Abstract
Fluid shear stress is a critical determinant of vascular remodeling and atherogenesis. Both integrins and the small GTPase Rho are implicated in endothelial cell responses to shear but the mechanisms are poorly understood. We now show that shear stress rapidly stimulates conformational activation of integrin αvβ3 in bovine aortic endothelial cells, followed by an increase in its binding to extracellular cell matrix (ECM) proteins. The shear-induced new integrin binding to ECM induces a transient inactivation of Rho similar to that seen when suspended cells are plated on ECM proteins. This transient inhibition is necessary for cytoskeletal alignment in the direction of flow. The results therefore define the role of integrins and Rho in a pathway leading to endothelial cell adaptation to flow.
Keywords: inside-out signaling/integrin affinity state/integrins/mechanotransduction
Introduction
Fluid shear stress due to blood flow is a major determinant of vascular remodeling, arterial tone and atherosclerosis (Davies et al., 1997). The endothelial monolayer in vivo acts as a signal transduction interface for hemodynamic forces; these forces determine the shape, cytoskeletal organization and function of endothelial cells, allowing the vessels to cope with physiological or pathological conditions (Davies et al., 1997). In regions of arteries where flow is disturbed and average shear stress is low, endothelial cells are polygonal and atherosclerosis develops (Caro et al., 1969, 1971; Girard and Nerem, 1995). In contrast, cells in regions of laminar shear elongate in the direction of flow and atherosclerosis is suppressed (Eskin et al., 1984; Davies, 1991, 1995; Girard and Nerem, 1993). Cell orientation and elongation are considered to be an adaptive process of endothelial cells to reduce the local mechanical load and subsequent injury. Despite the importance of mechanotransduction in vascular function and pathology, the molecular mechanisms by which endothelial cells sense and respond to fluid shear stress are not well understood.
A variety of data suggest a role for integrins in mechanotransduction. Endothelial cells must be anchored to their matrix in order to sense and transduce signals in response to shear stress (Takahashi and Berk, 1996). The attachment sites, termed focal adhesions, are complexes of integrins, cytoskeletal and signaling proteins that mediate signal transduction (Burridge and Chrzanowska-Wodnicka, 1996). A variety of published data implicate integrins in cellular responses to flow. Flow induces rapid remodeling of focal adhesion contacts, suggesting that these sites of cell attachment may be important in mechanotransduction (Davies et al., 1993, 1994). The β1- and β3-containing integrins are predominant in cultured bovine aortic endothelial cells (BAECs) (Dejana, 1993); interestingly, integrin αvβ3 is elevated in atherosclerotic plaques compared with quiescent regions of the same vessels (Hoshiga et al., 1995). In vivo, a peptide antagonist of integrins α5β1 and αvβ3 blocked flow-induced vasodilation (Muller et al., 1997). It was shown further that the endothelial responses elicited by a β1-activating antibody resemble some of those induced by shear stress (Ishida et al., 1996), and integrins transduce mechanical stimuli to biochemical signals via their association with Shc (Chen et al., 1999). More recently, it was shown that integrins function as intermediates in a shear stress-induced signaling cascade to activate Shc and c-Jun NH2-terminal kinases (JNKs) (Jalali et al., 2001).
Rho, Rac and Cdc42 (Nobes and Hall, 1994) are closely related small GTP-binding proteins that have also been implicated in mediating cellular responses to mechanical stimuli. Rho has been proposed as a mediator of mechanical signaling (Yano et al., 1996), based mainly on the ability of inhibitors of Rho to block cellular responses, including shear stress activation of JNK (Li et al., 1999). These treatments, however, may have indirect effects by altering the actin cytoskeleton. Li and co-workers showed that shear stress induced the translocation of Rho from cytosol to membrane, which was taken to indicate activation (Li et al., 1999). Membrane translocation of Rho family GTPases can, however, be regulated separately from activation (del Pozo et al., 2000). There is therefore a need to assess more directly the roles of Rho family GTPases in mediating mechanical signaling.
Integrins and Rho GTPases are intimately connected at multiple levels (for review see Schwartz and Shattil, 2000). Several groups have described biochemical regulation of Rho activity by integrins. Cells plated on fibronectin (FN) showed an initial downregulation of Rho activity during the phase of active spreading, when the cytoskeleton is highly dynamic. As spreading reaches completion, Rho GTP loading is increased, reaching a maximum as focal adhesion and stress fibers are assembled, and then slowly returning to baseline (Ren et al., 1999). These effects appear to be mediated by src-dependent phosphorylation of p190RhoGAP (Arthur et al., 2000) and require focal adhesion kinase (FAK) (Ren et al., 2000).
Despite numerous indications that integrins and Rho are in some way involved in mechanotransduction (Shyy and Chien, 1997; Chen et al., 1999; Li et al., 1999), the nature of their involvement is poorly understood. Jalali et al. (2001) found that shear stress induced an increase in endothelial monolayer staining with antibodies that recognize the ligand-occupied conformations of β1 and β3 integrins. Additionally, blocking new integrin binding to extracellular cell matrix (ECM) proteins prevented the shear-induced association of integrins with the adapter protein Shc. These results suggested that dynamic integrin–ligand binding contributed to shear stress signaling. To delineate further this pathway, we have tested the role of integrin activation in the endothelial cell response to shear. We report that shear stress induces conformational activation of integrins followed by their increased binding to ECM ligands. This de novo occupancy then triggers a transient decrease in Rho activity that is required for cellular alignment.
Results
Shear stress increases high affinity αvβ3
Application of fluid shear stress to resting BAECs induces integrin–ligand binding (Jalali et al., 2001). This result suggests that shear stress may upregulate integrin function, consistent with many studies demonstrating that cell adhesion can be regulated by a variety of intracellular signaling pathways (Hato et al., 1998). Integrin activation involves two complementary mechanisms, affinity and avidity modulation. Conformational changes in the integrin heterodimer lead to increased affinity for their ligands, whereas avidity modulation involves changes in lateral mobility and clustering of integrin heterodimers into oligomers that increase the binding of cells to multivalent matrices. Few reagents are available to directly assess conformational integrin activation separate from effects on clustering/avidity. A recently developed engineered Fab fragment WOW-1 (Pampori et al., 1999) reacts selectively with activated αvβ3 integrin and provides a suitable assay for activation of this integrin. WOW-1 contains an Arg-Gly-Asp (RGD) tract and binds only to unoccupied integrins in a ligand-mimetic fashion. Since WOW-1 is monovalent, it is relatively insensitive to changes in integrin clustering and reports mainly changes in integrin affinity.
To test whether shear stress altered activation of αvβ3 integrin, confluent monolayers of BAECs were subjected to an increase in shear stress from 0 to 12 dynes/cm2. Cells were then fixed, labeled with fragments of WOW-1 and counterstained with a polyclonal antiserum against the β3 integrin subunit to label the total pool of αvβ3. The majority of unsheared cells showed only weak WOW-1 staining, whereas the anti-β3 antibody stained both focal adhesions and diffusely over the cell surface as expected (Figure 1A). Shear stress caused the formation of lamellipodia, which correlated with increased WOW-1 staining, while total αvβ3 appeared unchanged. Some WOW-1 staining at the interface of neighboring cells was also observed (not shown). As a control, cells were labeled with WOW-1 in the presence of EDTA, which blocks specific ligand binding to αvβ3 and this showed minimal staining (not shown but see Figure 2A).
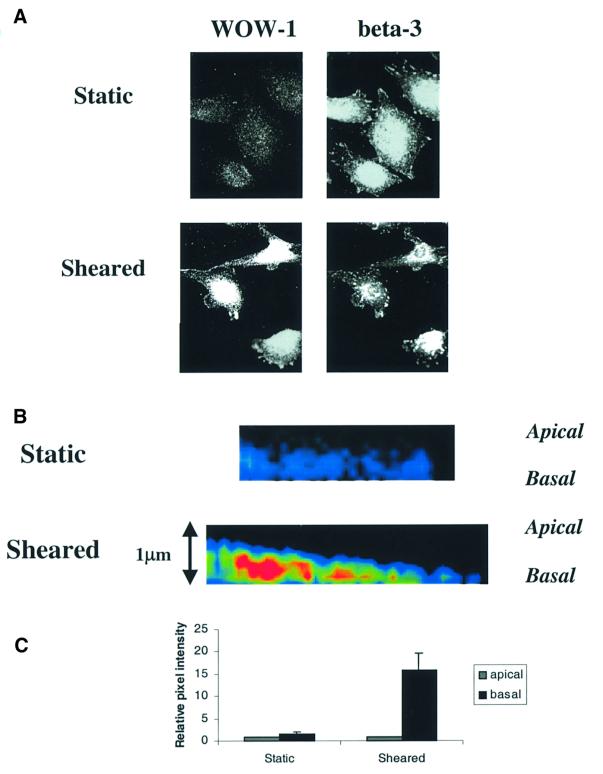
Fig. 1. Integrin αvβ3 activation by shear stress. (A) BAECs under static conditions or after shear stress at 12 dynes/cm2 for 5 min were fixed and stained with WOW-1 to label high-affinity unoccupied αvβ3 and double labeled with a polyclonal antibody that recognizes all αvβ3 integrins. (B) Color intensity profiles of representative XZ reconstructions of static and sheared cells that have been stained with WOW-1. Images show portions of single cells. The apical and basal surfaces are labeled. Red represents high and blue is low WOW-1 staining. (C) Histogram showing relative intensity of WOW-1 staining of basal compared with apical Z-sections of static and sheared cells. Cell edges were defined by TRITC– wheatgerm agglutinin (WGA) staining and were used as reference for quantitation of pixel intensity of WOW-1 staining; 45 cells were scored per condition.
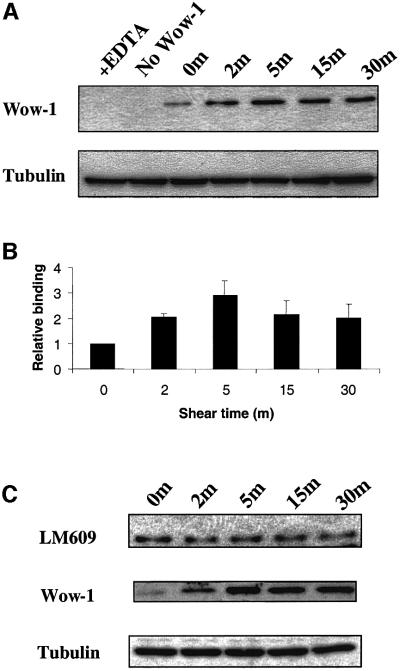
Fig. 2. Quantitation of high affinity αvβ3 integrin. (A) BAECs were subjected to shear for the indicated times and incubated with WOW-1 to label activated αvβ3. Bound antibody was quantitated by western blotting. As a control, EDTA was added to block specific binding. Blots were stripped and reprobed for tubulin to assess protein loading. (B) Histogram showing WOW-1 binding normalized to tubulin. Values are means ± SEM (n = 4 independent experiments) relative to cells at time 0, values are statistically significant as defined by a student’s t-test (P <0.01). (C) Coverslips were incubated with WOW-1 or LM609 and bound antibody detected by western blotting. Results are representative of three independent experiments. Blots were stripped and reprobed for tubulin to assess protein loading.
To determine whether the active integrin was localized to the basal or apical surface, samples were analyzed using laser scanning confocal microscopy. Endothelial cells are very flat (∼1 µm thick), which is sufficiently close to the limits of resolution for optical microscopy (∼0.4 µm) that the upper and lower surfaces cannot be fully resolved. However, examination of XZ reconstructions of the Z-sections showed low levels of WOW-1 staining on both apical and basal surfaces in static endothelial cells, whereas cells exposed to shear showed an increase in high affinity αvβ3 primarily in the basal sections (Figure 1B). Quantitation of staining intensity at the upper and lower surfaces (Figure 1C) showed that WOW-1 staining increased dramatically in the basal surface while barely changing at the apical surface. These results indicate that the shear-induced increase in activated αvβ3 is primarily basal in location. Previous studies using the anti-αvβ3 integrin antibody LM609 showed that shearing caused an enhanced staining at the peripheral region of the abluminal side of the cells and no change in the luminal side (Li et al., 1997), in agreement with our findings.
To quantitate total WOW-1 staining, cells were incubated with WOW-1 and rinsed; lysates were prepared and the amount of bound WOW-1 was determined by western blotting (Figure 2A). These results showed that shear stress stimulated an increase in high affinity αvβ3 that was approximately half maximal by 2 min; binding reached a maximum at 5 min that was ∼3-fold relative to baseline (P <0.01 compared with static control), then declined to ∼2-fold at 30 min (Figure 2B). EDTA abolished binding of WOW-1 to sheared cells, demonstrating specificity. LM609, an αvβ3-specific antibody that is insensitive to integrin activation state, showed no increase in binding to sheared cells compared with static controls (Figure 2C). This finding indicates that the increase in WOW-1 binding after shear stress represents a specific increase in the high affinity αvβ3 integrins, rather than a general increase in surface expression. WOW-1 is ligand mimetic and can therefore bind only unoccupied integrins (Pampori et al., 1999). The increase in WOW-1 staining shortly after application of shear stress and the subsequent decrease therefore suggests that the high-affinity unoccupied αvβ3 may rapidly bind to immobilized ECM, which would then prevent WOW-1 binding.
Shear stress stimulation of integrin binding to ECM
Affinity modulation as monitored by WOW-1 binding could induce integrin binding to ECM. In order to quantitate the increase in integrin–ligand binding in response to shear stress we used two markers for ligand-bound integrins. The ligand-induced binding site antibodies LIBS-6 and HUTS-21 have high affinities for ligand-occupied β3 (Hughes et al., 1997) and β1 integrins (Luque et al., 1996; Gómez et al., 1997), respectively. These antibodies also stabilize the ligand-occupied conformation of integrins and can therefore activate them, but at the low concentrations and short times used in these experiments they would serve primarily as a read-out for integrin without strongly perturbing integrin activation state.
It is well established that cells on FN and vitronectin (VN) for 2 h assemble a matrix and make focal adhesions similar to the endogenous matrix produced by cells cultured overnight (Hayman and Ruoslahti, 1979; Avnur and Geiger, 1981; Dejana et al., 1988). BAECs were sheared for the indicated times and the binding of the antibodies was assessed by western blotting. As predicted, shear stress increased LIBS-6 binding to BAECs that had been plated on VN, and HUTS-21 binding to cells on FN (Figure 3). Binding of LIBS-6 and HUTS-21 was detectable at 2 min but was <20% of maximal (Figure 3). In contrast to WOW-1, the binding of LIBS-6 and HUTS-21 continued to increase at later times of shear stress. To verify that increased staining with these antibodies indicates ligand binding, cells plated on FN or VN were stained with both reagents. Cells on VN showed marked LIBS-6 binding but no increase in HUT21 binding, consistent with the specificity of VN for αv integrins. Conversely, cells on FN showed a large increase in HUTS-21 binding but only a slight increase in LIBS-6 binding, again consistent with the preference of FN for α5β1 relative to αvβ3 (not shown). These observations, together with the WOW-1 binding time courses, suggest that integrin activation is followed temporally by the formation of integrin–ligand complexes.
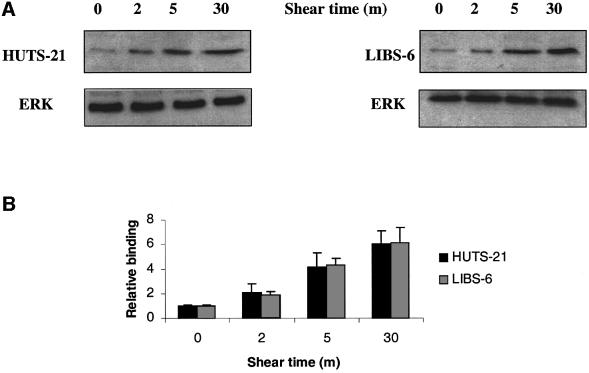
Fig. 3. Quantitation of integrin occupancy. (A) BAECs plated on VN or FN were sheared for the indicated times and incubated with 5 µg/ml LIBS-6 or HUTS-21, respectively. Bound antibody was assessed by western blotting. Blots were stripped and reprobed for extracellular signal regulator kinase (ERK) to assess protein loading. (B) Results from three independent experiments were quantitated. Values are means ± SEM.
Biphasic regulation of Rho activity during shear stress
New integrin–ligand binding would be predicted to induce many of the same signals seen in cells freshly plated on ECM proteins. As Rho has been suggested to play an important role in endothelial cell responses to shear, we assayed Rho GTP loading using a recently developed pull-down assay (Ren et al., 1999). BAECs were sheared for the indicated times and Rho-GTP loading assays were performed. Following a lag period of ∼2 min, shear triggered a decrease in Rho activity at 5–15 min that was followed by an increase that peaked at 60 min, which was statistically significant (P <0.05) (Figure 4B).
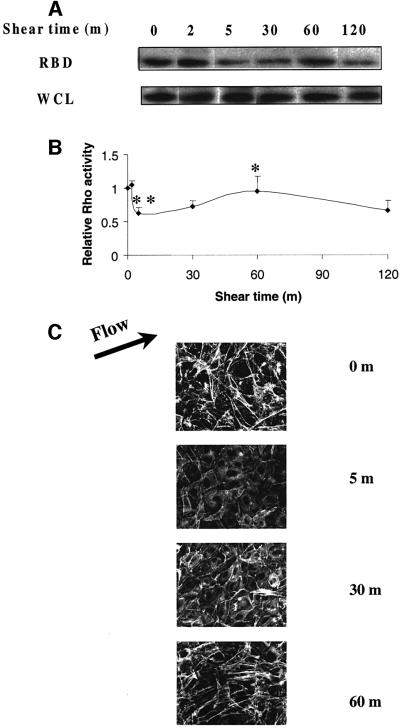
Fig. 4. Regulation of Rho activity by shear stress. (A) BAECs were subjected to shear stress for the indicated times. Rho activity is indicated by the amount of RBD-bound Rho, normalized to total Rho in whole cell lysates (WCL). (B) Quantitation of Rho activity relative to cells at time 0. Values are means ± SEM from three independent experiments, each of which was performed in duplicate. For the statistical analysis 5 min was compared with 0 min (**P <0.01) and 60 min was compared with 5 min (*P <0.05). (C) Time course of F-actin organization. Cells were subjected to shear stress, fixed and stained with rhodamine–phalloidin to detect actin stress fibers.
In order to correlate the effect of shear stress on Rho activity with its known actions on the cytoskeleton, sheared cells were fixed and stained with rhodamine– phalloidin to visualize F-actin. In sheared cells, the initial phase of low Rho activity is associated with a decrease in stress fibers, while the later restoration of Rho activity corresponds to an increase in stress fibers (Figure 4C). Reappearance of stress fibers at 60 min is accompanied by significant cell alignment in the direction of flow, although maximal alignment requires longer times of exposure to flow (Levesque and Nerem, 1985). Rho activity decreases again at 120 min, although actin stress fibers remain. We do not fully understand this result, although very similar effects were seen during adhesion to FN (Ren et al., 1999). It may be that Rho is highest during assembly of stress fibers and that lower levels are sufficient for their maintenance.
New ECM binding mediates stress-induced Rho activation
The response in Rho activity to shear is highly reminiscent of the effect of plating cells on FN (Ren et al., 1999; Arthur et al., 2000). Comparison of time courses of integrin–ligand binding (Figure 3) with that of Rho activation (Figure 4) in response to shear stress reveals that binding of integrins to ECM occurs slightly before Rho inhibition. We therefore tested whether the change in Rho activity requires new integrin binding to ECM by preventing formation of new integrin–ligand connections without affecting the existing adhesions. Endothelial cells were plated on FN for 2 h to allow completion of cell spreading and formation of adhesions. To study the effects of blocking the unoccupied FN sites, the cells were treated for 15 min with either blocking (16G3) or non-blocking (11E5) anti-FN antibodies. Shear stress was then applied to the cells and Rho assays were performed. No effect on cell shape or actin staining patterns could be detected after this treatment (not shown), indicating that the antibodies did not disrupt existing integrin–ligand bonds (not shown). Rho assays showed that the blocking antibody 16G3 strongly inhibited the shear stress-induced decrease in Rho activity, whereas the non-blocking antibody 11E5 had no effect (Figure 5). These results provide support for the hypothesis that regulation of Rho activity by shear stress is downstream of new integrin–ligand binding.
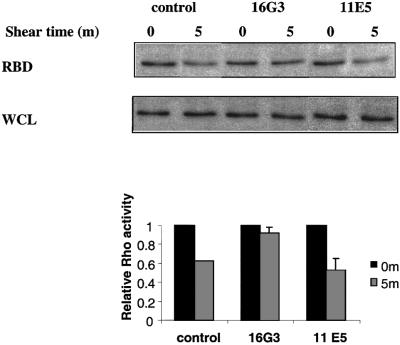
Fig. 5. Blocking free FN sites. BAECs were plated on FN-coated slides for 2 h in the absence of serum and then incubated with anti-FN Fab fragments for 15 min. Cells were either sheared (5 min, 12 dynes/cm2) or kept as static control and Rho assayed. Values are means ± SEM (n = 3, each in duplicate) relative to static samples.
To test whether integrin activation is sufficient to modulate Rho activity, its activity was assayed following integrin activation by two procedures. First, BAECs were treated with the activating anti-αvβ3 antibody LIBS-6 (Huang et al., 1993) at a concentration previously shown to activate αvβ3 integrin (Frelinger et al., 1990; O’Toole et al., 1994). Secondly, MnCl2 was added to activate all of the integrins. Although we did not observe changes in morphology, these treatments increase integrin binding to ECM and adhesion strength (Palecek et al., 1997; García et al., 1998). Both LIBS-6 and MnCl2 caused dramatic decreases in the activity of Rho, which showed partial recovery at 60 min (Figure 6). The regulation of Rho activity by artificial activation of integrins is similar to but more pronounced than that seen after the mechanical activation by shear stress (Figure 4). These results show that increased integrin activation in response to shear stress can account for the transient inhibition of Rho.
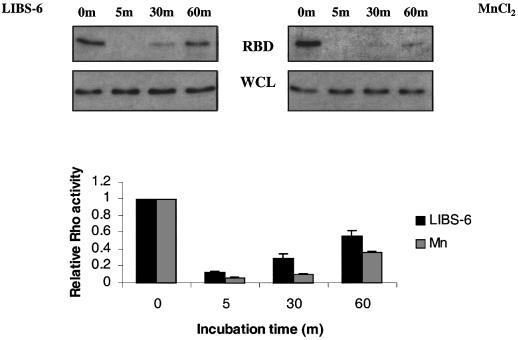
Fig. 6. Integrin activation mimics the shear-induced Rho activation. BAECs were incubated with 0.5 mM MnCl2 or 15 µg/ml LIBS-6 antibody for the indicated times and Rho activity was determined. Values are means ± SEM (n = 3, each in duplicate) and represent Rho activity relative to cells at time 0.
Downregulation of Rho is essential for the shear stress-induced cell alignment
Exposure of endothelial cells to shear stress results in their elongation along the direction of flow (Levesque and Nerem, 1985), the formation of stress fibers and alignment of microfilaments (Girard and Nerem, 1993). We hypothesized that the shear stress-induced decrease in Rho activity may be required for this response. To test this idea, BAECs were transiently transfected with expression vectors for constitutively activated Rho (V14Rho) together with green fluorescent protein (GFP) as a marker for transfected cells. Alternatively, cells received wild-type (WT) Rho or empty vector as controls. The cells were then sheared for 16 h, fixed and stained for actin. GFP-positive cells were then examined for elongation and orientation with the direction of fluid flow. As shown in Figure 7, few of the static cells were aligned. After 16 h of shear, BAECs expressing GFP alone or WT Rho aligned with the direction of flow and formed stress fibers similarly to untransfected cells. In contrast, expression of constitutively activated V14Rho strongly inhibited cell and cytoskeletal alignment in response to shear stress. These results demonstrate that the initial decline in Rho activity is required for cells to orient with the direction of flow at later times.
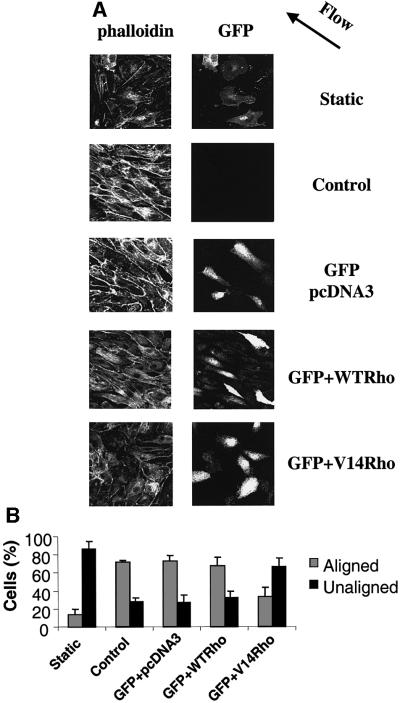
Fig. 7. Constitutively activated Rho blocks cell alignment. (A) Cells transiently transfected with empty vector, WT Rho or V14Rho plus GFP were subjected to shear stress or kept under static conditions for 16 h. The cells were fixed, stained with rhodamine–phalloidin and observed by confocal microscopy. The direction of flow is indicated by an arrow. (B) Quantification of cell alignment with the direction of flow. Values are means ± SEM, n = 3; >100 cells were scored per condition.
Discussion
In the present study, we have investigated the roles of integrins and Rho in endothelial shear stress signaling. Previous studies have established that endothelial cells respond to physiological levels of shear stress in the following manner: cells initially enhance their attachments to the ECM and neighboring cells, followed by a loss of the dense peripheral bands and an increase in motile behavior. Cells subsequently increase their stress fibers as cell alignment and elongation begin (Galbraith et al., 1998). This adaptation to flow is believed to contribute to a reduction of shear gradients along the endothelial cell surface (Barbee et al., 1994). It is possible that the shear stress-induced cytoskeletal changes are facilitated by integrin signaling. For example, shear stress causes activation of FAK (Li et al., 1997) and remodeling of focal adhesions in the direction of flow within several minutes (Davies et al., 1994). Indeed, the dynamic remodeling of the adhesion plaques in endothelial cells exposed to shear stress requires constant association and dissociation of integrins with the ECM (Davies et al., 1994). One of the general mechanisms for regulation of integrin function involves their activation. As a consequence of such activation, integrins enhance their apparent affinity or avidity for their extracellular ligands (Bazzoni and Hemler, 1998).
In this study, we show for the first time that integrin αvβ3 is activated by shear stress. Activation appears to be the result of affinity modulation because sheared cells show increased binding to a conformation-sensitive anti-αvβ3 antibody, WOW-1 Fab. Integrin α5β1 also shows increased binding to ECM following shear stress (Jalali et al., 2001); although no specific probe is available to assess α5β1 activity, it seems likely that it too is activated by shear stress. Analysis of the immunofluorescence data suggested that shear-induced increase of high affinity αvβ3 integrin was primarily on the basal surface of BAECs. Davies et al. (1994) showed that focal adhesion sites at the abluminal endothelial membrane are both acutely and chronically responsive to frictional shear stress forces applied to the opposite (luminal) cell surface. Our previous report that shear stress increases integrin– ligand binding on the basal surface of human umbilical vein endothelial cells (HUVECs) (Jalali et al., 2001) is in concert with the hypothesis that integrin activation is followed by the formation of integrin–ligand complexes on the basal surface of endothelial cells. However, the restriction of active αvβ3 to the basal surface may be a general feature of endothelial cell behavior rather than a specific consequence of shear stress signaling. Active integrins on the luminal surface of endothelia would be highly thrombogenic; thus, these cells may have evolved mechanisms to suppress high affinity integrins on the luminal side. Whether this spatial specificity is a feature of a cell’s response to shear stress or a general aspect of endothelial cells is therefore unclear.
We have shown that shear stress regulates Rho activity by performing GTP loading assays. These results implicate Rho as an important regulator of the shear-induced cytoskeletal reorganization. We suggest that the observed regulatory effects on Rho activity closely match the requirements for shear stress-induced cell alignment. Either inhibiting Rho by treatment of cells with C3 toxin or transfection with a dominant-negative form of Rho (Li et al., 1999) or constitutively activating Rho decreases cell alignment, just as it decreases cell migration (Takaishi et al., 1993; Allen et al., 1998; del Pozo et al., 1999). Thus, dynamic regulation of Rho is important for cell alignment. Importantly, our observations place Rho at a site downstream of integrins binding to their immobilized ligands. These findings are in agreement with those described in the study of Jalali et al. (2001), which showed that new connections between integrins and matrix proteins were needed for integrins to associate with Shc and activate JNK in response to shear stress.
The molecular mechanism(s) responsible for the cross-talk between integrins and the initial downregulation of Rho activity in response to shear stress are now becoming clearer. We have observed that fibroblasts from FAK–/– mice failed to transiently inhibit Rho activity when plated on FN, whereas re-expression of FAK restored normal Rho regulation (Ren et al., 2000). Another report has focused on the role of c-Src-mediated integrin signaling in modulating RhoA activity during cell adhesion through tyrosine phosphorylation of p190RhoGAP GTPase activating protein (Arthur et al., 2000). The same investigators also demonstrated the existence of a protein tyrosine phosphatase Shp-2, sensitive to calpeptin, acting upstream of RhoA (Schoenwaelder et al., 2000). Future studies aiming to determine the signaling events that activate Rho following its transient inhibition by shear stress are of particular interest in elucidating fundamental mechanisms in mechanotransduction.
In summary, the work presented here sheds new light on the mechanism by which integrins mediate mechanotransduction in response to shear stress. We have defined a pathway in which conversion of the integrin αvβ3 to its high affinity state leads to new integrin binding to the ECM. These new integrin–ECM connections generate a signaling cascade similar to cell adhesion and are essential for the downstream signaling to Rho, which allows endothelial cell alignment as a mechanism of adaptation to hemodynamic forces.
Materials and methods
Cell culture and shear stress
BAECs were maintained in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum, 1% penicillin/streptomycin and 1% l-glutamine (Gibco-BRL, Gaithersberg, MD). All cell cultures were kept in a humidified 5% CO2/95% air incubator at 37°C. BAECs cultured on 38 × 76 mm slides to confluence were either kept as static controls or subjected to shear stress in a parallel plate flow chamber (Frangos et al., 1985) modified so that multiple slides could be sheared simultaneously. A surface area of 14 cm2 on the BAEC-seeded slide was exposed to fluid shear stress generated by perfusing culture medium over the cells. The pH of the system was kept constant by gassing with 5% CO2/95% air and the temperature was maintained at 37°C. Shear stress at 12 dynes/cm2 was used for all experiments, which is relevant to the physiological range in human arteries.
Detection of activated integrins
BAECs grown on FN-coated slides were starved overnight in 0.5% serum then shear stress applied. Where indicated, cells were treated with 1 mM MnCl2 to activate all integrins or 15 µg/ml LIBS-6 (a gift from Dr M.H.Ginsberg) to activate β3 integrins, as previously described (Frelinger et al., 1990; O’Toole et al., 1994). Coverslips were incubated with His6-tagged recombinant WOW-1 Fab (30 µg/ml) or LM609 (15 µg/ml) (a gift from Dr D.A.Cheresh) for 30 min, washed in phosphate-buffered saline (PBS) and lysed in SDS sample buffer. Bound WOW-1 was detected by western blotting using a goat anti-rabbit antibody against His (Santa Cruz Biotechnology) and horseradish peroxidase (HRP)–protein A (Amersham Pharmacia Biotech).
For detection of occupied integrins, BAECs plated on FN- and VN-coated slides were starved overnight in 0.5% serum prior to application of shear stress for the indicated times. Cells were incubated with LIBS-6 (5 µg/ml) or HUTS-21 (5 µg/ml) (a gift from Drs F.Sánchez-Madrid and C.Cabañas) for 20 min at 37°C. Cells were rinsed with PBS, lysed in SDS sample buffer and bound IgG detected by western blotting using an HRP–goat anti-mouse antibody.
GTPase assays
BAECs were seeded on slides coated with FN (5 µg/cm2), grown to confluence and starved overnight in 0.5% serum. After shear stress, cells were chilled on ice, washed with ice-cold PBS and lysed in RIPA buffer [50 mM Tris pH 7.2, 1% Triton X-100, 0.5% sodium deoxycolate, 0.1% SDS, 500 mM NaCl, 10 mM MgCl2, 10 µg/ml each of leupeptin and aprotinin, 1 mM phenylmethyl sulfonyl fluoride (PMSF)] (Ren et al., 1999). Lysates were centrifuged at 13 000 g at 4°C for 10 min, and equal volumes were incubated with glutathione S-transferase–RBD (GST–RBD) beads (20 µg of protein per sample) at 4°C for 45 min. The beads were washed with buffer containing 50 mM Tris pH 7, 0.5% NP40, 500 mM NaCl, 1 mM MgCl2, 1 mM EGTA and 10 µg/ml each of leupeptin and aprotinin, 1 mM PMSF. Rho was detected by western blotting using a polyclonal antibody against RhoA (Santa Cruz Biotechnology).
Blocking of unoccupied FN
BAECs were serum starved overnight in 0.5% serum-containing media, plated on FN-coated glass slides and allowed to adhere for 2 h. The cells were incubated with 20 µg/ml Fab fragments of anti-FN monoclonal antibodies for 15 min, then shear was applied for 5 min. Fab fragments were produced as previously described (Harlow and Lane, 1988). The antibodies were: 16G3, which blocks both the αvβ3 and α5β1 binding sites for FN, and 11E5, which is a non-function blocking antibody (a generous gift from Dr K.M.Yamada).
Fluorescence microscopy
Cells were fixed for 30 min in 2% formaldehyde in PBS, permeabilized in 0.2% Triton X-100/PBS, and rinsed twice with PBS. Non-specific sites were blocked with 10% goat serum, incubated with WOW-1 Fab (30 µg/ml) and stained with 10 µg/ml ALEXA green-conjugated goat anti-mouse Fab′2 (Molecular Probes). They were also labeled with a polyclonal β3 serum (a gift of Dr Mark Ginsberg, Scripps Research Institute) at 1:100, followed by 1:100 CY5-conjugated goat anti-rabbit Fab′2 fragment (Sigma) and mounted in immunofluorescence mounting medium (ICN Immunobiologicals). Where indicated, cells were stained with TRITC–phalloidin (Sigma) or TRITC–wheatgerm agglutinin (WGA) (Sigma). Confocal serial sectioned images were acquired using a BioRad 1024 MRC scanning confocal microscope. Serially sectioned composite reconstructions were used to obtain a Z-section through the longitudinal plane of each cell. The pixel intensity profile of each image, comparing the apical and basal surfaces, was analyzed using Image Pro Software.
V14Rho transfections
BAECs were seeded on FN-coated slides and at ∼50% confluency were transfected with 2.25 µg of hemagglutinin-tagged V14 Rho or WT Rho in pcDNA3 plus 0.25 µg of vector encoding enhanced GFP using Effectene reagents (Qiagen), according to the manufacturer's instructions. After 10 h in growth medium cells were starved overnight in 0.5% serum. They were then subjected to shear stress for 16 h, fixed and stained with TRITC–phalloidin.
Acknowledgments
Acknowledgements
We thank Dr S.Y.Li for assistance with initial shear experiments, Dr N.Pampori for preparing the WOW-1 Fab and Dr W.B.Kiosses for technical imaging assistance. This work was supported by PHS grants P01 HL48728 (M.A.S.), P01 HL64382 (M.A.S./S.C.) and a fellowship from EMBO (ALTF 501-1997) (to M.A.d.P.).
References
- Allen W.E., Zicha,D., Ridley,A.J. and Jones,G.E. (1998) A role for Cdc42 in macrophage chemotaxis. J. Cell Biol., 141, 1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur W.T., Petch,L.A. and Burridge,K. (2000) Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol., 10, 719–722. [DOI] [PubMed] [Google Scholar]
- Avnur Z. and Geiger,B. (1981) The removal of extracellular fibronectin from areas of cell–substrate contact. Cell, 25, 121–132. [DOI] [PubMed] [Google Scholar]
- Barbee K.A., Davies,P.F. and Lal,R. (1994) Shear stress-induced reorganization of the surface topography of living endothelial cells imaged by atomic force microscopy. Circ. Res., 74, 163–171. [DOI] [PubMed] [Google Scholar]
- Bazzoni G. and Hemler,M.E. (1998) Are changes in integrin affinity and conformation overemphasized? Trends Biochem. Sci., 23, 30–34. [DOI] [PubMed] [Google Scholar]
- Burridge K. and Chrzanowska-Wodnicka,M. (1996) Focal adhesions, contractility and signaling. Annu. Rev. Cell. Dev. Biol., 12, 463–518. [DOI] [PubMed] [Google Scholar]
- Caro C.G., Fitz-Gerald,J.M. and Schroter,R.C. (1969) Arterial wall shear and distribution of early atheroma in man. Nature, 223, 1159–1160. [DOI] [PubMed] [Google Scholar]
- Caro C.G., Fitz-Gerald,J.M. and Schroter,R.C. (1971) Atheroma and arterial wall shear. Observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proc. R. Soc. Lond. B Biol. Sci., 177, 109–159. [DOI] [PubMed] [Google Scholar]
- Chen K.D., Li,Y.S., Kim,M., Li,S., Yuan,S., Chien,S. and Shyy,J.Y. (1999) Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins and Shc. J. Biol. Chem., 274, 18393–18400. [DOI] [PubMed] [Google Scholar]
- Davies P.F. (1991) Mechanical sensing mechanisms: shear stress and endothelial cells. J. Vasc. Surg., 13, 729–731. [DOI] [PubMed] [Google Scholar]
- Davies P.F. (1995) Flow-mediated endothelial mechanotransduction. Physiol. Rev., 75, 519–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P.F., Robotewskyj,A. and Griem,M.L. (1993) Endothelial cell adhesion in real time. Measurements in vitro by tandem scanning confocal image analysis. J. Clin. Invest., 91, 2640–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P.F., Robotewskyj,A. and Griem,M.L. (1994) Quantitative studies of endothelial cell adhesion. Directional remodeling of focal adhesion sites in response to flow forces. J. Clin. Invest., 93, 2031–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P.F. et al. (1997) Spatial relationships in early signaling events of flow-mediated endothelial mechanotransduction. Annu. Rev. Physiol., 59, 527–549. [DOI] [PubMed] [Google Scholar]
- Dejana E. (1993) Endothelial cell adhesive receptors. J. Cardiovasc. Pharmacol., 21, S18–S21. [DOI] [PubMed] [Google Scholar]
- Dejana E., Colella,S., Conforti,G., Abbadini,M., Gaboli,M. and Marchisio,P.C. (1988) Fibronectin and vitronectin regulate the organization of their respective Arg-Gly-Asp adhesion receptors in cultured human endothelial cells. J. Cell Biol., 107, 1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo M.A., Vicente-Manzanares,M., Tejedor,R., Serrador,J.M. and Sanchez-Madrid,F. (1999) Rho GTPases control migration and polarization of adhesion molecules and cytoskeletal ERM components in T lymphocytes. Eur. J. Immunol., 29, 3609–3620. [DOI] [PubMed] [Google Scholar]
- del Pozo M.A., Price,L.S., Alderson,N.B., Ren,X.D. and Schwartz,M.A. (2000) Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J., 19, 2008–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskin S.G., Ives,C.L., McIntire,L.V. and Navarro,L.T. (1984) Response of cultured endothelial cells to steady flow. Microvasc. Res., 28, 87–94. [DOI] [PubMed] [Google Scholar]
- Frangos J.A., Eskin,S.G., McIntire,L.V. and Ives,C.L. (1985) Flow effects on prostacyclin production by cultured human endothelial cells. Science, 227, 1477–1479. [DOI] [PubMed] [Google Scholar]
- Frelinger A.L., Cohen,I., Plow,E.F., Smith,M.A., Roberts,J., Lam,S.C. and Ginsberg,M.H. (1990) Selective inhibition of integrin function by antibodies specific for ligand-occupied receptor conformers. J. Biol. Chem., 265, 6346–6352. [PubMed] [Google Scholar]
- Galbraith C.G., Skalak,R. and Chien,S. (1998) Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil. Cytoskeleton, 40, 317–330. [DOI] [PubMed] [Google Scholar]
- García A.J., Takagi,J. and Boettiger,D. (1998) Two-stage activation for α5β1 integrin binding to surface-adsorbed fibronectin. J. Biol. Chem., 273, 34710–34715. [DOI] [PubMed] [Google Scholar]
- Girard P.R. and Nerem,R.M. (1993) Endothelial cell signaling and cytoskeletal changes in response to shear stress. Front. Med. Biol. Eng., 5, 31–36. [PubMed] [Google Scholar]
- Girard P.R. and Nerem,R.M. (1995) Shear stress modulates endothelial cell morphology and F-actin organization through the regulation of focal adhesion-associated proteins. J. Cell Physiol., 163, 179–193. [DOI] [PubMed] [Google Scholar]
- Gómez M., Luque,A., del Pozo,M.A., Hogg,N., Sánchez-Madrid,F. and Cabañas,C. (1997) Functional relevance during lymphocyte migration and cellular localization of activated β1 integrins. Eur. J. Immunol., 27, 8–16. [DOI] [PubMed] [Google Scholar]
- Harlow E and Lane,D. (1988) Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Hato T., Pampori,N. and Shattil,S.J. (1998) Complementary roles for receptor clustering and conformational change in the adhesive and signaling functions of integrin αIIb β3. J. Cell Biol., 141, 1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman E.G. and Ruoslahti,E. (1979) Distribution of fetal bovine serum fibronectin and endogenous rat cell fibronectin in extracellular matrix. J. Cell Biol., 83, 255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiga M., Alpers,C.E., Smith,L.L., Giachelli,C.M. and Schwartz,S.M. (1995) α-v β-3 integrin expression in normal and atherosclerotic artery. Circ. Res., 77, 1129–1135. [DOI] [PubMed] [Google Scholar]
- Huang M.M., Lipfert,L., Cunningham,M., Brugge,J.S., Ginsberg,M.H. and Shattil,S.J. (1993) Adhesive ligand binding to integrin αIIb β3 stimulates tyrosine phosphorylation of novel protein substrates before phosphorylation of pp125FAK. J. Cell Biol., 122, 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P.E., Renshaw,M.W., Pfaff,M., Forsyth,J., Keivens,V.M., Schwartz,M.A. and Ginsberg,M.H. (1997) Suppression of integrin activation: a novel function of a Ras/Raf-initiated MAP kinase pathway. Cell, 88, 521–530. [DOI] [PubMed] [Google Scholar]
- Ishida T., Peterson,T.E., Kovach,N.L. and Berk,B.C. (1996) MAP kinase activation by flow in endothelial cells. Role of β1 integrins and tyrosine kinases. Circ. Res., 79, 310–316. [DOI] [PubMed] [Google Scholar]
- Jalali S., del Pozo,M.M., Chen,K.D., Miao,H., Li,Y.S., Schwartz,M.A., Shyy,J.Y. and Chien,S. (2001) Integrin-mediated mechano transduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc. Natl Acad. Sci. USA, 98, 1042–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque M.J. and Nerem,R.M. (1985) The elongation and orientation of cultured endothelial cells in response to shear stress. J. Biomech. Eng., 107, 341–347. [DOI] [PubMed] [Google Scholar]
- Li S., Kim,M., Hu,Y.L., Jalali,S., Schlaepfer,D.D., Hunter,T., Chien,S. and Shyy,J.Y. (1997) Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. J. Biol. Chem., 272, 30455–30462. [DOI] [PubMed] [Google Scholar]
- Li S., Chen,B.P., Azuma,N., Hu,Y.L., Wu,S.Z., Sumpio,B.E., Shyy,J.Y. and Chien,S. (1999) Distinct roles for the small GTPases Cdc42 and Rho in endothelial responses to shear stress. J. Clin. Invest., 103, 1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque A., Gomez,M., Puzon,W., Takada,Y., Sanchez-Madrid,F. and Cabanas,C. (1996) Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355–425) of the common β1 chain. J. Biol. Chem., 271, 11067–11075. [DOI] [PubMed] [Google Scholar]
- Muller J.M., Chilian,W.M. and Davis,M.J. (1997) Integrin signaling transduces shear stress-dependent vasodilation of coronary arterioles. Circ. Res., 80, 320–326. [DOI] [PubMed] [Google Scholar]
- Nobes C. and Hall,A. (1994) Regulation and function of the Rho subfamily of small GTPases. Curr. Opin. Genet. Dev., 4, 77–81. [DOI] [PubMed] [Google Scholar]
- O’Toole T.E., Katagiri,Y., Faull,R.J., Peter,K., Tamura,R., Quaranta,V., Loftus,J.C., Shattil,S.J. and Ginsberg,M.H. (1994) Integrin cytoplasmic domains mediate inside-out signal transduction. J. Cell Biol., 124, 1047–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek S.P., Loftus,J.C., Ginsberg,M.H., Lauffenburger,D.A. and Horwitz,A.F. (1997) Integrin–ligand binding properties govern cell migration speed through cell–substratum adhesiveness. Nature, 385, 537–540. [DOI] [PubMed] [Google Scholar]
- Pampori N., Hato,T., Stupack,D.G., Aidoudi,S., Cheresh,D.A., Nemerow,G.R. and Shattil,S.J. (1999) Mechanisms and consequences of affinity modulation of integrin α(V)β(3) detected with a novel patch-engineered monovalent ligand. J. Biol. Chem., 274, 21609–21616. [DOI] [PubMed] [Google Scholar]
- Ren X.D., Kiosses,W.B. and Schwartz,M.A. (1999) Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J., 18, 578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Kiosses,W.B., Sieg,D.J., Otey,C.A., Schlaepfer,D.D. and Schwartz,M.A. (2000) Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J. Cell Sci., 113, 3673–3678. [DOI] [PubMed] [Google Scholar]
- Schoenwaelder S.M., Petch,L.A., Williamson,D., Shen,R., Feng,G. and Burridge,K. (2000) The protein tyrosine phosphatase shp-2 regulates RhoA activity. Curr. Biol., 10, 1523–1526. [DOI] [PubMed] [Google Scholar]
- Schwartz M.A. and Shattil,S.J. (2000) Signaling networks linking integrins and rho family GTPases. Trends Biochem. Sci., 25, 388–391. [DOI] [PubMed] [Google Scholar]
- Shyy J.Y. and Chien,S. (1997) Role of integrins in cellular responses to mechanical stress and adhesion. Curr. Opin. Cell Biol., 9, 707–713. [DOI] [PubMed] [Google Scholar]
- Takahashi M. and Berk,B.C. (1996) Mitogen-activated protein kinase (ERK1/2) activation by shear stress and adhesion in endothelial cells. Essential role for a herbimycin-sensitive kinase. J. Clin. Invest., 98, 2623–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi K., Kikuchi,A., Kuroda,S., Kotani,K., Sasaki,T. and Takai,Y. (1993) Involvement of rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) in cell motility. Mol. Cell. Biol., 13, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano Y., Saito,Y., Narumiya,S. and Sumpio,B.E. (1996) Involvement of rho p21 in cyclic strain-induced tyrosine phosphorylation of focal adhesion kinase (pp125FAK), morphological changes and migration of endothelial cells. Biochem. Biophys. Res. Commun., 224, 508–515. [DOI] [PubMed] [Google Scholar]


