Abstract
Programmed cell death (PCD) of epidermal cells that cover adventitious root primordia in deepwater rice (Oryza sativa) is induced by submergence. Early suicide of epidermal cells may prevent injury to the growing root that emerges under flooding conditions. Induction of PCD is dependent on ethylene signaling and is further promoted by gibberellin (GA). Ethylene and GA act in a synergistic manner, indicating converging signaling pathways. Treatment of plants with GA alone did not promote PCD. Treatment with the GA biosynthesis inhibitor paclobutrazol resulted in increased PCD in response to ethylene and GA presumably due to an increased sensitivity of epidermal cells to GA. Abscisic acid (ABA) was shown to efficiently delay ethylene-induced as well as GA-promoted cell death. The results point to ethylene signaling as a target of ABA inhibition of PCD. Accumulation of ethylene and GA and a decreased ABA level in the rice internode thus favor induction of epidermal cell death and ensure that PCD is initiated as an early response that precedes adventitious root growth.
Programmed cell death (PCD) is a genetically controlled suicide that occurs as an integral part of life of most multicellular organisms with the aim to eliminate undesirable cells. In plants, it occurs in response to abiotic and biotic factors and plays an essential role in plant development and survival (Greenberg, 1996). Cell death was described in reproductive processes as well as during vegetative growth (Greenberg, 1996; Beers, 1997), during stress adaptation, such as during low oxygen stress (Kawai et al., 1998; Samarajeewa et al., 1999), and as a defense mechanism against pathogens (Tenhaken et al., 1995; Ryerson and Heath, 1996; Chappell et al., 1997; Gechev et al., 2004).
While cell death is inducible by various internal and external signals, hormones frequently mediate these signals and regulate the processes leading to PCD. For example, during seed development, gibberellin (GA) promotes cell death in barley (Hordeum vulgare) aleurone cells, while abscisic acid (ABA) inhibits it (Bethke et al., 1999; Lovegrove and Hooley, 2000). Aerenchyma formation in roots is induced in adaptation to low oxygen stress as an environmental signal (Kawai et al., 1998; Samarajeewa et al., 1999). In maize (Zea mays) and other cereal plants, PCD of root cortex cells, which leads to aerenchyma formation, was shown to be mediated by ethylene (He et al., 1996a, 1996b).
In submergence-tolerant species, ethylene plays an important role in mediating many of the adaptive responses to flooding. Ethylene accumulates upon submergence through physical entrapment and enhanced biosynthesis (Kende et al., 1998). In Rumex palustris, ethylene promotes petiole elongation (Voesenek et al., 1993; Cox et al., 2004). In deepwater rice (Oryza sativa), elevated ethylene levels regulate such diverse responses as accelerated growth of mesocotyls, coleoptiles (Ohwaki and Nagao, 1967), internodes (Métraux and Kende, 1983; Raskin and Kende, 1984a, 1984b; Suge, 1985), and adventitious roots (Bleecker et al., 1986; Lorbiecke and Sauter, 1999) and cell death processes such as those underlying enhanced formation of aerenchyma (Justin and Armstrong, 1991; Inada et al., 2002) and epidermal cell death at the sites where adventitious roots emerge (Mergemann and Sauter, 2000). Since some of these processes need to be tightly coordinated in time and space, the question arises how the ethylene signal is perceived and interpreted by different organs and tissues to allow for these diverse responses in a timely fashion.
In the youngest internode of deepwater rice, it was shown that GA is the ultimate growth-promoting hormone. Submergence causes accumulation of ethylene, a decrease in ABA, and an increase in bioactive gibberellic acids (Hoffmann-Benning and Kende, 1992; Azuma et al., 1995; Van Der Straeten et al., 2001). The alteration in GA to ABA balance is induced by submergence in seedlings (Van Der Straeten et al., 2001) as well as in adult deepwater rice plants (Hoffmann-Benning and Kende, 1992). It can also be brought about by application of ethylene (Hoffmann-Benning and Kende, 1992), indicating that ethylene may be responsible for these changes in GA and ABA levels. In the youngest internode, GA has a growth promotive effect, whereas ABA acts as a growth inhibitor. Therefore, the altered GA to ABA balance strongly and rapidly promotes internodal growth (Kende et al., 1998). Similar changes in GA to ABA balance were reported for R. palustris, another well-adapted semiaquatic plant that displays hyponastic petiole growth in response to submergence (Cox et al., 2004).
Interactions between ethylene, GA, and ABA regulate many aspects of plant growth and development, and the hormones interact in different ways to achieve different responses. Root growth in Arabidopsis (Arabidopsis thaliana) is inhibited by ethylene and ABA and is promoted by GA. Genetic analysis indicated that inhibition of root growth by ABA depends on components of the ethylene signaling cascade defined by ETR1 (ethylene resistant), CTR1 (constitutive triple response), and EIN2 (ethylene insensitive; Beaudoin et al., 2000; Ghassemian et al., 2000). GA promotes root growth in Arabidopsis through proteasome-mediated degradation of the DELLA proteins RGA (repressor of gal-3) and GAI (gibberellic acid insensitive), which act as root growth repressors (Fu et al., 2002; Fu and Harberd, 2003; Fleet and Sun, 2005). Ethylene delays GA-promoted degradation of RGA in Arabidopsis roots and thus maintains root growth inhibition (Achard et al., 2003). Thus, DELLA proteins appear to be one point at which ethylene and GA signaling pathways meet. Apical hook formation in etiolated Arabidopsis is dependent on ethylene and was recently shown to require GA (Vriezen et al., 2004). Application of the ethylene precursor 1-aminocyclo-propane-1-carboxylic acid resulted in accumulation of RGA in nuclei, further pointing to a central role of DELLA proteins in ethylene and GA crosstalk.
Whereas ABA and ethylene act synergistically in regulation of root growth, they have antagonistic effects on seed germination with an inhibitory effect of ABA and a promotive effect of ethylene. GA is also known to promote seed germination. Signaling of all three hormones in the seed was suggested to converge on the ABA pathway (Beaudoin et al., 2000).
PCD in root cortex cells and in epidermal cells of nodes in deepwater rice was shown to be dependent on ethylene (He et al., 1996b; Mergemann and Sauter, 2000). Involvement of other plant hormones in regulating PCD in these tissues was so far not described. In this work, we provide evidence that death of epidermal cells is dependent on ethylene signaling and that GA and ABA are able to modulate this response. Hence, as with internodal elongation, PCD of epidermal cells may be controlled through an altered balance between ethylene, GA, and ABA. However, both processes are controlled through different networking between these hormones.
RESULTS
Ethylene-Induced Cell Death Is a Rapid Response
Previous work showed that treatment with the natural ethylene precursor 1-aminocyclo-propane-1-carboxylic acid induced death of epidermal cells in rice stem sections within 2 h, whereas inhibition of ethylene perception by 2,5-norbornadiene (bicyclo[2.2.1]hepta-2,5-diene) suppressed cell death (Mergemann and Sauter, 2000). In this study, we used ethephon, a synthetic ethylene-releasing compound, to induce epidermal cell death (Fig. 1). All measurements of PCD were done at the third node using Evans blue to stain dead cells (Fig. 1C).
Figure 1.
Adventitious root primordia at the third node of a rice stem. A, Twenty-centimeter-long stem segment including the third node. B, Root initials at the third node are covered by the epidermis. C, A stem section was treated with 150 μm ethephon for 24 h. Epidermal cell death above a root initial was observed by Evans blue staining.
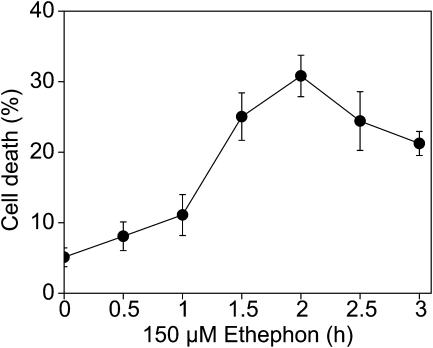
In a time-course experiment, we used ethephon at a concentration of 150 μm, which was shown to be optimal for promoting adventitious root growth (Lorbiecke and Sauter, 1999), and monitored ethephon-induced cell death (Fig. 2). The percentage of dead cells was measured every 30 min between 0 and 3 h as described. A significant increase from 5% to 11% cell death rate was determined at 1 h followed by an increase to 25% cell death at 1.5 h.
Figure 2.
Time course of death of epidermal cells treated with 150 μm ethephon. Cell death was measured every 30 min between 0 and 3 h. Means (±se) of two independent experiments each with six stem segments per time point are shown.
Ethylene Induces Cell Death, and GA and ABA Do Not
Internodal growth in deepwater rice is mediated through an altered balance between GA and ABA in that tissue (Hoffmann-Benning and Kende, 1992). At the third node, alterations in GA and ABA levels have not been studied. Nonetheless, we tested the possibility that regulation of cell death at the third node was also controlled by GA or ABA. We treated rice stem sections for 10 h with either ethephon, GA3, or ABA at concentrations between 0 and 150 μm (Fig. 3). Concentrations of 50 and 150 μm ethephon showed a significant increase in cell death (Fig. 3A). After 4 h, incubation with 150 μm ethephon resulted in 25% cell death (data not shown). After 10 h at 150 μm ethephon, the percentage of dead cells increased to about 43% (Fig. 3A). Treatment with GA3 (Fig. 3B) or ABA (Fig. 3C) did not promote cell death at the concentrations tested. ABA at 10 μm did appear to have a slightly inhibitory effect. Similar results were obtained in a long-term experiment in which PCD was determined after 48 h. Neither GA3 nor ABA at 10 or 100 μm showed a significant PCD-promoting effect (data not shown).
Figure 3.
Effect of ethylene, GA3, and ABA on epidermal cell death. Cell death was measured after treatment for 10 h with 0 to 150 μm ethephon (A), 0 to 100 μm GA3 (B), or 0 to 100 μm ABA (C). Bars indicate averages ± se from six to nine stem sections for each value.
Ethylene-Induced Cell Death Is Enhanced by GA
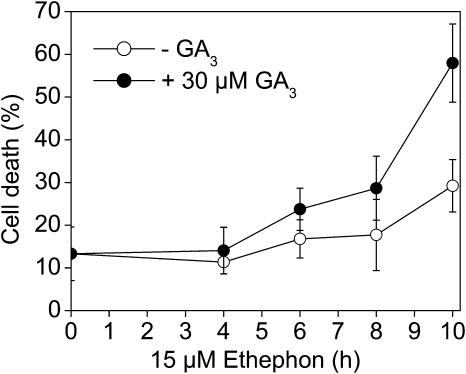
To test if GA3 had an effect on ethephon-induced cell death, we analyzed PCD at 15 μm ethephon with or without 30 μm GA3. PCD was measured between 0 and 10 h of treatment (Fig. 4). After 10 h treatment with 15 μm ethephon, the percentage of dead cells increased from 13% to about 30%. Stem sections treated with 15 μm ethephon and 30 μm GA3 showed an increase to about 58%. Thus, GA3 caused a near doubling of ethylene-induced PCD after 10 h.
Figure 4.
Time course of death of epidermal cells treated with 15 μm ethephon or treated with a combination of 15 μm ethephon and 30 μm GA3. Percentage of cell death was measured between 0 and 10 h. Bars indicate averages ± se from nine stem sections at each time point.
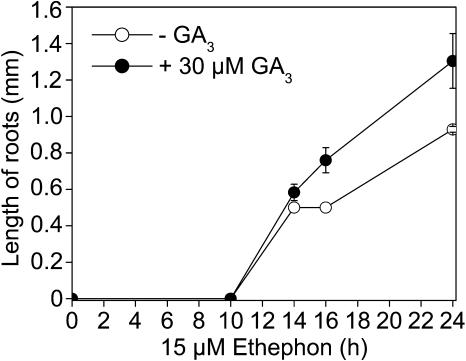
It was hypothesized that PCD of epidermal cells occurred in order to avoid damage to the emerging adventitious root tip (Mergemann and Sauter, 2000). Our studies showed that GA3 not only enhanced the rate of cell death but also the rate of adventitious root growth as described previously (Suge, 1985; Fig. 5). When stem sections were treated with 15 μm ethephon and 30 μm GA3, the lag phase for PCD was between 8 and 10 h (Fig. 4). On the other hand, adventitious root growth was induced between 10 and 14 h (Fig. 5). In an additional experiment, we measured the length of nonpenetrated adventitious root primordia in stems that were treated with 15 μm ethephon in the presence or absence of 30 μm GA3. To that end, cross sections were cut after 0, 2, 4, 6, 8, 10, and 16 h of treatment (data not shown). No increase in average root length was observed up to 10 h of either treatment, whereas roots were significantly longer after 16 h as shown also in Figure 5. Thus, PCD was a faster response to treatment with ethephon and GA3 than was adventitious root growth. This result is in accordance with the previously published idea that rapid induction of PCD is a prerequisite for proper root growth to occur.
Figure 5.
Time course of ethephon-induced adventitious root growth at the third node of rice stems treated with or without GA3. The lengths of penetrated roots were measured between 0 and 24 h of treatment with 15 μm ethephon and with 30 μm GA3 or without GA3 treatment. Averages ± se were calculated from 8 to 10 stem sections.
Paclobutrazol Inhibits Adventitious Root Growth
Paclobutrazol (PAC) is an inhibitor of GA biosynthesis that was shown to reduce internal GA levels in plants (Gianfagna et al., 1998; Rademacher, 2000). In deepwater rice, growth of adventitious roots is induced by ethylene. Furthermore, ethylene-induced adventitious root growth is strongly promoted by GA (Suge, 1985). In order to test if PAC effectively suppressed GA biosynthesis in rice, we employed growth of adventitious roots at the third node as a test system. To efficiently lower endogenous GA levels and de novo GA biosynthesis, plants were pretreated for 8 d either with 2 μm PAC or without PAC as a control. Higher concentrations of PAC were not used in our experiments in order to avoid unspecific or toxic side effects. After 8 d, stem sections were isolated, and treatment with or without PAC was continued and stem sections were incubated with or without ethephon and GA3.
In the absence of PAC, treatment with 15 μm ethephon resulted in an average adventitious root length of 1.2 mm after 48 h of treatment (Fig. 6) as compared to no growth in the absence of ethephon (data not shown). Incubation with 15 μm ethephon in combination with 100 μm GA3 increased root length to 6.7 mm. Treatment with 2 μm PAC resulted in a reduction of 15 μm ethephon-induced adventitious root growth by 33%. Inhibition of ethephon-induced growth by PAC was reverted by GA. Application of 100 μm GA3 to stems that were treated with 15 μm ethephon in the presence of PAC resulted in a growth rate that was higher than that obtained with ethephon alone, indicating that roots were responsive to GA after PAC treatment and that GA still exerted a synergistic effect. Thus, it appeared that the effect of PAC was reversed with GA. However, application of 100 μm GA3 and 15 μm ethephon in PAC-treated stem sections restored root growth to only about half of the growth achieved with 15 μm ethephon and 100 μm GA3 in the absence of PAC. Hence, reversibility of PAC-repressed growth by GA was only partially achieved. Application of even higher GA3 concentrations also did not fully restore root growth rate (data not shown). We conclude that PAC treatment not only reduced GA levels but also responsiveness to GA whereby responsiveness may be altered as a consequence of lowered GA.
Figure 6.
Effect of PAC on growth of adventitious roots at the third node. Rice stem sections were excised from plants that were pretreated with 2 μm PAC for 8 d or left without PAC. PAC treatment was continued throughout treatment with or without 15 μm ethephon (E) and with or without 100 μm GA3. Growth of adventitious roots was measured after 48 h. Bars indicate averages ± se from six stem sections.
PAC Promotes Cell Death
Plants were pretreated with 2 μm PAC for 8 d to inhibit GA biosynthesis. Stem sections were excised and treated with 15 μm ethephon and GA3 at concentrations between 0 and 300 μm (Fig. 7). PAC was also included during hormone incubation. For comparison, stem sections from plants that were not treated with PAC were studied. Hormone treatments were performed for 10 h.
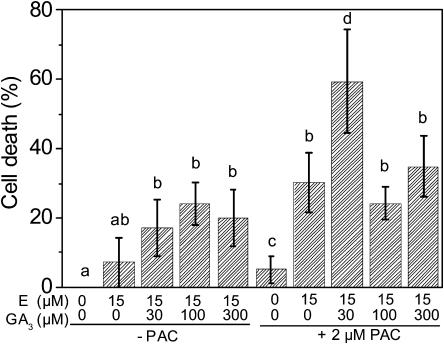
Figure 7.
Effect of internal and external GA on epidermal cell death. Stem sections were treated for 10 h with or without 15 μm ethephon (E) in combination with 0, 30, 100, or 300 μm GA3 as indicated. Experiments were performed with stem sections from plants that were pretreated with 2 μm PAC for 8 d and during experimental procedures or grown and incubated without PAC. Bars indicate averages ± se from four to seven stem sections. Values with different letters are significantly different from each other at P ≤ 0.05 (Mann and Whitney test).
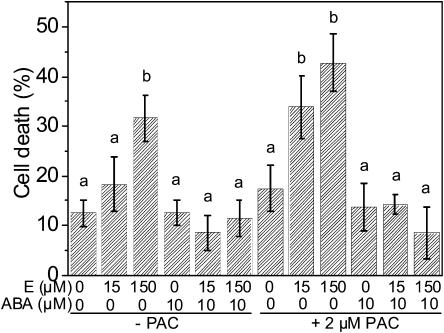
Since application of GA3 resulted in promotion of ethylene-induced cell death, the question arose if endogenous GA was a requirement for ethylene-induced PCD to occur. GA biosynthesis was efficiently reduced by PAC treatment as monitored through adventitious root growth (Fig. 6). In contrast to ethylene-induced root growth, which was reduced by PAC treatment, ethylene-induced PCD was promoted with PAC (Figs. 7 and 10). At a suboptimal ethephon concentration of 15 μm, the PCD rate was 7% in the absence of PAC. In the presence of the GA biosynthesis inhibitor, the rate of cell death was significantly and strongly elevated to 30% (Fig. 7). An elevated cell death rate in the presence of PAC was also observed at 150 μm ethephon (Fig. 10). This response to PAC was observed for PCD and for emergence of adventitious roots but not for root elongation rate (Steffens et al., 2005). It may indicate altered sensitivity of cells that undergo PCD toward GA.
Figure 10.
ABA inhibition of ethylene-induced PCD is not dependent on GA. Rice plants were pretreated with or without 2 μm PAC for 8 d. After harvesting of stem sections, incubation with or without PAC was continued for 10 h with addition of ethephon (E) and ABA as indicated. Bars indicate averages ± se from two independent experiments with five to six segments per treatment.
ABA Is a Potent Inhibitor of Ethylene-Induced and GA-Promoted PCD
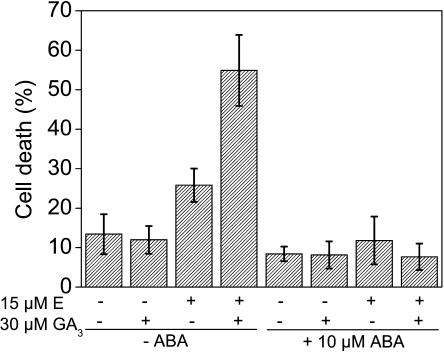
To test for a possible involvement of ABA in regulating epidermal PCD, stem sections were incubated for 10 h with combinations of 15 μm ethephon, 30 μm GA3, and 10 μm ABA (Fig. 8). In the absence of ABA, incubation with 15 μm ethephon resulted in an increase of PCD from 13% to about 26%. Application of 30 μm GA3 by itself had no effect. When 30 μm GA3 was combined with 15 μm ethephon, PCD was increased to 55%. When 10 μm ABA was applied in addition to either 15 μm ethephon or 30 μm GA3 or to a combination of 15 μm ethephon and 30 μm GA3, cell death rates were reduced in all cases to control levels (Fig. 8).
Figure 8.
Effect of ABA on ethephon- and GA3-induced cell death. Stem sections were treated for 10 h with combinations of 15 μm ethephon (E), 30 μm GA3, and 10 μm ABA as indicated. Bars indicate averages ± se from seven stem sections per treatment.
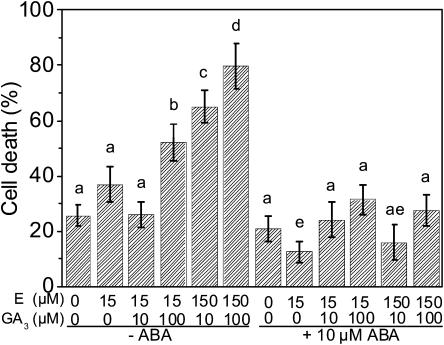
Since ABA at 10 μm was shown to efficiently inhibit cell death induced by 15 μm ethephon or 15 μm ethephon combined with 30 μm GA3 (Fig. 8), we next asked the question if this inhibition could be overcome either by high levels of ethylene or by high levels of GA. Stem sections were incubated with or without 10 μm ABA and in each case with an increasing concentration of ethephon. As shown before, increasing concentrations of ethephon induced elevated cell death rates. In the presence of ABA, induction of PCD was completely abolished even at high levels of ethephon (Fig. 9). Combined application of ethephon and GA3 resulted in a dose-dependent increase in PCD. In the presence of ABA, both ethephon-induced as well as GA3-promoted cell death rates were strongly reduced (Fig. 9).
Figure 9.
GA3 but not ethylene can partially counteract ABA inhibition of ethephon- and GA-induced cell death. Stem sections were treated for 10 h with combinations of ethephon (E), GA3, and ABA at the concentrations indicated. Bars indicate averages ± se from four to six segments per treatment. Values with different letters are significantly different from each other at P ≤ 0.05 (Mann and Whitney test).
An increasing level of GA3 supplied in addition to 15 μm ethephon resulted in partial recovery of PCD in the presence of ABA from 12% with 15 μm ethephon to 24% with 15 μm ethephon plus 10 μm GA3 and to 31% with 15 μm ethephon plus 100 μm GA3 (Fig. 9). On the other hand, increasing the concentration of ethephon from 15 to 150 μm in combination with either 10 or 100 μm GA3 did not promote PCD in the presence of ABA (Fig. 9). The data indicate that ABA is a potent inhibitor of ethylene-mediated epidermal PCD that can be partially overcome by physiological levels of GA3.
Does ABA Act on the Ethylene or on the GA Pathway to Inhibit PCD?
In order to approach the question of how ethylene, GA, and ABA interact to regulate epidermal cell death, we used PAC to inhibit GA biosynthesis. This way we intended to study the effect of ABA on ethylene-induced PCD without involvement of GA. However, as described above, PAC treatment resulted in greater responsiveness of PCD not only to GA but also to ethylene (Figs. 7 and 10). At 15 μm ethephon, the cell death response rose from 18% to 34% when 2 μm PAC was present. At 150 μm ethephon, an increase from 32% to 43% PCD was observed with PAC (Fig. 10).
When ABA was included at a concentration of 10 μM, ethylene-induced cell death was abolished even at an optimal ethylene concentration of 150 μM. Inhibition of ethylene-induced cell death was also observed in the presence of PAC (Fig. 10). Thus, inhibition of ethylene-induced PCD by ABA was not changed by modulating endogenous levels of GA.
DISCUSSION
Ethylene is known to promote cell death in defined tissues during plant development and in response to pathogens or to abiotic stress. Low oxygen stress induces at least two PCD responses, one of which is enhanced formation of aerenchyma, which relies on lysigenous death of cortical cells in roots. Hypoxia-induced aerenchyma formation was well described for cereal plant species and was shown to depend on the transduction of an ethylene signal (He et al., 1996a, 1996b; Kawai et al., 1998). The second PCD response induced by hypoxia is death of epidermal cells that cover adventitious root primordia at the nodes of the deepwater rice stem (Mergemann and Sauter, 2000). This response too was shown to depend on ethylene signaling. The root tip must be protected from mechanical injury when growing through parental shoot tissue. In Arabidopsis, a subtilisin-like protease was shown to be expressed specifically at the outer layers of the parental root at sites of lateral root emergence. Gene expression preceded lateral root protrusion, and it was hypothesized that the protease may digest structural cell wall proteins in order to facilitate lateral root emergence (Neuteboom et al., 1999). Emergence of lateral roots may in general be facilitated by cell and/or cell wall degradation.
Results reported here show that GA modulates epidermal cell death in rice. Even though GA is not effective on its own, it accelerates PCD induced by ethylene. Ethylene and GA act in a synergistic manner, indicating that they share a common signaling pathway. A similar synergistic effect between ethylene and GA on the development of nodal roots was described for the same deepwater rice cultivar by Suge (1985), supporting the idea that PCD and adventitious root emergence are coordinated by the same hormonal network. When rice stem sections were incubated with norbornadiene, an inhibitor of ethylene perception, in the presence of ethylene and GA, epidermal cell death was completely suppressed even after 2 d of incubation, indicating that ethylene signaling was required for cell death to proceed (data not shown). Thus, we conclude that ethylene signaling is the primary signaling pathway that leads to cell death.
Submergence induces 4-fold elevated levels of GA1 in the youngest internode of deepwater rice stems within 3 h (Hoffmann-Benning and Kende, 1992). GA1 is the major bioactive GA in rice (Kobayashi et al., 1989). A similar increase in GA levels was shown to occur in rice seedlings upon submergence (Van Der Straeten et al., 2001), indicating that the hormonal change was neither restricted to the adult stage nor to one particular stem part at that stage. Even though data on endogenous GA levels in nodes of adult plants are missing, we showed that a specific set of epidermal cells and adventitious roots at the nodes are competent to respond to elevated GA levels in the presence of ethylene.
How does GA modulate the ethylene response? In order to find out if endogenous GA was required for ethylene-induced cell death to take place, we pretreated rice plants with PAC, an inhibitor of GA biosynthesis (Rademacher, 2000). PAC reduced ethylene-induced adventitious root growth by 33%, and the effect of PAC was partly reversed by application of exogenous GA3, demonstrating that active GA was reduced by PAC treatment. Ethylene and GA also cooperate to break dormancy in beech (Fagus sylvatica) seeds (Calvo et al., 2004). In beech, a stimulatory effect of ethylene on seed germination was shown to be reverted by PAC (Calvo et al., 2004), indicating that the transition from seed dormancy to germination in beech may be regulated through similar hormone interactions as growth of adventitious roots in rice.
In contrast to adventitious root growth, ethylene-induced epidermal cell death at the same node was not diminished but rather enhanced in PAC-treated rice stems as compared to stems that were not depleted in GA. In the presence of PAC, exogenously applied GA should reflect exclusively the responsiveness of the cells to GA. In the presence of 15 μm ethephon, application of GA3 to GA-starved stems shifted the dose response curve of PCD to lower levels. An optimal cell death response in PAC-treated stems was obtained with 30 μm GA3. Control stems that were not depleted of GA showed an optimal cell death response at 100 μm GA3. At the same time, the maximal PCD response to ethylene and to ethylene applied in combination with GA was increased more than 2-fold after PAC treatment.
The observed changes in cell death rate may be explained in terms of increased sensitivity of epidermal cells. Lowering the GA content might increase the sensitivity toward residual GA. Assuming that the ethylene and GA signaling pathways are linked, it is also conceivable that lowering the GA content increased the sensitivity toward ethylene. This latter scenario could explain why ethylene when applied to PAC-treated tissue induced a stronger PCD response than when applied to tissue in which endogenous GA was not reduced.
In cereals, starchy endosperm cells die during kernel development. The onset of programmed death of starchy endosperm cells is regulated by ethylene (Young et al., 1997). In the maize kernel, ethylene receptors and signaling components were expressed at higher levels in the embryo, which does not undergo PCD, than in the endosperm, which undergoes PCD during kernel maturation. Based on the well-known fact that ethylene is a negative regulator that suppresses signaling, it was hypothesized that reduced receptor expression resulted in increased sensitivity toward ethylene (Gallie and Young, 2004). Hence, at comparable ethylene levels, endosperm cells were induced to die, whereas embryonic cells were not, even though they were competent to do so. It is conceivable that a reduced level of bioactive GA in rice alters the abundance of signaling components in epidermal cells to the effect that the PCD response is accelerated. This positive feedback regulation could affect either GA or ethylene signaling components.
Ethylene-induced adventitious root growth was reduced in the presence of PAC, and root growth in the presence of PAC was only partly restored by exogenously supplied GA3. Altering endogenous GA levels over a period of 8 d profoundly altered the responsiveness to ethylene and to GA at the third node. With respect to PCD, responsiveness of epidermal cells to ethylene and GA was increased. With respect to adventitious root growth, responsiveness to ethylene and GA was reduced. Hormonal regulation of adventitious root growth was described by Steffens et al. (2005). Comparison between PCD, adventitious root growth, internodal growth, and other aspects of submergence adaptation in deepwater rice will provide a better understanding of how these different responses are coordinated in a timely fashion by the same set of hormones. Our results so far demonstrate that the ethylene and GA signaling networks cooperate, and they cooperate differently in different tissues.
Many physiological responses that are promoted by GA are likewise inhibited by ABA. In germinating caryopses, GA is a promoter of PCD of cereal aleurone cells, while ABA inhibits death of these cells (Bethke et al., 1999; Lovegrove and Hooley, 2000). ABA can also inhibit ethylene-regulated PCD. During kernel maturation, death of starchy endosperm cells is induced by ethylene. When ABA perception or biosynthesis was impaired, PCD of maize endosperm cells was accelerated, indicating that the balance between ethylene and ABA is important for timing and progression of endosperm cell death (Young and Gallie, 2000a).
We tested the possibility that epidermal PCD might be regulated not only by ethylene and GA but might be under control of ABA as well. While ABA by itself had no effect on PCD, ABA did prevent cell death induced by ethylene. A cell death-delaying effect of ABA was also described in maize endosperm cells (Young and Gallie, 2000a), during androgenesis of microspores in barley (Wang et al., 1999), and in aleurone cells of germinating barley caryopses (Wang et al., 1996; Bethke et al., 1999). Our report supports the conclusion previously drawn by Young and Gallie (2000b) that ABA may play a general protective role in determining the timing and extent of PCD in cereal development.
Inhibition of ethylene-induced epidermal cell death by ABA was partially overcome by increasing levels of exogenously applied GA. A dose-dependent competitive effect of ABA and GA on ethylene-induced cell death was observed after 10 h of incubation as shown and more pronounced after 1 d of incubation (data not shown). The competitive activities of GA and ABA raised the possibility that ABA exerts its effect on PCD by acting on GA synthesis or GA signaling. Since addition of GA at saturating amounts that were in 10-fold excess to ABA overcame inhibition of PCD only partially, we conclude that GA levels were not limiting in overriding ABA inhibition of PCD.
One scenario that might explain our data could be this: GA acts downstream of ethylene, and GA signaling downstream of ethylene is required for ethylene to induce PCD. If ABA acted on GA signaling, we would expect inhibition of PCD independent of the ethylene concentration applied. This is in fact what we observed. Increasing ethylene levels were not able to overcome ABA inhibition of PCD. Increasing GA levels on the other hand did partly overcome inhibition of PCD by ABA. However, full recovery of PCD was not achieved even at high GA levels, possibly pointing to an alternative or additional mechanism of ABA activity that may circumvent GA signaling.
While NBD, an inhibitor of ethylene perception, completely blocked PCD, treatment with the GA biosynthesis inhibitor PAC did not inhibit PCD. Furthermore, GA by itself did not promote PCD, indicating that activation of the GA signaling pathway was not sufficient or possibly not required for PCD to take place. While GA appears to require one or several activated component(s) of the ethylene signaling pathway in order to promote PCD, ethylene may promote PCD in the absence of GA signaling. We hypothesize that ethylene-induced PCD can be altered by GA signaling but is not ultimately dependent on it. In this model, interaction of ABA with GA signaling would not fully explain inhibition of ethylene-induced PCD. Rather, interference of ABA with ethylene signaling would be required. It is conceivable that both interference of ABA with ethylene signaling and with GA signaling takes place.
Anderson et al. (2004) showed that expression of defense genes in Arabidopsis was induced by ethylene and gene expression was suppressed by ABA. Suppression of gene expression by ABA was not reversed by exogenous application of ethylene, pointing to a dominant effect of ABA. Full inhibition of ethylene-induced PCD by ABA in deepwater rice may be a similar dominant effect of ABA. We hypothesize that the inhibitory effect of ABA on epidermal cell death bypasses GA production. Rather, we propose that ABA interferes with ethylene and possibly GA signaling. A model showing possible sites of GA and ABA activity in affecting ethylene-induced PCD is given in Figure 11. Recent work revealed that ABA was an effective inhibitor not only of epidermal PCD but also of adventitious root growth in deepwater rice (Steffens et al., 2005).
Figure 11.
Model of possible sites of GA and ABA activity in affecting ethylene-induced PCD of epidermal cells covering adventitious root primordia.
Cell death was observed exclusively in epidermal patches that covered root primordia. Surrounding epidermal cells were not affected and stayed alive at any treatment. Such a high cell specificity was not always observed in cell death responses. In maize kernels, ethylene treatment caused ectopic induction of DNA fragmentation in the embryo. DNA degradation is characteristic of many cell death programs and was also observed in maize endosperm cells in the same kernels that are destined to die (Young et al., 1997). With ethylene treatment, cell death in the endosperm proceeded from the outside toward the center, while normal progression occurs the other way around. These results indicated that the competency to enter PCD was not restricted and that additional regulation was required to ensure proper cell death progression in cereal kernels. In rice, a locally defined region of epidermal cells that covers adventitious root primordia is predetermined to initiate PCD in response to ethylene. Ectopic PCD was not observed. This clear difference should allow us to identify the molecular components that determine competency for cell death. Experiments aimed at identifying differential gene expression in epidermal cells destined to enter a cell death program upon ethylene signaling are under way.
MATERIALS AND METHODS
Growth Conditions and Isolation of Rice Stem Sections
Deepwater rice plants (Oryza sativa Pin Gaew 56) were grown as described earlier (Sauter, 1997). Internodal stem sections were prepared from 12- to 14-week-old plants. Sections were cut 2 cm below the third node and had a total length of 20 cm (Mergemann and Sauter, 2000). All measurements of epidermal cell death and adventitious root growth were done at the third node from the top. Stem sections were placed in a 150-mL beaker containing 20 mL of aqueous solution of hormones or growth inhibitors. Plastic cylinders covered the beakers with the stem sections to assure high humidity. All experiments were performed at 27°C under permanent light at 150 μE m−2 s−1.
Ten- to eleven-week-old rice plants were pretreated for 8 d in hydroponic fertilizer containing 2 μm PAC (Duchefa), which inhibits GA biosynthesis (Gianfagna et al., 1998; Rademacher, 2000). Rice plants were then used for measurement of adventitious root length or cell death.
Measurement of Adventitious Root Length
After treatment of stem sections with 15 μm ethephon, with or without 30 μm GA3, the length of penetrated adventitious roots was measured with a ruler at 0, 10, 14, 16, and 24 h. Stem sections were treated for 48 h with ethephon and GA in the presence or absence of 2 μm PAC before adventitious root length was determined.
Cell Death Measurements
After incubation of stem sections, a 1-cm portion including the third node was stained with 2% Evans blue (w/v), a nontoxic water-soluble pigment, to identify dead epidermal cells as described previously (Mergemann and Sauter, 2000). The small segments were stained in Evans blue for 3 min and afterward washed with water. Staining patterns were observed with a binocular. Four different stages (I to IV) were classified as described by Mergemann and Sauter (2000). Briefly, stage I indicated no staining of epidermal cells covering an adventitious root primordium. In stage II, few cells stained blue above a root primordium. Stage III showed an area of epidermal cells that were stained. In stage IV, the epidermis has ruptured and in some cases a root initial is growing through the opening. Stages II to IV were taken together even though the majority of cell death events were in stage II. Each epidermal patch above a root initial counted as one cell death event. Cell death events were calculated as percentage of the total number of epidermal patches above root initials. Statistical significance of treatment differences was assessed using the Mann and Whitney test (U-test).
Hormone and Inhibitor Treatment
Aqueous solutions of ethephon (2-chloroethanephosphoric acid; Sigma-Aldrich), GA3 (Sigma-Aldrich), and ABA (Sigma-Aldrich) were used at the final concentrations indicated. PAC (Duchefa) was used at 2 μm as an inhibitor of GA biosynthesis.
Acknowledgments
The authors thank Dr. Guillaume Rzewuski for critical reading of the manuscript.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.064469.
References
- Achard P, Vriezen WH, Van Der Straeten D, Harberd NP (2003) Ethylene regulates Arabidopsis development via the modulation of DELLA protein growth repressor function. Plant Cell 15: 2816–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K (2004) Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16: 3460–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma T, Hirano T, Deki Y, Uchida N, Yasuda T, Yamaguchi T (1995) Involvement of the decrease in levels of abscisic acid in the internodal elongation of submerged floating rice. J Plant Physiol 146: 323–328 [Google Scholar]
- Beaudoin N, Serizet C, Gosti F, Giraudat J (2000) Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 12: 1103–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers EP (1997) Programmed cell death during plant growth and development. Cell Death Differ 4: 649–661 [DOI] [PubMed] [Google Scholar]
- Bethke PC, Lonsdale JE, Fath A, Jones RL (1999) Hormonally regulated programmed cell death in barley aleurone cells. Plant Cell 11: 1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Schuette JL, Kende H (1986) Anatomical analysis of growth and development patterns in the internode of deepwater rice. Planta 169: 490–497 [DOI] [PubMed] [Google Scholar]
- Calvo AP, Nicolas C, Lorenzo O, Nicolas G, Rodriguez D (2004) Evidence for positive regulation by gibberellins and ethylene of ACC oxidase expression and activity during transition from dormancy to germination in Fagus sylvatica L. seeds. J Plant Growth Regul 23: 44–53 [Google Scholar]
- Chappell J, Levine A, Tenhaken R, Lusso M, Lamb C (1997) Characterization of a diffusible signal capable of inducing defense gene expression in tobacco. Plant Physiol 113: 621–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MC, Benschop JJ, Vreeburg RA, Wagemaker CA, Moritz T, Peeters AJ, Voesenek LA (2004) The role of ethylene, auxin, abscisic acid, and gibberellin in the hyponastic growth of submerged Rumex palustris petioles. Plant Physiol 136: 2948–2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleet CM, Sun TP (2005) A DELLAcate balance: the role of gibberellin in plant morphogenesis. Curr Opin Plant Biol 8: 77–85 [DOI] [PubMed] [Google Scholar]
- Fu X, Harberd NP (2003) Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421: 740–743 [DOI] [PubMed] [Google Scholar]
- Fu X, Richards DE, Ait-ali T, Hynes LW, Ougham H, Peng J, Harberd NP (2002) Gibberellin-mediated proteasome-dependent degradation of the barley DELLA protein SLN1 repressor. Plant Cell 14: 3191–3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie DR, Young TE (2004) The ethylene biosynthetic and perception machinery is differentially expressed during endosperm and embryo development in maize. Mol Genet Genomics 271: 267–281 [DOI] [PubMed] [Google Scholar]
- Gechev TS, Gadjev IZ, Hille J (2004) An extensive microarray analysis of AAL-toxin-induced cell death in Arabidopsis thaliana brings new insights into the complexity of programmed cell death in plants. Cell Mol Life Sci 61: 1185–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P (2000) Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12: 1117–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfagna TJ, Logendra L, Durner EF, Janes HW (1998) Improving tomato harvest index by controlling crop height and side shoot production. Life Support Biosph Sci 5: 255–261 [PubMed] [Google Scholar]
- Greenberg JT (1996) Programmed cell death: a way of life of plants. Proc Natl Acad Sci USA 93: 12094–12097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CJ, Finlayson SA, Drew MC, Jordan WR, Morgan PW (1996. a) Ethylene biosynthesis during aerenchyma formation in roots of maize subjected to mechanical impedance and hypoxia. Plant Physiol 112: 1679–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CJ, Morgan PW, Drew MC (1996. b) Transduction of an ethylene signal is required for cell death and lysis in the root cortex of maize during aerenchyma formation induced by hypoxia. Plant Physiol 112: 463–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann-Benning S, Kende H (1992) On the role of abscisic acid and gibberellin in the regulation of growth in rice. Plant Physiol 99: 1156–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada N, Sakai A, Kuroiwa H, Kuroiwa T (2002) Three-dimensional progression of programmed death in the rice coleoptile. Int Rev Cytol 218: 221–258 [DOI] [PubMed] [Google Scholar]
- Justin SHFW, Armstrong W (1991) Evidence for the involvement of ethylene in aerenchyma formation in adventitious roots of rice (Oryza sativa L.). New Phytol 118: 49–62 [Google Scholar]
- Kawai M, Samarajeewa PK, Barrero RA, Nishiguchi M, Uchimiya H (1998) Cellular dissection of the degradation pattern of cortical cell death during aerenchyma formation in rice roots. Planta 204: 277–287 [Google Scholar]
- Kende H, van der Knaap E, Cho HT (1998) Deepwater rice: a model plant to study stem elongation. Plant Physiol 118: 1105–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Sakurai A, Saka H, Takahashi N (1989) Quantitative analysis of endogenous gibberellins in normal and dwarf cultivars of rice. Plant Cell Physiol 30: 963–969 [Google Scholar]
- Lorbiecke R, Sauter M (1999) Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiol 119: 21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovegrove A, Hooley R (2000) Gibberellin and abscisic acid signalling in aleurone. Trends Plant Sci 5: 102–110 [DOI] [PubMed] [Google Scholar]
- Mergemann H, Sauter M (2000) Ethylene induces epidermal cell death at the site of adventitious root emergence in rice. Plant Physiol 124: 609–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métraux JP, Kende H (1983) The role of ethylene in the growth response of submerged deepwater rice. Plant Physiol 72: 441–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuteboom LW, Veth-Tello LM, Clijdesdale OR, Hooykaas PJ, van der Zaal BJ (1999) A novel subtilisin-like protease gene from Arabidopsis thaliana is expressed at sites of lateral root emergence. DNA Res 6: 9–13 [DOI] [PubMed] [Google Scholar]
- Ohwaki Y, Nagao M (1967) Growth of rice coleoptiles in relation to oxygen concentration. Sci Rep Tohoku Univ Series V Biol 33: 1–5 [Google Scholar]
- Rademacher W (2000) Growth retardants: effects on gibberellin biosynthesis and other metabolic pathways. Annu Rev Plant Physiol Plant Mol Biol 51: 501–531 [DOI] [PubMed] [Google Scholar]
- Raskin I, Kende H (1984. a) Regulation of growth in stem sections of deep water rice. Planta 160: 66–72 [DOI] [PubMed] [Google Scholar]
- Raskin I, Kende H (1984. b) Role of gibberellin in the growth response of deep water rice. Plant Physiol 76: 947–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryerson DE, Heath MC (1996) Cleavage of nuclear DNA into oligonucleosomal fragments during cell death induced by fungal infection or by abiotic treatments. Plant Cell 8: 393–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarajeewa PK, Barrero RA, Umeda-Hara C, Kawai M, Uchimiya H (1999) Cortical cell death, cell proliferation, macromolecular movements and rTip1 expression pattern in roots of rice (Oryza sativa L.) under NaCl stress. Planta 207: 354–361 [Google Scholar]
- Sauter M (1997) Differential expression of a CAK (cdc2-activating kinase)-like protein kinase, cyclins and cdc2 genes from rice during the cell cycle and in response to gibberellin. Plant J 11: 181–190 [DOI] [PubMed] [Google Scholar]
- Steffens B, Wang J, Sauter M (2005) Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta doi/10.1007/s00425-005-0111-1 [DOI] [PubMed]
- Suge H (1985) Ethylene and gibberellin: regulation of internodal elongation and nodal root development in floating rice. Plant Cell Physiol 26: 607–614 [Google Scholar]
- Tenhaken R, Levine A, Brisson LF, Dixon RA, Lamb C (1995) Function of the oxidative burst in hypersensitive disease resistance. Proc Natl Acad Sci USA 92: 4158–4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Straeten D, Zhou Z, Prinsen E, Van Onckelen HA, Van Montagu MC (2001) A comparative molecular-physiological study of submergence response in lowland and deepwater rice. Plant Physiol 125: 955–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LA, Banga M, Their RH, Mudde CM, Harren FJ, Barendse GW, Blom CW (1993) Submergence-induced ethylene synthesis, entrapment, and growth in two plant species with contrasting flooding resistances. Plant Physiol 103: 783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriezen WH, Achard P, Harberd NP, Van Der Straeten D (2004) Ethylene-mediated enhancement of apical hook formation in etiolated Arabidopsis thaliana seedlings is gibberellin dependent. Plant J 37: 505–516 [DOI] [PubMed] [Google Scholar]
- Wang H, Hoekstra S, van Bergen S, Lamers GEM, Oppedijk BJ, van der Heiden MW, de Priester W, Schilperoort RA (1999) Apoptosis in developing anthers and the role of ABA in this process during androgenesis in Hordeum vulgare L. Plant Mol Biol 39: 489–501 [DOI] [PubMed] [Google Scholar]
- Wang H, Li J, Bostock RM, Gilchrist DG (1996) Apoptosis: functional paradigm for programmed plant cell death induced by a host-selective phytotoxin and invoked during development. Plant Cell 8: 375–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young TE, Gallie DR (2000. a) Regulation of programmed cell death in maize endosperm by abscisic acid. Plant Mol Biol 42: 397–414 [DOI] [PubMed] [Google Scholar]
- Young TE, Gallie DR (2000. b) Programmed cell death during endosperm development. Plant Mol Biol 44: 283–301 [DOI] [PubMed] [Google Scholar]
- Young TE, Gallie DR, DeMason DA (1997) Ethylene-mediated programmed cell death during maize endosperm development of wild-type and shrunken2 genotypes. Plant Physiol 115: 737–751 [DOI] [PMC free article] [PubMed] [Google Scholar]