Abstract
We analyzed expression of marker genes for three defense pathways during infection by Cauliflower mosaic virus (CaMV), a compatible pathogen of Arabidopsis (Arabidopsis thaliana), using luciferase reporter transgenes and directly by measuring transcript abundance. Expression of PR-1, a marker for salicylic acid signaling, was very low until 8 d postinoculation and then rose sharply, coinciding with the rise in virus levels. In contrast, as early as 2 h postinoculation, transcriptional up-regulation of GST1—a marker for reactive oxygen species—and PDF1.2—a marker for jasmonic acid/ethylene defense signaling—was detectable in the virus-inoculated leaf and systemically. In parallel with the activation of GST1, H2O2 accumulated locally and systemically in virus- but not mock-inoculated plants. However, in plants inoculated with infectious CaMV DNA rather than virus particles, the onset of systemic luciferase activity was delayed by 24 to 48 h, suggesting that virion structural proteins act as the elicitor. This phenomenon, which we term the rapid systemic response, preceded virus movement from the inoculated leaf; therefore, the systemic signal is not viral. Systemic, but not local, H2O2 accumulation was abolished in rbohDF double mutants and in etr1-1 and ein2-1 mutants, implicating NADPH oxidase and ethylene signaling in the generation and transduction of the response. Ethylene, but not rbohDF mutants, also showed reduced susceptibility to CaMV, whereas in NahG transgenics, virus levels were similar to wild type. These findings implicate reactive oxygen species and ethylene in signaling in response to CaMV infection, but suggest that salicylic acid does not play an effective role.
With the exception of gene silencing, host responses to compatible virus infections and the molecular mechanisms by which plants defend themselves against such infections are poorly characterized. During incompatible host-virus infections, the appropriate combination of viral gene (encoding the appropriate avirulence factor or elicitor) and host resistance gene (R gene) triggers rapid activation of defense responses (DeWit, 1997), leading to local and systemic acquired resistance (SAR; Hunt and Ryals, 1996), and often programmed cell death (PCD) at and adjacent to the initial sites of infection (Dangl et al., 1996). Virus spread is usually restricted to a small group of cells surrounding the initial site of infection. In the absence of an appropriate elicitor-R gene combination, the virus is able to spread systemically. How plants respond to infection by compatible viruses is less well understood despite the fact that it is compatible and not incompatible pathogens that cause disease and hence economic losses in agriculture.
Responses to infection by incompatible bacteria and fungi and the role of salicylic acid (SA) as a central signaling molecule in triggering defense have been well established (Hunt and Ryals, 1996; Bonas and Lahaye, 2002). The mechanisms that restrict spread of an incompatible virus to the area of local lesions are less well characterized, but the signaling pathways for virus defense appear to branch below SA from that for fungi and bacteria and are independent of NPR1 (Murphy et al., 1999, 2001). In resistant ecotypes of Arabidopsis (Arabidopsis thaliana), npr1 mutants retain resistance to Turnip crinkle virus (TCV; Kachroo et al., 2000) and one strain of Cucumber mosaic virus (CMV; Takahashi et al., 2002), although the same mutation leads to loss of resistance to some, but not all, isolates of Peronospera parasitica and Pseudomonas syringae (Glazebrook et al., 1996; Rairdan and Delaney, 2002). The virus-specific branch can be activated by antimycin A and cyanide and is inhibited by salicyl-hydroxamic acid, indicating a role for the mitochondrial alternative oxidase (Aox; Murphy et al., 2001).
Some of the defense responses that restrict spread of incompatible viruses may also act effectively against compatible viruses. Wong et al. (2002) reported that, in Arabidopsis, resistance to Turnip vein clearing tobamovirus (TVCV) was inducible by SA, cyanide, and antimycin A. Resistance to tobamoviruses appears to involve NPR1-independent inhibition of virus replication at the site of inoculation (Murphy and Carr, 2002; Wong et al., 2002). In contrast, SA-induced resistance to CMV appears to inhibit virus movement (Murphy et al., 2001), suggesting that, depending on the virus, defense may operate at different levels. All of these studies relied on treating plants with inducers or inhibitors of resistance. It remains undetermined whether the defense mechanisms with which they interact play any actual role in planta to control the spread or replication of compatible viruses.
Activation of the SA-mediated defense responses is associated with up-regulation of genes encoding pathogenesis-related (PR) proteins. Compatible pathogens are not usually associated with the induction of SAR. However, there are a number of reports of increased PR-1 expression in susceptible plants infected with viruses. For example, microarray analysis of Arabidopsis infected with five compatible RNA viruses showed greatly increased PR-1 transcript abundance (Whitham et al., 2003). The timing of the response is dependent on both host and virus. TCV infection activated PR-1 expression in both resistant and susceptible Arabidopsis ecotypes, but up-regulation occurred more rapidly and to a greater extent in ecotypes carrying the HRT resistance gene (Dempsey et al., 1997). In contrast, in Arabidopsis ecotypes systemically infected with Tobacco mosaic virus (TMV), levels of several PR gene transcripts were reported to be greatest in the most susceptible ecotypes (Dardick et al., 2000). Up-regulation of PR-1 is routinely used as a marker for SA and SAR (Volko et al., 1998). However, there is no evidence to indicate whether these responses function actively during systemic virus infections or indeed whether SA signaling is involved. Moreover, PR-1 is not believed to possess antiviral activity, and SA-mediated responses to incompatible virus infections are most likely activated via an NPR1-independent pathway (Murphy et al., 1999, 2001; Kachroo et al., 2000; Wong et al., 2002). Therefore, the relationship between increased PR-1 expression and defense against virus infection, compatible and incompatible, remains unclear.
Another well-known defense mechanism involves the generation of reactive oxygen species (ROS) following infection. ROS have been implicated as signaling intermediates during the PCD that typically occurs locally around sites of infection by incompatible pathogens (Dangl et al., 1996; Grant and Loake, 2000; Hancock et al., 2002). Locally increased activity of enzymes associated with the generation of ROS, such as NADPH oxidase, have been detected in resistant tobacco (Nicotiana tabacum) cultivars infected with TMV (Sagi and Fluhr, 2001). Increased levels of enzymes associated with the detoxification of ROS have also been demonstrated in bean (Phaseolus vulgaris) plants undergoing compatible infection with White clover mosaic virus (Clarke et al., 2002). Increases in ROS during incompatible infections with TMV have been detected directly using paramagnetic resonance spectroscopy (Fodor et al., 2001) and ROS-reactive fluorescent probes (Allan et al., 2001). In the latter case, a ROS burst was detected in tobacco epidermal cells within seconds of application of TMV to the outside of leaves of various tobacco cultivars. This burst was abolished by inhibitors of NADPH oxidase and was not dependent on virus replication, with virus coat protein acting as the elicitor. Interestingly, ROS bursts occurred with both resistant and susceptible tobacco cultivar-TMV isolate combinations. Although compatible isolates of TMV were effective inducers of ROS, no burst was detected at the early stages of infection by an unrelated (compatible) virus CMV.
The ROS burst appears to be an essential prerequisite for the hypersensitive response (HR) and local acquired resistance (Grant and Loake, 2000; Hancock et al., 2002), and there is some evidence that it may interact with the SA-signaling network. When infected with TMV, resistant tobacco cultivars carrying the NahG transgene showed reduced activation of several enzymes implicated in antioxidant activities (Kiraly et al., 2002). However, analysis of the ROS burst in a series of mutants suggested that it can function independently of ethylene, SA, and jasmonic acid (JA) signaling (Grant et al., 2000).
CaMV, which replicates and spreads systemically in Arabidopsis, is an excellent model with which to investigate host responses to infection by a compatible virus. We have previously characterized the infection phenotypes of 32 CaMV isolates in three Arabidopsis ecotypes (Cecchini et al., 1998). Here, we have used Arabidopsis transgenic reporter lines in which expression of LUC is driven by promoters from three defense-related genes to characterize defense responses activated in Arabidopsis following inoculation with CaMV. We identify responses that occur both early and late after inoculation. Within 2 h of exposure to CaMV, plants show systemic activation of marker genes for ROS and for JA/ethylene-mediated defense, and in parallel systemic accumulation of H2O2. Structural components of the virus appear to act as elicitors for the response. We also observe a sharp systemic increase in PR-1 expression, but this is delayed until about 8 d postinoculation (dpi), coincident with the increase in levels of replicating virus. We investigate the extent to which these responses are active in controlling or limiting levels of virus accumulation.
RESULTS
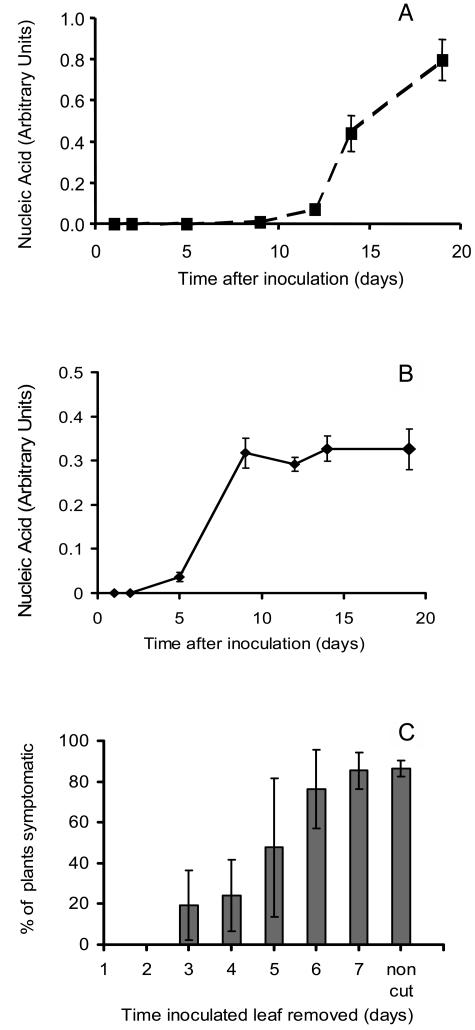
To determine the relationship between defense responses and virus replication/movement, we first established an infection time course (Fig. 1, A and B). Virus DNA (a measure of total virus accumulation) remained at a steady, low, but detectable level from 2 h postinoculation (hpi) up to 5 dpi (this presumably derives from the inoculum), and then increased rapidly from 8 dpi onward. Viral 19S and 35S RNA transcripts, which serve as a measure of actively replicating virus (Covey and Turner, 1991; Turner and Covey, 1993), were first detectable by hybridization at 5 dpi and increased greatly in abundance by 8 dpi; beyond this time, the increase in abundance was small (Fig. 1B). We assume that the number of cells supporting actively replicating virus does not increase greatly after 8 dpi, but virus continues to accumulate as the infection moves through the plant.
Figure 1.
A, Levels of CaMV DNA in plants at intervals after inoculation. Levels, measured by quantitative real-time PCR, are expressed in arbitrary units as a proportion of total DNA (see “Materials and Methods”). Error bars indicated sds of mean for triplicate samples each composed of DNA from three plants. B, Levels of CaMV 19S plus 35S RNA in plants at intervals after inoculation. Levels, measured by quantitative hybridization in slot blots and expressed in arbitrary units, are expressed as a proportion of total RNA (see “Materials and Methods”). Error bars indicated sds of mean for triplicate samples each composed of RNA from three plants. C, Percentage of plants that developed symptoms of infection following removal of the inoculated leaf at the time indicated. Non-cut indicates control in which the infected leaf was not removed. Infection was determined on the basis of symptom development by 16 dpi. Error bars indicate sd of mean based on three groups of 30 plants for each time point.
To establish the stage at which virus had begun to move systemically, we removed the inoculated leaf from groups of plants at intervals after inoculation and scored the percentage of plants that subsequently developed symptoms of infection (Fig. 1C). When the inoculated leaf was removed prior to 3 dpi, none of the plants developed systemic infections. Removal at or later than 6 dpi did not alter the proportion of infected plants compared to controls in which the inoculated leaf remained attached. We conclude that no infectious virus moves out of the inoculated leaf before 3 dpi.
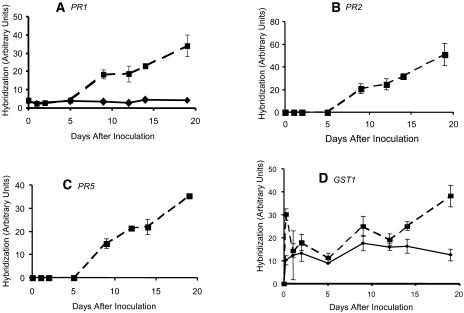
To identify whether inoculation with CaMV triggered defense responses, we quantitated transcripts of four genes, PR-1, PR-2 (encoding β-glucanase 2), and PR-5 (encoding thaumatin-like protein) that act as markers for SA-mediated defense, and GST1 (encoding glutathione S-transferase), which is activated by ROS (Grant et al., 2000). Total RNA was prepared from uninoculated leaves of CaMV- and mock-inoculated plants harvested at intervals from 2 hpi to 19 dpi, and transcript levels were estimated by quantitative slot-blot hybridization (Fig. 2). Up to 5 dpi, levels of PR-1, PR-2, and PR-5 transcripts were very low in both virus- and mock-inoculated plants, but from 8 dpi onward, we observed a rapid increase in the levels of all three transcripts in virus-infected plants. In contrast, levels of GST1 transcripts in virus-inoculated plants showed an immediate elevation at 2 hpi, compared to the preinoculation levels. We also observed a modest elevation in mock-inoculated plants, but to less than one-third that in virus-inoculated plants. At 1 dpi, levels of GST1 mRNA in virus-inoculated plants were lower than at 2 hpi, and they continued to fall over the period to 5 dpi, although they remained consistently higher than in mock-inoculated plants. In virus-infected plants, GST1 mRNA levels showed a second phase of increased abundance over the period from 8 to 19 dpi, but remained at a low and essentially constant level in mock-inoculated controls.
Figure 2.
Expression of defense-related genes PR-1 (A), PR-2 (B), PR-5 (C), and GST1 (D) at intervals after inoculation with CaMV. Levels of transcripts in noninoculated leaves were determined by quantitative hybridization in slot blots and are expressed in arbitrary units, which differ according to the probe used. Results were corrected for any differences in loading by quantitating the amount of RNA on each spot from the stained filter as described in “Materials and Methods.” ▪, CaMV inoculated; ♦, mock inoculated; PR-2 and PR-5 mRNAs were not detectable in mock-inoculated plants; zero values are not shown. Error bars indicate sds of mean for triplicate samples each composed of RNA from noninoculated leaves from three plants.
To analyze further the spatial and temporal patterns of expression of defense-related genes during infection by CaMV, we inoculated three lines of transgenic Arabidopsis plants carrying reporter constructs in which expression of LUC is driven by promoters from the PR-1a gene from tobacco (PR-1::LUC), the Arabidopsis GST1 gene (GST1::LUC), and the Arabidopsis PDF1.2 gene (PDF1.2::LUC), a marker for JA/ethylene-mediated defense (Manners et al., 1998; Penninckx et al., 1998; Yun et al., 2003). At times from 2 hpi to 19 dpi, plants were painted with beetle luciferin, and LUC activity was analyzed. Because luciferin has been reported as a possible inducer of PR-1 expression (Grant et al., 2000), individual plants were treated and analyzed for LUC activity only once; they were then retained until the end of the experiment to ensure that inoculated plants subsequently developed symptoms. Each experiment was carried out two or more times, using five plants for each time point. Patterns of luminescence were extremely consistent from plant to plant.
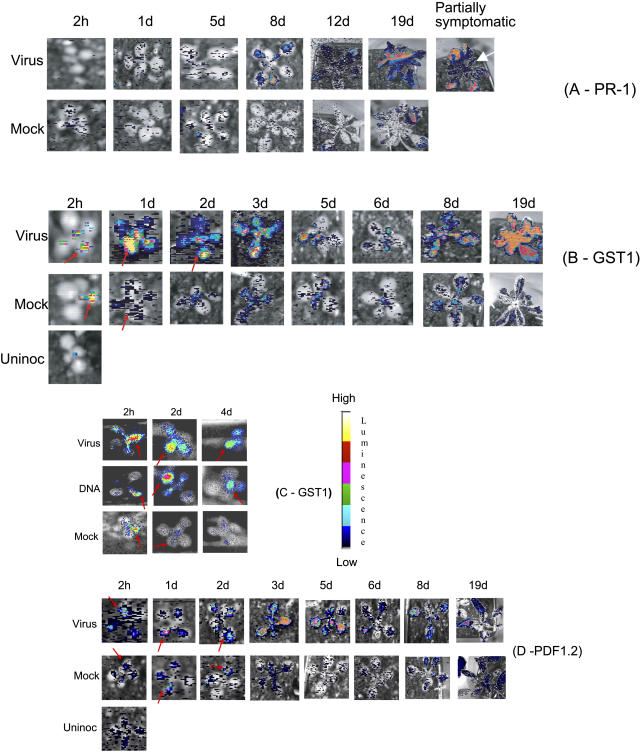
Figure 3A shows images of luminescence in virus- and mock-inoculated plants carrying PR-1::LUC, a reporter for SA-mediated defense (Grant et al., 2003). Uninoculated plants were similar to mock-inoculated (data not shown). By 8 dpi, moderate LUC activity was detectable in many of the leaves of plants inoculated with virus, and activity increased to a very high level by 19 dpi. In approximately 5% to 10% of plants, symptoms did not develop fully, the central apical region failing to show the extreme stunting and distortion typical with this isolate of CaMV. LUC activity at the central apical region of these partially symptomatic plants was low, although luminescence in the outer symptomatic leaves remained high (Fig. 3A, compare 19 d and partial symptoms).
Figure 3.
LUC activity in virus- and mock-inoculated plants. Luminescence was determined at the time after inoculation indicated above each image, as described in “Materials and Methods.” Luminescence levels are shown in pseudocolor using the logarithmic scale shown in the legend. Where appropriate, the inoculated leaf is indicated by a red arrow. A, Luminescence in PR-1a::LUC transgenic plants. The image labeled partially symptomatic shows the pattern of luminescence activity at 19 dpi in a plant that failed to develop symptoms in the apical region. For comparison, the adjacent image marked 19 d shows a comparable fully symptomatic plant. The shoot apical region is indicated with a white arrow. B, Luminescence in GST1::LUC transgenic plants. An image of an uninoculated plant is shown for one time point (2 hpi) only. At later time points, patterns of LUC activity in uninoculated plants were similar to mock-inoculated plants and are not shown. C, Luminescence in GST1::LUC transgenic plants after inoculation with CaMV virus and CaMV DNA. Mock-inoculated controls are shown for comparison. D, Luminescence in PDF1.2::LUC transgenic plants. An image of an uninoculated plant is shown for one time point (2 hpi) only. At later time points, patterns of LUC activity were similar to mock-inoculated plants and are not shown.
Figure 3B shows luminescence in plants carrying GST1::LUC, a reporter for ROS (Grant et al., 2000). Like with GST1 transcripts, we detected increased luminescence at 2 hpi. This was apparent in the inoculated leaf of both virus- and mock-inoculated plants compared to uninoculated controls, but luminescence in mock-inoculated plants was always distinctly lower than in virus-inoculated plants. Virus- but not mock-inoculated plants also showed a consistent moderate systemic LUC activity in both the cotyledons and in the apex. This systemic LUC activity was greatest from 1 to 2 dpi and then declined over the period to 5 dpi; at the same time, mock-inoculated controls showed very little LUC activity. Virus-inoculated plants showed a second phase of systemic LUC activity, beginning at 8 dpi and reaching very high levels by 19 dpi. We observed only a small increase in LUC activity in mock-inoculated controls over the same period. Except for the absence of wound-inducible luminescence in the inoculated leaf at the 2-h time point, uninoculated plants were similar to mock-inoculated controls (data not shown). Following LUC assay, plants were retained to determine whether they subsequently developed symptoms. Approximately 10% to 20% of virus-inoculated plants routinely failed to develop symptoms and accumulated little or no virus (e.g. Fig. 1C). However, over the period 2 hpi to 5 dpi, we observed no differences in the patterns of LUC activity between these escapes and plants that subsequently developed typical symptomatic infections.
To test whether virus replication was required to trigger the GST1-driven LUC activity, we inoculated with CaMV DNA (which is fully infectious) and compared the response to that in plants inoculated with purified virus particles (Fig. 3C). At 2 hpi, plants inoculated with DNA showed a similar pattern of LUC activity to mock-inoculated controls (slight luminescence in the inoculated leaf and little or no systemic LUC activity), whereas plants inoculated with virus particles showed the typical systemic LUC activity in the inoculated leaf, cotyledons, and apex, as described above. At 2 dpi, plants inoculated with CaMV DNA showed a pattern of local and systemic LUC activity that more closely resembled plants inoculated with virus particles, and, by 4 dpi, both virus- and DNA-inoculated plants showed near-identical patterns of continuing luminescence in the inoculated leaf, but reduced systemic luminescence. Since CaMV DNA is highly infectious, the systemic response at 2 hpi must be triggered by a component (other than the DNA) present in the virus particle inoculum, presumably one of the virus capsid proteins. In DNA-inoculated plants, where this elicitor is absent from the inoculum, the delayed systemic response presumably follows its synthesis de novo during replication.
To determine whether the JA/ethylene defense-signaling pathway (Penninckx et al., 1998) was being activated, we assayed luminescence in CaMV-inoculated plants carrying the PDF1.2::LUC reporter (Fig. 3D). Up to 5 dpi, patterns of LUC activity were similar to those in plants carrying GST1::LUC. At 2 hpi, both mock- and virus-inoculated plants showed increased activity in the inoculated leaf, but as with GST1::LUC, virus- but not mock-inoculated plants also showed a modest, but consistent, systemic LUC activity in cotyledons. In virus-inoculated plants, systemic and local LUC activity rose to a maximum by 3 dpi and then declined; in mock-inoculated plants, activity declined slowly over the same period. Unlike with GST1::LUC, we did not observe any late increase in LUC activity from 8 to 19 dpi, although both mock- and virus-inoculated plants showed a small, presumably age-related, increase.
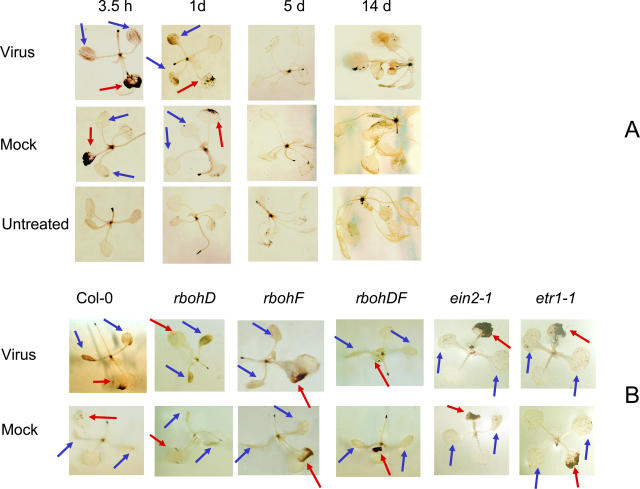
GST1 has previously been shown to respond to ROS (Grant et al., 2000). We stained for ROS accumulation in CaMV-inoculated plants using 3,3-diaminobenzidine (DAB). This gives a brown color in the presence of the H2O2 that accumulates in tissues following the generation of ROS. Results are shown in Figure 4A. In both mock- and virus-inoculated plants, the inoculated leaf consistently showed strong staining at 3.5 hpi, presumably at least partially as a response to wounding. However, in virus- but not mock-inoculated plants, we also observed staining of the cotyledons, which was greatest at 1 dpi and declined thereafter. Patterns of staining were very consistent. In three independent experiments (with 6–10 plants at each time point), we observed uniform staining of both cotyledons in all the virus-inoculated plants at both 2 hpi and 1 dpi. In mock-inoculated plants, we did not observe such staining, although in an occasional plant we observed partial staining of a single cotyledon. We did not observe any virus-specific staining at later stages of infection, although all plants showed uniform light staining by 14 dpi, presumably age related. DAB staining relies on endogenous peroxidase to allow development of the color in the presence of H2O2 (Thordal Christensen et al., 1997). Since we always observed staining of the inoculated leaf, and at least in virus-inoculated plants of the cotyledons also, sufficient peroxidase activity must be present to react to any H2O2 present. Thus, the early, but not the second phase, of GST1 activity is associated with both local and systemic accumulation of H2O2.
Figure 4.
Accumulation of H2O2 in tissues as determined by DAB staining. The presence of H2O2 results in the deposition of a brown pigment. Where appropriate, the inoculated leaf is indicated by a red arrow and the cotyledons are indicated by blue arrows. A, DAB staining of virus- and mock-inoculated Col-0 plants and untreated (uninoculated) controls at the times after inoculation indicated above each image. B, DAB staining of virus- and mock-inoculated Col-0 and mutants at 1 dpi. The genotype of the mutant is shown above each image. Like uninoculated Col-0, none of the uninoculated mutants exhibited any obvious staining and images are not shown.
PCD is associated with the generation of ROS (Dangl et al., 1996; Grant and Loake, 2000; Hancock et al., 2002). We have previously reported that the isolate of CaMV used in this study does not elicit necrotic lesions in Arabidopsis (Cecchini et al., 1998). To confirm that infection was not generating micro-hypersensitive responses (HRs), we carried out trypan blue staining on infected seedlings, but we were unable to identify any micro-HR lesions in any of the Arabidopsis genotypes used in this study.
NADPH oxidase has been implicated as a generator of ROS in plants undergoing HR (Torres et al., 1998; Sagi and Fluhr, 2001; Hancock et al., 2002). To test whether this might be the case here, we inoculated and then DAB stained NADPH oxidase mutants rbohD and rbohF, which contain T-DNA insertions in the genes encoding the respiratory-burst oxidase-homolog D and F polypeptides (Tissier et al., 1999) plus the double mutant (Torres et al., 2002). In two independent experiments, each with six plants, we observed very consistent patterns of staining (Fig. 4B). At 1 dpi, both rbohD and rbohF showed systemic DAB staining in response to inoculation with CaMV, although in rbohF, but not rbohD, the staining was considerably less intense than in wild type. The DF double mutant did not show any detectable systemic accumulation of H2O2 in cotyledons, and, although both the mock- and virus-inoculated leaf still showed some staining, this was less intense than in wild type. We observed no obvious difference between any of the mutants and wild-type plants in symptom severity, and with the DF double mutants, 80% to 90% of plants developed symptoms by 24 dpi, a similar proportion to wild type.
To determine whether ethylene signaling might be involved in the systemic ROS burst, we inoculated and then DAB stained two ethylene response mutants, etr1-1 and ein2-1 (Fig. 4B). Again, patterns of staining were very consistent. In contrast to wild type, we could detect no virus-dependent systemic accumulation of H2O2 in either of these mutants. However, the inoculated leaf still stained strongly. Therefore, an intact ethylene-signaling pathway appears to be required for propagation of the systemic ROS burst.
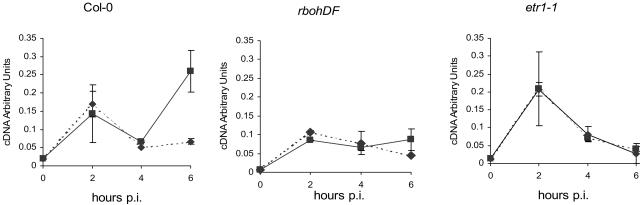
Since GST1 has been shown to be a good marker for ROS, we used real-time reverse transcription (RT)-PCR to quantify levels of GST1 mRNA in uninoculated leaves of ecotype Columbia (Col-0), etr1-1, and rbohDF double mutants at 2-h intervals from 0 to 6 hpi of a single leaf with CaMV. Results are shown in Figure 5. Mock-inoculated Col-0 plants showed an increase in GST1 transcript levels in systemic leaves at 2 hpi, with a steady reduction over the next 4 h. Virus-inoculated Col-0 showed a similar increase in GST1 transcripts at 2 hpi, followed by a further increase at 6 hpi. At 2 hpi, levels of GST1 transcripts in mock-inoculated plants varied considerably from sample to sample. However, in mock-inoculated plants, we never observed elevated transcript levels at 6 hpi, at which time levels were reproducibly about one-fifth those in virus-inoculated plants. We assume that the variable response at 2 hpi may result from wound induction. In contrast to wild-type plants, the virus-dependent elevation in GST1 transcripts at 6 hpi was absent in both etr1-1 and rbohDF mutants. In etr1-1, like in Col-0, levels of GST1 transcripts showed a somewhat variable elevation at 2 hpi, but then declined in both virus- and mock-inoculated plants. In rbohDF, GST1 transcript levels at 2 hpi were considerably lower than in wild type or etr1-1 and remained at a similar steady level in both CaMV- and mock-inoculated plants. These results provide further evidence linking GST expression with a ROS burst and suggest that an intact ethylene-signaling pathway and the rboh D and F genes are required for the virus-dependent, although not necessarily the wounding, response.
Figure 5.
Levels of GST1 mRNA at intervals after inoculation, assayed by quantitative real-time RT-PCR; ▪, CaMV-inoculated plants; ♦, mock-inoculated plants. From left to right, Col-0, rbohDF, and etr1-1. Each point represents the mean value (± sd) derived from duplicate assays of two batches of seven plants. Levels, expressed in arbitrary units, have been normalized using ACT2 as an internal reference.
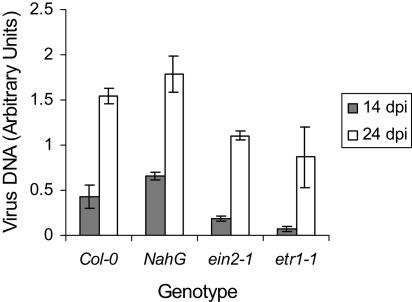
Although PR-1, PR-2, and PR-5 are not believed to play a direct role in defense against virus infection, they are markers for defense pathways that are activated by SA, and we have recently shown that up-regulation of PR-1 in CaMV-infected plants is SA dependent (Laird et al., 2004). To test for involvement of SA or ethylene in regulating defense against CaMV, we measured susceptibility to infection in NahG, a transgenic line expressing a bacterial salicylate hydroxylase that is unable to accumulate SA, and in etr1-1 and ein2-1. Symptoms in NahG were similar to wild type at 14 dpi, although by 24 dpi, NahG plants were very severely stunted. Symptoms in ein2-1, and in particular in etr1-1, were consistently milder than in wild type, and the first appearance of systemic symptoms showed a reproducible delay of 2 d. We extracted DNA from infected plants at 14 and 24 dpi and assayed levels of virus DNA by real-time PCR (Fig. 6). We were unable to identify any significant differences between virus DNA levels in wild-type (Col-0) and NahG at either 14 or 24 dpi. However, in ein2-1 and etr1-1, levels of CaMV DNA were significantly lower than in Col-0. Consistent with the delay in the appearance of symptoms, this difference was greater at 14 dpi. Thus, ethylene, but not SA, appears to regulate susceptibility to CaMV.
Figure 6.
Levels of CaMV DNA accumulating in uninoculated leaves from infected wild type and mutants. Levels of CaMV DNA at 14 dpi (gray bars) and 24 dpi (white bars), expressed in arbitrary units, were measured by quantitative real-time PCR. Values were corrected by normalization to a standard (Arabidopsis 18S rDNA; see “Materials and Methods”). Mean levels of virus DNA were determined from three independent experiments. For each experiment, DNA was quantified (for each genotype) in two biological samples each comprising the pooled tissue from three infected plants. Error bars show sds of the mean.
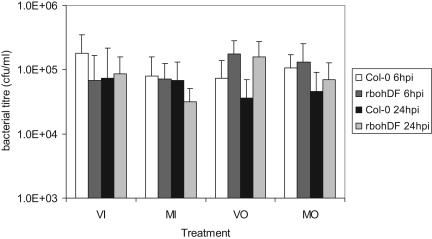
ROS act as essential signaling intermediates for defense against both virulent and avirulent pathogens (Yoshioka et al., 2003; Kunze et al., 2004; Rentel et al., 2004) and in regulating cell death (Delladonne et al., 2001; Torres et al., 2002; Overmyer et al., 2003). To test whether the CaMV-dependent ROS burst was capable of inducing resistance against other pathogens, we inoculated Arabidopsis seedlings (Col-0 and rbohDF) with CaMV on a single leaf (control plants were mock inoculated with water) and 6 or 24 h later vacuum infiltrated the whole seedlings with a virulent bacterial pathogen, P. syringae pv maculicola ES4236. After 64 h, bacterial growth was assayed in the inoculated and uninoculated leaves of virus- and mock-inoculated plants (Fig. 7), and the data were analyzed by multivariate ANOVA using the Bonferroni method. We found no significant effect (P > 0.05) of virus inoculation (at either 6 or 24 h prior to infiltration) on bacterial growth; this was the case for both the inoculated and noninoculated (opposite) leaf. We did find that, for all treatments, bacterial titers in the opposite leaves of the rbohDF mutants were on average 2.1-fold higher than in wild-type controls. This difference is highly statistically significant (P < 0.001).
Figure 7.
Bacterial titers (colony forming units/mL) in different leaves 64 h after vacuum infiltration with P. syringae pv maculicola strain ES4326. Col-0 or rbohDF seedlings were inoculated on a single true leaf with CaMV or mock inoculated with water and challenged with bacteria 6 hpi or 24 hpi later. VI, Virus-inoculated leaf; MI, mock-inoculated leaf; VO, virus-inoculated seedling—opposite leaf; MO mock-inoculated seedling—opposite leaf. Results show mean values from 10 plants. Error bars show sds of the mean.
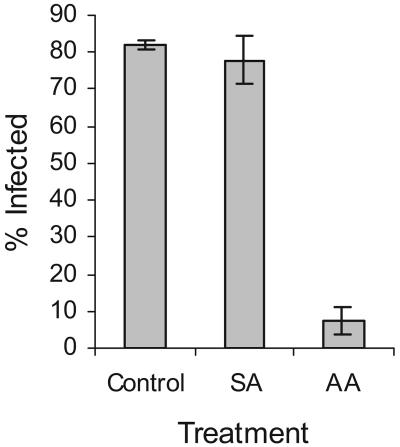
ROS have been implicated as potential signaling intermediates in SA-mediated defense against incompatible virus infections, possibly functioning via the mitochondrial Aox pathway (Chivasa et al., 1997; Murphy et al., 1999; Gilliland et al., 2003). Since we identified a ROS burst in response to inoculation with CaMV, we treated plants with SA or with antimycin A, an inhibitor of respiration and stimulator of the Aox pathway. Treatment with 1 mm SA, either by spraying or by inclusion in the inoculum, did not significantly affect the proportion of plants that developed symptoms of systemic infection (Fig. 8). In contrast, inclusion of 50 μm antimycin A in the inoculum dramatically reduced the proportion of plants that developed symptoms compared to dimethyl sulfoxide (DMSO)-treated controls (Fig. 8). In addition to inhibiting respiration, antimycin A has been reported to stimulate PCD (Yao et al., 2002). To exclude the possibility that this might be responsible for the induced resistance, we extracted DNA from inoculated leaves and analyzed for the presence of DNA ladders, markers for apoptosis. We detected no such laddering (data not shown). Also, we did not observe any visual evidence of tissue damage or necrosis. When plants were inoculated on one leaf with CaMV, and on a different leaf with 2 μL of 50 μm antimycin A, 79% developed symptoms of systemic infection compared to 82% in untreated controls. Thus, antimycin A must act locally rather than systemically.
Figure 8.
Effect of treatments designed to potentiate defense. Ler-0 plants were treated as described (“Materials and Methods”) and assessed visually for the presence of systemic symptoms of CaMV infection (vein clearing, stunting, mosaics) at 16 dpi. Shown: 0.1% DMSO (control); 1 mm salicylic acid (SA); 50 μm antimycin A in 0.1% DMSO (AA). Data shown are mean ± sd for duplicate groups of 40 plants.
DISCUSSION
Our results demonstrate that Arabidopsis responds to a compatible virus, CaMV, by activating expression of marker genes associated with three defense response pathways that utilize SA, JA/ethylene, and ROS as signaling intermediates. Each reporter showed a distinct temporal and spatial pattern of activation following inoculation. Up-regulation of PR-1 during infection by compatible plant viruses has been reported previously (Whitham et al., 2003), and there have also been reports that plant cells exposed to compatible, as well as incompatible, viruses can react by generating ROS (Allan et al., 2001). However, the systemic up-regulation of GST1 and the accumulation of H2O2 in tissues distant from the site of inoculation is a novel and unexpected finding. GST1 has previously been shown to be responsive to ROS (Grant et al., 2000); the local and systemic accumulation of H2O2 suggests that this is the likely trigger for its activation here. Mutations that interfere with ethylene signaling and perception abolished the systemic propagation of the response, and we also detected, in parallel, rapid and systemic activation of a marker for ethylene/JA-mediated defense, PDF1.2.
We interpret these findings as evidence that plants show a systemic response to an elicitor encoded by a compatible viral pathogen. We term the phenomenon the rapid systemic response (RSR). A recent report (Kunze et al., 2004) has identified a highly conserved motif at the n terminus of elongation factor (EF)-Tu from a variety of plant pathogenic bacteria as a pathogen-associated molecular pattern (PAMP) for several members of the Brassicaceae. EF-Tu, or a peptide comprising the 18 n-terminal amino acids, stimulated generation of ROS within minutes of application to Arabidopsis leaves. The elicitors not only induced an oxidative burst, but also stimulated production of ethylene. A rapid coronatine-dependent transcriptional activation of an ethylene response factor gene, elicited independently of the R-Avr interaction and triggered by virulence factors encoded by P. syringae, has also been reported (He et al., 2004), and a recent report (Desikan et al., 2005) identifies a direct role for ETR1 in H2O2 perception. These responses, the local ROS burst elicited in tobacco epidermal cells by TMV coat protein (Allan et al., 2001) and the RSR elicited by CaMV, may be aspects of a common response associatedwith PAMP-dependent activation of basal defense mechanisms. However, unlike with EF-Tu (Kunze et al., 2004), we were unable to demonstrate that CaMV could elicit any significant increase in basal resistance when plants were subsequently challenged with a virulent bacterial pathogen. Thus the ability of CaMV structural proteins to elicit defense may be limited functionally.
Although there was some discrepancy between the exact time course of GST1::LUC activity and GST1 transcript levels, this is most likely attributable to differences in the intracellular half-life of the GST1 and LUC mRNA protein. Also, direct quantification of GST1 transcripts did reveal a transient increase in levels in mock-inoculated plants that was not obvious on the basis of LUC activity, although this was very variable (in Col-0 and etr1, coefficients of variance at 2 hpi were about 3-fold greater than at 0 or 6 hpi; see Fig. 5). Perhaps, in addition to the consistent virus-induced response, some transient systemic activation of GST1 is triggered by the wounding associated with inoculation. Whichever of these techniques is the most reliable, there is no doubt that the onset of the virus-dependent response is rapid, probably within 2 h and certainly within 6 h of inoculation. Tobacco epidermal cells respond to both incompatible and compatible isolates of TMV within seconds of exposure (Allan et al., 2001). Our results indicate that by 2 to 6 h of inoculation with CaMV, an ROS burst occurs not only locally but also systemically. The nature of our inoculation technique, and the consistency of the response in both cotyledons of the virus-inoculated plants (routinely 100% for both GST1::LUC and DAB staining in a number of independent experiments), allows us to rule out cross-contamination of the inoculum as the elicitor. Thus, a systemic signal must be stimulating ROS generation in distant parts of the plant. The nature of this signal is unknown, but it cannot be infectious virus because our leaf removal experiments show that this does not leave the inoculated leaf until at least 3 dpi. Since the direction of signaling, from true leaf to cotyledon, is counter to the movement of photoassimilates, the signal probably does not move via the phloem and may be volatile.
Virus particles stimulated GST1-driven systemic LUC activity by 2 hpi, but this response was delayed in plants inoculated with virus DNA. The elicitor must therefore be a component already present in the virus inoculum, presumably one of the capsid-associated polypeptides encoded by open reading frames (ORFs) III and IV (Hohn and Futterer, 1997). TMV coat protein can directly elicit local ROS generation in tobacco (Allan et al., 2001); most likely, structural components of the CaMV virion function similarly in Arabidopsis. LUC activity at 2 dpi in DNA-inoculated plants is presumably activated by virus proteins synthesized de novo during replication.
How are these signaling responses generated, and what is their function? ROS have been identified as signaling molecules during PCD (Grant and Loake, 2000; Hancock et al., 2002) and the NADPH oxidase complex has been identified as a source of ROS (Torres et al., 1998; Grant and Loake, 2000), with both the D and F polypeptides implicated in defense-related ROS generation (Low and Merida, 1996; Grant et al., 2000; Torres et al., 2002). DAB staining in cotyledons of rboh mutants in response to CaMV, in particular the absence of systemic staining and low levels of GST1 transcripts in the rbohDF double mutant, identify NADPH oxidase as the principal generator of ROS at sites distant from the inoculated leaf. Local accumulation of H2O2, some of which may be attributable to wound responses, was reduced, but not abolished, in the double mutant; here, the NADPH oxidase cannot be the exclusive generator of ROS. Since single mutants still showed at least some systemic accumulation of H2O2, there must be a degree of functional redundancy between the D and F polypeptides, although rbohF had the stronger effect.
We observed no differences in the systemic GST1::LUC response between the majority of plants that subsequently went on to become systemically infected, and the minority that never developed symptoms. Furthermore, unlike the two ethylene-insensitive mutants, we found no obvious difference in susceptibility to CaMV between the rbohDF mutant and wild type. Thus, the early systemic ROS burst does not itself appear to influence the later outcome of the infection. Interestingly, Torres et al. (2002) report that, during infection by incompatible isolates of P. syringae and P. parasitica, the rbohD mutation abolished most of the ROS production but had only a modest effect on PCD, suggesting that the link between ROS generation by NADPH oxidase and defense against incompatible pathogens may also be indirect. Here, we found that a virulent isolate of P. syringae grew significantly better in rbohDF double mutants, linking NADPH oxidase to basal defense against virulent bacteria but not CaMV.
Recently, Gilliland et al. (2003) proposed a role for Aox in regulating ROS accumulation and hence ROS-mediated antivirus defense signaling. We found that antimycin A, which stimulates mitochondrial electron transport through the Aox pathway, was an effective inducer of resistance, implicating this source of local ROS generation with defense against the virus, but only when virus and antimycin A were coinoculated. Inoculation on separate leaves was ineffective, suggesting that there is no systemic signaling involved. Although we did not detect any obvious increase in susceptibility to CaMV in rboh mutants, this does not mean that the response is trivial. A number of plant viruses encode proteins that suppress defense mechanisms (e.g. silencing; Baulcombe, 2002), and preliminary evidence from our laboratory (A.J. Love, J.J. Milner, and J. Laird, unpublished data) suggests that a protein encoded by CaMV can suppress the Aox defense pathway. Defense suppression provides a potential mechanism by which the virus might be able to evade ROS-dependent basal defense responses triggered by PAMP-like domains in the virus capsid.
Only one previous report implicates ethylene signaling in response to a virus, possibly in conjunction with SA, RCY-dependent resistance to CMV strain Y being partially compromised in Arabidopsis etr1 mutants transgenic for NahG (Takahashi et al., 2002). Activation of GST1 and PAL1 by incompatible isolates of P. syringae pv tomato is independent of ethylene signaling in Arabidopsis (Grant et al., 2000), but ethylene perception is reported to be required for H2O2 production in tomato (Lycopersicon esculentum) cells (de Jong et al., 2002). Here, we have found three separate lines of evidence implicating ethylene signaling in responses to CaMV infection. (1) PDF1.2::LUC (a marker for the ethylene/JA defense-signaling pathway; Yun et al., 2003) is up-regulated from about 2 hpi to 5 dpi following inoculation with CaMV, a pattern of activity similar to GST1::LUC. Activation of both markers may therefore involve a common elicitor. In what appears to be a related phenomenon, Kunze et al. (2004) report that application of a bacterial protein stimulated the production of both ROS and ethylene in Arabidopsis leaves. (2) In ein2 and etr1 mutants, virus spread was delayed and virus levels were significantly reduced compared to wild type, direct evidence of a role for ethylene signaling in regulating susceptibility to CaMV. (3) The same mutants failed to show systemic H2O2 accumulation following inoculation, and the virus-dependent increase in GST1 mRNA levels was abolished in etr1 mutants. An intact ethylene perception/signaling pathway is therefore required for the systemic (but not necessarily the local) virus-dependent ROS burst (and the associated up-regulation of GST1). Since up-regulation of GST1 occurred rapidly, apparently triggered by the presence of viral coat proteins in the inoculum, reduced virus replication/spread in the ethylene mutants is unlikely to be a factor in compromised H2O2 accumulation; whether ethylene signaling is required for the generation, propagation, or perception of the systemic signal remains to be determined, although the rapidity of the signaling and its independence from vascular flow make the idea that ethylene itself might be the signal an attractive one. Further evidence of a role for ethylene comes from our recent identification of its involvement in the action of protein P6, the major symptom determinant of CaMV (Geri et al., 2004).
In contrast to PDF1.2 and GST1, markers of SA-mediated defense were activated late in infection, coinciding with the sharp rise in levels of CaMV DNA that accompanied systemic spread of virus. Incompatible pathogens activate SAR rapidly, and increased PR-1 expression is generally detectable within 24 h of inoculation (DeWit, 1997). CaMV stimulated very high levels of PR-1 expression, but not until much later in infection. The second phase of GST1 activity, which shows a pattern similar to PR-1 but is not associated with accumulation of ROS, may be activated by a related signaling pathway.
Several reports indicate that treating plants with SA can inhibit the cell-to-cell movement of compatible viruses (Naylor et al., 1998; Murphy and Carr, 2002; Wong et al., 2002). We did not find this with CaMV. Moreover in NahG, which is unable to accumulate SA, levels of CaMV DNA were not significantly higher than in wild type. Expression of PR-1 in CaMV-infected plants is SA dependent (Laird et al., 2004), but failure to accumulate SA does not lead to enhanced disease susceptibility. Although in NahG plants the accumulation of catechol increases susceptibility to infection (van Wees and Glazebrook, 2003) independently of the effect on SA-mediated defense, this would give rise to a type I rather than a type II error. Carr and coworkers have proposed that resistance to TMV in tobacco acts downstream of SA via the Aox-dependent virus-specific pathway (Chivasa and Carr, 1998; Murphy et al., 2001). We have found that application of antimycin A dramatically reduced the susceptibility of Arabidopsis to infection by CaMV. Here, however, the Aox-dependent pathway may be acting independently of SA signaling.
CONCLUSION
Infection of Arabidopsis by CaMV, a compatible virus, engages a series of defense pathways. These are activated in a manner that is spatially and temporally more complex than hitherto suspected. Within 2 h of inoculation, the RSR is triggered, a component of the virus capsid acting as the elicitor. ROS are generated and ROS- and ethylene-responsive marker genes are transcriptionally up-regulated both locally and in parts of the plant distant from the site of inoculation. Generation of ROS requires components of the NADPH oxidase. Propagation/perception of the systemic signal, which is not of viral origin, requires ethylene signaling. Ethylene also plays a role in regulating susceptibility to CaMV. In contrast, SA-mediated defense is activated late in infection and does not appear to play an effective role in controlling virus susceptibility.
MATERIALS AND METHODS
Virus Infection
CaMV isolate Cabb B-JI (Delseny and Hull, 1983; Al Kaff and Covey, 1995) was maintained and propagated in turnip (Brassica rapa-rapifera cv Just Right) as described (Cecchini et al., 1998). Virus DNA was prepared from purified virus as described (Al Kaff and Covey, 1994). Arabidopsis (Arabidopsis thaliana) plants were grown in compost in a controlled environment at a temperature of 22°C. Light was provided by Osram warm white fluorescent tubes at an intensity of 100 μmol m−2 for 10 h/d. After the emergence of the first true leaves, Arabidopsis seedlings were manually inoculated by pipetting onto one of the true leaves 2 μL of water in which was suspended a small quantity of celite as an abrasive and which contained 0.1 μg purified virus or 0.1 μg of purified CaMV DNA. The leaf was then rubbed gently with a gloved finger or a small glass rod (Cecchini et al., 1998). Controls were mock inoculated with water.
For treatment with SA, a hand-held mister was used to spray ecotype Landsberg erecta (Ler-0) plants with 1 mm SA, 10 mm sodium phosphate, pH 7.0, prior to inoculation. For antimycin A treatment, 50 μm antimycin A and 0.1% DMSO was included in the inoculum in addition to virus. Control plants were inoculated with virus in 0.1% DMSO.
Arabidopsis Mutants and Transgenic Lines
All mutants and transgenic Arabidopsis lines were in a Columbia background. Arabidopsis mutants ein2-1 and etr1-1 were obtained from Nottingham Arabidopsis Stock Centre. rbohD, rbohF, and the double mutant rbohDF, containing T-DNA insertions in the genes encoding the D and F polypeptides of NADPH oxidase (Tissier et al., 1999) were obtained from Professor J.G. Jones (Sainsbury Laboratory). NahG transgenic line B6 was obtained from Novartis. Transgenic reporter lines PR-1::LUC, GST1::LUC and PDF1.2::LUC, in which promoters from the tobacco (Nicotiana tabacum) PR-1a and Arabidopsis GST1 and PDF1.2 genes, respectively, drive expression of a LUC coding sequence have been described (Grant et al., 2000; Murray et al., 2002; Yun et al., 2003).
Assay of CaMV DNA
For each sample, approximately 50 mg of leaf tissue were harvested from three plants and pooled. DNA was purified with a nucleospin kit (CLONTECH), according to the manufacturer's instructions, but with an additional step of digestion with proteinase K (Cecchini et al., 2002). DNA concentration was measured fluorometrically using PicoGreen double-stranded DNA quantitation reagent (Molecular Probes), according to the manufacturer's protocol.
CaMV DNA was assayed by quantitative PCR using a Stratagene MX4000 real-time PCR machine (Stratagene). Reactions were carried out in a volume of 25 μL using Stratagene Brilliant kits, according to the manufacturer's protocol. Primer concentrations were 0.2 μm. Each reaction contained 50 pg of total DNA extracted from infected plants. PCR conditions were 10 min at 95°, then 40 cycles of 30 s at 95°C, 30 s at 55°C, and 1 min at 72°C.
For each sample, duplicate sets of parallel reactions were carried out. Primers AGCGGTCAAAATATTGCTTA complementary to part of ORF VII and AACTTACCGTATGCTAGATTACCT derived from the ORF I region of CaMV Cabb B-JI were used to amplify 141 bp of the CaMV genome. Primers CGTGATCGATGAATGCTACC and GGGGTTTGTTGCACGTATTA were used to amplify 199 bp of the Arabidopsis 18S ribosomal RNA gene. Ct values were determined from SYBR-Green fluorescence using software provided by Stratagene. Regression lines were generated and amplification products quantified using as an external standard a batch of total DNA extracted at 21 dpi from Arabidopsis Col-0 plants infected with CaMV Cabb B-JI. For each sample, the raw values for the quantity of CaMV DNA were normalized to the amount of Arabidopsis DNA present in the sample by dividing them by the values obtained (in parallel) for Arabidopsis 18S rDNA in the same sample. Since the Arabidopsis genome contains a fixed number of copies of the 18S rRNA genes, 18S rDNA provides the most accurate measure of the amount of total DNA present and its inclusion as a standard is essential to correct for dilution errors. Normalized amounts of CaMV DNA are expressed in arbitrary units relative to the level in the external standard.
Preparation of RNA from Tissue
RNA was extracted from approximately 50 mg of tissue as described previously (Cecchini et al., 1997), or using the Sigma Tri-Reagent kit (Sigma-Aldrich), according to the manufacturer's protocol. Total RNA concentration was determined spectrophotometrically. CaMV and Arabidopsis mRNAs were assayed by quantitative hybridization or by quantitative real-time RT-PCR.
Assay of mRNA by Quantitative Hybridization
Approximately 3.0 μg of each sample were applied to Hybond n membrane (Amersham) using a vacuum slot-blot apparatus (Geri et al., 1999). Loading of RNA was confirmed by staining with methylene blue. Hybridization was carried out using 32P-labeled probes as described previously (Cecchini et al., 1997). For CaMV 19S plus 35S RNA, pUC-BJI, which contains the CaMV ORF VI region (Cecchini et al., 1997), was used as probe. Plasmids containing full-length cDNAs for Arabidopsis PR-1 (encoding PR-1-like protein; accession no. M90508), PR-2 (encoding β-glucanase 2; accession no. M58464), and PR-5 (encoding thaumatin-like protein; accession no. AY059114), gifts from Novartis, and a plasmid containing the full-length cDNA for Arabidopsis GST1 (Grant et al., 2000), were used as hybridization probes for the appropriate mRNAs. We have recently shown that cDNA M90508 (corresponding to At2g14910) is the authentic SA-dependent PR-1 gene in Arabidopsis (Laird et al., 2004). Slot blots were developed using a Raytek FLA500 phosphorimager. Images were converted to TIFF files and levels of hybridization determined using Quantiscan for Windows (Cecchini et al., 2002). Values were corrected for differences in loading by quantitating the methylene blue-stained RNA.
Assay of mRNA by Quantitative Real-Time RT-PCR
RNA samples (50 ng) were transcribed to cDNA in 20-μL reactions using Qiagen Sensiscript kits (Qiagen), according to the manufacturer's protocol. cDNA was quantified in the MX4000 real-time PCR machine using Stratagene Brilliant kits as described above for the assay of CaMV DNA, but with minor modifications. Each 25-μL reaction contained 2 μL cDNA. PCR conditions were 10 min at 95°C, then 40 cycles of 30 s at 95°C, 30 s at 59°C, and 1 min at 72°C. Reactions were carried out in duplicate for each biological sample (each comprising cDNA derived from the pooled RNA from seven individual plants). For GST1, primers TAATAAAAGTGGCGATGACC and ACATTCAAATCAAACACTCG were used to amplify 101 bp of the GST1 cDNA sequence. For ACT2, primers CTAAGCTCTCAAGATCAAAGGCTTA and ACTAAAACGCAAAACGAAAGCGGTT were used to amplify 218 bp of the cDNA sequence. ACT2 was chosen as an internal reference because we have previously shown that it is expressed in a wide variety of cell types in Arabidopsis (Laval et al., 2002) and, from microarray data, that expression of ACT2 appears to be broadly constitutive: In particular, levels of ACT2 transcripts are unaffected by CaMV infection (V. Laval, J. Laird, P. Armengaud, and J.J. Milner, unpublished data). To avoid amplification of cDNAs encoded by gene homologs, primers were designed to generate an amplicon from the 3′ noncoding region of both transcripts. Regression lines were generated using, as external standards, DNA from plasmids containing full-length cDNA sequences of ACT2 and GST1 (Grant et al., 2000; Laval et al., 2002). For each sample, values for the concentration of GST1 cDNA were calculated by normalizing the raw values for GST1 using the concentration of ACT2 as an internal reference.
LUC Imaging
Imaging was carried out using an ultra-low-light imaging camera system (EG and G Berthold Luminograph 980 or Photek IFS 532), as described previously (Grant et al., 2000). Plants were lightly painted with 1 mm beetle luciferin (Promega), 0.01% (v/v) Triton X-100, 0.03% (v/v) Silwet (Union Carbide), and 1 mm sodium citrate, pH 5.8. A series of brightfield images were taken, the dark box was closed, and the photon emission captured over the following 4 s. The luminescence image was recorded in pseudocolor and the brightfield images were overlaid.
Staining for H2O2
H2O2 accumulation in planta was visualized by DAB staining (Thordal Christensen et al., 1997). Arabidopsis seedlings or plants were excised at soil level, placed in 50-mL tubes, covered with 0.1% (w/v) DAB, and incubated on an orbital shaker for 18 h. The stain was poured off and chlorophyll removed by boiling in 96% (v/v) ethanol for 10 to 40 min. DAB is rapidly absorbed by the plant and is polymerized locally in the presence of H2O2 and peroxidase giving a visible brown stain.
Bacterial Growth Assays
Seedlings at the two true leaf stages were manually inoculated on a single leaf with CaMV or water, as described above. Six or 24 h later, plants were challenged with Pseudomonas syringae pv maculicola strain ES4326. Fresh bacterial cultures were diluted to 105 colony forming units/mL in 10 mm MgCl2 and plants were inoculated by vacuum infiltration as described by Katagiri et al. (2002). After 64 h, leaves were detached and ground with 1.0 mL of 10 mm MgCl2. Bacterial titers were then determined by plating 50 μL of serial dilutions onto King's Broth agar, according to Glazebrook and Weigel (2002).
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requester.
Acknowledgments
We would like to thank Janet Laird for excellent technical assistance and Dr. C. Geri, Dr. A. Sadanandom, and Dr. S.E. Barnes for helpful discussions. We would also like to thank Novartis and Prof. J.G. Jones for gifts of transgenic lines and Arabidopsis mutants.
This work was supported in part by the Biotechnology and Biological Sciences Research Council (BBSRC; grant nos. 17P/09461, 17P/12855, and 15/P20067 to J.J.M., grant no. 17/P12855 to V.L., and grant no. 15/P20067 to G.J.L.). A.J.L. was the recipient of an Institute of Biological and Life Sciences postgraduate studentship and was supported for the later part of the work by the Bower Fire Insurance Fund.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.066803.
References
- Al Kaff NS, Covey SN (1994) Variation in biological properties of cauliflower mosaic-virus clones. J Gen Virol 75: 3137–3145 [DOI] [PubMed] [Google Scholar]
- Al Kaff NS, Covey SN (1995) Biological diversity of cauliflower mosaic-virus isolates expressed in 2 Brassica species. Plant Pathol 44: 516–526 [Google Scholar]
- Allan AC, Lapidot M, Culver JN, Fluhr R (2001) An early tobacco mosaic virus-induced oxidative burst in tobacco indicates extracellular perception of the virus coat protein. Plant Physiol 126: 97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulcombe D (2002) Viral suppression of systemic silencing. Trends Microbiol 10: 306–308 [DOI] [PubMed] [Google Scholar]
- Bonas U, Lahaye T (2002) Plant disease resistance triggered by pathogen-derived molecules: refined models of specific recognition. Curr Opin Microbiol 5: 44–50 [DOI] [PubMed] [Google Scholar]
- Cecchini E, Al Kaff NS, Bannister A, Giannakou ME, McCallum DG, Maule AJ, Milner JJ, Covey SN (1998) Pathogenic interactions between variants of cauliflower mosaic virus and Arabidopsis thaliana. J Exp Bot 49: 731–737 [Google Scholar]
- Cecchini E, Geri C, Love AJ, Coupland G, Covey SN, Milner JJ (2002) Mutations that delay flowering in Arabidopsis de-couple symptom response from cauliflower mosaic virus accumulation during infection. Mol Plant Pathol 3: 81–90 [DOI] [PubMed] [Google Scholar]
- Cecchini E, Gong ZH, Geri C, Covey SN, Milner JJ (1997) Transgenic Arabidopsis lines expressing gene VI from cauliflower mosaic virus variants exhibit a range of symptom-like phenotypes and accumulate inclusion bodies. Mol Plant Microbe Interact 10: 1094–1101 [DOI] [PubMed] [Google Scholar]
- Chivasa S, Carr JP (1998) Cyanide restores N gene-mediated resistance to tobacco mosaic virus in transgenic tobacco expressing salicylic acid hydroxylase. Plant Cell 10: 1489–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivasa S, Murphy AM, Naylor M, Carr JP (1997) Salicylic acid interferes with tobacco mosaic virus replication via a novel salicylhydroxamic acid-sensitive mechanism. Plant Cell 9: 547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SF, Guy PL, Burritt DJ, Jameson PE (2002) Changes in the activities of antioxidant enzymes in response to virus infection and hormone treatment. Physiol Plant 114: 157–164 [DOI] [PubMed] [Google Scholar]
- Covey SN, Turner DS (1991) Comparison of viral nucleic-acid intermediates at early and late stages of cauliflower mosaic-virus infection suggests a feedback regulatory mechanism. J Gen Virol 72: 2603–2606 [DOI] [PubMed] [Google Scholar]
- Dangl JL, Dietrich RA, Richberg MH (1996) Death don't have no mercy: cell death programs in plant-microbe interactions. Plant Cell 8: 1793–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardick CD, Golem S, Culver JN (2000) Susceptibility and symptom development in Arabidopsis thaliana to tobacco mosaic virus is influenced by virus cell-to-cell movement. Mol Plant Microbe Interact 13: 1139–1144 [DOI] [PubMed] [Google Scholar]
- de Jong AJ, Yakimova ET, Kapchina VM, Woltering EJ (2002) A critical role for ethylene in hydrogen peroxide release during programmed cell death in tomato suspension cells. Planta 214: 537–545 [DOI] [PubMed] [Google Scholar]
- Delladonne M, Zeier J, Marocco A, Lamb C (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA 98: 13454–13459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delseny M, Hull R (1983) Isolation and characterization of faithful and altered clones of the genomes of cauliflower mosaic-virus isolates Cabb B-JI, CM4–184, and Bari-1. Plasmid 9: 31–41 [DOI] [PubMed] [Google Scholar]
- Dempsey DA, Pathirana MS, Wobbe KK, Klessig DF (1997) Identification of an Arabidopsis locus required for resistance to turnip crinkle virus. Plant J 11: 301–311 [DOI] [PubMed] [Google Scholar]
- Desikan R, Hancock JT, Bright J, Harrison J, Weir L, Hooley R, Neill SJ (2005) A role for ETR1 in hydrogen peroxide signaling in stomatal guard cells. Plant Physiol 137: 831–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWit PJGM (1997) Pathogen avirulence and plant resistance: a key role for recognition. Trends Plant Sci 2: 452–458 [Google Scholar]
- Fodor J, Hideg E, Kecskes A, Kiraly Z (2001) In vivo detection of tobacco mosaic virus-induced local and systemic oxidative burst by electron paramagnetic resonance spectroscopy. Plant Cell Physiol 42: 775–779 [DOI] [PubMed] [Google Scholar]
- Geri C, Cecchini E, Giannakou ME, Covey SN, Milner JJ (1999) Altered patterns of gene expression in Arabidopsis elicited by cauliflower mosaic virus (CaMV) infection and by a CaMV gene VI transgene. Mol Plant Microbe Interact 12: 377–384 [DOI] [PubMed] [Google Scholar]
- Geri C, Love AJ, Cecchini E, Barrett SJ, Laird J, Covey SN, Milner JJ (2004) Arabidopsis mutants that suppress the phenotype induced by transgene-mediated expression of cauliflower mosaic virus (CaMV) gene VI are less susceptible to CaMV-infection and show reduced ethylene susceptibility. Plant Mol Biol 56: 111–124 [DOI] [PubMed] [Google Scholar]
- Gilliland A, Singh DP, Hayward JM, Moore CA, Murphy AM, Carr JP (2003) Genetic modification of alternative respiration has differential effects on antimycin A-induced versus salicylic acid-induced resistance to tobacco mosaic virus. Plant Physiol 132: 1518–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J, Rogers EE, Ausubel FM (1996) Isolation of Arabidopsis mutants with enhanced disease susceptibility by direct screening. Genetics 143: 973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J, Weigel D (2002) Arabidopsis: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 77–80
- Grant JJ, Chini A, Basu D, Loake GJ (2003) Targeted activation tagging of the Arabidopsis NBS-LRR gene, ADR1, conveys resistance to virulent pathogens. Mol Plant Microbe Interact 16: 669–680 [DOI] [PubMed] [Google Scholar]
- Grant JJ, Loake GJ (2000) Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol 124: 21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JJ, Yun BW, Loake GJ (2000) Oxidative burst and cognate redox signalling reported by luciferase imaging: identification of a signal network that functions independently of ethylene, SA and Me-JA but is dependent on MAPKK activity. Plant J 24: 569–582 [DOI] [PubMed] [Google Scholar]
- Hancock JT, Desikan R, Clarke A, Hurst RD, Neill SJ (2002) Cell signalling following plant/pathogen interactions involves the generation of reactive oxygen and reactive nitrogen species. Plant Physiol Biochem 40: 611–617 [Google Scholar]
- He P, Chintamanani S, Chen ZY, Zhu LH, Kunkel BN, Alfano JR, Tang XY, Zhou JM (2004) Activation of a COI1-dependent pathway in Arabidopsis by Pseudomonas syringae type III effectors and coronatine. Plant J 37: 589–602 [DOI] [PubMed] [Google Scholar]
- Hohn T, Futterer J (1997) The proteins and functions of plant pararetroviruses: knowns and unknowns. CRC Crit Rev Plant Sci 16: 133–161 [Google Scholar]
- Hunt MD, Ryals JA (1996) Systemic acquired resistance signal transduction. CRC Crit Rev Plant Sci 15: 583–606 [Google Scholar]
- Kachroo P, Yoshioka K, Shah J, Dooner HK, Klessig DF (2000) Resistance to turnip crinkle virus in Arabidopsis is regulated by two host genes and is salicylic acid dependent but NPR1, ethylene, and jasmonate independent. Plant Cell 12: 677–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri F, Thilmony R, He SY (2002) The Arabidopsis thaliana-Pseudomonas syringae interaction. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, pp 2–35 [DOI] [PMC free article] [PubMed]
- Kiraly Z, Barna B, Kecskes A, Fodor J (2002) Down-regulation of antioxidative capacity in a transgenic tobacco which fails to develop acquired resistance to necrotization caused by TMV. Free Radic Res 36: 981–991 [DOI] [PubMed] [Google Scholar]
- Kunze G, Zipfer C, Robatzek S, Niehaus K, Boller T, Felix G (2004) The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 16: 3496–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird J, Armengaud P, Laval V, Giuntini P, Milner JJ (2004) Inappropriate annotation of a key defence marker in Arabidopsis: will the real PR-1 please stand up? Planta 219: 1089–1092 [DOI] [PubMed] [Google Scholar]
- Laval V, Korolova OA, Murphy E, Lu C, Milner JJ, Hooks MA, Tomos AD (2002) Distribution of actin gene isoforms in the Arabidopsis leaf measured in microsamples from intact individual cells. Planta 215: 287–292 [DOI] [PubMed] [Google Scholar]
- Low PS, Merida JR (1996) The oxidative burst in plant defense: function and signal transduction. Physiol Plant 96: 533–542 [Google Scholar]
- Manners JM, Penninckx IAMA, Vermaere K, Kazan K, Brown RL, Morgan A, MacLean DJ, Curtis MD, Cammue BPA, Broekaert WF (1998) The promoter of the plant defensin gene PDF1.2 from Arabidopsis is systemically activated by fungal pathogens and responds to methyl jasmonate but not to salicylic acid. Plant Mol Biol 38: 1071–1080 [DOI] [PubMed] [Google Scholar]
- Murphy AM, Carr JP (2002) Salicylic acid has cell-specific effects on tobacco mosaic virus replication and cell-to-cell movement. Plant Physiol 128: 552–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AM, Chivasa S, Singh DP, Carr JP (1999) Salicylic acid-induced resistance to viruses and other pathogens: a parting of the ways? Trends Plant Sci 4: 155–160 [DOI] [PubMed] [Google Scholar]
- Murphy AM, Gilliland A, Eng Wong C, West J, Singh DP, Carr JP (2001) Signal transduction in resistance to plant viruses. Eur J Plant Pathol 107: 121–128 [Google Scholar]
- Murray SL, Thomson C, Chini A, Read ND, Loake GJ (2002) Characterization of a novel, defense-related Arabidopsis mutant, cir1, isolated by luciferase imaging. Mol Plant Microbe Interact 15: 557–566 [DOI] [PubMed] [Google Scholar]
- Naylor M, Murphy AM, Berry JO, Carr JP (1998) Salicylic acid can induce resistance to plant virus movement. Mol Plant Microbe Interact 11: 860–868 [Google Scholar]
- Overmyer K, Brosche M, Kangasjarvi J (2003) Reactive oxygen species and hormonal control of cell death. Trends Plant Sci 8: 335–342 [DOI] [PubMed] [Google Scholar]
- Penninckx IAMA, Thomma BPHJ, Buchala A, Metraux JP, Broekaert WF (1998) Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 10: 2103–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rairdan GJ, Delaney TP (2002) Role of salicylic acid and NIM1/NPR1 in race-specific resistance in Arabidopsis. Genetics 161: 803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentel MC, Lecourieux D, Ouaked F, Usher SL, Petersen L, Okamoto H, Knight H, Peck SC, Grierson CS, Hirt H, et al (2004) OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 427: 858–861 [DOI] [PubMed] [Google Scholar]
- Sagi M, Fluhr R (2001) Superoxide production by plant homologues of the gp91(phox) NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol 126: 1281–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Miller J, Nozaki Y, Takeda M, Shah J, Hase S, Ikegami M, Ehara Y, Dinesh-Kumar SP (2002) RCY1, an Arabidopsis thaliana RPP8/HRT family resistance gene, conferring resistance to cucumber mosaic virus requires salicylic acid, ethylene and a novel signal transduction mechanism. Plant J 32: 655–667 [DOI] [PubMed] [Google Scholar]
- Thordal Christensen H, Zhang ZG, Wei YD, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11: 1187–1194 [Google Scholar]
- Tissier AF, Marillonnet S, Klimyuk V, Patel K, Torres MA, Murphy G, Jones JDG (1999) Multiple independent defective suppressor-mutator transposon insertions in Arabidopsis: a tool for functional genomics. Plant Cell 11: 1841–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JDG (2002) Arabidopsis gp91(phox) homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Onouchi H, Hamada S, Machida C, Hammond-Kosack KE, Jones JDG (1998) Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91(phox)). Plant J 14: 365–370 [DOI] [PubMed] [Google Scholar]
- Turner DS, Covey SN (1993) Reverse transcription products generated by defective plus-strand synthesis during cauliflower mosaic virus replication. Virus Res 28: 171–185 [Google Scholar]
- van Wees SC, Glazebrook J (2003) Loss of non-host resistance of Arabidopsis NahG to Pseudomonas syringae pv phaseolicola is due to degradation products of salicylic acid. Plant J 33: 733–742 [DOI] [PubMed] [Google Scholar]
- Volko SM, Boller T, Ausubel FM (1998) Isolation of new Arabidopsis mutants with enhanced disease susceptibility to Pseudomonas syringae by direct screening. Genetics 149: 537–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham SA, Quan S, Chang HS, Cooper B, Estes B, Zhu T, Wang X, Hou YM (2003) Diverse RNA viruses elicit the expression of common sets of genes in susceptible Arabidopsis thaliana plants. Plant J 33: 271–283 [DOI] [PubMed] [Google Scholar]
- Wong CE, Carson RAJ, Carr JP (2002) Chemically induced virus resistance in Arabidopsis thaliana is independent of pathogenesis-related protein expression and the NPR1 gene. Mol Plant Microbe Interact 15: 75–81 [DOI] [PubMed] [Google Scholar]
- Yao N, Tada Y, Sakamoto M, Nakayashiki H, Park P, Tosa Y, Mayama S (2002) Mitochondrial oxidative burst involved in apoptotic response in oats. Plant J 30: 567–579 [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JDG, Doke N (2003) Nicotiana benthamiana gp91(phox) homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 15: 706–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun BW, Atkinson HA, Gaborit C, Greenland A, Read ND, Pallas JA, Loake GJ (2003) Loss of actin cytoskeletal function and EDS1 activity, in combination, severely compromises non-host resistance in Arabidopsis against wheat powdery mildew. Plant J 34: 768–777 [DOI] [PubMed] [Google Scholar]