Abstract
Objective
This study aimed to assess the dosimetric parameters and hematological toxicity (HT) associated with bone marrow‐sparing (BMS) intensity‐modulated radiation therapy (IMRT) in patients diagnosed with International Federation of Gynecology and Obstetrics (FIGO) stage IIIC cervical cancer undergoing extended‐field radiation therapy (EFRT).
Methods
Patients with cervical cancer presenting with common iliac or para‐aortic lymph node metastases require EFRT, which often results in grade 3 HT. Therefore, we retrospectively analyzed data of 84 patients with FIGO stage IIIC cervical cancer who underwent concurrent chemoradiotherapy (EFRT, brachytherapy, and weekly cisplatin 40 mg/m2) at Harbin Medical University Cancer Hospital, including 40 who received BMS‐IMRT and 44 who received normal IMRT. Dose–volume histogram (DVH) parameters and estimated treatment times were compared. We also compared acute HT between the normal and BMS groups.
Results
Dosimetric analysis demonstrated that BMS‐IMRT significantly reduced the mean volume of bone marrow receiving ≥10, ≥20, ≥30, and ≥40 Gy without affecting the target coverage of planning target volume and sparing the organs at risk. Within the BMS‐IMRT group, 37.5% of the patients developed grade ≥3 HT, with an increase in HT (HT3+ = 61.4%) in patients receiving normal‐IMRT (P = 0.029).
Conclusions
For patients with cervical cancer treated with EFRT, BMS‐IMRT represents a feasible treatment approach that may mitigate HT and facilitate the uninterrupted administration of concurrent chemoradiotherapy.
Keywords: cervical cancer, bone marrow sparing, extended‐field radiation therapy, hematological toxicity, intensity‐modulated radiation therapy
Bone Marrow Sparing Intensity Modulated Radiation Therapy is a feasible option for cervical cancer patients treated with extended‐field radiation therapy, it may reduce hematologic toxicity and ensure the continuity of concurrent chemoradiotherapy.
1. INTRODUCTION
Cervical cancer is a major public health concern worldwide. 1 Despite the introduction of cervical cancer screening and the human papillomavirus (HPV) vaccine, cervical cancer remains a significant health issue and tends to affect younger individuals in China. 2 To date, in cases of locally advanced cervical cancer (International Federation of Gynecology and Obstetrics [FIGO] IIB–IVA), the integration of chemotherapy with radiotherapy remains the gold‐standard treatment strategy.
Pelvic lymph node (PLN) and para‐aortic lymph node (PALN) metastases are important prognostic factors, 3 , 4 , 5 and the FIGO staging system for uterine cervical cancer has allowed the involvement of node status since 2018. 6 At present, extended‐field (pelvic and para‐aortic) radiotherapy (EFRT) is indicated for patients with positive common iliac or PALNs 7 and recommended for patients with multiple positive PLNs. Cisplatin‐based EFRT is the standard treatment for these patients. However, this approach is associated with a higher incidence of hematological and gastrointestinal toxicities compared to pelvic radiotherapy alone. 8 , 9 This is largely attributable to the fact that approximately 50% of active bone marrow (BM) is situated within the radiation field of conventional EFRT for cervical cancer (Figure 1A). Acute grade 3–4 hematological toxicities can result in missed cycles of concurrent chemotherapy, delays in treatment, and deterioration of clinical outcomes. 10 Therefore, reducing hematological toxicity during concomitant cisplatin‐based EFRT is an important goal for radiation therapists.
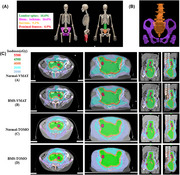
FIGURE 1.

(A) Distribution of most functional bone marrow in the radiation field of conventional EFRT in cervical cancer; (B) Typical figures showing contours for bone marrow cavity in EFRT of LS (orange) and OC (purple). (C) Typical dose distributions of the four plans in cervical cancer: normal‐VMAT, BMS‐VMAT, normal‐TOMO, and BMS‐VMAT plans. EFRT, extended‐field radiation therapy; LS, lumbosacral spine; OC, os coxae; VMAT, volume‐modulated arc therapy; BMS, bone marrow sparing; TOMO, helical tomotherapy.
Our previous dosimetric analysis has demonstrated that the bone marrow‐sparing (BMS) intensity‐modulated radiation therapy (IMRT) plan, which included the lumbosacral spine (LS) and os coxae (OC) as separate dose–volume constraints, significantly reduced the mean volume of BM receiving ≥10 Gy, ≥20 Gy, ≥30 Gy, and ≥40 Gy in patients with FIGO stage IIB cervical cancer. This approach may be a promising therapeutic option for reducing hematological toxicity in patients with cervical cancer. 11 Therefore, this study aimed to evaluate the dosimetric quality and hematological toxicity of BMS‐IMRT in patients with FIGO stage IIIC cervical cancer receiving EFRT.
2. METHODS
2.1. Patient selection and characteristics
Clinical data of 84 patients with biopsy‐confirmed cervical cancer recruited between January 2019 and January 2022 were retrospectively analyzed. Patients were administered concurrent cisplatin‐based EFRT owing to the presence of positive common iliac or PALNs. Clinical staging was performed according to the updated 2018 FIGO staging classification. To assess whether PLNs or PALNs were involved, imaging examinations were performed. The imaging modalities employed included computed tomography (CT) (17.9%), magnetic resonance imaging (52.4%), and positron emission tomography (PET) (29.8%). The eligibility criteria for our study were as follows: (1) radiologically confirmed clinical FIGO stage IIIC cervical cancer; (2) pathologically confirmed cervical squamous cell carcinoma or adenocarcinoma; (3) concurrent cisplatin‐based EFRT was administered at our hospital, and the clinical data were thoroughly collected; (4) no distant metastasis; (5) no prior history of other malignancies; (6) received either a volume‐modulated arc therapy (VMAT) plan or a helical tomotherapy (TOMO) plan; and (7) had not received any radiotherapy, chemotherapy, or immunotherapy prior to radiotherapy initiation. The study was approved by the Institutional Research Medical Ethics Committee of Harbin Medical University Cancer Hospital.
2.2. Treatment regimen
All patients underwent extended‐field external beam radiotherapy (pelvic + para‐aortic), concurrent chemotherapy (cisplatin, 30–40 mg/m2 weekly), and brachytherapy. Before initiating radiation treatment, individuals were positioned supine and stabilized using custom‐molded thermoplastic immobilization systems. They were then scanned in the head‐first position using a Philips 16‐slice Brilliance big‐bore CT scanner with a slice thickness of 5 mm. Before the simulation, all patients were instructed to have a full bladder and empty rectum and to undergo bowel preparation with meglumine diatrizoate.
Targets and organs at risk (OARs) were delineated according to the guidelines stipulated in the International Commission on Radiation Units and Measurements reports Report 62 and Radiation Therapy Oncology Group 0418 protocol. The gross tumor volume (GTV) included positive lymph nodes. The clinical target volume (CTV) encompassed the pelvic and para‐aortic regions, which were delineated as zones that may contain potential microscopic diseases. These include the GTV, cervix, parametria, uterus, uterosacral ligaments, and a portion of the vagina (depending on the extent of the primary tumor), as well as the pelvic and para‐aortic lymphatic drainage areas (para‐aortic, common iliac, external iliac, internal iliac, obturator, and presacral lymph nodes). The superior boundary of the CTV was placed at L1/L2. Using image‐guided radiotherapy techniques, the planning target volume (PTV) was defined as the CTV with an additional 5 mm margin, whereas the planning gross target volume (PGTV) was delineated as the GTV covering the positive lymph nodes, also with a 5 mm margin. A radiation dose of 45 Gy was prescribed to at least 95% of the PTV and delivered in 25 daily fractions via VMAT or TOMO. A minimum of 95% of the PGTV was escalated to 61.6 Gy in 28 daily fractions using the simultaneous integrated boost and late‐course boost (LCB) techniques. For patients presenting with bulky parametrial sidewall disease, an additional parametrial boost of 6 Gy was administered using the LCB. Furthermore, patients underwent brachytherapy using high‐dose‐rate Ir‐192 sources, with a total dose of 30–36 Gy delivered to each A point in 5–6 fractions, administered either once or twice weekly.
The bladder, small intestine, rectum, femoral head, kidneys, spinal cord, BM, OC, and LS were routinely delineated as OARs. Consistent with our previous research, 11 we delineated the marrow cavity of the intramedullary low‐density area based on the bone window in the CT simulation. This included the OC, defined as the region extending from the iliac crests to the ischial tuberosities, encompassing the ilium, pubis, ischium, and acetabula, but excluding the femoral heads, and the LS, which extends 2 cm above the upper boundary of the PTV to the coccyx. The contours of the marrow cavity are illustrated in Figure 1B, and the dose–volume constraints for the OARs are presented in Table 1.
TABLE 1.
Dose–volume constraints of the organs at risk in cervical cancer.
| Structures | Dose–volume constraints |
|---|---|
| Kidney | V20< 30%, Dmean < 12 Gy |
| Small bowel | V40 < 30%, Dmax < 52 Gy |
| Rectum | V40 < 40%, Dmax < 52 Gy |
| Bladder | V40 < 40 % |
| Femoral head | V40 < 5 % |
| Spinal cord | Dmax < 40 Gy |
| OC | V10 < 80%, V20 < 50 %, V30 < 30% |
| LS | V10 < 90%, V20 < 70 %, V30 < 40% |
Abbreviations: LS, lumbosacral spine; OC, os coxae.
2.3. Radiotherapy techniques
The VMAT plans were generated using Monaco 5.11 planning system. These plans utilized 6 MV X‐ray beams delivered by the Elekta Versa HD linear accelerator, with dose calculations performed using the Monte Carlo algorithm. Two 360° arc fields were designed for the VMAT, with each arc containing 200 control points. TOMO plans were generated using a tomotherapy planning system (Accuray Inc., Madison, WI, USA), employing 6 MV photon beams. The beamlet calculation parameters included a pitch value of 0.287, field width of 2.5 cm, modulation factor of 3, and normal dose calculation grid. We also redesigned 15 patients’ plan in BMS‐VMAT, BMS‐TOMO, Normal‐VAMT, and Normal‐TOMO to compare dosimetric difference. The PTV, PGTV, and OARs parameters were recorded after treatment planning completion. Target delineation for all patients, including dosimetric and hematological toxicity analyses, was performed by the same physician. In addition, the treatment plans were performed by a single physicist to ensure consistency and accuracy.
2.4. Evaluation of toxicity and adverse effects
All patients underwent weekly assessments for acute hematological toxicities during the course of treatment as well as at one and three months after treatment completion. Treatment‐related toxicities were classified according to the Common Terminology Criteria for Adverse Events (CTCAE v5.0). Severe toxic reactions were characterized as harmful manifestations observed throughout the clinical intervention phase or during the subsequent 3‐month monitoring window following therapeutic cessation.
2.5. Statistical analysis
To maintain consistency in the outcome evaluation, all therapeutic regimens were adjusted to ensure that 95% of the PTV was covered with a dose of 45 Gy. Following this standardization, dose–volume histogram metrics corresponding to the PTV, PGTV, and OARs were compared using a paired t‐test analysis. Hematological toxicity incidence rates across patient subgroups stratified by clinicopathological characteristics and therapeutic variables were analyzed using nonparametric statistical methods. The Mann–Whitney U test was applied to ordinal variables, and Pearson's chi‐square test was employed for categorical comparisons. Multivariate logistic regression analysis with covariate adjustment was performed to evaluate the association between hematologic adverse events (AEs) and the EFRT regimen (BM‐sparing or not).
3. RESULTS
3.1. Patient characteristics
A total of 84 participants fulfilled the inclusion criteria, with a median age of 55 years (range, 29–73 years). All participants received EFRT and had common iliac or para‐aortic lymph node metastases: 85.7% (72/84) had common iliac and 14.3% (12/84) had para‐aortic metastases. Of the 84 patients, 52 (61.9%) had tumors exceeding 4 cm in size. Patients with elevated SccAg levels >10 ng/ml prior to treatment constituted 39.3% of the study population (33/84). The clinical and pathological features of the patients are shown in Table 2.
TABLE 2.
Clinicopathological characteristics at baseline in the BMS and control groups.
| Variable | BMS group (N = 40) | Control group (N = 44) | P‐value |
|---|---|---|---|
| Age, (years) | 0.160 | ||
| ≤45 | 2 (5%) | 7 (15.9%) | |
| >45 | 38 (95%) | 37 (84.1%) | |
| Clinical stage | 0.040 a | ||
| IIIC1r | 31 (77.5%) | 41 (93.2%) | |
| IIIC2r | 9 (22.5%) | 3 (6.8%) | |
| Pathology | 0.224 | ||
| Squamous | 38 (95%) | 44 (100%) | |
| Adenocarcinoma | 2 (5%) | 0 (0%) | |
| Chemotherapy cycles | 0.261 | ||
| 1–4 | 10 (25%) | 16 (36.4) | |
| 5–6 | 30 (75%) | 28 (63.6) | |
| SCC‐Ag value,(ng/ml) | 0.053 | ||
| ≤10 | 27 (67.5%) | 22 (50%) | |
| >10 | 11 (27.5%) | 22 (50%) | |
| N/A | 2 (5.0%) | 0 (0%) | |
| Tumor size,(cm) | 0.314 | ||
| ≤4 | 13 (32.5%) | 19 (43.2%) | |
| >4 | 27 (67.5%) | 25 (56.8%) | |
| Original HGB,(g/dL) | 1.000 | ||
| ≤9 | 1 (2.5%) | 2 (4.5%) | |
| >9 | 39 (97.5%) | 42 (95.5%) |
Abbreviations: HGB, hemoglobin; BMS, bone marrow sparing.
* P value was computed using the chi‐square test.
Statistically significant
3.2. Dosimetric comparison
The original treatment plans exhibited outstanding coverage of the target volume. Specifically, 95% of the PTV and PGTV was exposed to radiation doses of 45 and 55 Gy, respectively. The mean volume measurements of PTV and PGTV were 1355.82±263.02 cubic centimeters (cc) and 46.29±46.9 cc, respectively. The measured mean volume of the entire BM was 811.39 ± 127.53 cc. Specifically, the mean volumes of the LS and OC regions were 418.23 ± 127.48 cc and 419.39 ± 75.89 cc, respectively. The dosimetric parameters of BM and comparisons between the two BMS‐IMRT plans (VMAT and TOMO) and the two conventional IMRT plans (VMAT and TOMO) are presented in Table 3 and Figures 2C and 3A. Within the two BMS‐IMRT groups, a notable decline was observed in both the mean volume of BM exposed to radiation doses of 10, 20, 30, and 40 Gy, and the average radiation dose. The following are the specific decreases in volume: 10.88% and 2.57% for V10 (BMS‐TOMO vs. normal TOMO: 84.99±1.63% vs. 95.31±2.62%, P<0.001; BMS‐VMAT vs. normal VMAT: 86.60±3.16% vs. 88.88±2.74%, P = 0.005), 22.58% and 19.10% for V20 (BMS‐TOMO vs. normal TOMO: 60.68±3.24% vs. 78.38±3.89%, P<0.001; BMS‐VMAT vs. normal VMAT: 62.05±3.29% vs. 76.70±3.15%, P<0.001), 31.66% and 31.86% for V30 (BMS‐TOMO vs. normal TOMO: 37.41±1.84% vs. 54.74±3.96%, P<0.001; BMS‐VMAT vs. normal VMAT: 37.04±2.56% vs. 54.36±4.99%, P<0.001), 23.91% and 30.96% for V40 (BMS‐TOMO vs. normal TOMO: 15.21±1.51% vs. 19.99±2.83%, P<0.001; BMS‐VMAT vs. normal VMAT: 14.16±2.03% vs. 20.51±4.22%, P<0.001). Moreover, significant mean dose reductions were observed in the BM, reaching 15.10% and 13.00% respectively, (BMS‐TOMO vs. normal TOMO: 25.31±0.85 vs. 29.81±1.12 Gy, P<0.001; BMS‐VMAT vs. normal VMAT: 25.00±0.86 vs. 29.09±1.25 Gy, P<0.001). Compared with the normal IMRT plans, BMS‐IMRT showed better BM protection in both the VMAT and TOMO plans. Therefore, BMS‐IMRT is a promising treatment modality for reducing hematological toxicity in patients diagnosed with FIGO stage IIIC cervical cancer who are currently receiving EFRT.
TABLE 3.
Dosimetric parameters and comparisons of PGTV, PTV, and OARs among the two BMS‐IMRT and two normal‐IMRT plans.
| P‐value | |||||||
|---|---|---|---|---|---|---|---|
| BMS‐VMAT | Normal‐VMAT | BMS‐TOMO | Normal‐TOMO | BMS‐VMAT VS Normal‐VMAT | BMS‐TOMO VS Normal‐TOMO | BMS‐VMAT VS BMS‐TOMO | |
| PGTV | |||||||
| Dmean (Gy) | 56.86±0.52 | 56.81±0.61 | 56.57±0.42 | 56.59±0.44 | 0.318 | 0.718 | 0.077 a |
| HI | 0.077±0.016 | 0.076±0.020 | 0.045±0.011 | 0.044±0.010 | 0.704 | 0.560 | <0.001 a |
| CI | 0.603±0.100 | 0.609±0.111 | 0.777±0.050 | 0.785±0.044 | 0.572 | 0.626 | <0.001 a |
| PTV | |||||||
| Dmean (Gy) | 48.64±0.82 | 48.71±0.91 | 47.03±0.69 | 47.08±0.83 | 0.261 | 0.430 | <0.001 a |
| HI | 0.230±0.033 | 0.226±0.031 | 0.239±0.033 | 0.238±0.033 | 0.141 | 0.552 | 0.130 |
| CI | 0.830±0.033 | 0.824±0.045 | 0.851±0.024 | 0.847±0.029 | 0.432 | 0.465 | 0.014 a |
| Bone marrow | |||||||
| V10 (%) | 86.60±3.16 | 88.88±2.74 | 84.99±1.63 | 95.31±2.62 | 0.005 a | <0.001 a | 0.059 |
| V20 (%) | 62.05±3.29 | 76.70±3.15 | 60.68±3.24 | 78.38±3.89 | <0.001 a | <0.001 a | 0.101 |
| V30 (%) | 37.04±2.56 | 54.36±4.99 | 37.41±1.84 | 54.74±3.96 | <0.001 a | <0.001 a | 0.445 |
| V40 (%) | 14.16±2.03 | 20.51±4.22 | 15.21±1.51 | 19.99±2.83 | <0.001 a | <0.001 a | 0.032 a |
| Dmean (Gy) | 25.00±0.86 | 29.09±1.25 | 25.31±0.85 | 29.81±1.12 | <0.001 a | <0.001 a | 0.104 |
| Lumbosacral spine (LS) | |||||||
| V10 (%) | 92.34±4.85 | 95.25±5.26 | 89.61±1.26 | 97.91±3.50 | <0.001 a | <0.001 a | 0.025 a |
| V20 (%) | 69.94±5.09 | 90.07±9.74 | 69.45±2.52 | 89.67±9.51 | <0.001 a | <0.001 a | 0.714 |
| V30 (%) | 42.08±3.85 | 67.70±11.25 | 43.01±1.28 | 67.33±9.82 | <0.001 a | <0.001 a | 0.393 |
| V40 (%) | 17.95±2.78 | 26.85±6.70 | 18.44±2.00 | 26.10±5.74 | <0.001 a | <0.001 a | 0.427 |
| Dmean (Gy) | 27.23±1.34 | 32.97±29.96 | 27.27±0.72 | 33.00±2.76 | <0.001 a | <0.001 a | 0.900 |
| Os coxae | |||||||
| V10 (%) | 80.64±3.78 | 83.52±4.95 | 79.98±0.40 | 92.69±4.15 | 0.011 a | <0.001 a | 0.517 |
| V20 (%) | 53.45±2.19 | 63.22±8.06 | 51.46±1.05 | 66.86±6.38 | <0.001 a | <0.001 a | 0.007 a |
| V30 (%) | 31.23±2.00 | 40.73±9.30 | 31.04±1.94 | 41.94±7.11 | 0.002 a | <0.001 a | 0.637 |
| V40 (%) | 10.19±1.77 | 14.16±6.13 | 11.28±1.78 | 13.63±3.80 | 0.010 a | 0.013 a | 0.013 a |
| Dmean (Gy) | 22.56±0.69 | 25.16±2.59 | 23.11±0.42 | 26.50±1.64 | 0.002 a | <0.001 a | 0.002 a |
| Small intestine | |||||||
| V10 (%) | 84.18±10.92 | 83.43±10.67 | 91.85±9.95 | 91.60±9.84 | 0.283 | 0.426 | <0.001 a |
| V20 (%) | 54.14±11.72 | 53.14±10.54 | 55.57±10.72 | 55.92±10.09 | 0.202 | 0.514 | 0.325 |
| V30 (%) | 30.89±9.62 | 33.17±10.20 | 31.00±8.95 | 32.37±9.13 | <0.001 a | <0.001 a | 0.871 |
| V40 (%) | 13.33±6.34 | 13.72±6.76 | 16.46±6.68 | 16.87±6.82 | 0.158 | 0.127 | 0.001 a |
| Dmean (Gy) | 23.19±3.87 | 23.20±3.76 | 24.31±3.64 | 24.46±3.58 | 0.979 | 0.052 | <0.001 a |
| Rectum | |||||||
| V10 (%) | 95.13±6.77 | 94.98±6.98 | 97.47±5.23 | 97.35±5.40 | 0.546 | 0.479 | 0.001 a |
| V20 (%) | 91.53±7.95 | 91.49±7.97 | 93.24±7.25 | 93.01±7.07 | 0.777 | 0.608 | 0.024 a |
| V30 (%) | 80.44±7.72 | 80.23±6.88 | 71.71±9.24 | 72.59±8.37 | 0.893 | 0.457 | 0.007 a |
| V40 (%) | 40.37±6.88 | 38.60±8.27 | 30.92±4.01 | 31.27±3.55 | 0.327 | 0.578 | <0.001 a |
| Dmean (Gy) | 35.50±2.11 | 37.30±8.63 | 34.24±1.97 | 34.35±1.80 | 0.418 | 0.598 | 0.006 a |
| Bladder | |||||||
| V10 (%) | 100.00±0.00 | 100.00±0.00 | 100.00±0.00 | 100.00±0.00 | – | – | – |
| V20 (%) | 94.50±6.17 | 94.76±6.57 | 100.00±0.00 | 99.52±1.81 | 0.649 | 0.318 | 0.004 a |
| V30 (%) | 70.47±5.38 | 70.19±4.91 | 84.73±7.34 | 84.21±8.87 | 0.741 | 0.673 | <0.001 a |
| V40 (%) | 41.32±5.88 | 40.60±6.13 | 35.81±4.37 | 36.09±4.32 | 0.244 | 0.640 | <0.001 a |
| Dmean (Gy) | 35.95±0.89 | 35.81±1.00 | 37.32±1.05 | 37.22±1.20 | 0.445 | 0.593 | 0.007 a |
| Spinal cord | |||||||
| Dmax (Gy) | 23.80±5.18 | 35.79±3.28 | 35.81±5.33 | 32.21±2.50 | <0.001 a | <0.001 a | 0.061 |
| Femoral head‐L | |||||||
| V10 (%) | 92.48±8.77 | 94.28±6.22 | 98.36±2.37 | 100.00±0.00 | 0.411 | 0.018 a | 0.028 a |
| V20 (%) | 34.98±8.85 | 37.50±6.13 | 68.23±13.22 | 82.07±14.31 | 0.125 | <0.001 a | <0.001 a |
| V30 (%) | 6.54±2.48 | 10.68±4.04 | 25.38±10.26 | 37.47±13.23 | 0.001 a | <0.001 a | <0.001 a |
| V40 (%) | 0.16±0.33 | 0.49±0.89 | 0.61±1.06 | 0.32±0.50 | 0.071 | 0.191 | 0.132 |
| Dmean (Gy) | 18.15±1.72 | 19.16±1.13 | 24.31±2.52 | 27.02±2.59 | 0.008 a | <0.001 a | <0.001 a |
| Femoral head‐R | |||||||
| V10 (%) | 90.19±8.47 | 91.36±8.18 | 98.31±2.85 | 100.00±0.00 | 0.566 | 0.038 a | 0.001 a |
| V20 (%) | 34.46±10.06 | 38.01±4.95 | 68.47±13.45 | 83.75±11.04 | 0.218 | <0.001 a | <0.001 a |
| V30 (%) | 6.67±4.28 | 10.16±3.99 | 24.64±7.89 | 37.21±9.44 | 0.040 a | <0.001 a | <0.001 a |
| V40 (%) | 0.07±0.15 | 0.37±0.55 | 0.43±0.65 | 0.49±1.25 | 0.043 a | 0.771 | 0.035 a |
| Dmean (Gy) | 17.95±1.84 | 18.82±1.16 | 24.25±2.18 | 27.19±1.89 | 0.131 | <0.001 a | <0.001 a |
| Kidney‐L | |||||||
| V10 (%) | 46.02±23.20 | 46.84±23.81 | 57.95±28.26 | 56.73±27.51 | 0.372 | 0.134 | <0.001 a |
| V20 (%) | 16.72±10.83 | 15.21±8.20 | 12.70±8.60 | 7.42±6.98 | 0.361 | 0.001 a | 0.058 |
| V30 (%) | 2.63±3.50 | 1.20±1.56 | 0.82±1.11 | 0.57±0.98 | 0.032 a | 0.158 | 0.026 a |
| V40 (%) | 0.14±0.33 | 0.03±0.10 | 0.02±0.04 | 0.02±0.05 | 0.195 | 1.000 | 0.139 |
| Dmean (Gy) | 10.60±4.43 | 10.41±4.20 | 11.31±4.69 | 10.58±4.31 | 0.280 | 0.004 a | 0.042 a |
| Kidney‐R | |||||||
| V10 (%) | 43.55±22.09 | 42.36±23.82 | 54.78±27.81 | 52.36±26.91 | 0.328 | 0.003 a | <0.001 a |
| V20 (%) | 16.28±8.48 | 11.63±7.18 | 13.28±7.57 | 6.62±5.31 | <0.001 a | <0.001 a | 0.029 a |
| V30 (%) | 2.07±2.15 | 0.66±0.87 | 0.90±1.13 | 0.33±0.56 | 0.003 a | 0.003 a | 0.016 a |
| V40 (%) | 0.02±0.07 | 0.01±0.03 | ‐ | ‐ | 0.334 | ‐ | 0.334 |
| Dmean (Gy) | 10.07±4.05 | 9.35±3.95 | 10.88±4.50 | 9.82±4.03 | 0.001 a | <0.001 a | 0.009 a |
Abbreviations: BMS, bone marrow‐sparing; PGTV, planning gross target volume; PTV, planning target volume; TOMO, helical tomotherapy; VMAT, volume‐modulated arc therapy.
*The P value was computed using a paired t test.
Statistically significant.
FIGURE 2.

The histogram shows the difference in dosimetric parameters of PTV and OARs among the two BMS‐IMRT (VMAT and TOMO) and two normal IMRT plans (VMAT and TOMO): (A) conformity index (CI) and (B) homogeneity index (HI) for planning target volume (PTV) and planning gross target volume (PGTV); Dose–volume parameters and comparisons of (C) BM and (D) other OARs. ***P<0.001, ****P<0.0001. ns: P > 0.05, no statistical significance. PTV, planning target volume; OAR, organs at risk, BMS‐IMRT, bone marrow‐sparing intensity‐modulated radiation therapy; VMAT, volume‐modulated arc therapy; TOMO, helical tomotherapy.
FIGURE 3.

(A) Dose–volume histograms (DVHs) of PTV, PGTV, and bone marrow in different plans. (B) Comparison of treatment time among different plans. (C) Multivariate logistic regression analyses of clinicopathological variables associated with grade 3 or 4 acute hematological toxicity. ****P < 0.0001. ns: P > 0.05, no statistical significance.
As depicted in Figures 2A and 2B and presented in Table 3, when evaluating the ability to attain a homogeneous and conformal dose distribution for the PTV/PGTV, the BMS‐IMRT plans displayed values of HI, CI, and Dmean that were comparable to those of the normal IMRT plans. This result demonstrated that BMS‐IMRT did not affect the target coverage of the PTV while protecting the BM and indicated the feasibility of its clinical application (Figure 1C).
In terms of other OARs, both the BMS‐IMRT and normal‐IMRT plans resulted in sufficient sparing of OARs. As shown in Figure 2D, BMS‐IMRT plans achieved comparable V30 and V40 for the small intestine, rectum, and bladder. Concurrently, they lead to a reduction in the V30 of the femoral heads, causing an elevation in the V20 of the kidneys. When compared with the normal IMRT plans in both TOMO and VMAT, although the BMS‐IMRT plan increased the V20 of the kidneys, the actual dose delivered was still within the predefined dose–volume constraints for the kidneys (V20 <30%, Dmean <12 Gy).
In addition, we also compared the difference between BMS‐TOMO and BMS‐VMAT plans, we discovered the BMS‐TOMO plans offered a significant benefit with a lower HI (BMS‐TOMO vs. BMS‐VMAT: 0.045±0.011 vs. 0.077±0.016, P<0.001) and higher CI (BMS‐TOMO vs. BMS‐VMAT: 0.777±0.050 vs. 0.603±0.100, P<0.001) were observed in the PGTV coverage, which is a small volume target. In terms of PTV coverage, BMS‐TOMO also exhibited better CI (BMS‐TOMO vs. BMS‐VMAT: 0.851±0.024 vs. 0.830±0.033, P = 0.014) and had similar HI, compared with BMS‐VAMT (Figures 1, 2, and 2B). Furthermore, the BMS‐TOMO plans demonstrated a substantial decrease in the volume of the bladder and rectum receiving a dose of 40 Gy (V40) compared with the BMS‐VMAT plans. Specifically, for the bladder, the V40 was 35.81% ± 4.37% for BMS‐TOMO, compared to 41.32% ± 5.88% for BMS‐VMAT (P<0.001). Similarly, for the rectum, V40 was 30.92% ± 4.01% for BMS‐TOMO, compared to 40.37% ± 6.88% for BMS‐VMAT (P<0.001). These results indicate superior sparing of the bladder and rectum with BMS‐TOMO (Figure 2D). In terms of BMS, both TOMO and VMAT demonstrated similar results in both high‐dose and low‐dose–volume areas, BMS‐VMAT only showed some advantage in V40 (BMS‐VMAT vs. BMS‐TOMO: 14.16±2.03 vs. 15.21±1.51, P = 0.032) (Figure 2C).
Treatment time was also evaluated, (Table 4 and Figure 3B). When compared with normal IMRT plans, the BMS‐IMRT plans had a longer mean treatment time (BMS‐TOMO vs. normal TOMO: 711.20±91.82 s vs. 680.40±89.02 s, P<0.001; BMS‐VMAT vs. normal VMAT: 225.95±25.52 s vs. 199.12±25.40 s, P<0.001). In the BMS‐IMRT plans, the mean treatment time of VMAT was significantly shorter than that of TOMO (BMS‐VMAT vs. BMS‐TOMO: 225.95±25.52 s vs. 711.20±91.82 s, P<0.001).
TABLE 4.
Treatment time in different plans.
| P‐value | |||||||
|---|---|---|---|---|---|---|---|
| BMS‐VMAT | Normal‐VMAT | BMS‐TOMO | Normal‐TOMO | BMS‐VMAT VS normal‐VMAT | BMS‐TOMO VS normal‐TOMO | BMS‐VMAT VS normal‐TOMO | |
| Treatment time (s) | 225.95±25.52 | 199.12±25.40 | 711.20±91.82 | 680.40±89.02 | 0.001 a | <0.001 a | <0.001 a |
Abbreviations: BMS, bone marrow‐sparing; VMAT, volume‐modulated arc therapy; TOMO, helical tomotherapy.
*The P value was computed using a paired t test.
Statistically significant
3.3. Hematologic AEs
The occurrence of grades ≥2 and ≥3 acute hematological toxicity is presented in Table 5. Most acute hematological toxicities were reversible, and no treatment‐related deaths were reported.
TABLE 5.
Incidence of grade ≥ 2 and grade ≥ 3 acute hematological toxicity in the BMS and control groups.
| Toxicity | BMS group n (%) |
Grade ≥ 2 Control group n (%) |
P‐value | BMS group n (%) |
Grade ≥ 3 Control group n (%) |
P‐value |
|---|---|---|---|---|---|---|
| HT | 34 (85) | 39 (88.6) | 0.622 | 15 (37.5) | 27 (61.4) | 0.029 a |
| Leukopenia | 34 (85) | 36 (81.8) | 0.698 | 13 (32.5) | 25 (56.8) | 0.026 a |
| Neutropenia | 18 (45) | 31 (70.5) | 0.033 a | 12 (30) | 20 (45.5) | 0.214 |
| Thrombocytopenia | 10 (25) | 8 (18.2) | 0.450 | 2 (5) | 4 (9.1) | 0.470 |
| Anemia | 8 (20) | 21 (47.7) | 0.008 a | 2 (5) | 3 (6.8) | 0.727 |
Abbreviations: BMS, bone marrow sparing; HT, hematological toxicity.
* The P value of grade ≥ 2 and grade ≥ 3 HT was computed by the chi‐square test. The P value of leukopenia, neutropenia, thrombocytopenia, and anemia was computed using the Mann–Whitney U rank test.
Statistically significant.
In the BMS‐IMRT group comprising 40 patients, 15 (37.5%) experienced grade 3 or 4 acute adverse effects. Specifically, 13 patients (32.5%) developed leukopenia, 12 (30.0%) developed neutropenia, two (5.0%) developed anemia, and two (5.0%) developed thrombocytopenia. Among the 44 patients in the normal‐IMRT group, 27 (61.4%) experienced grade 3 or grade 4 acute toxicities: 25 (56.8%) had leukopenia, 20 (45.4%) had neutropenia, 3 (6.8%) had anemia, and 4 (9.1%) had thrombocytopenia. The occurrence frequencies of ≥grade 3 acute hematological toxicity and leukopenia were remarkably greater in the normal‐IMRT group than in the BMS‐IMRT group (37.5% vs. 61.4%, P = 0.029; 32.5% vs. 56.8%, P = 0.026). The occurrence rates of ≥grade 2 neutropenia and anemia were lower in the BMS‐IMRT group than in the normal‐IMRT group (45% vs. 70.5%, P = 0.033; 20% vs. 47.7%, P = 0.008). The incidence of grades ≥2 and ≥3 thrombocytopenia did not show a significant difference. Additionally, the association between clinicopathological characteristics and severe hematologic adverse events (CTCAE v5.0 grades 3–4) was investigated using chi‐square tests and multivariate logistic regression models. As shown in Figure 3C and Table 6, BMS‐IMRT was associated with a decreased risk of grade 3 acute toxicities.
TABLE 6.
Incidence of different grades of acute hematological toxicity in different groups.
| Variable | G0–2, n (%) | G3–4, n (%) | P‐value |
|---|---|---|---|
| Age (years) | |||
| ≤45 | 5 (11.9) | 4 (9.5) | 1.000 |
| >45 | 37 (88.1) | 38 (90.5) | |
| Tumor size (cm) | |||
| ≤4 | 15 (35.7) | 17 (40.5) | 0.653 |
| >4 | 27 (64.3) | 25 (59.5) | |
| Pathologic types | |||
| Squamous | 40 (95.2) | 42 (100) | 0.494 |
| Adenocarcinoma | 2 (4.8) | 0 (0) | |
| Stage | |||
| IIIC1r | 37 (88.1) | 35 (83.3) | 0.533 |
| IIIC2r | 5 (11.9) | 7 (16.7) | |
| Chemotherapy cycles | |||
| 1–4 | 13 (31) | 13 (31) | 1.000 |
| 5–6 | 29 (69) | 29 (69) | |
| SCC‐Ag value,(ng/ml) | |||
| ≤10 | 27 (64.3) | 22 (52.4) | 0.163 |
| >10 | 13 (31.0) | 20 (47.6) | |
| N/A | 2 (4.7) | 0 (0) | |
| Original HB,(g/dL) | |||
| ≤9 | 1 (2.4) | 2 (4.8) | 0.557 |
| >9 | 41 (97.6) | 40 (95.2) | |
| BMS | |||
| No | 17 (40.5) | 27 (64.3) | 0.029 a |
| Yes | 25 (59.5) | 15 (35.7) | |
Abbreviations: BMS, bone marrow sparing; SCC, squamous cell carcinoma.
* The P value was computed using the chi‐square test.
Statistically significant.
4. DISCUSSION
Despite effective popularization of screening and HPV vaccination, an appreciable incidence of locally advanced cervical cancer (LACC) in China remains. EFRT combined with concurrent chemotherapy has become a commonly acknowledged treatment approach for patients with LACC with para‐aortic or common iliac nodes metastases. 12 , 13 , 14 Although EFRT yields encouraging survival outcomes for these patients, the radiation‐related hematological toxicity resulting from the large irradiation field of EFRT and substantial irradiated volume of the BM needs to be considered. 15 , 16 High‐grade acute hematological toxicity poses a significant concern, as it affects approximately 60% of patients undergoing EFRT, 17 which can lead to treatment breaks. Therefore, reducing hematological toxicity from EFRT with concurrent chemotherapy in patients with FIGO IIIC cervical cancer is of significant clinical interest and may further improve treatment efficacy.
BM is highly sensitive to irradiation. Radiation exposure significantly affects the continuous replenishment of circulating peripheral blood cells by hematopoietic stem cells residing in BM. In particular, the administration of radiation doses of 2–4 Gy over 1–3 days can substantially decrease both cellular density and proliferative activity of the BM. Another crucial factor is the volume of the BM exposed to irradiation. 18 , 19 The volume of BM exposed to both a low‐dose range of 10–20 Gy 20 , 21 , 22 , 23 and a high dose of 40 Gy are factors linked to ≥grade 2 hematological toxicity. 24 Approximately half of the active BM is situated in the lumbar sacrum, pubis, ilium, ischium, and proximal femur 25 and encompasses the radiation field of conventional EFRT in cervical cancer. Therefore, reducing BM damage during EFRT may reduce hematological toxicity in patients and improve their tolerance to treatment.
BM delineation methods in BMS‐IMRT include pelvic whole bone delineation, 26 , 27 BM cavity delineation, 17 , 28 , 29 and active BM delineation according to the functionally active areas using PET‐CT. Previous studies centered on PET‐guided functional BM have indicated that the majority of functional BM are situated within the marrow cavity. 30 , 31 , 32 Moreover, results from earlier studies have also suggested that when compared with the entire BM, IMRT plans that utilized the lumbosacral spine and hip bones as dose–volume constraints demonstrated the most effective sparing of the BM. 33 In our previous study on BMS‐IMRT in FIGO IIB cervical cancer, 11 we delineated low‐density intramedullary areas as BM regions and divided the pelvic BM into two distinct segments: the LS and OC. The IMRT plan for BMS, which employed LS and OC as distinct dose–volume constraints, attained the most efficient BMS. This plan resulted in the lowest BM volume for doses of ≥10 Gy, ≥20 Gy, ≥30 Gy, and ≥40 Gy. Therefore, we continued to use our previous BM delineation methods and dose–volume constraints in the current study and conducted a dosimetric study of BM‐sparing IMRT in EFRT. Compared to the normal IMRT plans, the BMS‐IMRT significantly decreased the volumes of BM exposed to doses of ≥10 Gy, ≥20 Gy, ≥30 Gy, and ≥40 Gy, without compromising the coverage of PTV and other OARs. Thus, applying BMS‐IMRT to EFRT may reduce hematological toxicity. In addition to volume dose limitations and delineation methods for BM, most studies have focused on radiotherapy techniques. Compared to conventional three‐dimensional conformal radiation therapy (3D‐CRT), IMRT achieves superior BM dose reduction through its characteristic beam configuration, employing multiple gantry angles or arcs, and demonstrating distinct dosimetric advantages for pelvic BM preservation. 34 , 35 , 36 Intensity‐modulated proton therapy has also demonstrated significance and robustness in BM sparing. 37 VMAT and TOMO are the most commonly used radiotherapy techniques for patients with cervical cancer in China. Therefore, these two radiotherapy techniques were included in our study. Both techniques were effective in sparing the BM, achieving comparable outcomes in terms of volume in both high‐ and low‐dose areas. In addition, TOMO planning achieved superior dose conformity to both the PTV and PGTV while simultaneously decreasing high‐dose irradiation volumes in the bladder and rectal tissues adjacent to the target region compared to VMAT plans; this finding is consistent with those of previous studies. 38 , 39 Although TOMO demonstrated better HI and CI of the target and better sparing of OARs, VMAT met our clinical demands. VMAT required a much shorter treatment time than TOMO when delivering EFRT. Therefore, VMAT may be more suitable for BMS‐IMRT in China, which is a developing country, considering its insufficient medical resources.
Recently, BMS‐IMRT has been increasingly adopted to manage diverse cancer types, and has demonstrated favorable clinical outcomes. One prospective trial on stage IB2–IIIB cervical cancer confirmed that BMS‐IMRT significantly reduced hematological toxicity. However, to date, studies on BMS‐IMRT and its hematological toxicity have been few. Therefore, this study aimed to identify the preliminary clinical advantages of BMS‐IMRT in patients with FIGO IIIC cervical cancer treated with EFRT. As previously noted, hematological toxicity is a significant concern in patients undergoing EFRT. Previous studies of normal EFRT with concurrent chemotherapy have demonstrated that grade ≥3 hematological toxicity rates can be high at 37.5%–77.5%. 9 Our retrospective analysis showed that patients treated with BMS‐IMRT were less likely to experience hematological toxicity (leukocytes, neutrophils, hemoglobin, and platelets) than those treated with normal IMRT. Compared with the normal‐IMRT group, the BMS‐IMRT group had a lower incidence rate of grade 3 or 4 acute hematological toxicity (37.5% vs. 61.4%, P = 0.029). Multivariate logistic regression analyses also demonstrated that BMS‐IMRT was associated with a reduced risk of grade 3–4 acute toxicities (odds ratio, 2.756; 95% confidence interval [CI], 1.020–7.452; P = 0.046). Therefore, using BMS‐IMRT is important in treating FIGO IIIC cervical cancer, particularly in patients receiving concurrent chemotherapy.
Our study had some limitations. Since we mainly focused on acute hematological toxicity using information from the past 2 years, long‐term side effects and patient survival data after treatment were lacking. In addition, this study was a retrospective, single‐institution investigation conducted on a restricted patient cohort, resulting in imperfect matching between the two groups. Therefore, prospective clinical trials incorporating more extensive clinical data and direct dosimetric parameters of the BM are necessary to reach a consensus on the efficacy of BMS‐IMRT in reducing hematological toxicity in patients undergoing EFRT.
5. Conclusion
BMS‐IMRT should be administered in patients with FIGO stage IIIC cervical cancer treated with EFRT, brachytherapy, and concurrent chemotherapy. BMS‐IMRT was associated with a clear improvement in grade ≥3 hematological toxicity rates. The findings of this study will guide the optimized clinical implementation of BMS‐IMRT in patients with FIGO stage IIIC cervical cancer undergoing EFRT.
AUTHOR CONTRIBUTIONS
SS. Y, DY. Y, and JN. W contributed to study design and interpretation. JN. W and X.Y contributed to manuscript writing, and SS. Y and LN. G contoured the target and OARs and DY. Y and XY. Hu designed the treatment plan; X.Y and WK.Y contributed to the dosimetric data collection. DM.L, QY.S, JQ.X, and X.L contributed to clinicopathologic data collection. SS. Y and DY. Y contributed to the critical manuscript revision. All authors agree with the final version and are accountable for the integrity of the published data.
CONFLICT OF INTEREST STATEMENT
All authors declare no potential conflicts of interest.
ETHICS APPROVAL
This study complied with the Declaration of Helsinki and was approved by the Ethics Committee of Harbin Medical University Cancer Hospital (Harbin, China). All patients provided written informed consent for publication of their images/data.
ACKNOWLEDGMENTS
This work was partly funded by the National Natural Science Foundation of China (82373207), the Climbing Program of Harbin Medical University Cancer Hospital (PDYS2024‐06), and the Medical Innovative Technology Fund of Harbin Medical University Cancer Hospital (CSJSGC‐2023‐24 and CSJSGC‐2023‐35). The authors thank all study participants.
Wang J, Yu X, Gu L, et al. Dosimetric and hematological toxicity analyses of bone marrow‐sparing intensity‐modulated radiation therapy for patients with cervical cancer treated with extended‐field radiation therapy. Prec Radiat Oncol. 2025;9:96–107. 10.1002/pro6.70019
Jia‐nan Wang, Xi Yu and Li‐na Gu equally contributed in this work
Contributor Information
Shan‐Shan Yang, Email: yangshanshan@hrbmu.edu.cn.
De‐Yang Yu, Email: yudeyang@hrbmu.edu.cn.
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Wang J, Lv H, Xue Z, Wang L, Bai Z. Temporal Trends of Common Female Malignances on Breast, Cervical, and Ovarian Cancer Mortality in Japan, Republic of Korea, and Singapore: Application of the Age‐Period‐Cohort Model. Biomed Res Int. 2018;2018(1):5307459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rose PG, Java J, Whitney CW, et al. Nomograms predicting progression‐free survival, overall survival, and pelvic recurrence in locally advanced cervical cancer developed from an analysis of identifiable prognostic factors in patients from NRG oncology/gynecologic oncology group randomized trials of chemoradiotherapy. J Clin Oncology. 2015;33(19):2136‐2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mohamud A, Høgdall C, Schnack T. Prognostic value of the 2018 FIGO staging system for cervical cancer. Gynecol Oncol. 2022;165(3):506‐513. [DOI] [PubMed] [Google Scholar]
- 5. Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. The Lancet. 2019;393(10167):169‐182. [DOI] [PubMed] [Google Scholar]
- 6. Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri: 2021 update. Int J Gynaecol Obstet. 2021;155:28‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abu‐Rustum NR, Yashar CM, Bean S, et al. NCCN guidelines insights: cervical cancer, version 1.2020: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2020;18(6):660‐666. [DOI] [PubMed] [Google Scholar]
- 8. Sood BM, Gorla GR, Garg M, et al. Extended‐field radiotherapy and high–dose‐rate brachytherapy in carcinoma of the uterine cervix: Clinical experience with and without concomitant chemotherapy. Cancer. 2003;97(7):1781‐1788. [DOI] [PubMed] [Google Scholar]
- 9. Kuji S, Hirashima Y, Komeda S, et al. Feasibility of extended‐field irradiation and intracavitary brachytherapy combined with weekly cisplatin chemosensitization for IB2–IIIB cervical cancer with positive paraaortic or high common iliac lymph nodes: a retrospective review. Int J Clin Oncol. 2014;19:341‐347. [DOI] [PubMed] [Google Scholar]
- 10. Chen SW, Liang JA, Yang SN, Ko HL, Lin FJ. The adverse effect of treatment prolongation in cervical cancer byhigh‐dose‐rate intracavitary brachytherapy. Radiother Oncol. 2003;67(1):69‐76. [DOI] [PubMed] [Google Scholar]
- 11. Yu DY, Bai YL, Feng Y, et al. Which bone marrow sparing strategy and radiotherapy technology is most beneficial in bone marrow‐sparing intensity modulated radiation therapy for patients with cervical cancer? Front Oncol. 2020;10:554241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Small W, Winter K, Levenback C, et al. Extended‐field irradiation and intracavitary brachytherapy combined with cisplatin and amifostine for cervical cancer with positive para‐aortic or high common iliac lymph nodes: results of arm II of Radiation Therapy Oncology Group (RTOG) 0116. Int J Gynecol Cancer. 2011;21(7):1266‐1275. [DOI] [PubMed] [Google Scholar]
- 13. Small Jr W, Winter K, Levenback C, et al. Extended‐field irradiation and intracavitary brachytherapy combined with cisplatin chemotherapy for cervical cancer with positive para‐aortic or high common iliac lymph nodes: results of ARM 1 of RTOG 0116. Int J Radiat Oncol Biol Phys. 2007;68(4):1081‐1087. [DOI] [PubMed] [Google Scholar]
- 14. Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin‐based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340(15):1144‐1153. [DOI] [PubMed] [Google Scholar]
- 15. Wang W, Liu X, Meng Q, Zhang F, Hu K. Prophylactic extended‐field irradiation for patients with cervical cancer treated with concurrent chemoradiotherapy: a propensity‐score matching analysis. Int J Gynecol Cancer. 2018;28(8):1584‐1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoon HI, Cha J, Keum KC, et al. Treatment outcomes of extended‐field radiation therapy and the effect of concurrent chemotherapy on uterine cervical cancer with para‐aortic lymph node metastasis. Radiat Oncol. 2015;10:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen M, Wang D, Bao Z, et al. The impact of bone marrow irradiation dose on acute haematologic toxicity in cervical cancer patients treated with concurrent chemoradiotherapy. Radiat Oncol. 2023;18(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lehar TJ, Kiely JM, Pease GL, Scanlon PW. Effect of focal irradiation on human bone marrow. Am J Roentgenol Radium Ther Nucl Med. 1966;96(1):183‐190. [DOI] [PubMed] [Google Scholar]
- 19. Everitt S, Hicks RJ, Ball D, et al. Imaging cellular proliferation during chemo‐radiotherapy: a pilot study of serial 18F‐FLT positron emission tomography/computed tomography imaging for non–small‐cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;75(4):1098‐1104. [DOI] [PubMed] [Google Scholar]
- 20. Marinescu ȘA, Toma R‐V, Trifănescu OG, et al. Predictors of Clinical Hematological Toxicities under Radiotherapy in Patients with Cervical Cancer—A Risk Analysis. Cancer. 2024;16(17):3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lukovic J, Hosni A, Liu A, et al. Evaluation of dosimetric predictors of toxicity after IMRT with concurrent chemotherapy for anal cancer. Radiat Oncol. 2023;178:109429. [DOI] [PubMed] [Google Scholar]
- 22. Li D, Wang D, Feng S, et al. Comparing dosimetric and cancer control outcomes after intensity‑modulated radiation therapy and tomotherapy for advanced cervical cancer. Oncol Lett. 2022;24(1):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rose BS, Aydogan B, Liang Y, et al. Normal tissue complication probability modeling of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2011;79(3):800‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klopp AH, Moughan J, Portelance L, et al. Hematologic toxicity in RTOG 0418: a phase 2 study of postoperative IMRT for gynecologic cancer. Int J Radiat Oncol Biol Phys. 2013;86(1):83‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hayman JA, Callahan JW, Herschtal A, et al. Distribution of proliferating bone marrow in adult cancer patients determined using FLT‐PET imaging. Int J Radiat Oncol Biol Phys. 2011;79(3):847‐852. [DOI] [PubMed] [Google Scholar]
- 26. Mell LK, Tiryaki H, Ahn K‐H, Mundt AJ, Roeske JC, Aydogan B. Dosimetric comparison of bone marrow‐sparing intensity‐modulated radiotherapy versus conventional techniques for treatment of cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71(5):1504‐1510. [DOI] [PubMed] [Google Scholar]
- 27. Bao Z, Wang D, Chen S, et al. Optimal dose limitation strategy for bone marrow sparing in intensity‐modulated radiotherapy of cervical cancer. Radiat Oncol. 2019;14:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murakami N, Okamoto H, Kasamatsu T, et al. A dosimetric analysis of intensity‐modulated radiation therapy with bone marrow sparing for cervical cancer. Anticancer Res. 2014;34(9):5091‐5098. [PubMed] [Google Scholar]
- 29. Mell LK, Sirák I, Wei L, et al. Bone marrow‐sparing intensity modulated radiation therapy with concurrent cisplatin for stage IB‐IVA cervical cancer: an international multicenter phase II clinical trial (INTERTECC‐2). Int J Radiat Oncol Biol Phys. 2017;97(3):536‐545. [DOI] [PubMed] [Google Scholar]
- 30. El Naby El‐Tawab ASMM, Barakat AF, Hussien FZ, Ghanam AAEA, Hakim MMA. Bone marrow sparing intensity modulated radiotherapy concurrent with chemotherapy for treatment of cervical malignancy. Onkologia i Radioterapia. 2023;17(3). [Google Scholar]
- 31. Elicin O, Callaway S, Prior JO, Bourhis J, Ozsahin M, Herrera FG. [18F] FDG‐PET standard uptake value as a metabolic predictor of bone marrow response to radiation: impact on acute and late hematological toxicity in cervical cancer patients treated with chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2014;90(5):1099‐1107. [DOI] [PubMed] [Google Scholar]
- 32. Wyss JC, Carmona R, Karunamuni RA, Pritz J, Hoh CK, Mell LK. [18F] Fluoro‐2‐deoxy‐2‐d‐glucose versus 3′‐deoxy‐3′‐[18F] fluorothymidine for defining hematopoietically active pelvic bone marrow in gynecologic patients. Radiother Oncol. 2016;118(1):72‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang J, Gu F, Ji T, Zhao J, Li G. Pelvic bone marrow sparing intensity modulated radiotherapy reduces the incidence of the hematologic toxicity of patients with cervical cancer receiving concurrent chemoradiotherapy: a single‐center prospective randomized controlled trial. Radiat Oncol. 2020;15:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seppenwoolde Y, Majercakova K, Buschmann M, et al. Early morbidity and dose–volume effects in definitive radiochemotherapy for locally advanced cervical cancer: a prospective cohort study covering modern treatment techniques. Strahlenther Onkol. 2021;197(6):505‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kumar A, Tudu R, Singh R, Raina P. Dose–volume analysis of acute gastrointestinal complications in cervical cancer undergoing definitive concurrent chemoradiation. J Cancer Res Ther. 2021;17(1):170‐173. [DOI] [PubMed] [Google Scholar]
- 36. Mundt AJ, Lujan AE, Rotmensch J, et al. Intensity‐modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;52(5):1330‐1337. [DOI] [PubMed] [Google Scholar]
- 37. Dinges E, Felderman N, McGuire S, et al. Bone marrow sparing in intensity modulated proton therapy for cervical cancer: efficacy and robustness under range and setup uncertainties. Radiother Oncol. 2015;115(3):373‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Engels B, De Ridder M, Tournel K, et al. Preoperative helical tomotherapy and megavoltage computed tomography for rectal cancer: impact on the irradiated volume of small bowel. Int J Radiat Oncol Biol Phys. 2009;74(5):1476‐1480. [DOI] [PubMed] [Google Scholar]
- 39. Lian J, Mackenzie M, Joseph K, et al. Assessment of extended‐field radiotherapy for stage IIIC endometrial cancer using three‐dimensional conformal radiotherapy, intensity‐modulated radiotherapy, and helical tomotherapy. Int J Radiat Oncol Biol Phys. 2008;70(3):935‐943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available on request from the corresponding author.


