Abstract
Recombinant preparations of the cytokine interferon (IFN)-α are increasingly used to treat a number of medical conditions, including chronic viral hepatitis and several malignancies. Although frequently effective, IFNα induces a variety of neuropsychiatric adverse effects, including an acute confusional state that develops rapidly after initiation of high-dose IFNα, a depressive syndrome that develops more slowly over weeks to months of treatment, and manic conditions most often characterised by extreme irritability and agitation, but also occasionally by euphoria. Acute IFNα-induced confusional states are typically characterised by disorientation, lethargy, somnolence, psychomotor retardation, difficulties with speaking and writing, parkinsonism and psychotic symptoms. Strategies for managing delirium should be employed, including treatment of contributing medical conditions, use of either typical or atypical antipsychotic agents and avoidance of medications likely to worsen mental status. Significant depressive symptoms occur in 21–58% of patients receiving IFNα, with symptoms typically manifesting over the first several months of treatment. The most replicated risk factor for developing depression is the presence of mood and anxiety symptoms prior to treatment. Other potential, but less frequently replicated, risk factors include a past history of major depression, being female and increasing IFNα dosage and treatment duration. The available data support two approaches to the pharmacological management of IFNα-induced depression: antidepressant pretreatment or symptomatic treatment once IFNα has been initiated. Pretreatment might be best reserved for patients already receiving antidepressants or for patients who endorse depression or anxiety symptoms of mild or greater severity prior to therapy. Several recent studies demonstrate that antidepressants effectively treat IFNα-induced depression once it has developed, allowing the vast majority of subjects to complete treatment successfully. Recent data suggest that IFNα-induced depression may be composed of two overlapping syndromes: a depression-specific syndrome characterised by mood, anxiety and cognitive complaints, and a neurovegetative syndrome characterised by fatigue, anorexia, pain and psychomotor slowing. Depression-specific symptoms are highly responsive to serotonergic antidepressants, whereas neurovegetative symptoms are significantly less responsive to these agents. These symptoms may he more effectively treated by agents that modulate catecholaminergic functioning, such as combined serotonin-noradrenaline (norepinephrine) antidepressants, bupropion, psychostimulants or modafinil. Additional factors to consider in selecting an antidepressant include potential drug-drug interactions and adverse effect profile. Finally. IFNα appears capable of inducing manic symptoms. Mania, especially when severe, is a clinical emergency. When this occurs, IFNα and antidepressants should be stopped, an emergency psychiatric consultation should he obtained, and treatment with a mood stabiliser should be initiated.
Over the last several decades, recombinant preparations of the cytokine interferon (IFN)-α have played an increasingly important role in the treatment of a number of medical conditions, including chronic viral hepatitis and several malignancies. Although frequently of benefit in each of these conditions, IFNα has been repeatedly observed to cause a variety of neuropsychiatric adverse effects in many patients who receive treatment. In addition to their detrimental effect on quality of life, these adverse effects increase the risk of poor treatment outcome, given their frequent association with dosage reduction and/or treatment discontinuation.[1–3] Fortunately, however, increasing evidence suggests that appropriate recognition and management of IFNα-induced neuropsychiatric adverse effects allows the majority of patients to continue receiving treatment.[4–6] Combined with increased recognition of the ubiquity of neuropsychiatric adverse effects, these new data on potential treatment strategies have prompted increased recognition among healthcare providers of the importance of learning to manage IFNα-induced psychiatric disturbance effectively. In an effort to aid this process, we review the prevalence, clinical presentation and treatment of IFNα-induced neuropsychiatric adverse effects. The article focuses on delirium and related cognitive/psychomotor impairments most often seen during treatment with high-dose IFNα and on mood and related symptoms (including anxiety and fatigue) that are frequently observed in patients receiving both high- and low-dose regimens of IFNα. Risk factors for the development of depression during IFNα treatment are reviewed, and strategies to prevent and/or treat neuropsychiatric adverse effects are discussed. Finally, increasing evidence suggests that IFNα is capable of inducing manic symptoms and occasionally full-blown manic episodes. Although mania and hypomania are less frequent than depressive presentations, we feel that the potential seriousness of these conditions warrants a more extended diagnostic and management discussion than would be justified on the basis of their prevalence rate alone.
1. Interferon (IFN)-α-Induced Neuropsychiatric Adverse Effects
1.1 Overview
IFNα has been repeatedly reported to induce a panoply of neuropsychiatric adverse effects, whether used in combination with the antiviral agent ribavirin for chronic hepatitis C virus (HCV) infection, or for the treatment of several malignancies, including malignant melanoma and renal cell carcinoma. Although depression is the most widely recognised of these adverse effects, the available literature suggests that IFNα is capable of inducing several distinct syndromes that can be meaningfully identified on the basis of clinical presentation, developmental time course and treatment response.[7] These syndromes include: (i) an acute confusional state that develops rapidly after the onset of high-dose IFNα administered by either intracerebroventricular (ICV) or intravenous (IV) routes: (ii) a depressive syndrome that develops more slowly over weeks to months of treatment; and, less commonly, (iii) manic conditions most often characterised by extreme irritability and agitation, but also occasionally by euphoria. Each of these syndromes, and their treatment, is discussed separately below.
1.2 IFNα-lnduced Acute Confusional States
Many patients receiving high doses of IFNα, either alone or in combination with other cytokines such as interleukin (IL)-2, for the treatment of cancer develop an acute confusional state frequently consisting of disorientation, lethargy, somnolence, psychomotor retardation, difficulties with speaking and writing, and psychotic symptoms, such as hallucinations.[8–16] parkinsonism has been observed in up to one-third of patients receiving ICV IFNα for cancer metastatic to the brain.[8] Seizures have also been reported.[8] These symptoms typically resolve with discontinuation of treatment, but may persist in some patients.[7] While the time course of these acute states strongly implicates IFNα as the causative agent, it should be noted that underlying illnesses (i.e. cancer metastatic to the brain) and concomitant treatments, such as brain radiation, likely contribute to symptom development. Consistent with this, one study of high-dose IFNα found that all patients who developed acute confusional/psychotic slates had previously unsuspected, pre-existing neurological abnormalities upon subsequent evaluation.[16] Other factors that have been associated with an increased risk of delirium development include severity of underlying illness process, pre-existing brain injury and older age.[3,7] Fortunately, given the large population of patients receiving IFNα therapy for HCV infection, acute confusional states appear to be infrequent in this treatment group, even at doses significantly higher than currently used.[7]
As discussed below, significant progress has been made in our understanding of the pathways by which IFNα may predispose toward the development of depressive symptoms. Far less is known about the potential mechanisms for IFNα-induced acute confusional states/delirium. Nonetheless, IFNα is known to stimulate CNS opioid receptors.[18] Supporting a possible role for the opioid system in IFNα-induced delirium are the frequency with which opiate medications produce delirium in the medically ill in general and the effectiveness of opioid antagonists (i.e. naloxone/naltrexone) in reversing IFNα-induced analgesia and fever in rodents and memory and concentration disturbances in humans.[19,20] Other potential contributors to IFNα-induced delirium include decreased dopamine turnover in the striatum and increased release in cortical areas, a pattern that might partially explain the parkinsonian and psychotic symptoms often reported during the acute confusional state.[18] Finally, IFNα and other inflammatory mediators induced by IFNα appear capable of damaging neuronal integrity via several pathways, including the induction of cerebral oedema, the production of free radicals and the promotion of glutamate release with resultant excitotoxic cell death.[18,21,22] IFNα has also been shown in tissue cultures to interfere with glutamate-mediated postsynaptic potentials and long-term potentiation, processes that are considered central to memory and learning.[23]
No published data are available to guide the clinician in the management of IFNα-induced delirium and related acute symptoms. Hence, our recommendations in this regard should be taken as provisional. However, it is logical to assume that strategies used to manage delirium in the medically ill are relevant. Potentially treatable medical contributions should be evaluated and treated. Concomitant medications that might induce or worsen delirium, including anticholinergic agents such as diphenhydramine, should be discontinued, and in general, the patient’s medication burden should be minimised as much as possible. Both conventional and newer atypical antipsychotics have been shown to improve mental status swiftly in many delirious patients and should probably be the treatment of choice in patients with IFNα-induced acute confusional states.[24,25] Consistent with this, olanzapine has been found useful in the treatment of IFNα-induced psychosis.[26] However, a possible concern with the use of antipsychotic agents for the treatment of IFNα-induced delirium is the potential of these agents to cause neutropenia, a condition already common in patients receiving IFNα. In contradistinction to antipsychotics, benzodiazepines, while occasionally useful for agitation, do not appear to improve delirium and may actually worsen mental functioning in medically ill patients.[25] In patients who develop parkinsonian symptoms, levodopa has been reported to be of benefit.[27–29] Finally, environmental manipulations that help reinforce orientation, such as contact with caregivers, well lit environments, access to a window with an outside view and maintenance of circadian light/dark patterns are frequently of benefit, especially in hospitalised patients with delirium.
1.3 IFNα-Induced Depression: Prevalence, Clinical Presentation and Risk Factors
1.3.1 Prevalence
Of the many studies that have addressed the issue of whether IFNα induces depression, the vast majority suggest that treatment with IFNα represents a significant risk factor for the development of both depressive symptoms and a syndrome that meets criteria for major depression as defined by the DSM-IV.[30] (Although by strict DSM-IV criteria, IFNα-induced depression should be diagnosed as a “Substance-induced mood disorder”, it should be noted that the symptoms for the latter are the same as those for major depression, with the exception that there is a clearly identified pharmacologic aetiological factor.) Indeed, depression and symptoms frequently comorbid with depression, such as anxiety and fatigue, have been cited as a primary reason for treatment discontinuation, highlighting both their ubiquity mid potential morbidity [1,2]
However, despite strong evidence that IFNα causes depression, reliably determining prevalence rates of mood disturbance during treatment has proven problematic for several reasons. As discussed more extensively below (see section 1.3.3), rates of IFNα-induced depression appear sensitive to dosage and duration of treatment, as well as to premorbid patient-related risk factors.[1,2] In addition, depression during IFNα treatment appears to follow the old biblical adage “Seek and ye shall find”, given that rates of depression are consistently higher in studies that specifically examine IFNα-induced mood disorders using prospective designs and depression-specific assessment instruments when compared with studies that only identify depression retrospectively and/or as part of a generalised adverse effects screening process.[1] Finally, the very construct of depression in the context of IFNα treatment has been given widely divergent interpretations, with definitions across studies ranging from the single symptom of depressed mood through conditions defined by elevated scores on screening instruments to the full categorical syndrome of major depression. In general, rates of depression are higher when the condition is identified symptomatically rather than categorically, because many patients receiving IFNα develop constellations of depression-related symptoms that, while clinically meaningful do not meet full criteria for major depression.[31] Given abundant evidence that such depressive symptoms, even when subsyndromal, can significantly impair outcome across a variety of medical conditions,[32] it is important to recognise that studies utilising major depression as the definition of IFNα-induced depressive morbidity probably under-report clinically significant mood disturbances during treatment.
When these factors are taken into consideration, a clear pattern emerges. Early studies (i.e. those published prior to the mid-1990s) tended to define depression as a single symptom based on patient self-report during general screening for IFNα-related adverse effects. Not surprisingly, rates of depression tended to be low in these studies. For example, in one of the few trials comparing IFNα-treated patients with HCV-positive controls, Davis and colleagues[33] found that only 9% of patients receiving 3 million units of IFNα per week and 14% of patients receiving 9 million units per week reported depressed mood during 24 weeks of treatment. These rates were not significantly greater than the 8% rate reported by control subjects. Similarly, only 23 of 987 patients (2%) evinced significant depressive symptoms during 22 weeks of treatment in a large Japanese study of patients receiving IFNα monotherapy (i.e. without concomitant ribavirin) for 24 weeks.[34] Furthermore, a meta-analysis of randomised controlled trials of various doses of IFNα for HCV published prior to 1996 found that, in the 21 studies for which adverse effect information was available, 7% of patients reported significant depressed mood during treatment.[35] In a more recent study of 912 patients with HCV in which depression was assessed by self-report, McHutchison et al.[36] reported that 11% of patients receiving IFNα monotherapy and 16% of patients receiving combined IFNα/ribavirin developed depressed mood during treatment.
In contrast, studies that have evaluated depression as a syndrome of related symptoms (including fatigue and other neurovegetative symptoms, such as changes in sleep and appetite) and/or those that have used depression-specific screening assessments have routinely reported far higher rates of IFNα-induced depression. For example, an early study that specifically screened for depression during treatment found a significant increase in depressive symptoms in hepatitis B virus (HBV)-positive patients receiving IFNα compared with controls, although it should be noted that the effect of IFNα was most striking in patients who were comorbid for HIV.[37] In a large trial that did not utilise depression-specific assessment instruments, Lindsay and colleagues[38] nonetheless observed a 57% rate of ‘CNS’ adverse effects that included irritability, depression, impaired concentration and insomnia. Consistent with this, in a large French trial that observed a mere 9% depression rate during IFNα treatment, rates of neurasthenia reached 50%.[39] It should be noted that the construct of neurasthenia contains many neurovegetative symptoms that contribute to increased scores on depression-specific rating scales and that are included in the diagnosis of major depression. Interestingly, even when evaluated as a single symptom during a generalised screening process, rates of depression seem to be increasing in more recent, clinical trials compared with older trials, probably reflecting increased awareness on the part of study clinicians of the significant risk for depression during IFNα treatment. [40–42]
Over the last several years, a number of studies specifically designed to assess the prevalence of mood disturbance during IFNα treatment have been conducted. Whether assessed as DSM-IV-diagnosed major depression or as elevated scores on standardised depression rating scales, depression has emerged as a significant concomitant of treatment in virtually all these studies, with prevalence rates varying between 16% and 58%.[4,5,43–54] Three of these studies included control groups and in each case IFNα therapy was associated with significant increases in depression-related symptoms.[47,51,53] The majority of these studies have evaluated patients receiving IFNα with or without ribavirin for HCV. However, one study of patients receiving significantly higher doses of IFNα for the treatment of malignant melanoma reported that 50% of patients not pretreated with an antidepressant met diagnostic criteria for major depression at some point during 3 months of treatment.[4] Rates of depressive symptoms in this study were even higher, with 60% of patients endorsing depressed mood and 80% of patients endorsing significant fatigue during treatment.[31]
In the last several years, treatment with pegylated forms of IFNα-2b (PEG Intron®)1 and IFNα-2a (Pegasys®) in combination with the antiviral agent ribavirin has become the standard of care for patients with HCV. Pegylation involves the addition of a polyetheleneglycol molecule to IFNα, a process that increases the half-life of IFNα, allows once-a-week administration and improves antiviral efficacy. However, despite being the current standard of care, little is known about the relative proclivity of either pegylated IFNα-2b or pegylated IFNα-2a to cause depression compared with treatment with older, thrice weekly preparations of IFNα. In large registration trials, the rate of depression during 52 weeks of treatment with pegylated IFNα-2b was 31%%[41] and the rate with pegylated IFNα-2a over 48 weeks was 22%.[42] However, it should be noted that in these trials, depression was defined as a single symptom and was assessed by self-report as part of a generalised adverse effect screening process. Rates of other depress ion-related symptoms for pegylated IFNα-2b were fatigue (64%). insomnia (40%), irritability (35%) and weight loss (29%). The rates for pegylated IFNα-2a were fatigue (54%), insomnia (37%). irritability (24%) and reduced appetite (21%). The only study that has directly compared rates of major depression between pegylated and non-pegylated forms of IFNα found no differences between the two agents.[6] However, these results must be considered tentative, given the small sample size (36 patients receiving pegylated IFNα-2a) and the fact that the study reported uniformly low rates of major depression (12%), probably as a result of the fact that the study was designed primarily to evaluate the efficacy of antidepressant treatment rather than to comprehensively determine the prevalence of IFNα-induced depression.
1.3.2 Clinical Presentation: Neurovegetative and Depression-Specific Sub-Syndromes
As conceived in current psychiatric nosology, depression is not the single symptom of depressed mood, but rather a syndrome comprised of emotional, cognitive and neurovegetative abnormalities that can present in different combinations, but that tend to co-occur often enough to be recognised as forming a single spectrum of disease activity.[55] It should be noted that DSM-IV criteria include a number of symptoms, including fatigue, psychomotor slowing and changes in sleep and appetite that are also common in the context of immune system activation, such as occurs during medical illness or cytokine therapy.[56] While properly conceived of as contributing to major depression during IFNα treatment, recent data from patients receiving high-dose therapy for malignant melanoma suggest that these neurovegetative symptoms represent a sub-syndrome that is distinct from the more depression-specific symptoms (i.e. symptoms not as commonly observed in the context of illness) of depressed mood, anhedonia, anxiety and subjective cognitive disturbance. In a dimensional analysis, Capuron and colleagues[31] observed that neurovegetative symptoms of fatigue, psychomotor slowing and anorexia (loss of appetite) occurred early in treatment and persisted, whereas depression-specific symptoms co-developed significantly later in treatment. Depression-specific symptoms were exquisitely responsive to treatment with the selective serotonin reuptake inhibitor (SSRI) paroxetine, whereas neurovegetative symptoms were minimally responsive. These findings confirm clinical impressions that fatigue and related physical symptoms, while counting toward a diagnosis of depression, frequently occur in isolation from more depression-specific complaints.[3] That is, many patients receiving IFNα complain of chronic exhaustion, malaise, loss of appetite and insomnia, without also reporting significant sadness, hopelessness, guilt or loss of pleasure in life. These observations have clear treatment implications that are discussed below.
1.3.3 Risk Factors for IFNα-lnduced Depression
The high rate of depression during IFNα therapy has led many clinicians to routinely pretreat patients with antidepressants before initiation of therapy, a practice supported by data indicating that antidepressant pretreatment protects against the development of major depression in patients receiving high-dose IFNα for malignant melanoma.[4] However, even when administered in high doses, IFNα does not induce symptoms severe enough to qualify for major depression in at least 50% of patients, suggesting that routine antidepressant pretreatment may expose a significant number of patients to an additional unnecessary medication burden. Although generally benign, antidepressants are not without their own risks and adverse effects, especially in the medically ill who are often especially susceptible to medication adverse effects. Thus, determining risk factors for the development of IFNα-induced depression might help identify patients who would be especially likely to benefit from close psychiatric follow-up and/or antidepressant pretreatment, while decreasing the burden of providing such intensive follow-up to all patients receiving IFNα.
Risk factors for the development of depression can be divided into those that are inherent to IFNα treatment itself and premorbid factors that reflect each patient’s past history and pretreatment physical and psychiatric condition. Risk factors inherent to treatment include dosage and duration, as well as mode of IFNα delivery, with adverse effects generally worsening as dosage increases and treatment duration extends.[1,57] All neuropsychiatric adverse effects appear to be more common and more severe in patients receiving ICV and IV IFNα (who typically also receive high doses) than in patients receiving IFNα subcutaneously (as occurs in the treatment of HCV). Hence, patients receiving high-dose therapy administered either ICV or IV may especially benefit from close psychiatric follow-up and/or antidepressant pretreatment. Although preliminary, some data suggest that ribavirin, an antiviral agent typically used in combination with IFNα for HCV, may synergistically increase the proclivity of IFNα to induce depression.[46] If confirmed in prospective trials, this finding may take on increasing importance in light of evidence that higher dosages of ribavirin increase rates of viral clearance.
A number of premorbid patient-related risk factors for the development of IFNα-induced depression have been reported. The most replicated risk factor appears to be the presence of psychiatric disturbance just prior to commencing IFNα treatment. The majority of studies that have looked at this issue find that baseline depression and/or anxiety, even when subclinical, predicts the development of psychiatric morbidity during treatment.[5,45,53,54,57,58] Consistent with this, patients receiving psychiatric treatment at the time of IFNα initiation appear to be at increased risk of developing depression.[53,54] On the other hand, although one study reported that a history of past psychiatric disturbance significantly increased the risk of depression during IFNα therapy,[48] other studies do not find that a past history of depression significantly increases the risk of neuropsychiatric disturbance or is associated with higher rates of treatment discontinuation.[5,7,43,51] Indeed, one study found that even patients with severe psychiatric disorders, such as schizophrenia, can be successfully treated with IFNα if they are psychiatrically stable prior to treatment.[59] Similarly, a past history of drug or alcohol abuse does not appear to increase the risk of IFNα-induced depression, provided patients remain abstinent during treatment.[5,46,54] Although women are typically more vulnerable than men to mood and anxiety disorders, studies with IFNα are split between those that do[48,52,54] and those that do not find gender to be a risk factor.[44–46] Likewise, although one study found that very elderly patients may experience increased IFNα-induced depression,[50] age has not emerged as a consistent risk factor.[45,46] Finally, an early study of patients receiving IFNα for HBV infection reported that lack of social support significantly increased the risk of developing depressive symptoms during treatment,[37] a finding in line with a voluminous literature documenting the detrimental health effects of social isolation.[60,61]
Several physiological factors appear to increase the risk of developing depression during IFNα treatment. An obvious factor shared by all patients undergoing treatment is that physical illness itself is a significant risk factor for the development of depression.[32] Indeed, many studies have documented elevated rates of depression in patients with a wide range of medical disorders, including cancers and viral infections, the conditions against which IFNα is most typically directed. Traditionally, the causes for this increased prevalence of depression have been ascribed to the multiple psychological stressors that typically accompany illness. However, accumulating data indicate that physiological processes inherent to most illnesses, and especially activation of the proinflammatory cytokine network, may directly predispose towards the pathophysiology of depression via effects on the CNS.[62] In humans and animals, the administration of proinflammatory cytokines, or of substances that induce cytokines, such as lipopolysaccharide, reliably induces a behavioural syndrome that has been termed sickness behaviour and that includes symptoms also commonly seen in depression, including fatigue, anhedonia, social isolation, psychomotor slowing, decreased food and water intake, decreased libido, hyperalgesia, altered sleep patterns and cognitive impairment.[63] These symptoms can be ameliorated or prevented by blocking cytokine activity in the CNS.[64] IFNα has been shown to be a potent inducer of proinflammatory cytokine production and thus may be especially likely to induce depression-related physical and emotional symptoms in patients already experiencing illness-related increases in proinflammatory cytokine activity.[65] Consistent with this, premorbid fatigue in patients with HCV has been reported to be a powerful predictor of the development of disabling fatigue during IFNα therapy.
In addition to the general vulnerability to depression posed by immune system activation in the context of medical illness, recent studies point to more specific physiological risk factors for the development of depression during IFNα treatment. For example, patients who responded to a first dose of IFNα with hyperactivity of corticotropin-releasing hormone (CRH)-mediated stress pathways, as assessed by increased production of corticotropin and cortisol, were significantly more likely to develop major depression during treatment than were patients with more modest stress system responses to the initial injection, even though none of the patients demonstrated major depression at baseline prior to treatment.[65] CRH activates both the hypothalamic-pituitary-adrenal axis and sympathetic nervous system and is widely recognised as the principal orchestrator of the mammalian stress response.[66] Significant data indicate that CRH hyperactivity is a central abnormality in major depression and is apparent in persons exposed to early life stress and/or who meet criteria for posttraumatic stress disorder.[67] Thus, the finding that CRH hyperactivity in response to an initial dose of IFNα predicts the later development of depression may point to a potential pathway by which psychological stress predisposes towards the development of mood disorders in the context of medical illness.
A second mechanism implicated in the development of depression during IFNα therapy involves CNS serotonergic neurotransmission. By activating the proinflammatory cytokine network, IFNα induces the enzyme indoleamine 2,3-dioxegenase (IDO). which shunts the metabolism of tryptophan away from serotonin and toward kynurenine.[68] Because tryptophan is an essential amino acid not produced by the body, IDO-induced tryptophan depletion results in reduced serotonergic availability, a condition that has been demonstrated to rapidly induce significant dysphoria in many patients vulnerable to mood disorders.[69] Several studies report a correlation between reduced tryptophan levels and depression in patients receiving IFNα.[22,70–72] For example, Capuron et al,[70] recently observed that patients who developed major depression during IFNα treatment had significantly reduced plasma concentrations of tryptophan and increased concentrations of kynurenine compared with patients who did not develop depression, implicating increased IFNα-induced IDO activity as a risk factor for the development of depression during treatment. Interestingly, dimensional analyses indicated that evidence of both CRH and IDO hyperactivity correlated with depression-specific symptoms (depressed mood, anxiety and subjective memory and attentional difficulties), but not with neurovegetative (fatigue, anorexia and pain) symptoms,[65,70] providing further evidence that depression in the context of IFNα therapy may represent two separable syndromes. The first of these is a depression-specific syndrome characterised by symptoms more common in the context of major depression than sickness. These symptoms include depressed mood and anxiety, which are associated with activity in CRH and serotonin pathways and respond to antidepressant treatment consistent with the well known effects of antidepressants on these pathways. The second syndrome is a neurovegetative condition characterised by symptoms such as fatigue and anorexia that are also common in the context of sickness, develop early in treatment and are minimally responsive to treatment with serotonergic antidepressants.
In addition to CRH and serotonin pathways, other neurotransmitter and neuroendocrine systems may contribute to psychiatric symptoms during IFNα treatment. Animal studies demonstrate that IFNα alters CNS opioid, dopamine and noradrenaline (norepinephrine) activity.[18] Like other inflammatory stimuli. IFNα also appears to increase free radical and glutamate production, which, in turn, promote neuronal damage.[18] Finally, recent data from humans suggest that carriers of the epsilon 4 allele of the apolipoprotein E gene may be at increased risk of developing many neuropsychiatric symptoms during IFNα treatment, including irritability, anxiety and depressive symptoms.[73]
1.4 Treatment of IFNα-lnduced Depression
1.4.1 Prophylactic versus Symptomatic Treatment Strategies
In the pharmacological management of IFNα-induced depression, clinicians have two general treatment strategies at their disposal (see figure 1). Patients can be pretreated with an antidepressant to prevent or attenuate the development of depression. Alternatively, patients can be monitored for signs of depression during IFNα therapy and treatment commenced only when indicated.
Fig. 1.
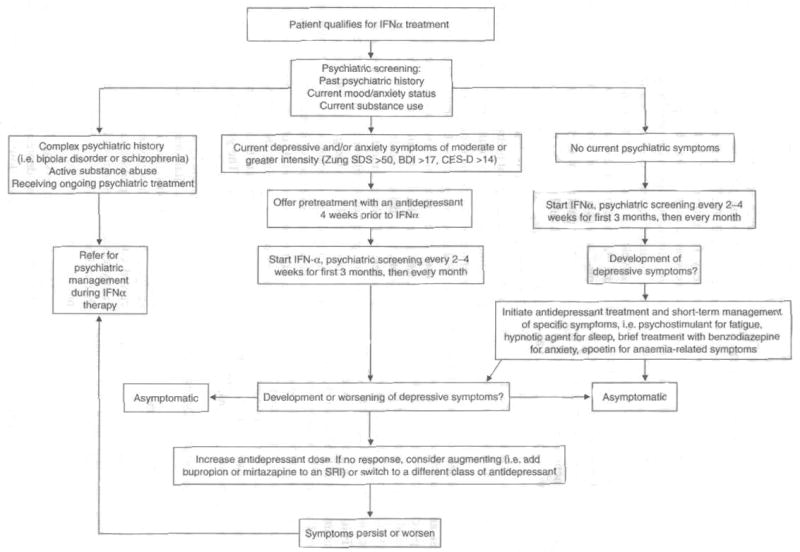
Algorithm for pharmacological treatment of interferon (IFN)-α-induced depression. BDI = Beck Depression Inventory; CES-D = Center for Epidemiological Studies Depression Rating Scale: SRI = serotonin reuptake inhibitor; Zung SDS = Zung Self Rating Depression Scale Index score.
Antidepressant pretreatment is clearly preferable for patients who are already taking an antidepressant for a psychiatric condition; necessary psychiatric medications should not be discontinued prior to initiation of IFNα. Pretreatment is also a reasonable strategy for patients at high risk for developing depression during IFNα therapy. As discussed above, premorbid depressive and/or anxiety symptoms (which frequently coexist) are a significant risk for developing clinical depression during IFNα treatment. Therefore, most patients with depressive symptom scores in the mild range or higher prior to treatment, or patients who meet criteria for major depression, should probably be offered pretreatment with an antidepressant prior to initiation of IFNα. This recommendation is based on an often-cited study demonstrating that pretreatment with the SSRI paroxetine significantly diminished the development of major depression in patients receiving high-dose IFNα for malignant melanoma.[4] In this study, 40 patients were randomly assigned to receive either paroxetine or placebo beginning 2 weeks before, and continuing during, treatment with IFNα. After 12 weeks of IFNα treatment, 45% of patients in the placebo group met criteria for major depression, compared with 11% in the group receiving paroxetine, a highly significant difference. Paroxetine treatment was also effective in preventing treatment discontinuation as a result of neuropsychiatric adverse effects, suggesting that antidepressant pretreatment may contribute to compliance by improving IFNα tolerability. The generalisability of these findings to other IFNα-based treatment regimens has been recently upheld by an open trial of pretreatment with the SSRI citalopram in patients receiving pegylated IFNα plus ribavirin for HCV. In this study, patients with a psychiatric history who received citalopram pretreatment developed significantly less depression during IFNα/ribavirin therapy than did patients with a psychiatric history who did not receive pretreatment.[74]
Most patients receiving IFNα do not develop clinically significant depression, especially when IFNα is used in lower doses, or in pegylated preparations, for the treatment of HCV. Thus, routine antidepressant pretreatment runs the risk of exposing the majority of patients to an additional medication burden that they will not require. Moreover, although safe and effective, the newer antidepressants are not without adverse effects and risks (including the potential induction of mania, see section 2). Therefore, in many patients, it makes clinical sense to initiate antidepressant treatment only if depressive symptoms begin to emerge once IFNα therapy has commenced. Symptomatic treatment strategies are most appropriate for patients without premorbid depressive or anxiety symptoms and in treatment settings in which routine depression screening is available during IFNα treatment. This latter point is especially important, given the rapidity with which IFNα-induced depressive symptoms often emerge and the typical time lag of 4–6 weeks for a full therapeutic response to antidepressants. It is interesting to note in this regard that only 2 weeks of symptoms are required to meet DSM-IV criteria for major depress ion,[55] pointing to the fact that even in the context of idiopathic depression, symptoms need not be present for an extended period for treatment to be indicated. This is even truer in the context of IFNα therapy where the known depressogenic factor will continue to adversely affect the patient, often for months or years of treatment. In general, therefore, we recommend commencing antidepressant treatment when a patient receiving IFNα has had ≥7 days of continuous depressive symptoms of mild or greater severity.
The rationale for treating patients symptomatically once IFNα-induced depression has developed is provided by recent data demonstrating that depressive symptoms can be effectively treated in patients receiving IFNα/ribavirin treatment for chronic hepatitis C.[50] In a study of 39 patients with chronic hepatitis C, 85% of subjects who developed major depression during treatment responded to the SSRI citalopram.[5] Similarly, in a larger study, 79% of subjects who developed depression during IFNα/ribavirin treatment were able to complete therapy following the addition of paroxetine.[6] Consistent with this finding, depression scores declined significantly in all patients within 4 weeks of antidepressant initiation, even in the context of ongoing IFNα treatment.[6] A recent study that found no increased risk of treatment discontinuation or other serious adverse events in patients with a past psychiatric history also observed a higher rate of antidepressant usage during IFNα treatment in this group compared with non-psychiatric controls, suggesting that judicious antidepressant use may have accounted for the good outcome during IFNα treatment in patients with pre-existing psychiatric disorders.[43] The results of these larger studies are consistent with those of many smaller open-label trials and case reports, suggesting that all antidepressants are effective in the treatment of IFNα-induced depression once it has emerged. But these findings must be considered preliminary, given that none of these studies employed a placebo control for antidepressant use.[75–81] Taken together, the available literature provides support for both prophylactic and symptomatic treatment of IFNα-induced depression with antidepressants; however, it is clear that a tremendous need exists for further placebo-controlled, double-blind trials.
Despite these encouraging data, there are circumstances when clinical judgement may dictate that IFNα treatment be suspended until the resultant depression has been adequately treated. A general rule of thumb is that IFNα therapy should be halted whenever the risks and liabilities of the depression outweigh the benefits of uninterrupted treatment. Although such a decision must be made on a case-by-case basis, several clinical scenarios are probably best dealt with by suspending IFNα treatment. These include cases in which depression is associated with significant suicidal ideation, especially if the patient has a plan and the means to accomplish it. It should be noted that, although not common, IFNα treatment has been associated with both suicide attempts and completions, often in patients with no prior psychiatric history.[82,83] Thus, all patients who endorse depressive symptoms during treatment should be screened for suicidal ideation. Although less pressing, other circumstances in which it might be best to suspend IFNα treatment include the presence of severe depression or depressive symptoms that pose an immediate threat to central aspects of the patient’s life, including disruption of family relationships or failure in a work environment. In all such cases, IFNα should be restarted only following adequate pretreatment with an antidepressant.
1.5 Factors to Consider when Selecting an Antidepressant
In patients who are at risk for depression or in whom IFNα-induced depression has already developed, any antidepressant is better than none, and newer agents are safe and effective, with benign adverse effect profiles. At present, SSRIs (especially paroxetine) have been studied most frequently, but this likely represents an historical accident and probably does not reflect any inherent superiority of these agents compared with other recently developed antidepressants. Because the available evidence suggests that all antidepressants are likely to be effective,[75–81] it makes sense to approach antidepressant administration for IFNα-induced depression using the same principles that guide the treatment of depression in general. Factors frequently useful in selecting an antidepressant for a particular patient include drug-drug interactions, adverse effect profile, and efficacy (see table I).
Table 1.
Characteristics of selected antidepressants (reproduced from Demetrashvili et al.,[84] with permission)
| Agent | Starting dose (mg/day) | Therapeutic dose (mg/day) | Effects on CYP system | Adverse effects | Comments |
|---|---|---|---|---|---|
| Sertraline | 25–50 | 50–200 | Intermediate inhibition | Anxiety, GI complaints, insomnia, sexual dysfunction, sweating, headache, weight gain (with long-term use) | SSRI (this class of agent has been more thoroughly studied than other newer antidepressants). Note varying likelihood of effects on CYP system |
| Fluoxetine | 10–20 | 20–80 | Potent inhibition | As above | As above |
| Fluvoxamine | 100 | 100–300 | Potent inhibition | As above | As above |
| Paroxetine | 10–20 | 20–80 | Potent inhibition | As above | As above |
| Citalopram | 20 | 20–60 | Few | As above | As above |
| Venlafaxine | 37.5 | 75–450 | Few | GI complaints, anxiety, sexual dysfunction, sweating, headache, potential increased blood pressure (at doses >300 mg/day) | Combined serotonin/noradrenaline (norepinephrine) agent. May be superior to SSRIs alone |
| Duloxetine | 30–60 | 60 | Intermediate inhibition | Same as venlafaxine, except no blood pressure increase | Combined serotonin/norepinephrine agent. May be superior to SSRIs alone |
| Mirtazapine | 15–30 | 15–60 | Few | Weight gain, somnolence, rare agranulocytosis | No sexual dysfunction. May help to counter IFNα-induced insomnia |
| Nefazodone | 50 | 300–600 | Potent inhibition | Somnolence, nausea, dizziness, very rare fatal liver failure (1 in 300 000) | May be contraindicated in hepatitis C patients. Low rate of sexual dysfunction. May help to counter IFNα-induced insomnia |
| Bupropion | 75 | 300–450 | Few | Anxiety, GI upset, potential for increased seizure risk in patients receiving IFNα | Acts on noradrenergic and dopaminergic systems. Not as effective as SSRIs for primary anxiety disorders. No sexual dysfunction. No weight gain |
| Methylphenidate | 5–10 | 10–60 | Potent inhibition | Nervousness, insomnia, others | Psychostimulant. Useful for fatigue, anhedonia, depressed mood. Can accelerate antidepressant response. Potential for abuse. Cases of abnormal liver function reported |
| Modafinil | 100 | 200–400 | Intermediate inhibition | Headache, nausea, anxiety, infection, insomnia | Wakefulness promoter. May be useful for fatigue. Reduce dose in elderly; 100 mg/day for hepatic impairment. No weight gain. No change in blood pressure. Generally benign adverse effect profile |
CYP = cytochrome P450; GI = gastrointestinal; IFN = interferon; SSRI = selective serotonin reuptake inhibitor.
1.5.1 Drug-Drug Interactions
The relevance of drug-drug interactions to the selection of an antidepressant is an area of active debate within psychiatry, but it seems clear that these interactions become increasingly important in patients with medical illnesses who require multiple medications, especially if any of these other medications has a narrow therapeutic index. In medically complicated patients on multiple medications, it is probably wise to avoid treatment with older antidepressants, such as TCAs and MAOIs that are lethal in overdose. Of the newer antidepressants, fluoxetine, paroxetine, fluvoxamine and nefazodone are the most likely to inhibit the metabolism, and hence raise blood levels, of other medications.[85,86] Venlafaxine, mirtazapine and citalopram appear to have few effects on the cytochrome P450 (CYP) enzyme system, although their metabolism can be affected by other medications that interfere with liver metabolism, such as protease inhibitors.[85,86] The ability of sertraline, bupropion and duloxetine to inhibit the metabolism of other medications appears to lie between potent CYP enzyme inhibitors such as paroxetine and less potent inhibitors such as citalopram.[85] Although patients with severe liver disease may require lower doses of antidepressants because of decreased metabolic capacity, a recent study in patients with HCV but without cirrhosis or liver failure found normal blood levels of citalopram when the medication was administered at standard doses,[87] suggesting that many patients with HCV will be best served by receiving standard therapeutic doses of antidepressants during treatment with IFNα/ribavirin.
1.5.2 Adverse Effects
In general, antidepressant adverse effects are related to the specific mechanism of action of each antidepressant. Thus, typical adverse effects of agents that block the serotonin reuptake site (fluoxetine, sertraline, paroxetine, citalopram, fluoxeamine, venlafaxine and duloxetine) include sexual dysfunction, gastrointestinal distress, anxiety, sweating and headaches. Venlafaxine is also associated with a low rate of hypertension at doses of ≥300 mg/day.[88] In addition to these general concerns, a recent report indicates that HCV-positive patients with cirrhosis and either portal hypertension or hepatic failure may be at increased risk for bleeding events when treated with an SSRI antidepressant, especially when SSRIs are combined with NSAIDs. These findings, although not conclusive, justify caution when combining SSRIs with NSAIDs in HCV-positive patients receiving IFNα, especially those with decompensated cirrhosis.[89]
Mirtazapine is frequently associated with weight gain and somnolence, although it does not cause significant sexual dysfunction. Like mirtazapine, nefazodone does not cause sexual dysfunction, but has recently been associated with a low incidence of potentially fatal liver failure.[90] For this reason, nefazodone is probably contraindicated in patients receiving IFNα for HCV. Bupropion acts on noradrenergic and dopaminergic systems in the brain. This agent may actually enhance sexual functioning and is not associated with weight gain. However, adverse effects include gastrointestinal distress and anxiety. In addition, use of bupropion with agents that block CYP isoenzymes (such as certain antiretroviral agents) has been associated with seizures,[91] which is of potential concern given that IFNα may lower seizure threshold. In this regard, a recent report in patients receiving pegylated IFNα-2b and ribavirin for HCV suggests that, although the incidence of seizures was low, a disproportionate number of patients who seized during treatment were also taking bupropion.[92]
Adverse effects are a frequent cause of discontinuation of antidepressant therapy, but in the context of IFNα therapy, these adverse effects may also be therapeutically employed. For example, as noted above, mirtazapine is frequently associated with somnolence and weight gain, both significant problems in many patients. But in the context of IFNα-induced insomnia and anorexia, these adverse effects may actually benefit certain patients.
With all antidepressants, adverse effects can be minimised by following the maxim to ‘start low and go slow’ in the early stages of treatment. This advice is especially pertinent for patients with a history of panic attacks, chronic anxiety or past sensitivity to other medications; these individuals are at increased risk of developing either panic or anxiety early in the course of treatment if antidepressants are initiated at standard doses. However, even among patients who require gradual initial titration, it is essential that full therapeutic doses are eventually achieved and maintained.
1.5.3 Antidepressant Efficacy in Patients Receiving IFNα
Several lines of evidence suggest that antidepressant strategies that combine serotonin and catecholamine (noradrenaline/dopamine) activity may be somewhat more effective than selective serotonin agents in the treatment of major depression. A number of studies indicate that addition of the noradrenergic TCA desipramine to an SSRI improves both the rate and depth of response.[93] Similar results have been reported with the addition of bupropion, which also enhances dopaminergic neurotransmission.[94] Consistent with this, data suggest that the combined serotonin/noradrenaline reuptake inhibitors venlafaxine, duloxetine and milnacipran may be more effective than SSRIs in the acute remission of depression.[95]
Patients receiving IFNα may especially benefit from agents with noradrenergic/dopaminergic activity. Such agents have been shown to be more effective than SSRIs in diminishing chronic pain and may be more effective in the treatment of fatigue, both of which are frequent symptoms in patients receiving IFNα, even when other depressive symptoms are absent.[96–99] In addition to venlafaxine, duloxetine, bupropion and mirtazapine, other noradrenergic/dopaminergic modulating strategies currently available in the US include treatment with psychostimulants, such as methylphenidate, or with the novel agent modafinil.[38–40] A small open-label trial showed that methylphenidate, when combined with aerobic exercise, significantly improved fatigue in patients receiving high-dose IFNα for malignant melanoma.[100] This finding is consistent with several recent controlled trials demonstrating that agents that enhance dopamine functioning, including psychostimulants and modafinil, are effective in treating fatigue in the context of medical illness.[101–103]
These findings, when combined with data indicating that SSRIs are not particularly effective for treating IFNα-induced fatigue and other neurovegetative symptoms,[31] support the first-line use of agents that augment noradrenaline/dopamine functioning in patients with significant depression-related neurovegetative symptoms (i.e. fatigue, anorexia and pain), but without additional depression-specific symptoms such as sadness, anxiety and guilt, etc. These agents include the antidepressant bupropion, the psychostimulants methylphenidate and dextro-amphetamine and the novel agent modafinil. First-line use of these agents in the context of isolated neurovegetative symptoms, especially fatigue, also has the advantage of sparing patients the sexual dysfunction that frequently accompanies treatment with SSRIs and that has the potential to exacerbate IFNα-associated decrements in sexual functioning. Nonetheless, it should be pointed out that, despite their increasing use in the clinical treatment of IFNα-related fatigue, psycho-stimulants remain an empiric treatment without controlled data to support their use.
Finally, recent data indicate that anaemia during IFNα treatment (which occurs in up to 36% of patients) adversely affects emotional and social functioning, independently of other treatment-related risk factors. [104] Moreover, treatment of anaemia with epoetin-α significantly improved multiple health-related quality-of-life domains, including mental health.[105]
2. IFNα-lnduced Mania: When Irritability is No Longer Depression
One would search in vain to find ‘irritability’ under the list of symptoms that comprise major depression in the DSM-IV. Instead, irritability is a classic symptom of mania.[55] Given that irritability is a common psychiatric adverse effect of IFNα, it should not be surprising that many reports in the literature confirm that IFNα is capable of inducing mania.[106–111] While previously considered a relatively rare occurrence, a recent study from Europe found that 20% of patients receiving pegylated IFNα plus ribavirin for HCV-evinced manic or hypomanic symptoms at some point during 24 weeks of treatment.[112] Nonetheless, because manic phenomena span a spectrum from relatively mild hypomanic conditions to full-blown psychotic manias, determining the true prevalence of IFNα-induced mania will very much depend on the criteria by which mania is defined. Up to 77% of patients receiving IFNα plus ribavirin for HCV complain of significant fatigue during treatment;[43] however, it is not currently known what percentage of patients with irritability are better thought of as experiencing dysphoric mania or a depressive or fatigue-related condition. In this regard, it should be noted that both depression and states of chronic fatigue are frequently associated with irritability.[113,114]
Consistent with the notion that most cases of IFNα-induced irritability are best conceptualised as belonging to a depressive spectrum disorder, clinical experience and some published data suggest that most cases of IFNα-induced irritability respond well to antidepressant treatment.[4,46] consistent with evidence that antidepressants, and especially SSRIs, have the capacity to decrease irritability and anger. Nonetheless, mania, when it occurs, is a psychiatric emergency requiring immediate action and a treatment strategy quite different from depression. Because time is frequently of the essence in dealing with manic patients, it has become incumbent upon physicians who treat with IFNα to recognise the symptoms of mania.
In its classic form, mania is not difficult to differentiate from depression because patients are euphoric, rather than sad. However, many patients with mania demonstrate extreme irritability rather than euphoria. These cases of dysphoric mania can frequently be a diagnostic challenge, because most physicians are accustomed to assuming that dysphoric individuals are depressed, especially within the context of IFNα treatment in which all neuropsychiatric complaints are typically attributed to ‘depression’. This misdiagnosis can have grave consequences, given evidence that antidepressants are capable of inducing or worsening mania.[89] Thus, notwithstanding the fact that many patients who report IFNα-induced irritability appear to benefit from initiation of an antidepressant, if irritability occurs within the context of a manic episode, the addition of an antidepressant may potentially worsen symptoms.
Key symptoms that tend to differentiate dysphoric mania from depression are listed in table II. Risk factors for mania include a family history of bipolar disorder or past manic or hypomanic episodes in the individual. Evidence suggests that many patients with recurrent depressions that start early in life may also be at increased risk for mania. Manic patients frequently have impaired insight into their condition and are much more likely than patients with depression to deny that ‘anything is wrong’. For this reason, spouses and others who know the patient well can be important sources of information and should be taken with utmost seriousness if they report bizarre, agitated or aggressive behaviour in the patient, even if the patients denies these symptoms.
Table II.
Differentiating dysphoric mania from depression (reproduced from Demetrashvili et al.,[84] with permission)
| Dysphoric mania | Depression |
|---|---|
| Rage | Mild Irritability |
| Poor insight into condition | Good insight into condition |
| Increased energy | Fatigued |
| Hypersexuality | Loss of sexual interest |
| Grandiose plans | Diminished expectations |
| Increased speech production | Diminished speech production |
| Increased rate of speech | Decreased rate of speech |
| Increased use of telephone | Decreased desire for social contact |
| Flamboyant style of dress | Drab Clothing |
| Psychosis relatively common | Psychosis relatively uncommon |
Emergency psychiatric consultation should be obtained if a mania appears to have developed in a patient while receiving IFNα. If the patient is taking an antidepressant, it should be discontinued immediately. Similarly, given the multiple risks attendant upon mania, we recommend that IFNα also be stopped. Effective antimanic agents include mood stabilisers such as lithium, valproic acid and carbamazepine, as well as atypical antipsychotics, of which olanzapine has been the best studied. Many manic patients will recover more rapidly if prolonged periods of sleep are induced. Benzodiazepines are useful for this purpose, especially in combination with other antimanic agents, although large doses of sedative medications may be required to induce sleep in acutely manic patients. Finally, it should be noted that mania in the context of withdrawal from IFNα treatment has also been reported.[107]
3. Conclusion
IFNα therapy is associated with a wide range of neuropsychiatric adverse effects. Symptoms of depression, anxiety and fatigue are the most widely recognised treatment sequelae, but IFNα is also capable of inducing delirium (especially when used at high doses for cancer) and states of extreme irritability and/or agitation that meet symptom criteria for mania. Treatment with IFNα, at high and low doses, has also been rarely associated with the development of frank psychosis. Because of this, psychiatric evaluation should be a part of the screening process for patients being considered for IFNα treatment, and patients should receive regular assessments for psychiatric symptoms during therapy. The best available data support antidepressant pretreatment for patients with baseline depression and anxiety and symptomatic treatment for patients without baseline psychiatric disturbance who develop IFNα-induced depression. Patients who develop extreme irritability during treatment should be evaluated for mania. Manic patients should receive an emergency psychiatric referral.
Acknowledgments
The authors would like to thank Bobbi J. Woolwine for her support in the preparation of this article. This work was supported in part by the National Institute of Mental Health (MH64619, MH069124 and MH60723) and the Centers for Disease Control and Prevention. Potential conflicts of interest: Charles L. Raison, MD, Speakers’ Bureau for Schering Plough, Wyeth and Eli Lilly; Marina Demetrashvili, no relationships to disclose; Lucile Capuron, no relationships to disclose; Andrew H. Miller, no relationships to disclose.
Footnotes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
- 1.Schaefer M, Engelbrecht MA, Gut O, et al. Interferon α (IFNa) and psychiatric syndromes: a review. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26 (4):731–46. doi: 10.1016/s0278-5846(01)00324-4. [DOI] [PubMed] [Google Scholar]
- 2.Dieperink E, Willenbring M, Ho SB. Neuropsychiatric symptoms associated with hepatitis C and interferon α: a review. Am J Psychiatry. 2000;157 (6):867–76. doi: 10.1176/appi.ajp.157.6.867. [DOI] [PubMed] [Google Scholar]
- 3.Trask P, Esper P, Riba M, et al. Psychiatric side effects of interferon therapy: prevalence, proposed mechanisms, and future directions. J Clin Oncol. 2000;18(11):2316–26. doi: 10.1200/JCO.2000.18.11.2316. [DOI] [PubMed] [Google Scholar]
- 4.Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344 (13):96–6. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 5.Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;9 (7):942–7. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- 6.Kraus MR, Schafer A, Faller H, et al. Paroxetine for the treatment of interferon-α-induced depression in chronic hepatitis C. Aliment Pharmacol Ther. 2002;16 (6):1091–9. doi: 10.1046/j.1365-2036.2002.01265.x. [DOI] [PubMed] [Google Scholar]
- 7.Renault PF, Hoofnagle JH, Park Y, et al. Psychiatric complications of long term interferon alfa therapy. Arch Intern Med. 1987;147(9):1577–80. [PubMed] [Google Scholar]
- 8.Meyers CA, Obbens EA, Scheibel RS, et al. Neurotoxicity of intraventricularly administered α-interferon for leptomeningeal disease. Cancer. 1991;68 (1):88–92. doi: 10.1002/1097-0142(19910701)68:1<88::aid-cncr2820680118>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Smedley H, Katrak M, Sikora K, et al. Neurological effects of recombinant human interferon. Br Med J (Clin Res Ed) 1983;286(6361):262–4. doi: 10.1136/bmj.286.6361.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohatiner AZ, Prior PF, Burton AC, et al. Central nervous system toxicity of interferon. Br J Cancer. 1983;47 (3):419–22. doi: 10.1038/bjc.1983.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niiranen A, Laaksonen R, Iivanainen M, et al. Behavioral assessment of patients treated with α-interferon. Acta Psychiatr Scand. 1988;78 (5):622–6. doi: 10.1111/j.1600-0447.1988.tb06395.x. [DOI] [PubMed] [Google Scholar]
- 12.Mattson K, Niiranen A, Iivanainen M, et al. Neurotoxicity of interferon. Cancer Treat Rep. 1983;67 (10):958–61. [PubMed] [Google Scholar]
- 13.Poutiainen E, Hokkanen L, Niemi ML, et al. Reversible cognitive decline during high-dose α-interferon treatment. Pharmacol Biochem Behav. 1994;47 (4):901–5. doi: 10.1016/0091-3057(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 14.Iivanainen M, Laaksonen R, Niemi ML, et al. Memory and psychomotor impairment following high-dose- interferon treatment in amyotrophic lateral sclerosis. Acta Neurol Scand. 1985;72(5):475–80. doi: 10.1111/j.1600-0404.1985.tb00904.x. [DOI] [PubMed] [Google Scholar]
- 15.Farkkila M, Iivanainen M, Roine R, et al. Neurotoxic and other side effects of high-dose interferon in amyotrophic lateral sclerosis. Acta Neurol Scand. 1984;70 (1):42–6. doi: 10.1111/j.1600-0404.1984.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 16.Adams F, Fernandez F, Mavligit G. Interferon-induced organic mental disorders associated with unsuspected pre-existing neurologic abnormalities. J Neurooncol. 1988;6 (4):355–9. doi: 10.1007/BF00177432. [DOI] [PubMed] [Google Scholar]
- 17.Meyers CA, Scheibel RS, Forman AD. Persistent neurotoxicity of systemically administered interferon-α. Neurology. 1991;41 (5):672–6. doi: 10.1212/wnl.41.5.672. [DOI] [PubMed] [Google Scholar]
- 18.Schaefer M, Schwaiger M, Pich M, et al. Neurotransmitter changes by interferon-α and therapeutic implications. Pharmacopsychiatry. 2003;36 (Suppl 3):S203–6. doi: 10.1055/s-2003-45131. [DOI] [PubMed] [Google Scholar]
- 19.Blalock JE, Smith EM. Human leukocyte interferon (HuIFNα): potent endorphin-like opioid activity. Biochem Biophys Res Commun. 1981;101 (2):472–8. doi: 10.1016/0006-291x(81)91284-5. [DOI] [PubMed] [Google Scholar]
- 20.Valentine AD, Meyers CA, Talpaz M. Treatment of neurotoxic side effects of interferon-α with naltrexone. Cancer Invest. 1995;13 (6):561–6. doi: 10.3109/07357909509024923. [DOI] [PubMed] [Google Scholar]
- 21.Licinio J, Kling MA, Hauser P. Cytokines and brain function: relevance to interferon-α-induced mood and cognitive changes. Semin Oncol. 1998;25 (1 Suppl 1):30–8. [PubMed] [Google Scholar]
- 22.Wichers MC, Maes M. The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-α-induced depression. J Psychiatry Neurosci. 2004;29 (1):11–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Mendoza-Fernandez V, Andrew RD, Barajus-Lopez C. Interferon-α inhibits long-term potentiation and unmasks a long-term depression in the rat hippocampus. Brain Res. 2000;885 (1):14–24. doi: 10.1016/s0006-8993(00)02877-8. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz TL, Masand PS. The role of atypical antipsychotics in the treatment of delirium. Psychosomatics. 2002;43 (3):171–4. doi: 10.1176/appi.psy.43.3.171. [DOI] [PubMed] [Google Scholar]
- 25.Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry. 1996;153(2):231–7. doi: 10.1176/ajp.153.2.231. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman RG, Cohen MA, Alfonso CA, et al. Treatment of interferon-induced psychosis in patients with comorbid hepatitis C and HIV. Psychosomatics. 2003;44 (5):417–20. doi: 10.1176/appi.psy.44.5.417. [DOI] [PubMed] [Google Scholar]
- 27.Mizoi Y, Kaneko H, Oharazawa A, et al. Parkinsonism in a patient receiving interferon α therapy for chronic hepatitis C [in Japanese] Rinsho Shinkeigaku. 1997;37 (1):54–6. [PubMed] [Google Scholar]
- 28.Sarasombath P, Sumida K, Kaku DA. Parkinsonism associated with interferon α therapy for chronic myelogenous leukemia. Hawaii Med J. 2002;61(3):48. 57. [PubMed] [Google Scholar]
- 29.Sunami M, Nishikawa T, Yorogi A, et al. Intravenous administration of levodopa ameliorated a refractory akathisia case induced interferon-α. Clin Neuropharmacol. 2000;23 (1):59–61. doi: 10.1097/00002826-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 30.American Psychiatric Association, Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC. American Psychiatric Association, 1994
- 31.Capuron L, Gumnick JF, Musselman DL, et al. Neurobehavioral effects of interferon-α in cancer patients; phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26 (5):643–52. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 32.Evans DL, Staab JP, Petitto JM, et al. Depression in the medical setting: biopsychological interactions and treatment considerations. J Clin Psychiatry. 1999;60 (Suppl 4):40–55. [PubMed] [Google Scholar]
- 33.Davis GL, Balart LA, Schiff ER, et al. Treatment of chronic hepatitis C with recombinant interferon alfa: a multicenter randomized, controlled trial. Hepatitis Interventional Therapy Group. N Engl J Med. 1989;321 (22):1501–6. doi: 10.1056/NEJM198911303212203. [DOI] [PubMed] [Google Scholar]
- 34.Okanoue T, Sakamoto S, Itoh Y, et al. Side effects of high-dose interferon therapy for chronic hepatitis C. J Hepatol. 1996;25 (3):283–91. doi: 10.1016/s0168-8278(96)80113-9. [DOI] [PubMed] [Google Scholar]
- 35.Poynard T, Leroy V, Cohard M, et al. Meta-analysis of interferon randomized trials in the treatment of viral hepatitis C: effects of dose and duration. Hepatology. 1996;24 (4):778–89. doi: 10.1002/hep.510240405. [DOI] [PubMed] [Google Scholar]
- 36.McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339 (21):1485–92. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 37.McDonald EM, Mann AH, Thomas HC. Interferons as mediators of psychiatric morbidity: an investigation in a trial of recombinant α-interferon in hepatitis-B carriers. Lancet. 1987;II(8569):1175–8. doi: 10.1016/s0140-6736(87)91319-5. [DOI] [PubMed] [Google Scholar]
- 38.Lindsay KL, Davis GL, Schiff ER, et al. Response to higher doses of interferon alfa-2b in patients with chronic hepatitis C: a randomized multicenter trial. Hepatitis Interventional Therapy Group. Hepatology. 1996;24 (5):1034–40. doi: 10.1002/hep.510240509. [DOI] [PubMed] [Google Scholar]
- 39.Poynard T, Bedossa P, Chevallier M, et al. A comparison of three interferon alfa-2b regimens for the long-term treatment of chronic non-A, non-B hepatitis. Multicenter Study Group. N Engl J Med. 1995;332 (22):1457–62. doi: 10.1056/NEJM199506013322201. [DOI] [PubMed] [Google Scholar]
- 40.Davis GL, Esteban-Mur R, Rustgi V, et al. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339(21):1493–9. doi: 10.1056/NEJM199811193392102. [DOI] [PubMed] [Google Scholar]
- 41.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358 (9286):958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 42.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347 (13):975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 43.Schaefer M, Schmidt F, Folwaczny C, et al. Adherence and mental side effects during hepatitis C treatment with interferon alfa and ribavirin in psychiatric risk groups. Hepatology. 2003;37 (2):443–51. doi: 10.1053/jhep.2003.50031. [DOI] [PubMed] [Google Scholar]
- 44.Bonaccorso S, Marino V, Biondi M, et al. Depression induced by treatment with interferon-α in patients affected by hepatitis C virus. J Affect Disord. 2002;72 (3):237–41. doi: 10.1016/s0165-0327(02)00264-1. [DOI] [PubMed] [Google Scholar]
- 45.Miyaoka H, Otsubo T, Kamijima K, et al. Depression from interferon therapy in patients with hepatitis C [letter] Am J Psychiatry. 1999;156(7):1120. doi: 10.1176/ajp.156.7.1120. [DOI] [PubMed] [Google Scholar]
- 46.Kraus MR, Schafer A, Faller H, et al. Psychiatric symptoms in patients with chronic hepatitis C receiving interferon alfa-2b therapy. J Clin Psychiatry. 2003;64 (6):708–14. doi: 10.4088/jcp.v64n0614. [DOI] [PubMed] [Google Scholar]
- 47.Malaguarnera M, Di Fazio I, Restuccia S, et al. Interferon α-induced depression in chronic hepatitis C patients: comparison between different types of interferon α. Neuropsychobiology. 1998;37 (2):93–7. doi: 10.1159/000026485. [DOI] [PubMed] [Google Scholar]
- 48.Gohier B, Goeb J, Rannou-Dubas K, et al. Hepatitis C, α interferon, anxiety and depression disorders. World J Biol Psychiatry. 2003;4 (3):115–8. doi: 10.1080/15622970310029904. [DOI] [PubMed] [Google Scholar]
- 49.Lang JP, Michel L, Halleguen O. Treatment of affective disorder in hepatitis C: a prospective study in 50 patients. Ann Med Interne (Paris) 2002;153 (7):22–30. [PubMed] [Google Scholar]
- 50.Horikawa N, Yamazaki T, Izumi N, et al. Incidence and clinical course of major depression in patients with chronic hepatitis type C undergoing interferon-α therapy: a prospective study. Gen Hosp Psychiatry. 2003;25 (1):34–8. doi: 10.1016/s0163-8343(02)00239-6. [DOI] [PubMed] [Google Scholar]
- 51.Pariante CM, Orru MG, Baita A, et al. Treatment with interferon-α in patients with chronic hepatitis and mood or anxiety disorders. Lancet. 1999;354 (9173):131–2. doi: 10.1016/S0140-6736(98)04793-X. [DOI] [PubMed] [Google Scholar]
- 52.Koskinas J, Merkouraki P, Manesis E, et al. Assessment of depression in patients with chronic hepatitis: effect of interferon treatment. Dig Dis. 2002;20 (3–4):284–8. doi: 10.1159/000067682. [DOI] [PubMed] [Google Scholar]
- 53.Dieperink E, Ho SB, Thuras P, et al. A prospective study of neuropsychiatric symptoms associated with interferon-a-2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics. 2003;44 (2):104–12. doi: 10.1176/appi.psy.44.2.104. [DOI] [PubMed] [Google Scholar]
- 54.Fontana RJ, Schwartz SM, Gebremariam A, et al. Emotional distress during interferon-α-2B and ribavirin treatment of chronic hepatitis C. Psychosomatics. 2002;43 (5):378–85. doi: 10.1176/appi.psy.43.5.378. [DOI] [PubMed] [Google Scholar]
- 55.Raison CL, Miller AH. The neuroimmunology of stress and depression. Semin Clin Neuropsychiatry. 2001;6 (4):277–94. doi: 10.1053/scnp.2001.0060277. [DOI] [PubMed] [Google Scholar]
- 56.Cotler SJ, Wartelle CF, Larson AM, et al. Pretreatment symptoms and dosing regimen predict side-effects of interferon therapy for hepatitis C. J Viral Hepat. 2000;7 (3):211–7. doi: 10.1046/j.1365-2893.2000.00215.x. [DOI] [PubMed] [Google Scholar]
- 57.Capuron L, Ravaud A. Prediction of the depressive effects of interferon alfa therapy by the patient’s initial affective state [letter] N Engl J Med. 1999;340 (17):1370. doi: 10.1056/NEJM199904293401716. [DOI] [PubMed] [Google Scholar]
- 58.Van Thiel DH, Friedlander L, De Maria N, et al. Treatment of chronic hepatitis C in individuals with pre-existing or confounding neuropsychiatric disease. Hepatogastroenterology. 1998;45(20):328–30. [PubMed] [Google Scholar]
- 59.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241 (4865):540–5. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 60.Capuron L, Ravaud A, Miller AH, et al. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-α cancer therapy. Brain Behav Immun. 2004 May;18(3):205–13. doi: 10.1016/j.bbi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 61.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–65. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 62.Kent S, Bluthe RM, Kelley KW, et al. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13 (1):24–8. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- 63.Bluthe RM, Dantzer R, Kelley KW. Effects of interleukin-I receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Brain Res. 1992;573 (2):318–20. doi: 10.1016/0006-8993(92)90779-9. [DOI] [PubMed] [Google Scholar]
- 64.Capuron L, Raison CL, Musselman DL, et al. Association of exaggerated HPA axis response to the initial injection of interferon-α with development of depression during interferon-α therapy. Am J Psychiatry. 2003;160 (7):1342–5. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- 65.Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991;43 (4):425–73. [PubMed] [Google Scholar]
- 66.Penza KM, Heim C, Nemeroff CB. Neurobiological effects of childhood abuse: implication for the pathophysiology of depression and anxiety. Arch Women Ment Health. 2003;6 (1):15–22. doi: 10.1007/s00737-002-0159-x. [DOI] [PubMed] [Google Scholar]
- 67.Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20(10):469–73. doi: 10.1016/s0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 68.Moreno FA, Heninger GR, McGahuey CA, et al. Tryptophan depletion and risk of depression relapse: a prospective study of tryptophan depletion as a potential predictor of depressive episodes. Biol Psychiatry. 2000;48 (4):327–9. doi: 10.1016/s0006-3223(00)00893-3. [DOI] [PubMed] [Google Scholar]
- 69.Capuron L, Neurauter G, Musselman DL, et al. Interferon-α-induced changes in tryptophan metabolism: relationship to depression and paroxetine treatment. Biol Psychiatry. 2003 Nov I;54(9):906–14. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 70.Capuron L, Ravaud A, Neveu PJ, et al. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;5 (7):468–73. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- 71.Bonaccorso S, Marino V, Puzella A, et al. Increased depressive ratings in patients with hepatitis C receiving interferon-α-based immunotherapy are related to interferon-α-induced changes in the serotonergic systems. J Clin Psychopharmacol. 2002;12 (1):86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 72.Gochee PA, Powell EE, Purdie DM, et al. Association between apolipoprotein E epsilon and neuropsychiatric symptoms during interferon α treatment for chronic hepatitis C. Psychosomatics. 2004;45(1):49–57. doi: 10.1176/appi.psy.45.1.49. [DOI] [PubMed] [Google Scholar]
- 73.Schafer M, Schwaiger M, Berg T. Citalopram for the prevention of interferon-α associated depression in psychiatric risk patients. Hepatology. 2003;38 (4 Suppl 1):320A. doi: 10.1016/j.jhep.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 74.Schafer M, Schmidt F, Amann B, et al. Adding low-dose antidepressants to interferon α treatment for chronic hepatitis C improved psychiatric tolerability in a patient with schizoaffective psychosis. Neuropsychobiology. 2000;42 (Suppl 1):43–5. doi: 10.1159/000054852. [DOI] [PubMed] [Google Scholar]
- 75.Farah A. Interferon-induced depression treated with citalopram. J Clin Psychiatry. 2002;63 (2):166–7. doi: 10.4088/jcp.v63n0213c. [DOI] [PubMed] [Google Scholar]
- 76.Gleason OC, Yates WR. Five cases of interferon-α-induced depression treated with antidepressant therapy. Psychomatics. 1999;40 (6):510–2. doi: 10.1016/S0033-3182(99)71190-4. [DOI] [PubMed] [Google Scholar]
- 77.Gleason OC, Yates WR, Isbell MD, et al. An open-label trial of citalopram for major depression in patients with hepatitis C. J Clin Psychiatry. 2002;63 (3):194–8. doi: 10.4088/jcp.v63n0304. [DOI] [PubMed] [Google Scholar]
- 78.Levenson JL, Fallon HJ. Fluoxetine treatment of depression caused by interferon-α. Am J Gastroenterol. 1993;88 (5):760–1. [PubMed] [Google Scholar]
- 79.Schramm TM, Lawford BR, MacDonald GA, et al. Sertraline treatment of interferon--alfa-induced depressive disorder. Med J Aust. 2000;173 (7):359–61. doi: 10.5694/j.1326-5377.2000.tb125687.x. [DOI] [PubMed] [Google Scholar]
- 80.Yoshida K, Higuchi H, Takahashi H, et al. Favorable effect of milnacipran on depression induced by interferon-α. J Neuropsychiatry Clin Neurosci. 2003;15 (2):242–3. doi: 10.1176/jnp.15.2.242. [DOI] [PubMed] [Google Scholar]
- 81.Janssen HL, Brouwer JT, Van Der Mast RC, et al. Suicide associated with alfa interferon therapy for chronic viral hepatitis. J Hepatol. 1994;21 (2):241–3. doi: 10.1016/s0168-8278(05)80402-7. [DOI] [PubMed] [Google Scholar]
- 82.Weiss K. Safety profile of interferon-α therapy. Semin Oncol. 1998;25 (1 Suppl 1):9–13. [PubMed] [Google Scholar]
- 83.Preskorn SH. Clinically relevant pharmacology of selective serotonin reuptake inhibitors: an overview with emphasis on pharmaeokinetics and effects on oxidative drug metabolism. Clin Pharmacokinet. 1997;32 (Suppl 1):1–21. doi: 10.2165/00003088-199700321-00003. [DOI] [PubMed] [Google Scholar]
- 84.Demetrashvili MD, Miller AH, Raison CL. Managing psychiatric side effects of interferon. Proceedings of the 1st Annual Clinical Care Options for Hepatitis Symposium; 2003 Jun 19–22: Laguna Niguel (CA) [online]. Available from URL: http://clinicaloptions.com/hep [Accessed 2004 Nov 15]
- 85.Owen JR, Nemeroff CB. New antidepressants and the cytochrome P450 system: focus on venlafaxine, nefazodone, and mirtazapine. Depress Anxiety. 1998;7 (Suppl 1):24–32. [PubMed] [Google Scholar]
- 86.Gleason OC, Yates WR, Philipsen MA, et al. Plasma levels of citalopram in depressed patients with hepatitis C. Psychosomatics. 2004;45(1):29–33. doi: 10.1176/appi.psy.45.1.29. [DOI] [PubMed] [Google Scholar]
- 87.Thase ME. Effects of venlafaxine on blood pressure: a meta-analysis of original data from 3744 depressed patients. J Clin Psychiatry. 1998;10 (59):502–8. doi: 10.4088/jcp.v59n1002. [DOI] [PubMed] [Google Scholar]
- 88.Ahmed F, Rovner D, Jacobson JM, et al. Seizures during pegylated interferon and ribavirin therapy for chronic hepatitis C in the WIN-R trial [abstract] Hepatology. 2003;38 (4 Suppl 1):734A. [Google Scholar]
- 89.Weinrieb R, Auriacombe M, Lynch KG, et al. A critical review of selective serotonin reuptake inhibitor-associated bleeding: balancing the risk of treating hepatitis C-infected patients. J Clin Psychiatry. 2003;64:1502–10. doi: 10.4088/jcp.v64n1215. [DOI] [PubMed] [Google Scholar]
- 90.Edwards IR. Withdrawing drugs: nefazodone, the start of the latest saga. Lancet. 2003;361 (9365):1240. doi: 10.1016/S0140-6736(03)13030-9. [DOI] [PubMed] [Google Scholar]
- 91.Park-Wyllie LY, Antoniou T. Concurrent use of bupropion with CYP2B6 inhibitors, nelfinavir, ritonavir and efavirenz: a case series. AIDS. 2003;17 (4):638–40. doi: 10.1097/00002030-200303070-00025. [DOI] [PubMed] [Google Scholar]
- 92.Nelson JC, Mazure CM, Bowers Jr MB, et al. A preliminary, open study of the combination of fluoxetine and desipramine for rapid treatment of major depression. Arch Gen Psychiatry. 1991;48 (4):303–7. doi: 10.1001/archpsyc.1991.01810280019002. [DOI] [PubMed] [Google Scholar]
- 93.Debatista C, Solvason HB, Poirier J, et al. A prospective trial of bupropion SR augmentation in partial and non-responders to serotonergic antidepressants. J Psychopharmacol. 2003;23 (1):27–30. doi: 10.1097/00004714-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 94.Tran PV, Bymaster FP, Mcnamara RK, et al. Dual monoamine modulation for improved treatment of major depressive disorder. J Clin Psychopharmacol. 2003;23 (1):78–86. doi: 10.1097/00004714-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 95.Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326 (19):1250–6. doi: 10.1056/NEJM199205073261904. [DOI] [PubMed] [Google Scholar]
- 96.Goodnick PJ. Treatment of chronic fatigue syndrome with venlafaxine [letter] Am J Psychiatry. 1996;153 (2):294. doi: 10.1176/ajp.153.2.294a. [DOI] [PubMed] [Google Scholar]
- 97.Goodnick PJ. Bupropion in chronic fatigue syndrome [letter] Am J Psychiatry. 1990;147 (8):1091. doi: 10.1176/ajp.147.8.1091a. [DOI] [PubMed] [Google Scholar]
- 98.Goodnick PJ, Sandoval R, Brickman A, et al. Bupropion treatment of fluoxetine-resistant chronic fatigue syndrome. Biol Psychiatry. 1992;32 (9):834–8. doi: 10.1016/0006-3223(92)90087-g. [DOI] [PubMed] [Google Scholar]
- 99.Schwartz AL, Thompson JA, Masood N. Interferon-induced fatigue in patients with melanoma: a pilot study of exercise and methylphenidate. Oncol Nurs Forum Online. 2002;29 (7):E85–90. doi: 10.1188/02.ONF.E85-E90. [DOI] [PubMed] [Google Scholar]
- 100.Sugawara Y, Akechi T, Shima Y, et al. Efficacy of methylphenidate for fatigue in advanced cancer patients: a preliminary study. Palliat Med. 2002;16 (3):261–3. doi: 10.1191/0269216302pm547xx. [DOI] [PubMed] [Google Scholar]
- 101.Sarhill N, Walsh D, Nelson KA, et al. Methylphenidate for fatigue in advanced cancer: a prospective open-label pilot study. Am J Hosp Palliat Care. 2001;18 (3):187–92. doi: 10.1177/104990910101800310. [DOI] [PubMed] [Google Scholar]
- 102.Zifko UA, Rupp M, Schwarz S, et al. Modafinil in treatment of fatigue in multiple sclerosis: results of an open-label study. J Neurol. 2002;249 (8):983–7. doi: 10.1007/s00415-002-0765-6. [DOI] [PubMed] [Google Scholar]
- 103.Afdhal NH, Klopfer A, Tang L, et al. Hemoglobin increase is independently associated with increased health-related quality of life (HRQL) in anemic interferon/ribavirin (IFN/RBV) treated hepatitis C virus (HCV)-infected patients treated with epoetin alfa. Hepatology. 2003;38 (4 Suppl 1):312A. [Google Scholar]
- 104.Afdhal NH, Goon B, Smith K, et al. Epoetin alfa (EPO) improves and maintains health-related quality of life (HRQL) in anemic HCV-infected patients receiving interferon/ribavirin (IFN/RBV): HRQL results from the proactive study. Hepatology. 2003;38 (4 Suppl 1):302A. [Google Scholar]
- 105.Altindag A, Ozbulut O, Ozen S, et al. Interferon-α-induced mood disorder with manic features. Gen Hosp Psychiatry. 2001;23 (3):168–70. doi: 10.1016/s0163-8343(01)00135-9. [DOI] [PubMed] [Google Scholar]
- 106.Carpiniello B, Orru MG, Baita A, et al. Mania induced by withdrawal of treatment with interferon alfa. Arch Gen Psychiatry. 1998;55(1):88–9. doi: 10.1001/archpsyc.55.1.88. [DOI] [PubMed] [Google Scholar]
- 107.Kanno A, Yamada M, Abe M, et al. A case of interferon α-induced manic psychosis in chronic hepatitis C. Tohoku J Exp Med. 1999;187 (1):79–82. doi: 10.1620/tjem.187.79. [DOI] [PubMed] [Google Scholar]
- 108.Monji A, Yoshida I, Tashiro K, et al. A case of persistent manic depressive illness induced by interferon-alfa in the treatment of chronic hepatitis C. Psychosomatics. 1998;39 (6):562–4. doi: 10.1016/s0033-3182(98)71296-4. [DOI] [PubMed] [Google Scholar]
- 109.Strite D, Valentine AD, Meyers CA. Manic episodes in two patients treated with interferon α. J Neuropsychiatry Clin Neurosci. 1997;9 (2):273–6. doi: 10.1176/jnp.9.2.273. [DOI] [PubMed] [Google Scholar]
- 110.Onyike CU, Bonner JO, Lyketsos CG, et al. Mania during treatment of chronic hepatitis C with pegylated interferon and ribavirin. Am J Psychiatry. 2004;61 (3):429–35. doi: 10.1176/appi.ajp.161.3.429. [DOI] [PubMed] [Google Scholar]
- 111.Castera L, Constant A, Henry C, et al. Incidence, risk factors and treatment of mood disorders associated with peginterferon and ribavirin therapy in patients with chronic hepatitis C: results of a prospective study [abstract] Hepatology. 2003;38 (4 Suppl 1):735A. [Google Scholar]
- 112.Gould RA, Ball S, Kaspi SP, et al. Prevalence and correlates of anger attacks: a two site study. J Affect Disord. 1996;39 (1):31–8. doi: 10.1016/0165-0327(96)00017-1. [DOI] [PubMed] [Google Scholar]
- 113.Appels A. Why do imminent victims of a cardiac event feel so tired? Int J Clin Pract. 1997;51 (7):447–50. [PubMed] [Google Scholar]
- 114.Altshuler LL, Post RM, Leverich GS, et al. Antidepressant-induced mania and cycle acceleration: a controversy revisited. Am J Psychiatry. 1995;152 (8):1130–8. doi: 10.1176/ajp.152.8.1130. [DOI] [PubMed] [Google Scholar]


