Abstract
Arabidopsis (Arabidopsis thaliana) mutants lacking a functional ERA1 gene, which encodes the β-subunit of protein farnesyltransferase (PFT), exhibit pleiotropic effects that establish roles for protein prenylation in abscisic acid (ABA) signaling and meristem development. Here, we report the effects of T-DNA insertion mutations in the Arabidopsis GGB gene, which encodes the β-subunit of protein geranylgeranyltransferase type I (PGGT I). Stomatal apertures of ggb plants were smaller than those of wild-type plants at all concentrations of ABA tested, suggesting that PGGT I negatively regulates ABA signaling in guard cells. However, germination of ggb seeds in response to ABA was similar to the wild type. Lateral root formation in response to exogenous auxin was increased in ggb seedlings compared to the wild type, but no change in auxin inhibition of primary root growth was observed, suggesting that PGGT I is specifically involved in negative regulation of auxin-induced lateral root initiation. Unlike era1 mutants, ggb mutants exhibited no obvious developmental phenotypes. However, era1 ggb double mutants exhibited more severe developmental phenotypes than era1 mutants and were indistinguishable from plp mutants lacking the shared α-subunit of PFT and PGGT I. Furthermore, overexpression of GGB in transgenic era1 plants partially suppressed the era1 phenotype, suggesting that the relatively weak phenotype of era1 plants is due to partial redundancy between PFT and PGGT I. These results are discussed in the context of Arabidopsis proteins that are putative substrates of PGGT I.
Protein prenylation involves the formation of a thioether bond between a farnesyl (C15) or geranylgeranyl (C20) group and a C-terminal Cys residue (Clarke, 1992; Zhang and Casey, 1996). This posttranslational modification promotes protein-membrane and, in many cases, protein-protein interactions. Three protein prenyltransferases are known to catalyze protein prenylation in plants and other eukaryotes: (1) a heterodimeric protein farnesyltransferase (PFT) that consists of α- and β-subunits and transfers a farnesyl group from farnesyl pyrophosphate (FPP) to proteins bearing a C-terminal CaaX motif, where “C” is Cys, “a” is often an aliphatic amino acid, and “X” is generally Met, Ala, Gln, Ser, or Cys; (2) a heterodimeric type I protein geranylgeranyltransferase (PGGT I) that shares a common α-subunit with PFT but possesses a distinct β-subunit and transfers a geranylgeranyl group from geranylgeranyl pyrophosphate (GGPP) to proteins bearing a C-terminal CaaX motif, where “X” is Leu; and (3) a heterotrimeric type II protein geranylgeranyltransferase (PGGT II or Rab PGGT) that consists of distinct α- and β-subunits, as well as a third subunit called the Rab escort protein, and transfers a geranylgeranyl group from GGPP to Rab protein substrates (Clarke, 1992; Zhang and Casey, 1996; Crowell, 2000). PFT subunits have been identified and characterized from pea (Pisum sativum; Yang et al., 1993; Qian et al., 1996), tomato (Lycopersicon esculentum; Yalovsky et al., 1997), and Arabidopsis (Arabidopsis thaliana; Cutler et al., 1996; Pei et al., 1998; Running et al., 2004), and PGGT I subunits have been identified and characterized from Arabidopsis (Caldelari et al., 2001). These and other studies establish the functional similarities between plant, yeast, and animal protein prenyltransferases (Crowell, 2000).
Most prenylated proteins undergo further modifications. For example, following the attachment of a prenyl group to the Cys residue of a CaaX motif, the three amino acids at the C terminus of the protein are proteolytically removed and the prenylcysteine residue is carboxyl methylated (Gutierrez et al., 1989; Yamane et al., 1990; Clarke, 1992; Boyartchuk et al., 1997). These modifications are necessary steps in the maturation, targeting, and function of prenylated proteins (Hancock et al., 1991; Sapperstein et al., 1994; Parish et al., 1995; Bergo et al., 2000; Rodríguez-Concepción et al., 2000). Two distinct CaaX proteases, encoded by the STE24 and RCE1 genes, and a single prenylcysteine α-carboxyl methyltransferase, encoded by the STE14 gene, catalyze the proteolysis and carboxyl methylation of prenylated proteins in Saccharomyces cerevisiae (Hrycyna et al., 1991; Sapperstein et al., 1994; Boyartchuk et al., 1997; Tam et al., 1998). Moreover, orthologs of these genes have been described in Arabidopsis, including AtSTE24, AtFACE-2 (an ortholog of RCE1), AtSTE14A, and AtSTE14B (Crowell et al., 1998; Crowell and Kennedy, 2001; Bracha et al., 2002; Narasimha Chary et al., 2002; Cadiñanos et al., 2003). Thus, protein prenylation, proteolysis, and methylation are conserved protein modifications in eukaryotes.
In Arabidopsis, loss-of-function mutations in the ERA1 gene, which encodes the β-subunit of PFT, cause an enhanced response to abscisic acid (ABA) in seed germination and stomatal closure assays (Cutler et al., 1996; Pei et al., 1998). This finding implicates one or more farnesylated proteins in negative regulation of ABA signaling. The era1 phenotype, which is associated with increased anion channel activation following ABA treatment of guard cells, is sufficient to cause reduced water loss during drought stress (Pei et al., 1998). era1 is epistatic to abi1 and abi2, suggesting that ERA1 acts at or downstream of the ABI1 and ABI2 phosphatases. However, ERA1 acts at or upstream of the ABI3 and ABI5 transcription factors, and transcription of the ABI3 gene has been shown to be elevated in era1 plants (Brady et al., 2003). In addition to an enhanced response to ABA, era1 mutants also exhibit enlarged meristems and supernumerary floral organs (Running et al., 1998; Bonetta et al., 2000; Yalovsky et al., 2000; Ziegelhoffer et al., 2000). This finding implicates protein farnesylation in regulation of meristem size. Similarly, loss-of-function mutations in the Arabidopsis PLP gene, which encodes the shared α-subunit of PFT and PGGT I, cause an enhanced response to ABA, dramatically larger meristems with disruptions in the pattern of cell layers, and flowers with significantly greater numbers of floral organs, especially petals (Running et al., 2004). Together, these observations establish roles for PFT in negative regulation of ABA signaling and meristem size.
To date, no phenotypic analyses of PGGT I mutants (i.e. mutants lacking the β-subunit of PGGT I) have been reported. It is tempting to infer the phenotypic consequences of PGGT I β-subunit mutations by comparing the phenotypes of era1 and plp plants. For example, era1 flowers exhibit extra floral organs, especially sepals and petals. However, the phenotype is relatively weak, and many era1 flowers have the normal number of four sepals and four petals (Running et al., 1998; Bonetta et al., 2000). plp flowers, on the other hand, exhibit a much stronger phenotype, averaging 5.1 sepals and 8.7 petals per flower (Running et al., 2004). This result can be interpreted in two ways: either floral organ number is governed by both farnesylated and geranylgeranylated proteins, or floral organ number is governed only by farnesylated proteins and PGGT I partially compensates for loss of PFT activity in era1 flowers by prenylating a subset of PFT substrate proteins. The only reasonable way to discriminate between these possibilities is to examine the phenotypes of mutants lacking PGGT I, but not PFT, activity. The ABA phenotypes of era1 and plp mutants present similar difficulties (Running et al., 2004) and suggest that PGGT I may play a role in ABA signaling distinct from that of PFT. For example, PGGT I may positively regulate ABA signaling.
To address the role of PGGT I in plant development and elucidate its relationship to PFT, we identified PGGT I β-subunit loss-of-function mutants (ggb) and generated transgenic era1 plants that overexpress the PGGT I β-subunit. ggb mutants exhibited no obvious developmental phenotypes but showed increased stomatal closure in response to ABA and increased lateral root formation in response to auxin. Importantly, era1 ggb double mutants phenocopied plp mutants and displayed several defects in meristem function, floral patterning, and growth rate. This result demonstrates that ERA1 and GGB do not function independently of PLP and supports the hypothesis that PGGT I partially compensates for loss of PFT in era1 mutants. This conclusion is further supported by the observation that overexpression of GGB partially rescued the era1 phenotype.
RESULTS
Identification of T-DNA Insertions in the Arabidopsis PGGT I β-Subunit Gene
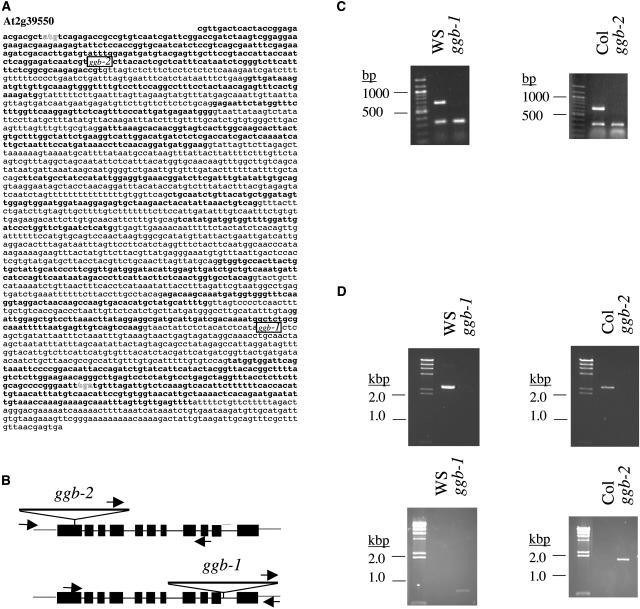
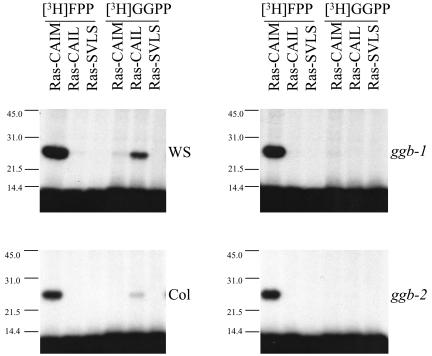
At2g39550, which was given the gene class symbol GGB by The Arabidopsis Information Resource, is the only gene in the Arabidopsis genome with significant sequence similarity to known PGGT I β-subunit genes. Furthermore, a cDNA corresponding to GGB (AtGGT-1B) was shown to encode a functional PGGT I β-subunit (Caldelari et al., 2001). As shown in Figure 1, A and B, GGB consists of 11 exons and 10 introns. Two distinct T-DNA insertion mutations were identified in the GGB coding region: one from the University of Wisconsin Arabidopsis Knockout Facility (Krysan et al., 1999; Sussman et al., 2000) in the Wassilewskija (Ws) background called ggb-1 and one from the Salk Institute Genomic Analysis Laboratory (Alonso et al., 2003) in the Columbia (Col) background called ggb-2. In ggb-1, the T-DNA is located in intron 10, whereas in ggb-2, the T-DNA is located in exon 1. These insertions abolished detectable PGGT I β-subunit mRNA accumulation, as judged by semiquantitative RT-PCR analysis (Fig. 1C). Genomic PCR analysis using gene-specific primers that bind to sequences flanking the two T-DNA insertion sites produced PCR products of the expected sizes in Ws and Col but did not produce products in the mutant lines (Fig. 1D, top panels). However, genomic PCR analysis using one T-DNA-specific primer and one gene-specific primer produced PCR products of the expected sizes for each of the mutant lines but not for the corresponding wild types (Fig. 1D, bottom panels). These results, together with segregation analyses (data not shown), confirmed the presence, location, and homozygosity of the T-DNA insertions in ggb-1 and ggb-2. As shown in Figure 2, PGGT I activity in the presence of the PGGT I substrate Ras-CAIL (Randall and Crowell, 1999) was detectable in floral extracts of wild-type plants but was not detectable in extracts of ggb plants. In contrast, PFT activity was detectable in floral extracts of wild-type and ggb plants using the PFT substrate Ras-CAIM (Randall and Crowell, 1999). Weak geranylgeranylation of Ras-CAIM was also observed in the presence of Ws floral extracts. Geranylgeranylation of PFT protein substrates was described previously for plant cell extracts containing protein prenyltransferase activities (Randall et al., 1993) and was attributed either to utilization of GGPP by PFT or recognition of Ras-CAIM by PGGT I. The results shown in Figure 2 support the former hypothesis because weak geranylgeranylation of Ras-CAIM was observed in the ggb-1 extract, which lacks PGGT I activity. Similar cross-specificities for isoprenoid and protein substrates have been reported for protein prenyltransferases from other organisms (Trueblood et al., 1993).
Figure 1.
Structure of the GGB (At2g39550) gene and molecular characterization of ggb mutants. A, The genomic sequence of the GGB gene is shown. Exons are in bold type. The ATG initiation and TGA termination codons are in shadow type. The locations of the T-DNA inserts in ggb-1 and ggb-2 are indicated. B, The intron-exon structure of the GGB gene, the locations of T-DNA inserts in ggb-1 and ggb-2, and the primer binding sites used for genomic PCR analysis are shown. C, RT-PCR analysis of GGB transcripts is shown for Ws, ggb-1, Col, and ggb-2, along with 100 bp Mr markers. The 18S rRNA competimer/primer pairs were included in each RT-PCR reaction as an internal control. The 18S RT-PCR product is approximately 300 bp in length, whereas the GGB RT-PCR product is 657 bp in length. D, Genomic PCR of Ws, ggb-1, Col, and ggb-2 is shown along with HindIII-cut λ DNA markers. The top two panels show the results of PCR analysis using genomic DNA from the indicated lines and two GGB gene-specific primers that bind to sequences flanking the T-DNA insertion sites. The bottom two panels show the results of genomic PCR analysis using one T-DNA-specific primer and one GGB gene-specific reverse primer.
Figure 2.
PFT and PGGT I activities in floral extracts of wild-type and ggb mutants of Arabidopsis. Protein prenyltransferase reactions were performed in the presence of floral extracts from the indicated plants, recombinant Ras protein substrates expressed in Escherichia coli, and either [3H]FPP or [3H]GGPP. Ras-CAIM is a well-characterized substrate for PFT, Ras-CAIL is a well-characterized substrate for PGGT I, and Ras-SVLS is a control protein that cannot be prenylated by any known protein prenyltransferase (Randall and Crowell, 1999).
Growth and Morphology of ggb Mutant Lines
The growth and morphology of ggb plants were not significantly different from the wild type. As shown in Figure 3, the rosettes, inflorescences, and flowers of ggb plants were indistinguishable from the corresponding wild-type plants. The lack of a floral phenotype in ggb plants supported the hypothesis that the weak floral phenotype of era1 plants compared to plp plants was due to the ability of PGGT I to partially compensate for loss of PFT in era1 flowers by prenylation of PFT substrate proteins. This hypothesis was further supported by partial suppression of the era1 phenotype in transgenic era1 plants that constitutively overexpressed a full-length GGB cDNA (Fig. 4). GGB overexpression caused no detectable phenotype in a wild-type background (data not shown) but suppressed the reduced stature, overinitiation of flower primordia, and floral organ number defects of the era1-4 mutant, suggesting partial restoration of normal meristematic function. era1 ggb double mutants phenocopied plp plants and exhibited a strong plp phenotype (Fig. 4). Like plp mutants, era1-4 ggb-2 double mutants produced greatly enlarged meristems, extra floral organs (especially petals), and exhibited retarded growth and reduced stature compared to the wild type (Running et al., 2004).
Figure 3.
Growth and morphology of wild-type and ggb mutants of Arabidopsis. Rosettes, inflorescences, and flowers of Ws, ggb-1, Col, and ggb-2 are shown.
Figure 4.
Effects of ggb-2 or constitutive GGB overexpression on the era1-4 phenotype. The floral phenotypes of ggb-2 and era1-4 ggb-2 plants and inflorescence and floral phenotypes of era1-4 and era1-4 35S::GGB plants are shown.
ABA Signaling in ggb Mutant Lines
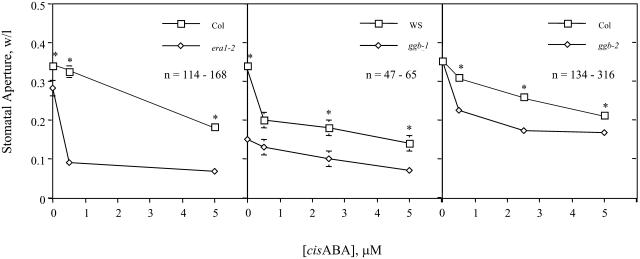
Because plp and era1 seeds exhibit an enhanced response to ABA, PFT and PGGT I may play distinct roles in ABA signaling. To examine the role of PGGT I in ABA signaling, excised rosette leaves of wild-type and ggb plants were floated for 2 h under high-light conditions in buffer containing various concentrations of ABA. Epidermal peels were then prepared, stained with toluidine blue, and immediately photographed at a magnification of 40×. As shown in Figure 5, the apertures of ggb stomata were smaller than the wild type over a range of ABA concentrations. The stomata of the ggb-1 line were more severely affected and exhibited almost complete closure in the absence of exogenous ABA. Stomatal apertures in the ggb-2 line were similar to the wild type in the absence of exogenous ABA but exhibited an enhanced response to exogenous ABA. These results are consistent with the hypothesis that guard cells of ggb mutants exhibit an enhanced response to ABA, although the response of era1-2 guard cells to ABA was greater than that of ggb-2 guard cells (both are in the Col background; Figure 5). The Ws ecotype of Arabidopsis appeared to be more sensitive to ABA than the Col ecotype, which may explain why the ggb-1 line exhibited the more severe phenotype, with almost complete stomatal closure in the absence of exogenous ABA.
Figure 5.
Analysis of Ws, ggb-1, Col, ggb-2, and era1-2 stomata in response to exogenous ABA. Epidermal peels were prepared from excised leaves treated for 2 h with various concentrations of ABA under high-light conditions. Stomatal apertures were measured from photographic images and recorded as the ratio of width per length. Standard error of the mean is shown for all data points. Asterisks indicate significant differences determined by Student's t test (P < 0.05).
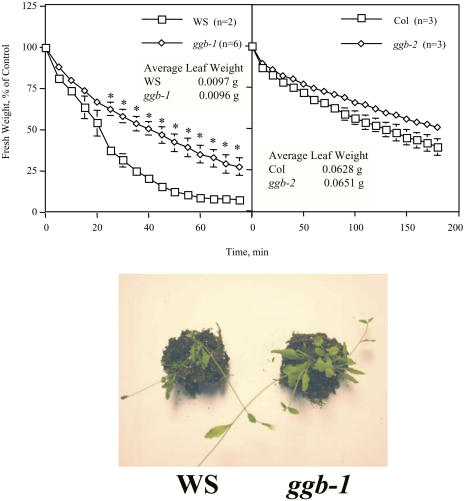
The results described above indicate that ggb plants exhibit an enhanced response to ABA in guard cells. To confirm these results and evaluate the physiological consequences of this phenotype, dehydration experiments were performed on excised leaves and whole plants. As shown in Figure 6, excised leaves from the ggb mutants lost water by transpiration at a slower rate than the corresponding wild types. These results correlated well with the results shown in Figure 5. Specifically, the ggb-1 mutant exhibited almost complete stomatal closure in the absence of exogenous ABA and differed significantly from the Ws wild type with respect to leaf water loss. The ggb-2 line, on the other hand, did not exhibit stomatal closure in the absence of exogenous ABA and differed only slightly from the Col wild type with respect to leaf water loss. The decreased apertures of ggb-1 stomata also resulted in reduced wilting of ggb-1 plants under drought stress conditions compared to Ws control plants (Fig. 6). In contrast, reduced wilting was not observed in the ggb-2 line compared to the Col control under drought stress conditions (data not shown), suggesting that the relatively weak stomatal phenotype of this line does not confer drought tolerance on whole plants.
Figure 6.
Leaf and whole-plant dehydration of wild-type and ggb mutants of Arabidopsis. Top two panels: water loss from excised leaves was monitored as a function of time for the indicated plants. Standard error of the mean is shown for all data points. Asterisks indicate significant differences determined by Student's t test (P < 0.05). Bottom panel: individually potted Ws and ggb-1 plants were denied water for approximately 10 d, beginning 1 to 2 d after bolting, and photographed. The data shown are representative of three independent experiments.
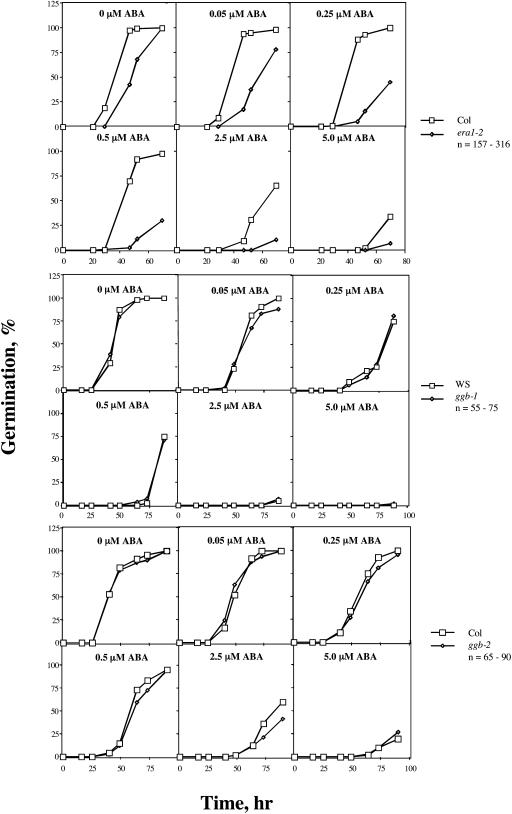
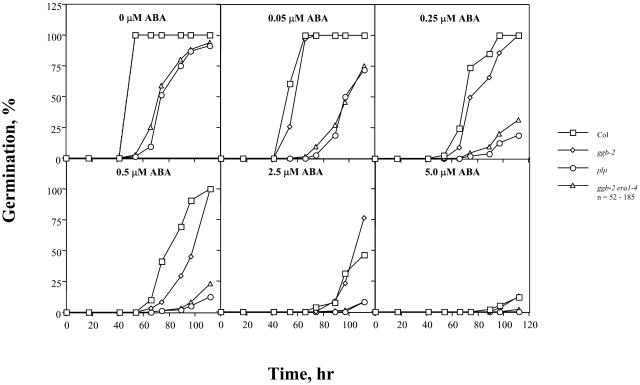
To further investigate ABA signaling in ggb mutant plants, ABA inhibition of seed germination was examined. In contrast to the results obtained for stomatal closure, no significant differences in seed germination were observed between the ggb mutants and the corresponding wild-type lines at any concentration of ABA (Fig. 7). This observation suggested that PGGT I negatively regulates ABA signaling specifically in guard cells. era1 seeds, on the other hand, exhibited a significantly enhanced response to exogenous ABA (Fig. 7), as previously reported by Cutler et al. (1996). Not surprisingly, ggb-2 era1-4 seeds exhibited an enhanced response to ABA and were indistinguishable from plp seeds at all concentrations of ABA tested (Fig. 8). Together with the results shown in Figure 4, these results confirm the hypothesis that ggb era1 double mutants are functionally identical to plp mutants.
Figure 7.
Analysis of Ws, ggb-1, Col, ggb-2, and era1-2 seed germination in response to exogenous ABA. Seeds were imbibed in the presence of the indicated concentrations of cisABA and scored for germination as a function of time. The data indicate the percentage of seeds from which the radical penetrated the seed coat. The ggb-1, ggb-2, and era1-2 data shown are representative of four, two, and four independent experiments, respectively.
Figure 8.
Analysis of Col, ggb-2, plp, and ggb-2 era1-4 seed germination in response to exogenous ABA. Seeds were imbibed in the presence of the indicated concentrations of cisABA and scored for germination as a function of time. The data indicate the percentage of seeds from which the radical penetrated the seed coat.
Auxin Signaling in ggb Mutant Lines
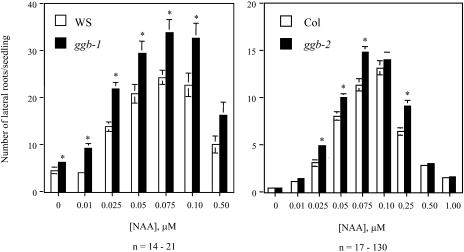
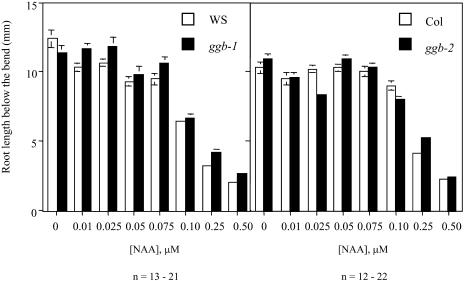
Because ABA and auxin signaling are related and both are modulated by protein farnesylation (Brady et al., 2003), the auxin responsiveness of wild-type and ggb seedlings was examined. As shown in Figure 9, stimulation of lateral root formation in response to exogenous auxin was enhanced in the ggb lines compared to the corresponding wild-type lines. As was observed for the ABA phenotype, the ggb-1 line in the Ws background exhibited the more severe auxin phenotype, with increased lateral root formation at all concentrations of auxin tested. These results suggested that PGGT I plays a role in negative regulation of auxin signaling during lateral root initiation. To determine whether PGGT I negatively regulates other aspects of auxin signaling, auxin inhibition of primary root growth was examined for the ggb mutant and wild-type lines. As shown in Figure 10, no change in auxin inhibition of primary root growth was observed in ggb mutants, suggesting that PGGT I specifically affects auxin signaling during lateral root initiation but does not generally affect all auxin responses.
Figure 9.
Lateral root formation in response to auxin treatment of wild-type and ggb seedlings. Ws, ggb-1, Col, and ggb-2 seedlings were grown on vertically oriented plates in the presence of various concentrations of α-naphthalene acetic acid (α-NAA) for 4 d and scored for number of lateral roots. Standard error of the mean is shown for all data points. Asterisks indicate significant differences determined by Student's t test (P < 0.01, right panel; P < 0.05, left panel).
Figure 10.
Inhibition of primary root growth in response to auxin treatment of wild-type and ggb seedlings. Ws, ggb-1, Col, and ggb-2 seedlings were grown on vertically oriented plates in the presence of various concentrations of α-NAA for 2 d, rotated 90°, grown for two more days, and the length of the root from the bend to the tip was measured. This procedure was followed to rule out differences in germination time. Standard error of the mean is shown for all data points. No significant differences were observed between wild-type and ggb seedlings.
DISCUSSION
T-DNA insertion mutations in the GGB gene did not noticeably affect the growth, stature, or morphology of ggb plants. This observation leads to two conclusions: (1) PGGT I is not a direct regulator of plant development, and (2) the relatively weak developmental phenotypes of era1 plants compared to plp plants are the result of PGGT I-mediated prenylation of PFT substrate proteins. The latter conclusion is supported by the observation that overexpression of GGB in transgenic era1 plants partially suppressed the era1 phenotype. Moreover, the data shown in Figures 5 and 9 establish roles for PGGT I in phytohormone signaling. ggb mutations caused enhanced stomatal closure at various concentrations of exogenous ABA without significantly affecting ABA inhibition of seed germination. In addition, ggb mutations caused an enhanced response to auxin-induced lateral root formation, but did not affect auxin inhibition of primary root growth. These phenotypes suggest that PGGT I plays specific roles in negative regulation of ABA and auxin signaling. Because ggb-2 phenotypes were consistently less severe than ggb-1 phenotypes, it is possible that PFT compensates for loss of PGGT I in the Col background more effectively than in the Ws background. However, differential sensitivities to phytohormones, or quantitative differences in the functions of PGGT I substrate proteins, also potentially explain the difference in severity between ggb phenotypes in the Col and Ws backgrounds.
The enhanced ABA response of ggb guard cells is consistent with the observation that Rop6 is a negative regulator of ABA signaling in guard cells (Lemichez et al., 2001). This small GTP-binding protein has a consensus CSIL motif at its C terminus with an upstream polybasic domain, which qualifies it as a putative substrate of PGGT I. Rop2, which possesses a C-terminal CAFL motif and can be geranylgeranylated in vitro (D.N. Crowell, unpublished results), is also a negative regulator of ABA signaling (Li et al., 2001). Given this, it is surprising that ggb mutants exhibited no significant phenotype with respect to ABA inhibition of seed germination (Fig. 7). These results suggest that different PGGT I substrate proteins may have different effects on ABA signaling and different patterns of expression in Arabidopsis plants. For example, it is possible that certain PGGT I substrate proteins are positive regulators of ABA signaling in seeds. It is also possible that PGGT I substrate proteins function specifically in negative regulation of ABA signaling in guard cells. Future experiments will elucidate why PGGT I appears to be involved in negative regulation of ABA-induced stomatal closure, but not ABA inhibition of seed germination.
The enhanced response of ggb mutants to auxin-induced lateral root formation is consistent with the prediction that AtAux2-11 is geranylgeranylated. Caldelari et al. (2001) demonstrated that glutathione S-transferase fused to the C-terminal CGGL motif of AtAux2-11 was geranylgeranylated in vitro in the presence of recombinant PGGT I from Arabidopsis. Furthermore, AtAux2-11 expression (i.e. promoter activity) was localized to sites of lateral root initiation, the root cap, and the elongating side of the hypocotyl during gravitropic curvature (Wyatt et al., 1993). AtAux2-11, also called IAA4, is a member of the auxin/indole-3-acetic acid (Aux/IAA) family of transcriptional repressors, which repress auxin response factor-mediated transcription of auxin-responsive genes (Dharmasiri and Estelle, 2002). Auxin signals for the ubiquitination and degradation of Aux/IAA proteins via the SCFTIR1 ubiquitin ligase complex, thereby removing Aux/IAA-mediated repression of auxin-responsive gene expression. Interestingly, Aux/IAA genes are themselves auxin-responsive due to the presence of auxin response elements in their promoters. Thus, auxin signals for Aux/IAA protein degradation, which leads to transcriptional activation of auxin-responsive genes, including Aux/IAA genes. This feedback regulation ensures transient activation of auxin-responsive genes in response to auxin. Because AtAux2-11 is a negative regulator of auxin signaling and is likely to be geranylgeranylated, ggb mutants were expected to exhibit an enhanced response to auxin-induced lateral root formation. However, other PGGT I substrates, including members of the Rop family of GTPases, are positive regulators of auxin signaling (Tao et al., 2002). Thus, PGGT I plays multiple roles in auxin signaling, both positive and negative, which may account for the observation that ggb mutants did not exhibit an enhanced response to auxin inhibition of primary root growth. Future studies will elucidate whether PGGT I substrates are involved in specific branches of the auxin signaling pathway or have redundant and/or offsetting effects on auxin signaling.
Because PFT and PGGT I prenylate multiple proteins, era1, ggb, and plp mutants are pleiotropic. At least 48 different Arabidopsis proteins are predicted to be substrates of PGGT I (http://www.danforthcenter.org/ptmdb/results.htm). These include eight Rop GTPases, which regulate actin organization (Fu et al., 2001, 2002; Gu et al., 2003), pollen tube tip growth (Lin et al., 1996; Fu et al., 2001; Gu et al., 2003), ABA and auxin signaling (Lemichez et al., 2001; Li et al., 2001; Tao et al., 2002), and other processes; AIG1, which is involved in RPS2- and avrRpt2-dependent responses to Pseudomonas infection (Reuber and Ausubel, 1996); Aux2-11, a negative regulator of auxin signaling (Wyatt et al., 1993; Caldelari et al., 2001; Dharmasiri and Estelle, 2002); and other proteins. Thus, it is surprising that era1, ggb, and plp phenotypes are not more severe. Data shown in this article suggest that the relatively weak phenotypes of era1 and ggb mutants are due to functional redundancy between PFT and PGGT I, but plants also appear to lack an absolute requirement for CaaX prenyltransferase activity (i.e. plp mutants and era1 ggb double mutants are viable and fertile). In contrast, Saccharomyces cerevisiae, Schizosaccharomyces pombe, and Drosophila melanogaster require PFT and/or PGGT I for survival (Ohya et al., 1991, 1993; Diaz et al., 1993; Trueblood et al., 1993; Therrien et al., 1995). Thus, plants are unusual in their ability to survive and reproduce without PFT or PGGT I.
MATERIALS AND METHODS
Plant Materials
Plant seed stocks were obtained from the following suppliers: ggb-1 was identified by PCR screening the T-DNA knockout collection at the University of Wisconsin Arabidopsis Knockout Facility (Krysan et al., 1999; Sussman et al., 2000) and obtained along with the corresponding wild-type line (Ws); ggb-2 was identified by the Salk Institute Genomic Analysis Laboratory (Alonso et al., 2003) by thermal asymmetric interlaced PCR and obtained from the Arabidopsis Biological Resource Center along with the corresponding wild-type line (Col). ggb-1 and ggb-2 were backcrossed to Ws and Col, respectively, and recovered as homozygotes in the F2 generation. era1-2, era1-4 (formerly known as wig-1), and plp were previously described (Cutler et al., 1996; Running et al., 1998, 2004). era1-4 ggb-2 double mutants were generated by crossing homozygous era1-4 and ggb-2 plants, allowing the F1 plants to self-fertilize, and collecting seed from F2 plants that resembled era1-4 mutants. Several F3 plants from self-fertilized F2 parents exhibiting the era1-4 phenotype showed a plp-like phenotype and were confirmed to be era1-4 ggb-2 double mutants by PCR genotyping. The 35S::GGB transgenic plants were generated by inserting a full-length GGB cDNA into the binary vector pCHF3, which is a derivative of pPZP211, downstream of the cauliflower mosaic virus 35S promoter and introducing the T-DNA portion of the plasmid into Arabidopsis (Arabidopsis thaliana) plants by the floral dip method using Agrobacterium tumefaciens strain C58. The 35S::GGB plants were then crossed to era1-4 plants. F1 progeny were allowed to self-fertilize, and F2 plants that survived kanamycin selection were observed to show either a wild-type phenotype or a mild phenotype less severe than that of era1-4 mutants. Crosses of affected plants to era1-4 confirmed their identity as era1-4 35S::GGB plants.
Analysis of ggb Mutants by PCR and RT-PCR
Genomic DNA was isolated from ggb and wild-type plants using Plant DNAzol Reagent according to the manufacturer's instructions (Invitrogen/Life Technologies). Genomic analysis of ggb mutants and the corresponding wild-type lines was then performed by PCR using 0.4 ng of genomic DNA, 12 pmol of forward primer, 12 pmol of reverse primer, and Ex-Taq polymerase (TaKaRa Bio) in a total reaction volume of 50 μL. PCR conditions varied, but generally consisted of a 5-min hot start presoak at 96°C and 36 cycles of the following PCR program: 94°C, 15 s; 65°C, 30 s; 72°C, 3 min. A post-soak was performed at 72°C for 7 min to complete product synthesis. Two different PCR analyses were performed. The first used two gene-specific primers, which for the ggb-1 and Ws lines were as follows: GGTB RT-5′, 5′-ATC GTC TTA CAC TCG CTC ATT TC-3′, and GGTB-R, 5′-CAA AGC GAA ACT GCA ATC TTA CAA TAG TC-3′. For the ggb-2 and Col lines, the two gene-specific primers were as follows: GGTB-F, 5′-TAA ATG AAT TAG CAG CAT GCG GTG GAA AG-3′, and GGTB RT-3′, 5′-CAT CAT TTG CTT GTC TCT GTA GG-3′. The second PCR analysis used one T-DNA-specific primer and one gene-specific reverse primer. The T-DNA-specific primer for the ggb-1 and Ws lines was JL-202, 5′-CAT TTT ATA ATA ACG CTG CGG ACA TCT AC-3′, and that for the ggb-2 and Col lines was T-DNA SALK-LBb1, 5′-GCG TGG ACC GCT TGC TGC AAC T-3′. The gene-specific reverse primers used in this analysis were GGTB-R for ggb-1 and Ws and GGTB RT-3′ for ggb-2 and Col, as described above.
Total RNA was isolated from rosette leaves of ggb and wild-type plants using TRIzol Reagent according to the manufacturer's instructions. RT-PCR was then performed to analyze GGB transcript levels in ggb and wild-type plants using 1 μg of total RNA, 5 pmol of GGTB RT-5′ primer (see above), 5 pmol of GGTB RT-3′ primer (see above), 18S rRNA competimer/primer pairs as an internal standard (Ambion), and the Platinum Quantitative RT-PCR Thermoscript One-Step System (Invitrogen/Life Technologies) in a total reaction volume of 25 μL. RT-PCR conditions included a 20-min reverse transcription step at 50°C, followed by a 5-min presoak at 95°C, and 32 cycles of the following PCR program: 95°C, 15 s; 50°C, 30 s; 72°C, 1 min. A post-soak was performed at 72°C for 4 min to complete product synthesis.
Plant Growth Conditions
Seeds were surface sterilized and imbibed on 0.5× Murashige and Skoog plates containing 1% Suc and 0.8% phytagar. For most applications, imbibed seeds were stratified at 4°C for 3 to 4 d before transfer to a Conviron Plant Growth Chamber at 22°C under long-day conditions (18 h of white light at 50 to 150 μmol m−2 s−1 followed by 6 h of darkness). After 4 to 7 d in the growth chamber, seedlings were transferred to soil (ProMix) and grown under the same conditions for propagation of seed or analysis of growth, morphology, stomatal function, or drought tolerance. Plants were irrigated from below with a standard mixture of macronutrients and micronutrients.
Seed Germination and Root Growth Analyses
For germination analyses, seeds were sterilized and imbibed on 0.5× Murashige and Skoog plates containing 1% Suc, 0.8% phytagar, and various concentrations of ABA, transferred directly to a 22°C growth chamber in the dark without stratification, and scored for germination at approximately 12-h intervals. For root growth assays, seeds were sterilized, imbibed, stratified, and grown under long-day conditions at 22°C on vertically oriented 0.5× Murashige and Skoog plates containing 1% Suc, 0.8% phytagar, and various concentrations of α-NAA. After 2 d, the plates were rotated 90°, and roots were visualized after two additional days of growth with a Nikon SMZ-1B dissecting microscope. Root length from the bend to the tip was measured to rule out differences in germination time. Lateral root numbers were also recorded.
Stomatal Closure and Dehydration Assays
Mature rosette leaves were excised from ggb and wild-type plants, floated for 2 h under high-light conditions (150 μmol m−2 s−1) in 5 mm MES, pH 6.15, 20 mm KCl, and 1 mm CaCl2 (Pei et al., 1997), and then for another 2 h in the same buffer containing various concentrations of ABA. Epidermal peels were prepared as described (Pei et al., 1997), stained with toluidine blue, and immediately photographed at a magnification of 40× using a Nikon Eclipse E600 microscope interfaced to a SPOT digital camera. Data were recorded as the ratio of aperture width to length.
Leaf dehydration was monitored by placing excised leaves on a Mettler balance and recording water loss (i.e. decreased mass) over time. Whole-plant dehydration was examined by withholding water from individually potted plants for approximately 10 d, after which they were photographed using a digital camera.
Protein Prenylation Reactions
Extracts were prepared from floral buds of ggb and wild-type Arabidopsis plants by grinding in a mortar and pestle at 4°C in the presence of 50 mm HEPES (pH 7.4), 500 mm mannitol, 5 mm EDTA, and Complete protease inhibitors (Roche Diagnostics). Extracts were strained through four layers of cheesecloth, centrifuged at 4°C for 10 min at 10,000g, and the resulting protein supernatants used for protein prenyltransferase assays. Protein prenyltransferase reactions were performed essentially as described (Randall et al., 1993) and consisted of 40 μg of Escherichia coli protein extract containing recombinant Ras-CAIM, Ras-CAIL, or Ras-SVLS, 210 μg of Arabidopsis protein extract as a source of protein prenyltransferase activities, 50 mm HEPES (pH 7.5), 20 mm MgCl2, 5 mm dithiothreitol, and 0.01 μCi μL−1 of either [1-3H]FPP (16.1 Ci mmol−1; Perkin-Elmer Life and Analytical Sciences) or [1-3H]GGPP (23.0 Ci mmol−1; Perkin-Elmer Life and Analytical Sciences) in a total volume of 125 μL. Reactions were incubated for 60 min at 30°C, resolved by 12% SDS-PAGE, and analyzed by fluorography using Amplify fluorographic reagent (Amersham Biosciences) and Kodak XAR-5 film (Eastman Kodak).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number NM_129513.
Acknowledgments
The authors wish to thank Angela Erwin for critical reading of the manuscript.
This work was supported by U.S. Department of Agriculture grants 00–03367 and 03–02151 to D.N.C. and by National Science Foundation grant IOB–0344261 to M.P.R.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.065045.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657; erratum Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Science 301: 1849 [DOI] [PubMed] [Google Scholar]
- Bergo MO, Leung GK, Ambroziak P, Otto JC, Casey PJ, Young SG (2000) Targeted inactivation of the isoprenylcysteine carboxyl methyltransferase gene causes mislocalization of K-Ras in mammalian cells. J Biol Chem 275: 17605–17610 [DOI] [PubMed] [Google Scholar]
- Bonetta D, Bayliss P, Sun S, Sage T, McCourt P (2000) Farnesylation is involved in meristem organization in Arabidopsis. Planta 211: 182–190 [DOI] [PubMed] [Google Scholar]
- Boyartchuk VL, Ashby MN, Rine J (1997) Modulation of Ras and a-factor function by carboxyl-terminal proteolysis. Science 275: 1796–1800 [DOI] [PubMed] [Google Scholar]
- Bracha K, Lavy M, Yalovsky S (2002) The Arabidopsis AtSTE24 is a CAAX protease with broad substrate specificity. J Biol Chem 277: 29856–29864 [DOI] [PubMed] [Google Scholar]
- Brady SM, Sarkar SF, Bonetta D, McCourt P (2003) The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant J 34: 67–75 [DOI] [PubMed] [Google Scholar]
- Cadiñanos J, Varela I, Mandel DA, Schmidt WK, Díaz-Perales A, López-Otín C, Freije JM (2003) AtFACE-2, a functional prenylated protein protease from Arabidopsis thaliana related to mammalian Ras-converting enzymes. J Biol Chem 278: 42091–42097 [DOI] [PubMed] [Google Scholar]
- Caldelari D, Sternberg H, Rodríguez-Concepción M, Gruissem W, Yalovsky S (2001) Efficient prenylation by a plant geranylgeranyltransferase-I requires a functional CaaL box motif and a proximal polybasic domain. Plant Physiol 126: 1416–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S (1992) Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu Rev Biochem 61: 355–386 [DOI] [PubMed] [Google Scholar]
- Crowell DN (2000) Functional implications of protein isoprenylation in plants. Prog Lipid Res 39: 393–408 [DOI] [PubMed] [Google Scholar]
- Crowell DN, Kennedy M (2001) Identification and functional expression in yeast of a prenylcysteine α-carboxyl methyltransferase gene from Arabidopsis thaliana. Plant Mol Biol 45: 469–476 [DOI] [PubMed] [Google Scholar]
- Crowell DN, Sen SE, Randall SK (1998) Prenylcysteine α-carboxyl methyltransferase in suspension-cultured tobacco cells. Plant Physiol 118: 115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler S, Ghassemian M, Bonetta D, Cooney S, McCourt P (1996) A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science 273: 1239–1241 [DOI] [PubMed] [Google Scholar]
- Dharmasiri S, Estelle M (2002) The role of regulated protein degradation in auxin response. Plant Mol Biol 49: 401–409 [PubMed] [Google Scholar]
- Diaz M, Sanchez Y, Bennett T, Sun CR, Godoy C, Tamanoi F, Duran A, Perez P (1993) The Schizosaccharomyces pombe cwg2+ gene codes for the β subunit of a geranylgeranyltransferase type I required for β-glucan synthesis. EMBO J 12: 5245–5254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Li H, Yang Z (2002) The ROP2 GTPase controls the formation of cortical fine F-actin and the early phase of directional cell expansion during Arabidopsis organogenesis. Plant Cell 14: 777–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Wu G, Yang Z (2001) Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. J Cell Biol 152: 1019–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Vernoud V, Fu Y, Yang Z (2003) ROP GTPase regulation of pollen tube growth through the dynamics of tip-localized F-actin. J Exp Bot 54: 93–101 [DOI] [PubMed] [Google Scholar]
- Gutierrez L, Magee AI, Marshall CJ, Hancock JF (1989) Post-translational processing of p21ras is two-step and involves carboxyl-methylation and carboxy-terminal proteolysis. EMBO J 8: 1093–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JF, Cadwallader K, Marshall CJ (1991) Methylation and proteolysis are essential for efficient membrane binding of prenylated p21K-ras(B). EMBO J 10: 641–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrycyna CA, Sapperstein SK, Clarke S, Michaelis S (1991) The Saccharomyces cerevisiae STE14 gene encodes a methyltransferase that mediates C-terminal methylation of a-factor and RAS proteins. EMBO J 10: 1699–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysan PJ, Young JC, Sussman MR (1999) T-DNA as an insertional mutagen in Arabidopsis. Plant Cell 11: 2283–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemichez E, Wu Y, Sanchez JP, Mettouchi A, Mathur J, Chua NH (2001) Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev 15: 1808–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Shen JJ, Zheng ZL, Lin Y, Yang Z (2001) The Rop GTPase switch controls multiple developmental processes in Arabidopsis. Plant Physiol 126: 670–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Wang Y, Zhu J-k, Yang Z (1996) Localization of a Rho GTPase implies a role in tip growth and movement of the generative cell in pollen tubes. Plant Cell 8: 293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimha Chary S, Bultema RL, Packard CE, Crowell DN (2002) Prenylcysteine α-carboxyl methyltransferase expression and function in Arabidopsis thaliana. Plant J 32: 735–747 [DOI] [PubMed] [Google Scholar]
- Ohya Y, Goebl M, Goodman LE, Petersen-Bjorn S, Friesen JD, Tamanoi F, Anraku Y (1991) Yeast CAL1 is a structural and functional homologue to the DPR1 (RAM) gene involved in ras processing. J Biol Chem 266: 12356–12360 [PubMed] [Google Scholar]
- Ohya Y, Qadota H, Anraku Y, Pringle JR, Botstein D (1993) Suppression of yeast geranylgeranyl transferase I defect by alternative prenylation of two target GTPases, Rho1p and Cdc42p. Mol Biol Cell 4: 1017–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish CA, Smrcka AV, Rando RR (1995) Functional significance of βγ-subunit carboxymethylation for the activation of phospholipase C and phosphoinositide 3-kinase. Biochemistry 34: 7722–7727 [DOI] [PubMed] [Google Scholar]
- Pei ZM, Ghassemian M, Kwak CM, McCourt P, Schroeder JI (1998) Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 282: 287–290 [DOI] [PubMed] [Google Scholar]
- Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI (1997) Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell 9: 409–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian D, Zhou D, Ju R, Cramer CL, Yang Z (1996) Protein farnesyltransferase in plants: molecular characterization and involvement in cell cycle control. Plant Cell 8: 2381–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall SK, Crowell DN (1999) Protein isoprenylation in plants. Crit Rev Biochem Mol Biol 34: 325–338 [DOI] [PubMed] [Google Scholar]
- Randall SK, Marshall MS, Crowell DN (1993) Protein isoprenylation in suspension-cultured tobacco cells. Plant Cell 5: 433–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuber TL, Ausubel FM (1996) Isolation of Arabidopsis genes that differentiate between resistance responses mediated by the RPS2 and RPM1 disease resistance genes. Plant Cell 8: 241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Concepción M, Toledo-Ortiz G, Yalovsky S, Caldelari D, Gruissem W (2000) Carboxyl-methylation of prenylated calmodulin CaM53 is required for efficient plasma membrane targeting of the protein. Plant J 24: 775–784 [DOI] [PubMed] [Google Scholar]
- Running MP, Fletcher JC, Meyerowitz EM (1998) The WIGGUM gene is required for proper regulation of floral meristem size in Arabidopsis. Development 125: 2545–2553 [DOI] [PubMed] [Google Scholar]
- Running MP, Lavy M, Sternberg H, Galichet A, Gruissem W, Hake S, Ori N, Yalovsky S (2004) Enlarged meristems and delayed growth in plp mutants result from lack of CaaX prenyltransferases. Proc Natl Acad Sci USA 101: 7815–7820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapperstein S, Berkower C, Michaelis S (1994) Nucleotide sequence of the yeast STE14 gene, which encodes farnesylcysteine carboxyl methyltransferase, and demonstration of its essential role in a-factor export. Mol Cell Biol 14: 1438–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman MR, Amasino RM, Young JC, Krysan PJ, Austin-Phillips S (2000) The Arabidopsis knockout facility at the University of Wisconsin-Madison. Plant Physiol 124: 1465–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam A, Nouvet FJ, Fujimura-Kamada K, Slunt H, Sisodia SS, Michaelis S (1998) Dual roles for Ste24p in yeast a-factor maturation: NH2-terminal proteolysis and COOH-terminal CAAX processing. J Cell Biol 142: 635–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao LZ, Cheung AY, Wu HM (2002) Plant Rac-like GTPases are activated by auxin and mediate auxin-responsive gene expression. Plant Cell 14: 2745–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therrien M, Chang HC, Solomon NM, Karim FD, Wassarman DA, Rubin GM (1995) KSR, a novel protein kinase required for RAS signal transduction. Cell 83: 879–888 [DOI] [PubMed] [Google Scholar]
- Trueblood CE, Ohya Y, Rine J (1993) Genetic evidence for in vivo cross-specificity of the CaaX-box protein prenyltransferases farnesyltransferase and geranylgeranyltransferase-I in Saccharomyces cerevisiae. Mol Cell Biol 13: 4260–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt RE, Ainley WM, Nagao RT, Conner TW, Key JL (1993) Expression of the Arabidopsis AtAux2–11 auxin-responsive gene in transgenic plants. Plant Mol Biol 22: 731–749 [DOI] [PubMed] [Google Scholar]
- Yalovsky S, Kulukian A, Rodríguez-Concepción M, Young CA, Gruissem W (2000) Functional requirement of plant farnesyltransferase during development in Arabidopsis. Plant Cell 12: 1267–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky S, Trueblood CE, Callan KL, Narita JO, Jenkins SM, Rine J, Gruissem W (1997) Plant farnesyltransferase can restore yeast Ras signaling and mating. Mol Cell Biol 17: 1986–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane HK, Farnsworth CC, Xie HY, Howald W, Fung BK, Clarke S, Gelb MH, Glomset JA (1990) Brain G protein γ subunits contain an all-trans-geranylgeranylcysteine methyl ester at their carboxyl termini. Proc Natl Acad Sci USA 87: 5868–5872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Cramer CL, Watson JC (1993) Protein farnesyltransferase in plants. Molecular cloning and expression of a homolog of the β subunit from the garden pea. Plant Physiol 101: 667–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FL, Casey PJ (1996) Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem 65: 241–269 [DOI] [PubMed] [Google Scholar]
- Ziegelhoffer EC, Medrano LJ, Meyerowitz EM (2000) Cloning of the Arabidopsis WIGGUM gene identifies a role for farnesylation in meristem development. Proc Natl Acad Sci USA 97: 7633–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]