Abstract
The seeds of parasitic plants of the genera Striga and Orobanche will only germinate after induction by a chemical signal exuded from the roots of their host. Up to now, several of these germination stimulants have been isolated and identified in the root exudates of a series of host plants of both Orobanche and Striga spp. In most cases, the compounds were shown to be isoprenoid and belong to one chemical class, collectively called the strigolactones, and suggested by many authors to be sesquiterpene lactones. However, this classification was never proven; hence, the biosynthetic pathways of the germination stimulants are unknown. We have used carotenoid mutants of maize (Zea mays) and inhibitors of isoprenoid pathways on maize, cowpea (Vigna unguiculata), and sorghum (Sorghum bicolor) and assessed the effects on the root exudate-induced germination of Striga hermonthica and Orobanche crenata. Here, we show that for these three host and two parasitic plant species, the strigolactone germination stimulants are derived from the carotenoid pathway. Furthermore, we hypothesize how the germination stimulants are formed. We also discuss this finding as an explanation for some phenomena that have been observed for the host-parasitic plant interaction, such as the effect of mycorrhiza on S. hermonthica infestation.
Parasitic weeds are a serious problem in agriculture, causing large crop losses in many parts of the world. Orobanche spp. (broomrapes; Orobanchaceae) are holoparasites and acquire all nutrients and water from their host through a root connection. The Striga spp. (witchweeds; Orobanchaceae) are hemiparasites but, although they have chlorophyll and a basal photosynthetic activity, basically also behave as holoparasites (Parker and Riches, 1993). Crops infected by witchweeds and broomrapes can be heavily damaged even before the parasites emerge above the soil. Striga spp. parasitize mainly tropical cereal crops, such as maize (Zea mays), sorghum (Sorghum bicolor), pearl millet (Pennisetum glaucum), and upland rice (Oryza sativa) (Striga hermonthica and Striga asiatica), but also cowpea (Vigna unguiculata; Striga gesnerioides; Press et al., 2001). Orobanche spp. parasitize more-temperate climate crops, such as sunflower (Helianthus annuus; Orobanche cumana), tomato (Lycopersicon esculentum), potato (Solanum tuberosum), tobacco (Nicotiana tabacum), rapeseed (Brassica napus) (Orobanche ramosa), and legumes (Orobanche crenata and Orobanche aegyptiaca).
The life cycles of Striga and Orobanche spp. are very similar, and a number of mechanisms ensure the coordination of the parasites' life cycles to that of their host (Bouwmeester et al., 2003). The important steps in the life cycle are germination, radicle growth to the host root, haustorium formation and attachment to the host root, the successful establishment of a xylem connection and compatible interaction, and production of seeds. The interaction between host and parasite begins with the secretion of secondary metabolites from the roots of the host (and some false nonhosts) that induce the germination of the parasite seeds. The seeds of Orobanche and Striga spp. contain only little reserves, and they can survive for a few days only after germination unless they reach a host root and a xylem connection is established.
For Striga spp. several germination stimulants have been identified in the root exudates of host and nonhost plants. Most of these are collectively described as the strigolactones (Fig. 1). Strigol was the first Striga germination stimulant to be identified in the false host cotton (Gossypium hirsutum; Cook et al., 1972) and later also in maize (Siame et al., 1993), sorghum (Hauck et al., 1992; Siame et al., 1993), and millet (Siame et al., 1993). Sorgolactone is another member of the family and was identified in sorghum (Hauck et al., 1992). Some germination stimulant(s) also have been identified for the Orobanche spp., and these also belong to the strigolactones. Alectrol, orobanchol, and a third unidentified compound were isolated from the root exudate of red clover (Trifolium pretense; Yokota et al., 1998) and induced germination of O. minor. Interestingly, alectrol is also a germination stimulant produced by cowpea and induces the germination of S. gesnerioides (Muller et al., 1992). Recently, the isolation of strigol-like germination stimulants of O. minor from the root exudates of tomato was reported (Yoneyama et al., 2004). Although so far there is no proof for this, it has also been postulated that germination stimulant concentration gradient may be responsible for the direction of radicle growth toward the root (Dube and Olivier, 2001).
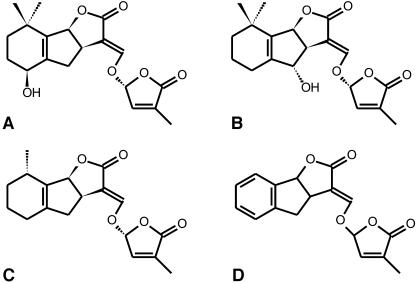
Figure 1.
Structure of identified strigolactone germination stimulants. A, (+)-Strigol; B, orobanchol; C, sorgolactone; D, synthetic germination stimulant GR24.
The induction of germination of parasitic plants by germination stimulants has been studied by many groups as a possible target for control measures. For example, Zwanenburg and coworkers worked on the development of synthetic germination stimulants to induce suicidal germination under field conditions (Wigchert and Zwanenburg, 1999; Mwakaboko, 2003). Ejeta and coworkers selected sorghum lines with reduced induction of germination (Mohamed et al., 2001). Also, the wide use of trap crops, used in monoculture or in intercropping, and catch crops is a control measure partly based on the (suicidal) induction of germination (Chittapur et al., 2001).
Although the germination stimulants so far identified were isolated from a wide variety of (host) crops and induce germination of a range of parasitic plant species, the compounds themselves are strikingly similar and are obviously derived from the same biosynthetic pathway (Bouwmeester et al., 2003). The strigolactones have been described as sesquiterpene lactones by many authors (Butler, 1995; Yokota et al., 1998). However, the structures also bear similarities to higher terpenoids/isoprenoids and may be derived from them (Bouwmeester et al., 2003).
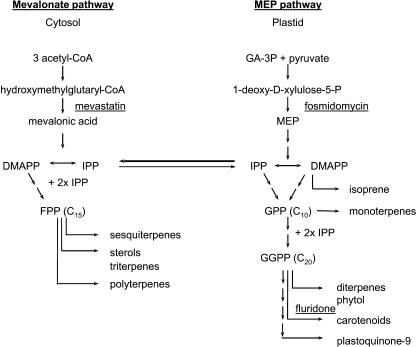
Isoprenoids have a carbon skeleton that is composed of C5-isoprene units (Fig. 2). They are subdivided into groups based on their carbon number, e.g. the 10-carbon monoterpenes, 15-carbon sesquiterpenes, 20-carbon diterpenes, 25-carbon sesterterpenes, 30-carbon triterpenes, 40-carbon tetraterpenes, and C5n polyterpenes (Fig. 2). Isoprenoids are biosynthesized from isopentenyl diphosphate and the isomeric dimethylallyl diphosphate via two independent pathways: the cytosolic mevalonic acid (MVA) pathway and the plastidic, nonmevalonate, methylerythritol phosphate pathway (Fig. 2). The plastidic methylerythritol phosphate pathway produces isopentenyl diphosphate (IPP) and dimethylallyl diphosphate for the biosynthesis of monoterpenes, diterpenes, carotenoids, the plant hormones gibberellin and abscisic acid, and the side chains of chlorophylls, plastoquinones, and phylloquinones. The cytosolic MVA pathway generates the precursors for sesquiterpenes, sterols, and triterpenes. Although there is a clear subcellular separation between the two precursor pathways, there is more and more evidence that some exchange of common precursors, such as IPP, occurs between the two compartments (Laule et al., 2003; Fig. 2).
Figure 2.
Isoprenoid biosynthetic pathway in higher plants redrawn from Lichtenthaler (1999). GA-3P, d-glyceraldehyde-3-P; MEP, 2-C-methyl-d-erythritol 4-P; IPP, isopentenyl diphosphate; DMAPP, dimethylallyl diphosphate; GPP, geranyl diphosphate; FPP, farnesyl diphosphate; GGPP, geranylgeranyl diphosphate.
If the strigolactones are sesquiterpenes, then they must be derived from farnesyl diphosphate (de Kraker et al., 1998). However, there is also some structural resemblance between the strigolactones and abscisic acid and other compounds, which are derived from the carotenoid pathway (Parry and Horgan, 1992; Tan et al., 1997; Figs. 1 and 3). In the latter case, the germination stimulants would be derived from the plastidic isoprenoid pathway (Fig. 2). Because of the extremely low concentrations of germination stimulants produced by hosts (in the order of 10−7 to 10−15 m; Joel, 2000), analytical techniques such as the use of stable-isotope-labeled precursors to decide on the biosynthetic origin are not sensitive enough. For example, despite the use of modern technologies, detection and quantification of orobanchol in the root exudate of red clover seedlings using liquid chromatrography-tandem mass spectrometry still required 400 seedlings (Sato et al., 2003). Here, we describe the use of mutants and isoprenoid pathway inhibitors in maize, cowpea, and sorghum and the use of a germination bioassay with S. hermonthica and O. crenata seeds to analyze germination stimulant formation on single plants. We can now report on the biosynthetic origin of the strigolactone germination stimulants of Striga and Orobanche spp. in the roots of a number of their hosts.
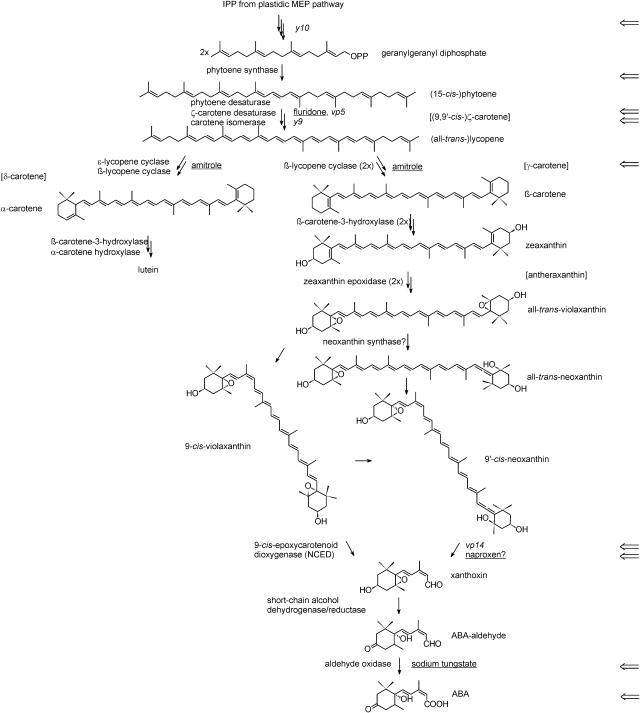
Figure 3.
Carotenoid and abscisic acid biosynthetic pathway in maize. Intermediates that are not shown are in brackets. Carotenoid maize mutants (italics) and inhibitors (underlined) at different steps in the pathway that are described in this article are indicated (⇐). IPP, Isopentenyl diphosphate; MEP, 2-C-methyl-d-erythritol 4-P. Redrawn from Cunningham and Gantt (1998), Hirschberg (2001), and Seo and Koshiba (2002).
RESULTS
To assess the involvement of the isoprenoid biosynthetic pathways, we investigated the induction of S. hermonthica seed germination by root exudates of seedlings treated with inhibitors and of a series of maize mutants. Root exudates of control maize seedlings always induced germination of preconditioned S. hermonthica seeds regardless of lines and cultivars used. Water alone did not induce any germination of S. hermonthica seeds.
Inhibitors of the Isoprenoid Pathway
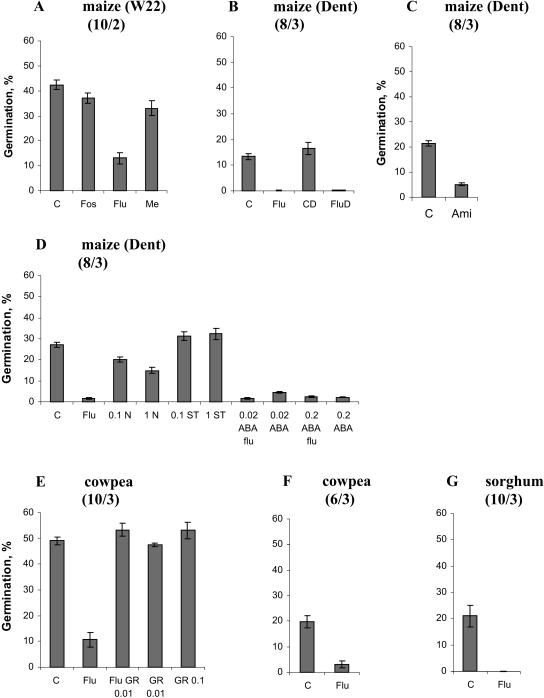
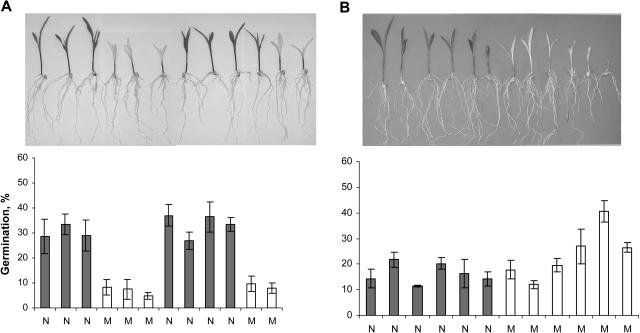
Mevastatin, an inhibitor of 3-hydroxy-3-methylglutaryl CoA reductase, catalyzing the formation of MVA from 3-hydroxy-3-methylglutaryl, was used to reduce cytosolic isoprenoid formation (Fig. 2). Fosmidomycin, blocking 1-deoxy-d-xylulose 5-phosphate reductoisomerase, was used to inhibit plastidic isoprenoid formation, and fluridone was used to block carotenoid formation in the plastids (Fig. 2). Inhibitors were applied from 3 d after germination when the root length was about 3 to 5 cm. Exudates from individual control plants of inbred line W22 (Fig. 4A) induced a high and reproducible germination score (42% ± 2%; Fig. 5A). Mevastatin did not affect the morphology or appearance of the plants in contrast to fosmidomycin, which induced slightly pale-yellow leaves (Fig. 4, B and D). Mevastatin and fosmidomycin slightly but nonsignificantly reduced maize root exudate-induced S. hermonthica germination (37% ± 2% for fosmidomycin and 33% ± 3% for mevastatin-treated plants compared with the control; Fig. 5A). Leaves of maize seedlings grown in the presence of the carotenoid inhibitor fluridone were white with green tips (Fig. 4C), showing the proportion of newly formed tissue that was affected by fluridone treatment. Germination induced by the root exudates of fluridone-treated maize was about 80% lower than for control maize (Fig. 5A). Fluridone specifically inhibits the second dedicated enzyme in the carotenoid pathway, phytoene desaturase (Li et al., 1996; Fig. 3). The highly reduced root exudate-induced germination of S. hermonthica by fluridone-treated plants strongly suggests that the germination stimulant produced by maize is derived from the carotenoid pathway.
Figure 4.
Phenotypes of inhibitor-treated maize seedlings and phenotypes of carotenoid maize mutant seedlings. Inbred W22: nontreated control (A), 100 μm fosmidomycin (B), 25 μm fluridone (C), and 10 μm mevastatin (D). Inbred Dent: nontreated control (E), 10 μm fluridone (F), nontreated control grown under dim light (G), 10 μm fluridone grown under dim light (H), inbred Dent nontreated control grown at 21°C (I), 200 μm amitrole grown at 21°C (J), 0.1 mm naproxen (K), 1 mm naproxen (L), 0.1 mm sodium tungstate (M), 1 mm sodium tungstate (N), 0.02 mm abscisic acid (O), and 0.02 mm abscisic acid + 10 μm fluridone (P). Carotenoid mutants cl1 (Q), lw1 (R), y10 (S), al1y3 (T), vp5 (U), y9 (V), and vp14 (W).
Figure 5.
Germination of S. hermonthica seeds induced by root exudates of maize (W22 or Dent, as indicated in the graphs; A–D), cowpea (E and F), and sorghum (G) as affected by treatment of the host seedlings with (A) 100 μm fosmidomycin (Fos), 25 μm fluridone (Flu), and 10 μm mevastatin (Me) compared with untreated control (C). B, 10 μm Fluridone (Flu) applied to plants growing under normal light (Flu) and dim light (FluD) compared with their respective untreated controls (C and CD). C, 200 μm Amitrole (Ami) compared with untreated control (C). Seedlings were grown at 21°C. D, Untreated control (C), 10 μm fluridone (Flu), 0.1 mm naproxen (0.1 N), 1 mm naproxen (1 N), 0.1 mm sodium tungstate (0.1 ST), 1 mm sodium tungstate (1 mm ST), 0.02 mm abscisic acid and 10 μm fluridone (0.02 ABA Flu), 0.02 mm abscisic acid (0.02 ABA), 0.2 mm abscisic acid and 10 μm fluridone (0.2 ABA Flu), and 0.2 mm abscisic acid (0.2 ABA). E, 10 μm Fluridone and nontreated control (C), 10 μm fluridone (Flu), and root exudate of fluridone-treated seedlings combined with 0.01 mg/L GR24 (Flu GR 0.01) compared with 0.01 and 0.1 mg/L GR24 alone (GR 0.01 and GR 0.1). F, 10 μm Fluridone (Flu) on O. crenata germination compared with nontreated control (C). G, 4 μm Fluridone (Flu) compared with nontreated control (C). Within each experiment, the concentrations of root exudates were equalized, by dilution, for differences in root fresh weight. Data represent average germination ± standard error of 8 to 10 individual plants and two or three disks (indicated in each diagram by 8/3, etc.), with about 50 S. hermonthica seeds each per plant. Statistical analysis showed significant difference (P < 0.05) for all treatments, except the treatments with 100 μm fosmidomycin,10 μm mevastatin, and 0.1 mm naproxen.
Germination of S. hermonthica is also induced by cowpea root exudates, and a strigolactone germination stimulant of S. gesnerioides, alectrol, has been identified in cowpea root exudates (Muller et al., 1992). Therefore, we assayed the effect of fluridone also on cowpea. The root exudates of nontreated cowpea induced 49% ± 1.5% germination of S. hermonthica seeds compared with 11% ± 3% by fluridone-treated cowpea root exudates (Fig. 5E). To exclude the possibility that fluridone treatment leads to the formation of inhibitors of germination (rather than a lower production of the germination stimulant), the root exudates of fluridone-treated cowpea were also assessed in combination with suboptimal concentrations of the synthetic germination stimulant GR24. The resulting germination of S. hermonthica induced by fluridone-treated cowpea root exudates with GR24 was slightly higher (53% ± 2.5%) than germination induced by GR24 alone (48% ± 1%; Fig. 5E). These results show that the germination stimulant exuded from the roots of cowpea is also derived from the carotenoid pathway and that lower germination induced by fluridone-treated root exudates is caused by a lower concentration of germination stimulants in the root exudate and not by the production of inhibitors in fluridone-treated plants. In addition to S. hermonthica, cowpea root exudates also induced the germination of seeds of O. crenata (Fig. 5F). Just like for S. hermonthica, germination of O. crenata seeds with fluridone-treated cowpea root exudate was lower than that induced by the control (Fig. 5F), showing that also for O. crenata the germination stimulant(s) in cowpea root exudate is (are) carotenoid derived.
Fig. 4C shows that fluridone, through the inhibition of carotenoid formation in the leaves, also caused photodestruction of the chlorophyll. Therefore, in the next experiment, maize seedlings were grown under dim light in order to prevent photodestruction of chlorophyll while carotenoid biosynthesis was inhibited. Figure 4, E to H, shows the phenotypes of Dent seedlings grown for 9 d under normal and low light without and with fluridone treatment. Control plants grown under normal light were green, and root exudates induced germination of S. hermonthica seeds (13% ± 1%; Fig. 5B). Fluridone-treated plants exhibited the typical white phenotype and induced virtually no germination. Control seedlings grown under dim light were pale green but they induced similar germination of S. hermonthica seeds as control seedlings grown under normal light conditions. Under dim light conditions, fluridone-treated seedlings did not show the typical bleaching visible under normal light but exhibited a pale-green phenotype similar to control seedlings under dim light (Fig. 4, F and H). Nevertheless, germination of S. hermonthica seeds induced by root exudates of fluridone-treated seedlings was also negligible (Fig. 5B). This demonstrates that the degradation/absence of chlorophyll itself is not causing the reduced germination induced by root exudates of maize in which carotenoid formation is disturbed. Finally, an additional possibility to discriminate between the direct effect of the absence of carotenoids and the indirect effect through destruction of chlorophyll is the use of a chlorophyll mutant. The maize mutant chlorophyll1 (cl1; 311AA) exhibits an albino phenotype (Fig. 4Q) and was first described by Everett (see Robertson et al., 1966) to be a chlorophyll mutant. Later, Robertson et al. (1966) described a group of cl1 mutants as mutants that are impaired in both chlorophyll and carotenoid formation to different levels depending on a clm (gene modifier; Robertson et al., 1966). Therefore, we analyzed both the induction of germination by the cl1 311AA mutant as well as its carotenoid content. Mutant phenotype siblings contained strongly reduced carotenoid levels in the shoots (Table I). In the roots, only violaxanthin was detectable, and there was no difference in the concentration of this compound between wild-type and mutant phenotype siblings (Table I). The root exudate of cl1 311AA seedlings induced normal germination, comparable to the wild-type phenotype seedlings (Fig. 6B).
Table 1.
Carotenoid composition of shoots and roots of 10-d-old maize seedlings treated with inhibitors of abscisic acid biosynthesis or abscisic acid itself and carotenoid mutants al1y3 and cl1 311AA
Carotenoid content in μg·g−1 fresh weight shoot/root. n.d., Not detected.
| Neoxanthin | Violaxanthin | Lutein | β-Carotene | |
|---|---|---|---|---|
| Shoots | ||||
| Control | 153.40 | 72.65 | 88.56 | 65.51 |
| 10 μm Fluridone | n.d. | n.d. | n.d. | n.d. |
| 1 mm Naproxen | 111.81 | 52.08 | 68.58 | 47.56 |
| 0.1 mm Sodium tungstate | 147.58 | 72.19 | 83.94 | 64.29 |
| 0.02 mm Abscisic acid | 115.29 | 50.84 | 73.01 | 47.35 |
| Roots | ||||
| Control | n.d. | 0.11 | n.d. | 0.02 |
| 10 μm Fluridone | n.d. | n.d. | n.d. | n.d. |
| 1 mm Naproxen | n.d. | 0.29 | n.d. | 0.04 |
| 0.1 mm Sodium tungstate | n.d. | 0.22 | n.d. | 0.03 |
| 0.02 mm Abscisic acid | n.d. | 0.07 | n.d. | 0.01 |
| Carotenoid mutants | ||||
| Shoots | ||||
| al1y3 | 32.43 | 31.06 | 55.72 | 46.47 |
| Mutant al1y3 | 2.64 | 3.15 | 6.17 | 4.68 |
| cl1 311AA | 28.06 | 26.77 | 50.95 | 36.44 |
| Mutant cl1 311AA | n.d. | 1.14 | 1.08 | n.d. |
| Roots | ||||
| al1y3 | n.d. | n.d. | n.d. | n.d. |
| Mutant al1y3 | n.d. | n.d. | n.d. | n.d. |
| cl1 311AA | n.d. | 0.07 | n.d. | 0.01 |
| Mutant cl1 311AA | n.d. | 0.08 | n.d. | 0.01 |
Figure 6.
A, Phenotypes of mutant y10 seedlings (white) and corresponding wild-type siblings (gray) and germination of S. hermonthica induced by the root exudates of these seedlings. B, Phenotypes of mutant cl1 311AA seedlings (M; white) and corresponding wild-type siblings (N; gray) and germination of S. hermonthica induced by root exudates of these seedlings.
An additional interesting inhibitor of carotenoid biosynthesis is the bleaching herbicide amitrole that inhibits carotenoid biosynthesis by blocking lycopene cyclase in maize seedlings (Fig. 3; Dalla Vecchia et al., 2001). Amitrole-treated inbred Dent plants had a yellow-green phenotype (Fig. 4J). Root exudates of amitrole-treated seedlings induced lower germination of S. hermonthica seeds than control seedlings (Fig. 5C).
Mutants in Carotenoid/Abscisic Acid Biosynthesis
To further investigate the germination stimulant biosynthetic pathway, we tested several other maize carotenoid mutants and inhibitors for their effect on induction of S. hermonthica seed germination (Fig. 3). From the Maize Genetic COOP Stock Center we obtained a number of mutants that are characterized by a change in the accumulation of carotenoids, depending on the step in the biosynthetic pathway that is affected by the mutation. Some of the mutants we could obtain only had a limited viability, and in some cases this restricted our experiments.
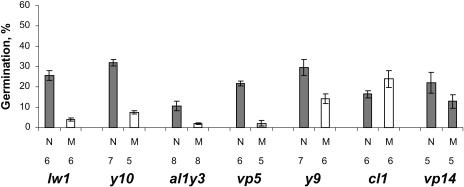
The mutation causing the reduced carotenoid level in the lemon white1 (lw1) carotenoid mutant is not defined yet. Seedlings of lw1 exhibit an albino phenotype (Fig. 4R). Root exudates collected from lw1 albino seedlings induced significantly lower germination of S. hermonthica in comparison to corresponding seedlings with nonmutant phenotype (Fig. 7).
Figure 7.
Average germination of S. hermonthica induced by root exudates of maize mutants lw1, y10, al1y3, vp5, y9, cl1 311AA, and vp14 and their corresponding wild-type siblings (the number of seedlings used for each bioassay is indicated). Statistical analysis showed that lw1, y10, al1y3, vp5, and y9 mutant phenotype induced significantly lower (P < 0.05) germination than the corresponding nonmutant phenotype seedlings. The difference in germination induced by the vp14 mutant was not significant (P = 0.09).
Carotenoid mutant y10 is characterized by a pale-yellow endosperm color and albino seedlings (Fig. 4S) Although the exact position of the mutation in the carotenoid pathway has not been clarified, the defect in the y10 mutant is supposed to affect a step in the isoprenoid pathway preceding the biosynthesis of geranylgeranyl diphosphate (Fig. 3), which results in a reduced content of carotenoids in the endosperm and carotenoids and chlorophylls in the leaves of the mutant seedlings (Janick-Buckner et al., 1999). Root exudates of y10 mutant seedlings induced a significantly lower germination than wild-type sibling plants (Figs. 6 and 7).
The al1y3 mutant (variation of al1 albescent plant 1) seedlings have a white to pale-green phenotype (Fig. 4T). According to Wurtzel (2004), it is unclear which gene is affected, but the root exudates of mutant al1y3 seedlings induced lower germination of S. hermonthica seeds than the sibling seedlings from a segregating ear that exhibit a nonmutant phenotype (Fig. 7). Carotenoid analysis showed 10-fold lower concentration of all detected carotenoids in the shoots of mutant seedlings than in seedlings with nonmutant phenotype (Table I). Since lutein content also is strongly reduced in the mutant phenotype, it is highly likely that al1y3 is indeed a mutant associated with phytoene synthase. In roots, carotenoids were all below the detection level.
Mutant viviparous5 (vp5) is known to be associated with phytoene C-12,13 desaturation, the same step that is blocked by fluridone (Fig. 3), which causes an accumulation of phytoene (Li et al., 1996). vp5 mutant seedlings are white (Fig. 4U), and root-exudates-induced S. hermonthica germination was much lower than for wild-type seedlings (Fig. 7).
Mutant pale yellow9 (y9) exhibits a slightly pale-green phenotype (Fig. 4V). y9 is associated with the conversion of ζ-carotene to lycopene in the maize endosperm (Fig. 3). Chlorophyll and carotenoids could still be detected in pale-green leaves of homozygous y9 plants, albeit in reduced amounts (Janick-Buckner et al., 2001). Although y9 mutant seedlings also induced significantly lower germination of S. hermonthica seeds than seedlings with nonmutant phenotype, germination was higher than for the albino mutants, lw1, y10, and vp5, described above showing that the mutation in y9 indeed is not 100% effective (Fig. 7).
Finally, maize mutant vp14-2274 was assessed. vp14 is a mutation in 9-cis-epoxycarotenoid dioxygenase (NCED), an important step in the abscisic acid biosynthetic pathway (Scwartz et al., 1997; Fig. 3). The mutation is slightly leaky. vp14 does not have an albino phenotype, suggesting that carotenoid formation is not affected by the mutation (Fig. 4W). Nevertheless, the mutant induced lower germination than the control (P = 0.09; Fig. 7).
It is unlikely that with all inhibitors and carotenoid mutants we have assayed secretion of the germination stimulants, rather than their production, is decreased. However, to exclude this possibility, we analyzed root extracts, made with several organic solvents, of fluridone-treated and nontreated (control) maize plants for induction of S. hermonthica germination. The effect of the solvents and their toxicity on S. hermonthica seeds in combination with GR24 were tested as well. Extracts of fluridone-treated roots also induced significantly lower germination than extracts of nontreated roots (data not shown).
Involvement of Abscisic Acid
To assess whether abscisic acid is perhaps a precursor of the germination stimulants, maize seedlings were grown in the absence or presence of fluridone in combination with 0.02 or 0.2 mm (±)-abscisic acid. The roots of abscisic-acid-treated plants were slightly thicker and more brittle than in nontreated seedlings, and the shoots were green (Fig. 4O). Independent of the presence of fluridone, plants supplied with abscisic acid induced very low germination of S. hermonthica (Fig. 5D). Subsequently, the effects of naproxen, a putative inhibitor of NCED (Lee and Milborrow, 1997), and sodium tungstate, an inhibitor blocking oxidation of the C-1 aldehyde group of xanthoxin (Lee and Milborrow, 1997), were assessed (Fig. 3). Plants treated with naproxen had a normal green phenotype with slight inhibition of root growth at the highest concentration (Fig. 4, K and L). Naproxen reduced germination of S. hermonthica seeds to 20% ± 1% (0.1 mm) or 15% ± 1.5% (1 mm), which represents a 44% reduction compared with nontreated plants (27% ± 1.5%; Fig. 5D). Sodium tungstate used in concentrations of 0.1 and 1 mm (Fig. 4, M and N) did not affect germination (Fig. 5D), even though 1 mm sodium tungstate did affect maize root morphology. Roots were much shorter, thicker, and more fragile than in control seedlings.
To exclude a possible direct effect of abscisic acid on germination, we did two germination bioassays. In the first bioassay, we tested root exudates of abscisic-acid-treated plants in combination with a suboptimal concentration of GR24. In the second experiment, we applied abscisic acid (in the same concentration as used for maize treatment) in combination with a suboptimal concentration of GR24. In both cases, germination of S. hermonthica as induced by GR24 was not reduced, which shows that there is no direct effect of this abscisic acid concentration on germination of S. hermonthica seeds.
Carotenoid Analysis
Plants treated with fluridone, naproxen, sodium tungstate, and abscisic acid were analyzed for carotenoid contents of roots and shoots. In fluridone-treated roots, phytoene accumulated but other carotenoids could not be detected (Table I). In naproxen-treated roots, the concentration of violaxanthin was about 3-fold and of β-carotene 2-fold higher than in control roots (Table I). In sodium tungstate-treated roots, the amount of violaxanthin was 2-fold higher than in control plants. In contrast, in abscisic-acid-treated roots, violaxanthin and β-carotene concentrations were 37% and 50% lower than in control roots, respectively.
Also, in the shoot of fluridone-treated plants, phytoene accumulated, but other carotenoids were below detection level (Table I). Sodium tungstate did not affect carotenoid accumulation in shoots. Both naproxen and abscisic acid decreased accumulation of β-carotene, violaxanthin, neoxanthin, and lutein (Table I).
DISCUSSION
Biosynthetic Origin of Strigolactone Germination Stimulants
Our results demonstrate that the germination stimulants of S. hermonthica present in the root exudates of maize, cowpea, and sorghum are derived from the carotenoid biosynthetic pathway, and this also holds for the germination stimulant(s) of O. crenata in the root exudate of cowpea. For these three host species, maize, cowpea, and sorghum, the germination stimulants have been isolated and identified to be strigolactones, viz strigol, alectrol, and sorgolactone (Fig. 1; Hauck et al., 1992; Muller et al., 1992; Siame et al., 1993). Our results with O. crenata suggest that this species also responds to a strigolactone germination stimulant. O. crenata is not a parasite of cowpea but parasitizes legumes in more-temperate climates such as North Africa and Spain. The germination stimulant(s) of O. crenata has not been identified yet. Root exudates of cowpea readily induced germination of O. crenata, and fluridone treatment decreased this germination by about the same percentage as for S. hermonthica. This makes it likely that the germination stimulants of Orobanche spp. that parasitize legumes in temperate regions of the world are also strigolactones and are hence also derived from the carotenoid pathway. This hypothesis is supported by the detection of orobanchol, alectrol, and a third unidentified strigolactone in the legume red clover (Yokota et al., 1998) and alectrol in cowpea (Muller et al., 1992).
The fact that we demonstrated the carotenoid origin of the germination stimulants for two parasitic plant species and three monocotyledonous and dicotyledonous hosts and the (tentative) identification of strigolactones in the root exudates of other plant species, such as red clover and tomato (Yokota et al., 1998; Yoneyama et al., 2004), suggest that carotenoid-derived germination stimulant formation occurs throughout the plant kingdom. The finding that sorghum root-exudate-induced S. hermonthica germination is completely blocked by fluridone sheds an interesting light on the discussion about the true nature of the sorghum germination stimulant. Lynn and coworkers have claimed that the nonstrigolactone sorgoleone is the natural sorghum germination stimulant (Keyes et al., 2001), but our results make it very likely that the natural sorghum germination stimulant is sorgolactone.
The germination stimulants are produced in extremely low concentrations, which causes large difficulties for an analytical approach to discover the biosynthetic origin of the germination stimulants. Due to the high sensitivity of parasitic plant seeds to the germination stimulants, the germination bioassay-guided approach has proven to be a powerful tool to unravel the pathway. Using this bioassay, we showed that germination stimulant formation is effectively blocked by fluridone. Both mevastatin and fosmidomycin also slightly, but not significantly, reduced root-exudate-induced germination (Fig. 5A). Both compounds have been demonstrated to be quite efficiently inhibiting the cytosolic or plastidic isoprenoid biosynthetic pathway, respectively (Laule et al., 2003); hence, we might expect a clear effect of fosmidomycin, which in principle should also block carotenoid biosynthesis. However, fosmidomycin and mevastatin have also been used to prove exchange of isoprenoid intermediates between cytosol and cytoplasm, likely in the form of IPP (Fig. 2; Hemmerlin et al., 2003; Botella-Pavia et al., 2004). Recently, it was also shown that the exchange from cytosol to plastids occurs particularly in the dark (Botella-Pavia et al., 2004). All this suggests that the inhibition by fosmidomycin of carotenoid formation was less effective than by fluridone, perhaps as a consequence of isoprenoid precursor supply from the cytosol (Fig. 2). This assumption is supported by the fact that fosmidomycin-treated seedlings did not exhibit the bleached phenotype that fluridone-treated seedlings showed (Fig. 4, B and C). The inhibition of root-exudate-induced germination by mevastatin is possibly due to inhibition of cytosolic IPP supply to the plastids or because IPP is now exported more easily from the plastids into the cytosol. Alternatively, it could be that part of the germination stimulant biosynthesis occurs in the cytosol. In abscisic acid biosynthesis, xanthoxin is also transported out of the plastids into the cytosol, where it is further processed to form abscisic acid (Seo and Koshiba, 2002). Possibly, the tricyclic strigolactone backbone is also exported to the cytosol after carotenoid cleavage. There, several additional enzymatic reactions will still be required (see below), and the D-ring will have to be coupled. The origin of the D-ring is as yet completely unknown but could perhaps arise from coupling of the tricyclic skeleton to an isoprenoid unit, such as dimethylallyl diphosphate or 4-hydroxydimethylallyl diphosphate, by the action of a prenyl transferase. If this occurs in the cytosol, then mevastatin treatment could also lead to a lower formation of germination stimulants, as the D-ring is required for biological activity (Wigchert and Zwanenburg, 1999).
Fluridone effectively blocks the activity of phytoene desaturase, which corresponds to the vp5 locus (Li et al., 1996; Hable et al., 1998). The vp5 mutant has a lesion in the phytoene desaturase gene (Hable et al., 1998), and reduced phytoene desaturase transcript in maize endosperm was detected in this mutant (Li et al., 1996). Both fluridone-treated maize and the vp5 mutant root exudates induced significantly lower germination of S. hermonthica (Figs. 5 and 7). These results indicate that the biosynthetic pathway leading to the production of the strigolactone germination stimulant branches from the main carotenoid pathway below phytoene (vp5 mutant and fluridone). Our results in germination bioassays with root exudates of the y9 mutant and amitrole-treated plants (Fig. 4) show that the branch point is below ξ-carotene and lycopene.
Carotenoid Cleavage Enzymes
In a next step, we tested the effect of naproxen, a putative inhibitor of epoxy-carotenoid cleavage, to give xanthoxin (Lee and Milborrow, 1997; Fig. 3). The formation of the germination stimulant of maize was indeed reduced by the action of naproxen (Fig. 5D). This suggests that carotenoid cleavage is involved in its biosynthesis, which is logical because the C40-carotenoids need to be cleaved in order to lead to the C14 (excluding the D-ring) strigolactones. Naproxen has been used to inhibit the NCED, a subcategory of the carotenoid cleavage dioxygenases, that catalyzes the oxidative cleavage of 9-cis-epoxy carotenoids to apocarotenoids (C25) and xanthoxin (C15), the precursor of abscisic acid in higher plants (Schwartz et al., 1997). Our bioassay with vp14, a mutant of NCED, confirmed the result obtained with naproxen (Fig. 7). Both naproxen and vp14 were not fully effective in inhibiting root-exudate-induced germination but only gave about 44% inhibition compared with the corresponding control/wild-type seedlings (Figs. 5D and 7). This agrees quite well with results obtained by others. Lee and Milborrow reported that in a cell-free system of avocado (Persea americana), naproxen reduced radiolabel accumulation from [14C]-MVA in abscisic acid by 43% (Lee and Milborrow, 1997). In the maize vp14 mutant, the mutation in NCED reduced abscisic acid accumulation in embryos by 40% to 60%, depending on embryo developmental stage, and abscisic acid accumulation in water-stressed leaves by 45% (Tan et al., 1997).
From the maize mutant vp14-2274, NCED was cloned and the substrate specificity characterized (Schwartz et al., 2003). The enzyme requires a 9-cis double bond adjacent to the site of cleavage (the 11–12 bond) and cleaves 9-cis-violaxanthin, 9-cis-neoxanthin, and 9-cis-zeaxanthin (Schwartz et al., 2003). However, the structural requirements in the carotenoid ring for cleavage have not been investigated; thus, it is conceivable that NCED is nonspecific and can cleave other 9-cis-carotenoids, such as 9-cis-β-carotene (Fig. 8). Other 9-cis-carotenoids, such as 9-cis-β-carotene and 9-cis-zeaxanthin, have been reported to occur in a range of plant species (Ben-Amotz and Fishler, 1998). vp14 is expressed constitutively in maize embryos and roots, and in Southern blots, at least nine bands were detected when hybridizing with vp14 cDNA (Tan et al., 1997). This may also explain why the mutation in vp14 is not 100% effective in inhibiting abscisic acid (Tan et al., 1997) or germination stimulant formation (Fig. 7), as NCED family members may partly compensate for the NCED activity lost in vp14. NCED cDNAs have been cloned from several other plant species, such as Phaseolus vulgaris (Qin and Zeevaart, 1999), cowpea (Iuchi et al., 2000), avocado (Chernys and Zeevaart, 2000), and Arabidopsis (Arabidopsis thaliana; Neill et al., 1998). Just like in maize, in all these species, NCEDs were found to belong to a gene family.
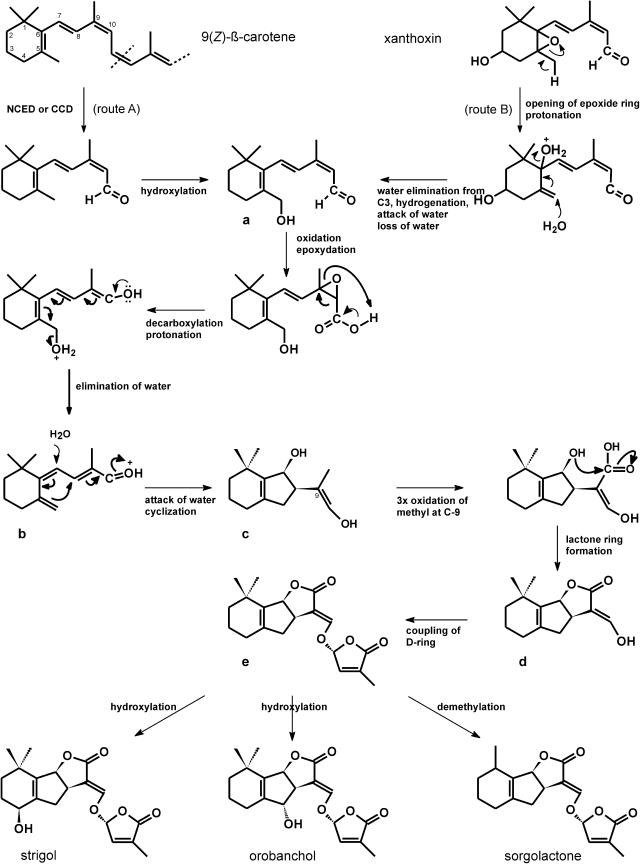
Figure 8.
Putative biogenetic scheme for the formation of strigol, orobanchol, and sorgolactone. CCD, Carotenoid cleavage dioxygenase.
Carotenoid cleavage dioxygenases are probably involved in the cleavage of a carotenoid precursor to a C14 dialdehyde, the likely precursor of mycorradicin, the major component of yellow pigment observed in roots colonized by arbuscular mycorrhiza in a range of plant species such as maize, sorghum, other cereals, and tomato (Maier et al., 1997; Strack et al., 2003). Interestingly, several groups have reported that mycorrhiza can reduce S. hermonthica infection of sorghum and maize (Lendzemo and Kuyper, 2001; Gworgwor and Weber, 2003; Lendzemo, 2004). This effect of mycorrhiza may simply be due to the positive effect on plant growth that will make plants more resistant or tolerant to parasitism, but another explanation could be that mycorradicin formation competes for carotenoid substrate with germination stimulant formation. Indeed, in preliminary experiments, Lendzemo found that root exudates of mycorrhizal sorghum induced less germination than the exudates of nonmycorrhizal sorghum (Lendzemo, 2004), and we found the same for maize (data not shown). Other possible explanations for the negative effect of mycorrhiza on S. hermonthica germination could be the presence of (germination) inhibitors derived from or induced by the mycorrhizal fungus or the production by mycorrhiza of abscisic acid (Hartung and Gimmler, 1994; Cutler and Krochko, 1999), which could inhibit germination stimulant formation (Fig. 5D).
Biosynthetic Fate after Carotenoid Cleavage
The inhibitor of abscisic acid aldehyde oxidation, sodium tungstate, did not have an effect, but abscisic acid strongly reduced root-exudate-induced S. hermonthica germination (Fig. 5). This shows that the germination stimulants are not derived from intermediates below abscisic acid aldehyde or from abscisic acid itself. The reduction of root-exudate-induced germination by abscisic acid is most probably due to feedback inhibition by the exogenously applied abscisic acid on the carotenoid pathway (Table I). Consequently, the formation of germination stimulant branching from the carotenoid biosynthetic pathway is also decreased. The levels of violaxanthin and β-carotene were indeed reduced by about 35% and 65%, respectively, in abscisic-acid-treated roots, and in the shoot a similar trend can be observed (Table I). Very little is known about the effects of exogenously applied abscisic acid on carotenoid and abscisic acid biosynthesis in roots and other nonphotosynthetic tissues. Feedback regulation of phytoene desaturase in green tissue by accumulation of abscisic acid, the end product of the carotenoid pathway, was proposed (Corona et al., 1996). In Arabidopsis seedlings, exogenously applied abscisic acid did not affect the transcript level of NCED (Xiong et al., 2001b). In contrast, positive feedback regulation by abscisic acid at the transcriptional level was observed for AAO3, encoding abscisic acid aldehyde oxidase (Seo et al., 2000), and abscisic acid3, encoding the molybdenum cofactor sulfurase, which catalyzes sulfurization of MoCo, a cofactor required by aldehyde oxidase (Xiong et al., 2001a, 2001b).
Our results suggest that the biosynthetic pathway of the strigolactone germination stimulants branches off from the carotenoid pathway at an intermediate that is a product of NCED action. This may be xanthoxin but could more likely be an analog derived from cleavage of other substrates, such as 9-cis-β-carotene. A biogenetic scheme can be postulated starting from such a 9-cis-β-carotene cleavage product and from xanthoxin (Fig. 8), but also downstream derivatives of xanthoxin or alternative carotenoids could serve as substrate in this biogenetic scheme. Starting from 9-cis-β-carotene, C11-12 cleavage by a dioxygenase leads to a C15-aldehyde, which upon hydroxylation yields intermediate a (Fig. 8). Alternatively, xanthoxin, upon opening of the epoxyde ring followed by protonation, elimination of water at C3 followed by hydrogenation, attack of water, and loss of water, could also lead to intermediate a. Oxidation followed by epoxydation and decarboxylation, protonation, and elimination of water leads to intermediate b. This intermediate, upon attack of water at C7, cyclizes to intermediate c or its tautomeric aldehyde. After triple oxidation of the methyl at C9, a lactone ring will be formed with the C7 hydroxyl group to produce intermediate d. Alternatively, the methyl at C9 could already be oxidized to form a carboxyl group in compound a (not shown in Fig. 8). Instead of attack of water at C7, that carboxyl group could then attack the carbocation at C7 leading directly to intermediate d (A. de Groot, personal communication). As an alternative to lactone ring formation as described above, intermediate c could undergo keto-enol tautomerization, followed by lactol ring formation with the hydroxy at C7 and subsequent oxidation to a lactone ring (not shown in Fig. 8). Double oxidation of the methyl at C9 would then also lead to intermediate d (B. Zwanenburg, personal communication). From intermediate d, coupling of the D-ring will lead to intermediate e, and allylic hydroxylation in ring A or B or demethylation in ring A, and to strigol, orobanchol, and sorgolactone, respectively (Fig. 8). The structure of alectrol is still under debate (B. Zwanenburg, personal communication). It is not unlikely that our biogenetic scheme may help to postulate a new structure for alectrol that should subsequently be proven using chemical synthesis. Of course the order of the reactions as we propose may be different in vivo.
Conclusion
In conclusion, our results show that a carotenoid cleavage dioxygenase, most likely NCED, is involved in the biosynthesis of the strigolactone germination stimulants in hosts of Orobanche and Striga spp. After this cleavage step, a number of other enzymatic reactions, such as hydroxylation, epoxydation, oxidation, etc., are involved in the further modification of the primary apocarotenoid skeleton to the different strigolactones. Currently, our work is focusing on the further biochemical and molecular characterization of the pathway.
MATERIALS AND METHODS
Plant Material and Chemicals
Maize (Zea mays) inbred W22 was obtained from Vicky Child (Long Ashton Research Station, Bristol, UK), and inbred line Dent was obtained from J.C. Robinson Seeds. Maize seeds deficient in chlorophyll and carotenoid biosynthesis were obtained from the Maize Genetics COOP Stock Center (Urbana, IL; mutants lw1, y10, vp5, vp14, y9, al1y3, and cl1 311AA). Cowpea (Vigna unguiculata L. Walp) seeds were obtained from Sériba Katilé (Institut d'Économie Rurale, Mali). Sorghum bicolor variety CSH-1 was obtained from Bob Vasey (Sheffield University, Sheffield, UK). Striga (Striga hermonthica Del. Benth) seeds were collected from a maize field in 1994 (Kibos, Kenya), and S. hermonthica seeds used in germination bioassay with sorghum root exudates were collected from a sorghum field in Sudan in 1995 and were obtained from Bob Vasey. Orobanche crenata seeds were obtained from D.M. Joel (Newe-Ya'ar Research Center, Ramat Yishay, Israel).
The following inhibitors of isoprenoid pathways were used: mevastatin (Sigma-Aldrich), fosmidomycin (Molecular Probes), fluridone (Ducheva), amitrole (3-amino-1,2,4-triazole), naproxen [(S)-6-methoxy-α-methyl-2-naphthaleneacetic acid sodium salt], sodium tungstate, dihydrate, and (±)-abscisic acid (all from Sigma-Aldrich). The synthetic germination stimulant strigolactone analog GR24 was kindly provided by B. Zwanenburg (University of Nijmegen, Nijmegen, The Netherlands).
Root Exudate Production
Seeds of maize were sterilized in 4% sodium hypochlorite containing 0.02% (v/v) Tween 20, rinsed thoroughly with sterile water, and imbibed for 24 h on moistened filter paper at 25°C. Plants were grown in autoclaved perlite in separate tubes or in 1-L pots (15-cm diameter) at 25°C in a climate room with a 16/8-h photoperiod at 28 μmol photons·m−2·s−1. To grow plants under dim light, plants were grown in a box covered by several layers of cheesecloth and aluminum foil with holes (0.5 μmol photons·m−2·s−1). The plants were watered with tap water (control, carotenoid mutants) or with solutions of abscisic acid or inhibitors. Depending on the vigor, mutants were grown for 8 to 11 d. After several days, depending on the experiment, seedlings were removed from perlite and carefully cleaned from perlite. Plants were put into about 8 mL of tap water in glass tubes at 25°C to collect root exudates. Tubes were covered with aluminum foil to exclude light. After 6 to 24 h, root exudates were collected, and root fresh weight of each seedling was determined. Exudates were diluted to equal concentrations (milliliters of exudate per gram of roots) within each experiment and subsequently used in a bioassay. Seeds of cowpea were imbibed for 24 h on moistened filter paper at 25°C in darkness. Imbibed seeds were sown in pots containing a mixture of vermiculite and perlite (1:1). Plants were grown in a climate room with a 16/8-h photoperiod at 28 μmol photons·m−2·s−1 at 25°C. The plants were regularly watered for 4 d. Subsequently, seedlings were watered with water (control) or with 10 μm fluridone for 5 d. Root fresh weight and amount of root exudate were determined. Exudates were diluted to equal concentration (milliliters of exudate per gram of roots) and used for bioassay. Seeds of sorghum were germinated for 3 d at 28°C in autoclaved vermiculate and watered with 40% modified Long Ashton nutrient solution (Gurney et al., 2002). Small seedlings were transferred to glass tubes to grow hydroponically in a phytotron 14/10-h photoperiod at 250 μmol photons·m−2·s−1 at 28/23°C. Twelve-day-old seedlings were transferred into clean tubes containing 40% Long Ashton nutrient solution without/with 4 μm fluridone. Five days later, plants were transferred into glass tubes with sterile demineralized water, and root exudates were collected for 24 h. The exudates were diluted to the same concentration of grams of root fresh weight per milliliter root exudates, and induction of S. hermonthica (collected from sorghum field) germination was tested.
Experiments with Inhibitors
W22 seedlings were grown in perlite in separate tubes for 3 d at 25°C. Tubes were covered with aluminum foil, and the plants were watered with tap water. Solutions of inhibitors were applied, and seedlings were grown in the presence of inhibitors for an additional 5 d. Mevastatin (10 μm) and fosmidomycin (100 μm) were used to inhibit isoprenoid formation in the cytosol and plastids, respectively, and 25 μm fluridone was used to block carotenoid formation. After 5 d, seedlings were taken from the perlite, and any perlite clinging to the roots was carefully removed. Plants were put into tap water in glass tubes for 6 h at 25°C. A dilution of 70 mg root fresh weight/mL root exudate was used for the bioassay. Root exudates from 10 individual plants for each treatment were tested. Exudates were assessed in duplicate, 50 μL/disk. For amitrole experiments, germinated seeds of maize inbred line Dent were sown in perlite in pots and grown at 21°C for 11 d. Control plants were watered with tap water, and amitrole-treated plants were watered with 200 μm amitrole. Root exudates were collected in tap water for 8 h. Naproxen- and sodium-tungstate-treated maize inbred line Dent plants were sown in perlite in pots and grown at 25°C for 10 d. Control plants were watered with tap water, and naproxen-treated plants were watered with 0.1 and 1 mm naproxen. Sodium-tungstate-treated plants were watered with 0.1 and 1 mm solutions. Abscisic-acid-treated plants were watered with 0.02 and 0.2 mm abscisic acid without/with 10 μm fluridone. Root exudates from eight individual seedlings/treatment were collected in tap water for 18 h.
Germination Bioassay
Root exudates produced by individual plants were tested by bioassay. The seeds of Orobanche and Striga spp. require preconditioning (or warm stratification) for a certain period of time at a suitable temperature before the seeds become responsive to germination stimulants (Matusova et al., 2004). Preconditioning was performed under sterile conditions. The seeds were surface sterilized in 2% sodium hypochlorite containing 0.02% (v/v) Tween 20 for 5 min and rinsed thoroughly with sterile demineralized water. Subsequently, the seeds were dried for 30 min in a laminar air flow cabinet. Approximately 50 to 100 seeds were spread on a glass fiber filter paper disk (9-mm diameter) and put into sterile petri dishes (9-cm diameter) lined with Whatman filter paper wetted with 3 mL of demineralized water. Petri dishes were sealed with parafilm and incubated for preconditioning. S. hermonthica seeds were preconditioned at 30°C in darkness for 10 to 12 d, and O. crenata seeds were preconditioned at 21°C in darkness for 14 d. After the preconditioning period, the glass fiber filter paper disks with S. hermonthica or O. crenata seeds were removed from the petri dish and dried for 20 min to remove surplus moisture. The disks were transferred to another petri dish within a filter paper ring (outer diameter of 9 cm; inner diameter of 8 cm) wetted with 0.9 mL of water, which maintained a moist environment during the germination bioassay. Fifty microliters of the test solutions were added to duplicate or triplicate disks, depending on the experiment. The synthetic germination stimulant GR24 (0.01 and 0.1 mg·L−1 GR24) as a positive control and water as a negative control were included in each bioassay. Seeds were incubated at 30°C (S. hermonthica) or 25°C (O. crenata) in darkness for 2 d (S. hermonthica) or 6 d (O. crenata). The germinated and nongerminated seeds were counted using a binocular. Seeds were considered germinated when the radicle protruded through the seed coat. To test any inhibitory effect of root exudates from fluridone-treated seedlings, a control combining root exudates of maize, cowpea, and sorghum with GR24 was included in the germination bioassays. Root exudates and GR24 were diluted with water to the same final concentration of root exudates used without GR24 and a nonsaturating concentration of GR24. Use of nonsaturated concentration of GR24 ensures that any inhibitory (negative) effect of root exudates from fluridone-treated cowpea seedlings will be visible.
Carotenoid Analysis
The shoots and roots of maize seedlings were frozen in liquid nitrogen and ground to a fine powder. Carotenoids were extracted from 1.5 g of roots and 0.5 g of shoots essentially as described before (Bino et al., 2005). HPLC analysis was performed according to Fraser et al. (2000). Identification of carotenoids was based on retention time and spectral characteristics of the standards.
Statistical Analysis
The generalized linear mixed model method was used for statistical analysis of all germination bioassay results using Genstat, procedure IRREML (Payne and Lanne, 1993).
Note Added in Proof
During the reviewing process of this paper, a paper by Akiyama et al. appeared in Nature (K. Akiyama, K. Matsuzaki, H. Hayashi [2005] Nature 435: 824–827). In their paper, the authors describe the isolation of the strigolactone, 5-deoxy-strigol, as the mycorrhizal branching factor from Lotus japonicus. Also, other strigolactones tested, such as strigol, and even GR24 exhibited mycorrhizal branching factor activity at extremely low concentrations. This means that the strigolactones play an important role in establishing the beneficial relationship between plants and mycorrhiza. It shows that the strigolactones do have an important function in plants and explains why these compounds were not selected against during evolution, which would be expected if they were only responsible for the induction of germination of parasitic plants. It also shows that the relationship between mycorrhizal colonization and the reduction of germination of parasitic plant seeds, as we discuss it in the present paper, may reflect a more direct causal relationship than we here postulate, for example, down-regulation of mycorrhizal branching factor formation after mycorrhizal colonization.
Acknowledgments
The authors thank Vicky Child for maize and S. hermonthica seeds as well as many helpful suggestions, the Maize Genetic COOP Stock Center for supplying seeds of maize mutants, Piet Arts of J.C. Robinson Seeds for Dent maize seeds, Bob Vasey for his kind help in supplying many different batches of host as well as parasite seeds, Danny Joel for supplying O. crenata seeds, Ric de Vos and Harry Jonker for their help with carotenoid analyses, Jacques Withagen for his help with statistical analysis, Binne Zwanenburg for supplying GR24 and helpful comments on the biogenetic scheme, and Aede de Groot for helpful comments on the biogenetic scheme.
This work was supported in part by the European Commission (the FP5 European Union project Improved Striga Control in Maize and Sorghum [International Collaboration with Developing Countries, ICA4–CT–2000–30012; to H.J.B.] and the FP6 European Union Project Grain Legumes [FOOD–CT–2004–506223; to H.J.B. and R.M.]); by the Dutch Ministry of Agriculture, Nature Management, and Fisheries in the form of an International Agricultural Centre fellowship (to R.M.) and the North–South program (to H.J.B.); by the Netherlands Organization for Scientific Research (North Atlantic Treaty Organization visiting scientist fellowships to R.M. and K.R.); and by the Organization for Economic Cooperation and Development (a fellowship under the Cooperative Research Program: Biological Resource Management for Sustainable Agriculture Systems [to R.M.]).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.061382.
References
- Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827 [DOI] [PubMed] [Google Scholar]
- Ben-Amotz A, Fishler R (1998) Analysis of carotenoids with emphasis on 9-cis beta-carotene in vegetables and fruits commonly consumed in Israel. Food Chem 62: 515–520 [Google Scholar]
- Bino RJ, de Vos RCH, Lieberman M, Hall RD, Bovy A, Jonker HH, Tikunov Y, Lommen A, Moco S, Levin I (2005) The light-hyperresponsive high pigment-2dg mutation of tomato: alterations in the fruit metabolome. New Phytol 166: 427–438 [DOI] [PubMed] [Google Scholar]
- Botella-Pavia P, Besumbes O, Phillips MA, Carretero-Paulet L, Boronat A, Rodriguez-Concepcion M (2004) Regulation of carotenoid biosynthesis in plants: evidence for a key role of hydroxymethylbutenyl diphosphate reductase in controlling the supply of plastidial isoprenoid precursors. Plant J 40: 188–199 [DOI] [PubMed] [Google Scholar]
- Bouwmeester HJ, Matusova R, Zhongkui S, Beale MH (2003) Secondary metabolite signalling in host-parasitic plant interactions. Curr Opin Plant Biol 6: 358–364 [DOI] [PubMed] [Google Scholar]
- Butler LG (1995) Chemical communication between the parasitic weed Striga and its crop host. A new dimension in allelochemistry. In K Inderjit, FA Einhellig, eds, Insights into Allelopathy, ACS Symposium Series. ACS Books, Washington, DC, pp 158–168
- Chernys JT, Zeevaart JAD (2000) Characterization of the 9-cis-epoxycarotenoid dioxygenase gene family and the regulation of abscisic acid biosynthesis in avocado. Plant Physiol 124: 343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittapur BM, Hunshal CS, Shenoy H (2001) Allelopathy in parasitic weed management: role of catch and trap crops. Allelopathy J 8: 147–160 [Google Scholar]
- Cook CE, Whichard LP, Wall ME, Egley GH, Coggon P, Luhan PA, McPhail AT (1972) Germination stimulants. 2. The structure of strigol—a potent seed germination stimulant for witchweed (Striga lutea Lour.). J Am Chem Soc 94: 6198–6199 [Google Scholar]
- Corona V, Aracri B, Kosturkova G, Bartley GE, Pitto L, Giorgetti L, Scolnik PA, Giuliano G (1996) Regulation of a carotenoid biosynthesis gene promoter during plant development. Plant J 9: 505–512 [DOI] [PubMed] [Google Scholar]
- Cunningham FX, Gantt E (1998) Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 49: 557–583 [DOI] [PubMed] [Google Scholar]
- Cutler AJ, Krochko JE (1999) Formation and breakdown of ABA. Trends Plant Sci 4: 472–478 [DOI] [PubMed] [Google Scholar]
- Dalla Vecchia F, Barbato R, La Rocca N, Moro I, Rascio N (2001) Responses to bleaching herbicides by leaf chloroplasts of maize plants grown at different temperatures. J Exp Bot 52: 811–820 [DOI] [PubMed] [Google Scholar]
- de Kraker JW, Franssen MCR, de Groot A, Konig WA, Bouwmeester HJ (1998) (+)-Germacrene A biosynthesis: the committed step in the biosynthesis of bitter sesquiterpene lactones in chicory. Plant Physiol 117: 1381–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube MP, Olivier A (2001) Striga gesnerioides and its host, cowpea: interaction and methods of control. Can J Bot 79: 1225–1240 [Google Scholar]
- Fraser PD, Pinto MES, Holloway DE, Bramley PM (2000) Application of high-performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. Plant J 24: 551–558 [DOI] [PubMed] [Google Scholar]
- Gurney AL, Press MC, Scholes JD (2002) Can wild relatives of sorghum provide new sources of resistance or tolerance against Striga species? Weed Res 42: 317–324 [Google Scholar]
- Gworgwor NA, Weber HC (2003) Arbuscular mycorrhizal fungi-parasite-host interaction for the control of Striga hermonthica (Del.) Benth. in sorghum [Sorghum bicolor (L.) Moench]. Mycorrhiza 13: 277–281 [DOI] [PubMed] [Google Scholar]
- Hable WE, Oishi KK, Schumaker KS (1998) Viviparous-5 encodes phytoene desaturase, an enzyme essential for abscisic acid (ABA) accumulation and seed development in maize. Mol Gen Genet 257: 167–176 [DOI] [PubMed] [Google Scholar]
- Hartung W, Gimmler H (1994) A stress physiological role for abscisic acid (ABA) in lower plants. Prog Bot 55: 157–173 [Google Scholar]
- Hauck C, Muller S, Schildknecht H (1992) A germination stimulant for parasitic flowering plants from Sorghum bicolor, a genuine host plant. J Plant Physiol 139: 474–478 [Google Scholar]
- Hemmerlin A, Hoeffler JF, Meyer O, Tritsch D, Kagan IA, Grosdemange-Billiard C, Rohmer M, Bach TJ (2003) Cross-talk between the cytosolic mevalonate and the plastidial methylerythritol phosphate pathways in tobacco Bright Yellow-2 cells. J Biol Chem 278: 26666–26676 [DOI] [PubMed] [Google Scholar]
- Hirschberg J (2001) Carotenoid biosynthesis in flowering plants. Curr Opin Plant Biol 4: 210–218 [DOI] [PubMed] [Google Scholar]
- Iuchi S, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K (2000) A stress-inducible gene for 9-cis-epoxycarotenoid dioxygenase involved in abscisic acid biosynthesis under water stress in drought-tolerant cowpea. Plant Physiol 123: 553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janick-Buckner D, Hammock JD, Johnson JM, Osborn JM, Buckner B (1999) Biochemical and ultrastructural analysis of the y10 mutant of maize. J Hered 90: 507–513 [Google Scholar]
- Janick-Buckner D, O'Neal JM, Joyce EK, Buckner B (2001) Genetic and biochemical analysis of the y9 gene of maize, a carotenoid biosynthetic gene. Maydica 46: 41–46 [Google Scholar]
- Joel DM (2000) The long-term approach to parasitic weeds control: manipulation of specific developmental mechanisms of the parasite. Crop Prot 19: 753–758 [Google Scholar]
- Keyes WJ, Taylor JV, Apkarian RP, Lynn DG (2001) Dancing together. Social controls in parasitic plant development. Plant Physiol 127: 1508–1512 [PMC free article] [PubMed] [Google Scholar]
- Laule O, Furholz A, Chang H-S, Zhu T, Wang X, Heifetz PB, Gruissem W, Lange M (2003) Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 6866–6871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Milborrow BV (1997) Endogenous biosynthetic precursors of (+)-abscisic acid. IV. Biosynthesis of ABA from [H-2(n)]carotenoids by a cell-free system from avocado. Aust J Plant Physiol 24: 715–726 [Google Scholar]
- Lendzemo VW (2004) The tripartite interaction between sorghum, Striga hermonthica, and arbuscular mycorrhizal fungi. PhD thesis. Wageningen University, Wageningen, The Netherlands
- Lendzemo VW, Kuyper TW (2001) Effects of arbuscular mycorrhizal fungi on damage by Striga hermonthica on two contrasting cultivars of sorghum, Sorghum bicolor. Agr Ecosyst Environ 87: 29–35 [Google Scholar]
- Li ZH, Matthews PD, Burr B, Wurtzel ET (1996) Cloning and characterization of a maize cDNA encoding phytoene desaturase, an enzyme of the carotenoid biosynthetic pathway. Plant Mol Biol 30: 269–279 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK (1999) The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 50: 47–65 [DOI] [PubMed] [Google Scholar]
- Maier W, Hammer K, Dammann U, Schulz B, Strack D (1997) Accumulation of sesquiterpenoid cyclohexenone derivatives induced by an arbuscular mycorrhizal fungus in members of the Poaceae. Planta 202: 36–42 [Google Scholar]
- Matusova R, van Mourik T, Bouwmeester HJ (2004) Changes in the sensitivity of parasitic weed seeds to germination stimulants. Seed Sci Res 14: 335–344 [Google Scholar]
- Mohamed A, Rich PJ, Housley TL, Ejeta G (2001) In vitro techniques for studying mechanisms of Striga resistance in sorghum. In A Fer, P Thalouarn, LJ Musselman, C Parker, JAC Verkleij, eds, Proceedings of the 7th International Parasitic Weed Symposium. Faculté des Sciences et des Techniques, Université de Nantes, Nantes, France, pp 96–100
- Muller S, Hauck C, Schildknecht H (1992) Germination stimulants produced by Vigna unguiculata Walp cv. Saunders Upright. J Plant Growth Regul 11: 77–84 [Google Scholar]
- Mwakaboko AS (2003) Synthesis and biological evaluation of new strigolactone analogues as germination stimulants for the seeds of the parasitic weeds Striga and Orobanche spp. PhD thesis. University of Nijmegen, Nijmegen, The Netherlands
- Neill SJ, Burnett EC, Desikan R, Hancock JT (1998) Cloning of a wilt-responsive cDNA from an Arabidopsis thaliana suspension culture cDNA library that encodes a putative 9-cis-epoxy-carotenoid dioxygenase. J Exp Bot 49: 1893–1894 [Google Scholar]
- Parker C, Riches CR (1993) Parasitic Weeds of the World: Biology and Control. CAB International, Wallingford, UK, pp 111–163
- Parry AD, Horgan R (1992) Abscisic-acid biosynthesis in roots. 1. The identification of potential abscisic-acid precursors, and other carotenoids. Planta 187: 185–191 [DOI] [PubMed] [Google Scholar]
- Payne RW, Lanne PW (1993) Genstat 5 Release 3 Reference Manual. Clarendon Press, Oxford
- Press MC, Scholes JD, Riches CR (2001) Current status and future prospects for management of parasitic weeds (Striga and Orobanche). In CR Riches, ed, The World's Worst Weeds. British Crop Protection Council, Brighton, UK, pp 71–90
- Qin XQ, Zeevaart JAD (1999) The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc Natl Acad Sci USA 96: 15354–15361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DS, Bachmann MD, Anderson IC (1966) Role of carotenoids in protectiong chlorophyll from photodestruction. II. Studies on the effect of four modifiers of the albino cl1 mutant of maize. Photochem Photobiol 5: 797–805 [Google Scholar]
- Sato D, Awad AA, Chae SH, Yokota T, Sugimoto Y, Takeuchi Y, Yoneyama K (2003) Analysis of strigolactones, germination stimulants for Striga and Orobanche, by high-performance liquid chromatography/tandem mass spectrometry. J Agric Food Chem 51: 1162–1168 [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Tan BC, Gage DA, Zeevaart JAD, McCarty DR (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276: 1872–1874 [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Tan BC, McCarty DR, Welch W, Zeevaart JAD (2003) Substrate specificity and kinetics for VP14, a carotenoid cleavage dioxygenase in the ABA biosynthetic pathway. Biochim Biophys Acta 1619: 9–14 [DOI] [PubMed] [Google Scholar]
- Seo M, Koshiba T (2002) Complex regulation of ABA biosynthesis in plants. Trends Plant Sci 7: 41–48 [DOI] [PubMed] [Google Scholar]
- Seo M, Peeters AJM, Koiwai H, Oritani T, Marion-Poll A, Zeevaart JAD, Koornneef M, Kamiya Y, Koshiba T (2000) The Arabidopsis aldehyde oxidase 3 (AA03) gene product catalyzes the final step in abscisic acid biosynthesis in leaves. Proc Natl Acad Sci USA 97: 12908–12913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siame BA, Weerasuriya Y, Wood K, Ejeta G, Butler LG (1993) Isolation of strigol, a germination stimulant for Striga asiatica, from host plants. J Agric Food Chem 41: 1486–1491 [Google Scholar]
- Strack D, Fester T, Hause B, Schliemann W, Walter MH (2003) Arbuscular mycorrhiza: biological, chemical, and molecular aspects. J Chem Ecol 29: 1955–1979 [DOI] [PubMed] [Google Scholar]
- Tan BC, Schwartz SH, Zeevaart JAD, McCarty DR (1997) Genetic control of abscisic acid biosynthesis in maize. Proc Natl Acad Sci USA 94: 12235–12240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigchert SCM, Zwanenburg B (1999) A critical account on the inception of Striga seed germination. J Agric Food Chem 47: 1320–1325 [DOI] [PubMed] [Google Scholar]
- Wurtzel ET (2004) Genomics, genetics and biochemistry of maize carotenoid biosynthesis. In J Romeo, ed, Recent Advances in Phytochemistry, Vol 38. Elsevier, Amsterdam, pp 85–110
- Xiong LM, Gong ZZ, Rock CD, Subramanian S, Guo Y, Xu WY, Galbraith D, Zhu JK (2001. a) Modulation of abscisic acid signal transduction and biosynthesis by an Sm-like protein in Arabidopsis. Dev Cell 1: 771–781 [DOI] [PubMed] [Google Scholar]
- Xiong LM, Ishitani M, Lee H, Zhu JK (2001. b) The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell 13: 2063–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Sakal H, Okuno K, Yoneyama K, Takeuchi Y (1998) Alectrol and Orobanchol, germination stimulants for Orobanche minor, from its host red clover. Phytochemistry 49: 1967–1973 [Google Scholar]
- Yoneyama K, Takeuchi Y, Sato D, Sekimoto H, Yokota T (2004) Determination and quantification of strigolactones. In DM Joel, ed, Proceedings of the 8th International Parasitic Weed Symposium. International Parasitic Plant Society, Amsterdam, p 9