassett KL. Blindness and eye diseases in Tibet: Findings from a randomised, population based survey. British Journal of Ophthalmology. 2003;87:1443–1448. doi: 10.1136/bjo.87.12.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cerebral Cortex. 1997;7:181–192. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- Engel SA, Rumelhart DE, Wandell BA, Lee AT, Glover GH, Chichilnisky EJ, Shadlen MN. fMRI of human visual cortex. Nature. 1994;369:525. doi: 10.1038/369525a0. [DOI] [PubMed] [Google Scholar]
- Ergun E, Maár N, Radner W, Barbazetto I, Schmidt-Erfurth U, Stur M. Scotoma size and reading speed in patients with subfoveal occult choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2003;110:65–69. doi: 10.1016/s0161-6420(02)01566-x. [DOI] [PubMed] [Google Scholar]
- Eye Disease Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Archives of Ophthalmology. 2004a;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- Eye Disease Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Archives of Ophthalmology. 2004b;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- Fahle M, Schmid M. Naso-temporal asymmetry of visual perception and of the visual cortex. Vision Research. 1988;28:293–300. doi: 10.1016/0042-6989(88)90157-5. [DOI] [PubMed] [Google Scholar]
- Fine EM, Peli E. Scrolled and rapid serial visual presentation texts are read at similar rates by the visually impaired. Journal of the Optical Society of America A: Optics, Image Sciences, and Vision. 1995;12:2286–2292. doi: 10.1364/josaa.12.002286. [DOI] [PubMed] [Google Scholar]
- Fine EM, Rubin GS. Reading with simulated scotomas: Attending to the right is better than attending to the left. Vision Research. 1999;39:1039–1048. doi: 10.1016/s0042-6989(98)00208-9. [DOI] [PubMed] [Google Scholar]
- Fletcher DC, Schuchard RA. Preferred retinal loci relationship to macular scotomas in a low-vision population. Ophthalmology. 1997;104:632–638. doi: 10.1016/s0161-6420(97)30260-7. [DOI] [PubMed] [Google Scholar]
- Fletcher DC, Schuchard RA, Livingstone CL, Crane WG, Hu SY. Scanning laser ophthalmoscope macular perimetry and applications for low vision rehabilitation clinicians. Low Vision and Vision Rehabilitation. 1994;7:257–265. [Google Scholar]
- Fletcher DC, Schuchard RA, Watson G. Relative locations of macular scotomas near the PRL: Effect on low vision reading. Journal of Rehabilitation Research and Development. 1999;36:356–364. [PubMed] [Google Scholar]
- Fox PT, Miezin FM, Allman JM, Van Essen DC, Raichle ME. Retinotopic organization of human visual cortex mapped with positron-emission tomography. Journal of Neuroscience. 1987;7:913–922. doi: 10.1523/JNEUROSCI.07-03-00913.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Mintun MA, Raichle ME, Herscovitch P. A noninvasive approach to quantitative functional brain mapping with H215O and positron emission tomography. Journal of Cerebral Blood Flow and Metabolism. 1984;4:329–333. doi: 10.1038/jcbfm.1984.49. [DOI] [PubMed] [Google Scholar]
- Fox PT, Mintun MA, Raichle ME, Miezin FM, Allman JM, Van Essen DC. Mapping human visual cortex with positron emission tomography. Nature. 1986;323:806–809. doi: 10.1038/323806a0. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proceedings of the National Academy of Sciences of the U.S.A. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- F uchs, W. (1938). Pseudo-fovea. In A Source Book of Gestalt Psychology, ed. & trans. Ellis, W.D., pp. 357–365. London: Kegan Paul. (Original work published 1922)
- Fujii GY, De Juan E, Jr, Humayun MS, Sunness JS, Chang TS, Rossi JV. Characteristics of visual loss by scanning laser ophthalmoscope microperimetry in eyes with subfoveal choroidal neovascularization secondary to age-related macular degeneration. American Journal of Ophthalmology. 2003;136:1067–1078. doi: 10.1016/s0002-9394(03)00663-9. [DOI] [PubMed] [Google Scholar]
- Gilbert CD. Adult cortical dynamics. Physiological Reviews. 1998;78:467–485. doi: 10.1152/physrev.1998.78.2.467. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Receptive field dynamics in adult primary visual cortex. Nature. 1992;356:150–152. doi: 10.1038/356150a0. [DOI] [PubMed] [Google Scholar]
- Girard P, Hupé JM, Bullier J. Feedforward and feedback connections between areas V1 and V2 of the monkey have similar rapid conduction velocities. Journal of Neurophysiology. 2001;85:1328–1331. doi: 10.1152/jn.2001.85.3.1328. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Wurtz RH. Activity of superior colliculus in behaving monkey. I. Visual receptive fields of single neurons. Journal of Neurophysiology. 1972a;35:542–559. doi: 10.1152/jn.1972.35.4.542. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Wurtz RH. Activity of superior colliculus in behaving monkey. II. Effect of attention on neuronal responses. Journal of Neurophysiology. 1972b;35:560–574. doi: 10.1152/jn.1972.35.4.560. [DOI] [PubMed] [Google Scholar]
- Guez JE, Le Gargasson JF, Rigaudière F, O’Regan JK. Is there a systematic location for the pseudo-fovea in patients with central scotoma? Vision Research. 1993;33:1271–1279. doi: 10.1016/0042-6989(93)90213-g. [DOI] [PubMed] [Google Scholar]
- Hassan SE, Lovie-Kitchin JE, Woods RL. Vision and mobility performance of subjects with age-related macular degeneration. Optometry and Vision Science. 2002;79:697–707. doi: 10.1097/00006324-200211000-00007. [DOI] [PubMed] [Google Scholar]
- He S, Cavanagh P, Intriligator J. Attentional resolution and the locus of visual awareness. Nature. 1996;383:334–337. doi: 10.1038/383334a0. [DOI] [PubMed] [Google Scholar]
- Heinen SJ, Skavenski AA. Recovery of visual responses in foveal V1 neurons following bilateral foveal lesions in adult monkey. Experimental Brain Research. 1991;83:670–674. doi: 10.1007/BF00229845. [DOI] [PubMed] [Google Scholar]
- Holmes G. Disturbances of vision by cerebral lesions. British Journal of Ophthalmology. 1918;2:353–383. doi: 10.1136/bjo.2.7.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G. The cortical localization of vision. British Medical Journal. 1919;2:193–199. doi: 10.1136/bmj.2.3059.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G. The organization of the visual cortex in man. Proceedings of the Royal Society B (London) 1945;132:348–361. [Google Scholar]
- Holmes G, Lister WT. Disturbances of vision from cerebral lesions, with special reference to the cortical representation of the macula. Brain. 1916;39:34–73. [PMC free article] [PubMed] [Google Scholar]
- Horton JC, Hoyt WF. The representation of the visual field in human striate cortex. A revision of the classic Holmes map. Archives of Ophthalmology. 1991;109:816–824. doi: 10.1001/archopht.1991.01080060080030. [DOI] [PubMed] [Google Scholar]
- Hsu WM, Cheng CY, Liu JH, Tsai SY, Chou P. Prevalence and causes of visual impairment in an elderly Chinese population in Taiwan: The Shihpai Eye Study. Ophthalmology. 2004;111:62–69. doi: 10.1016/j.ophtha.2003.05.011. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Anatomical demonstration of columns in the monkey striate cortex. Nature. 1969;221:747–750. doi: 10.1038/221747a0. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. Journal of Physiology. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa NP, Trachtenberg JT, Chapman B, Zahs KR, Stryker MP. The critical period for ocular dominance plasticity in the Ferret’s visual cortex. Journal of Neuroscience. 1999;19:6965–6978. doi: 10.1523/JNEUROSCI.19-16-06965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Collins CE. Anatomic and functional reorganization of somatosensory cortex in mature primates after peripheral nerve and spinal cord injury. Advances in Neurology. 2003;93:87–95. [PubMed] [Google Scholar]
- Kaas JH, Krubitzer LA, Chino YM, Langston AL, Polley EH, Blair N. Reorganization of retinotopic cortical maps in adult mammals after lesions of the retina. Science. 1990;248:229–231. doi: 10.1126/science.2326637. [DOI] [PubMed] [Google Scholar]
- Kelly JP, Weiss AH, Zhou Q, Schmode S, Dreher AW. Imaging a child’s fundus without dilation using a handheld confocal scanning laser ophthalmoscope. Archives of Ophthalmology. 2003;121:391–396. doi: 10.1001/archopht.121.3.391. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ. Recasting the smooth pursuit eye movement system. Journal of Neurophysiology. 2004;91:591–603. doi: 10.1152/jn.00801.2003. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Cheng H-M, Brady TJ, Rosen BR. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proceedings of the National Academy of Sciences of the U.S.A. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legge GE, Ross JA, senberg LM, LaMay JM. Psychophysics of reading. XII. Clinical predictors of low-vision reading speed. Investigative Ophthalmology and Visual Science. 1992;33:677–687. [PubMed] [Google Scholar]
- Legge GE, Rubin GS, Pelli DG, Schleske MM. Psychophysics of reading. II. Low vision. Vision Research. 1985;25:253–265. doi: 10.1016/0042-6989(85)90118-x. [DOI] [PubMed] [Google Scholar]
- Lei H, Schuchard RA. Using two preferred retinal loci for different lighting conditions in patients with central scotomas. Investigative Ophthalmology and Visual Science. 1997;38:1812–1818. [PubMed] [Google Scholar]
- Levi DM, Klein SA, Aitsebaomo AP. Vernier acuity, crowding and cortical magnification. Vision Research. 1985;25:963–977. doi: 10.1016/0042-6989(85)90207-x. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annual Review of Physiology. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Lott LA, Schneck ME, Haegerström-Portnoy G, Brabyn JA, Gildengorin GL, West CG. Reading performance in older adults with good acuity. Optometry and Vision Science. 2001;78:316–324. doi: 10.1097/00006324-200105000-00015. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Kaas JH. Connectional evidence for dorsal and ventral V3, and other extrastriate areas in the prosimian primate, Galago garnetti. Brain, Behavior and Evolution. 2002;59:114–129. doi: 10.1159/000064159. [DOI] [PubMed] [Google Scholar]
- Markowitz SM, Muller C. Macular perimetry in low vision. Canadian Journal of Ophthalmology. 2004;39:56–60. doi: 10.1016/s0008-4182(04)80053-x. [DOI] [PubMed] [Google Scholar]
- McFadzean RM, Hadley DM, Condon BC. The representation of the visual field in the occipital striate cortex. Neuro-Ophthalmology. 2002;27:55–78. doi: 10.1136/bjo.78.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morland AB, Baseler HA, Hoffmann MB, Sharpe LT, Wandell BA. Abnormal retinotopic representations in human visual cortex revealed by fMRI. Acta Psychologica. 2001;107:229–247. doi: 10.1016/s0001-6918(01)00025-7. [DOI] [PubMed] [Google Scholar]
- Munoz DP. Saccadic eye movements: Overview of neural circuitry. Progress in Brain Research. 2002;140:89–96. doi: 10.1016/S0079-6123(02)40044-1. [DOI] [PubMed] [Google Scholar]
- Nilsson UL, Frennesson C, Nilsson SEG. Location and stability of a newly established eccentric retinal locus suitable for reading, achieved through training of patients with a dense central scotoma. Optometry and Vision Science. 1998;75:873–878. doi: 10.1097/00006324-199812000-00009. [DOI] [PubMed] [Google Scholar]
- Nilsson UL, Frennesson C, Nilsson SEG. Patients with AMD and a large absolute central scotoma can be trained successfully to use eccentric viewing, as demonstrated in a scanning laser ophthalmoscope. Vision Research. 2003;43:1777–1787. doi: 10.1016/s0042-6989(03)00219-0. [DOI] [PubMed] [Google Scholar]
- Obata S, Obata J, Das A, Gilbert CD. Molecular correlates of topographic reorganization in primary visual cortex following retinal lesions. Cerebral Cortex. 1999;9:238–248. doi: 10.1093/cercor/9.3.238. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim S-G, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proceedings of the National Academy of Sciences of the U.S.A. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantev C, Oostenveld R, Engelien A, Ross B, Roberts LE, Hoke M. Increased auditory cortical representation in musicians. Nature. 1998;392:811–814. doi: 10.1038/33918. [DOI] [PubMed] [Google Scholar]
- Petre KL, Hazel CA, Fine EM, Rubin GS. Reading with eccentric fixation is faster in inferior visual field than in left visual field. Optometry and Vision Science. 2000;77:34–39. doi: 10.1097/00006324-200001000-00011. [DOI] [PubMed] [Google Scholar]
- Rovamo J, Virsu V. An estimation and application of the human cortical magnification factor. Experimental Brain Research. 1979;37:495–510. doi: 10.1007/BF00236819. [DOI] [PubMed] [Google Scholar]
- Rovamo J, Virsu V, Näsänen R. Cortical magnification factor predicts the photopic contrast sensitivity of peripheral vision. Nature. 1978;271:54–56. doi: 10.1038/271054a0. [DOI] [PubMed] [Google Scholar]
- Rovner BW, Casten RJ. Activity loss and depression in age-related macular degeneration. American Journal of Geriatric Psychiatry. 2002;10:305–310. [PubMed] [Google Scholar]
- Sansbury RV, Skavenski AA, Haddad GM, Steinman RM. Normal fixation of eccentric targets. Journal of the Optical Society of America. 1973;63:612–614. doi: 10.1364/josa.63.000612. [DOI] [PubMed] [Google Scholar]
- Schneider KA, Richter MC, Kastner S. Retinotopic organization and functional subdivisions of the human lateral geniculate nucleus: A high-resolution functional magnetic resonance imaging study. Journal of Neuroscience. 2004;24:8975–8985. doi: 10.1523/JNEUROSCI.2413-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchard RA, Fletcher DC. Preferred retinal locus. A review with applications in low vision rehabilitation. Low Vision and Vision Rehabilitation. 1994;7:243–256. [Google Scholar]
- Schuchard RA, Naseer S, de Castro K. Characteristics of AMD patients with low vision receiving visual rehabilitation. Journal of Rehabilitation Research and Development. 1999;36:294–302. [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RBH. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Sharp PF, Manivannan A. The scanning laser ophthalmoscope. Physics in Medicine and Biology. 1997;42:951–966. doi: 10.1088/0031-9155/42/5/014. [DOI] [PubMed] [Google Scholar]
- Sharp PF, Manivannan A, Xu A, Forrester JV. The scanning laser ophthalmoscope–A review of its role in bioscience and medicine. Physics in Medicine and Biology. 2004;49:1085–1096. doi: 10.1088/0031-9155/49/7/001. [DOI] [PubMed] [Google Scholar]
- Sunness JS, Applegate CA, Haselwood D, Rubin GS. Fixation patterns and reading rates in eyes with central scotomas from advanced atrophic age-related macular degeneration and Stargardt disease. Ophthalmology. 1996;103:1458–1466. doi: 10.1016/s0161-6420(96)30483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunness JS, Gonzalez-Baron J, Applegate CA, Bressler NM, Tian Y, Hawkins B, Barron Y, Bergman A. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology. 1999;106:1768–1779. doi: 10.1016/S0161-6420(99)90340-8. [DOI] [PubMed] [Google Scholar]
- Sunness JS, Liu T, Yantis S. Retinotopic mapping of the visual cortex using functional magnetic resonance imaging in a patient with central scotomas from atrophic macular degeneration. Ophthalmology. 2004;111:1595–1598. doi: 10.1016/j.ophtha.2003.12.050. [DOI] [PubMed] [Google Scholar]
- Sunness JS, Schuchard RA, Shen N, Rubin GS, Dagnelie G, Haselwood DM. Landmark-driven fundus perimetry using the scanning laser ophthalmoscope. Investigative Ophthalmology and Visual Science. 1995;36:1863–1874. [PMC free article] [PubMed] [Google Scholar]
- Timberlake GT, Mainster MA, Peli E, Augliere RA, Essock EA, Arend LE. Reading with a macular scotoma. I. Retinal location of scotoma and fixation area. Investigative Ophthalmology and Visual Science. 1986;27:1137–1147. [PubMed] [Google Scholar]
- Timberlake GT, Mainster MA, Webb RH, Hughes GW, Trempe CL. Retinal localization of scotomata by scanning laser ophthalmoscopy. Investigative Ophthalmology and Visual Science. 1982;22:91–97. [PubMed] [Google Scholar]
- Tootell RBH, Switkes E, Silverman MS, Hamilton SL. Functional anatomy of macaque striate cortex. II. Retinotopic organization. Journal of Neuroscience. 1988;8:1531–1568. doi: 10.1523/JNEUROSCI.08-05-01531.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turano KA, Broman AT, Bandeen-Roche K, Munoz B, Rubin GS, West SK, the SEE Project Team. Association of visual field loss and mobility performance in older adults: Salisbury Eye Evaluation Study. Optometry and Vision Science. 2004;81:298–307. doi: 10.1097/01.opx.0000134903.13651.8e. [DOI] [PubMed] [Google Scholar]
- Virsu V, Rovamo J. Visual resolution, contrast sensitivity, and the cortical magnification factor. Experimental Brain Research. 1979;37:475–494. doi: 10.1007/BF00236818. [DOI] [PubMed] [Google Scholar]
- Wandell BA, Chial S, Backus BT. Visualization and measurement of the cortical surface. Journal of Cognitive Neuroscience. 2000;12:739–752. doi: 10.1162/089892900562561. [DOI] [PubMed] [Google Scholar]
- Wandell BA, Engel SA, Hel-Or HZ. Creating images of the flattened cortical sheet. Investigative Ophthalmology and Visual Science. 1996;37:S1081. [Google Scholar]
- Webb RH, Hughes GW. Scanning laser ophthalmoscope. IEEE Transactions on Biomedical Engineering. 1981;28:488–492. doi: 10.1109/TBME.1981.324734. [DOI] [PubMed] [Google Scholar]
- Webb RH, Hughes GW, Pomerantzeff O. Flying spot TV ophthalmoscope. Applied Optics. 1980;19:2991–2997. doi: 10.1364/AO.19.002991. [DOI] [PubMed] [Google Scholar]
- Wertheim T. Peripheral visual acuity: Th. Wertheim, trans. Dunsky, I.L. American Journal of Optometry and Physiological Optics. 1980;57:915–924. (Original work published 1891) [PubMed] [Google Scholar]
- Westcott MC, Garway-Heath DF, Fitzke FW, Kamal D, Hitchings RA. Use of high spatial resolution perimetry to identify scotomata not apparent with conventional perimetry in the nasal field of glaucomatous subjects. British Journal of Ophthalmology. 2002;86:761–766. doi: 10.1136/bjo.86.7.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker SG, Budd J, Cummings RW. Eccentric fixation with macular scotoma. Investigative Ophthalmology and Visual Science. 1988;29:268–278. [PubMed] [Google Scholar]
- W ilson, H.R., Levi, D., Maffei, L., Rovamo, J. & DeValois, R. (1990). The perception of form: Retina to striate cortex. In Visual Perception: The Neurophysiological Foundations, ed. Spillmann, L. & Werner, J.S., Chap. 10. San Diego, California: Academic Press.
- Wurtz RH, Goldberg ME. Activity of superior colliculus in behaving monkey. III. Cells discharging before eye movements. Journal of Neurophysiology. 1972a;35:575–586. doi: 10.1152/jn.1972.35.4.575. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME. Activity of superior colliculus in behaving monkey. IV. Effects of lesions on eye movements. Journal of Neurophysiology. 1972b;35:587–596. doi: 10.1152/jn.1972.35.4.587. [DOI] [PubMed] [Google Scholar]


