Abstract
The temperature response of net CO2 assimilation rate (A), the rate of whole-chain electron transport, the activity and activation state of Rubisco, and the pool sizes of ribulose-1,5-bisphosphate (RuBP) and 3-phosphoglyceric acid (PGA) were assessed in sweet potato (Ipomoea batatas) grown under greenhouse conditions. Above the thermal optimum of photosynthesis, the activation state of Rubisco declined with increasing temperature. Doubling CO2 above 370 μbar further reduced the activation state, while reducing CO2 by one-half increased it. At cool temperature (<16°C), the activation state of Rubisco declined at CO2 levels where photosynthesis was unaffected by a 90% reduction in O2 content. Reduction of the partial pressure of CO2 at cool temperature also enhanced the activation state of Rubisco. The rate of electron transport showed a pronounced temperature response with the same temperature optimum as A at elevated CO2. RuBP pool size and the RuBP-to-PGA ratio declined with increasing temperature. Increasing CO2 also reduced the RuBP pool size. These results are consistent with the hypothesis that the reduction in the activation state of Rubisco at high and low temperature is a regulated response to a limitation in one of the processes contributing to the rate of RuBP regeneration. To further evaluate this possibility, we used measured estimates of Rubisco capacity, electron transport capacity, and the inorganic phosphate regeneration capacity to model the response of A to temperature. At elevated CO2, the activation state of Rubisco declined at high temperatures where electron transport capacity was predicted to be limiting, and at cooler temperatures where the inorganic phosphate regeneration capacity was limiting. At low CO2, where Rubisco capacity was predicted to limit photosynthesis, full activation of Rubisco was observed at all measurement temperatures.
In C3 plants exposed to optimal temperature and saturating light, the rate of net CO2 assimilation (A) at CO2 levels below the current ambient partial pressure of 370 μbar is typically limited by the capacity of Rubisco; by contrast, above 370 μbar, one of the processes contributing to the ribulose-1,5-bisphosphate (RuBP) regeneration capacity typically limits A (von Caemmerer, 2000). At temperatures below the thermal optimum, the capacity for RuBP regeneration often becomes limiting for photosynthesis, in particular, the component of RuBP regeneration associated with the regeneration of inorganic phosphate (Pi) during starch and Suc synthesis (Sharkey, 1985; Sage and Sharkey, 1987; Savitch et al., 1997; Hendrickson et al., 2004). Above the thermal optimum, the situation is less clear. Analyses of the CO2 response of photosynthesis (the A/intercellular CO2 partial pressure [Ci] curve) in a number of species indicate that Rubisco capacity is limiting for A above the thermal optimum, even at elevated CO2 levels (von Caemmerer and Farquhar, 1981; Sage and Sharkey, 1987; Sage et al., 1990a; Salvucci and Crafts-Brandner, 2004a). The increased control of Rubisco at higher temperature occurs in part because CO2 solubility and Rubisco specificity for CO2 decline (Jordan and Ogren, 1984). In addition, the activation state of Rubisco decreases at high temperature, possibly to the extent that Rubisco capacity in vivo becomes limiting for photosynthesis (Weis, 1981b; Kobza and Edwards, 1987; Crafts-Brandner et al., 1997; Vu et al., 1997; Feller et al., 1998; Salvucci et al., 2001; Haldimann and Feller, 2004; Salvucci and Crafts-Brandner, 2004a, 2004b, 2004c). The reduction in the activation state of Rubisco at elevated temperature is proposed to occur because the rate of inhibitor formation via misprotonation events increases and the activity of Rubisco activase declines (Portis, 2003; Salvucci and Crafts-Brandner, 2004a, 2004b, 2004c; Kim and Portis, 2005). Loss of Rubisco activase activity appears to occur in two stages (Crafts-Brandner et al., 1997; Salvucci et al., 2001; Portis, 2003; Salvucci and Crafts-Brandner, 2004a, 2004b). At temperatures just above the thermal optimum of A (typically 30°C to 40°C), activase complexes dissociate, forming inactive aggregates of one to two subunits from active aggregates of up to 16 subunits. This phase is reversible upon cooling (Crafts-Brandner and Law, 2000). Above about 42°C, activase subunits denature and aggregate into large, insoluble complexes. Formation of insoluble complexes is not readily reversible, and, as a result, A remains low upon cooling (Crafts-Brandner and Law, 2000).
The thermal optimum of photosynthesis at elevated CO2 has also been reported to correspond to the thermal optimum of electron transport in vitro, indicating that the reduction in photosynthesis above the thermal optimum may be related to increased control by RuBP regeneration capacity, rather than Rubisco limitations caused by misprotonation or a loss of activase activity (Sage et al., 1995; June et al., 2004; Schrader et al., 2004; Wise et al., 2004; Sharkey, 2005). At elevated temperature, protons leak through the thylakoid membrane at a higher rate, indicating the coupling of ATP synthesis to electron transport is impaired (Pastenes and Horton, 1996; Bukhov et al., 1999; Sharkey, 2005). Additionally, RuBP pools can decline after rapid increases in temperature, possibly in response to heat-induced reductions in the redox potential of the chloroplast (Oja et al., 1988; Schrader et al., 2004; Sharkey, 2005).
At moderate temperature, the reduction in the activation state of Rubisco is a regulated response to limitations in one of the processes contributing to RuBP regeneration capacity (Mott et al., 1984; Sage et al., 1988, 1990b, 1993; Portis, 2003). As the RuBP regeneration capacity becomes limiting, ATP:ADP levels and the redox potential of the chloroplast decline, causing a loss of activase activity and, in turn, a reduction in the activation state of Rubisco (Price et al., 1998; Zhang and Portis, 1999; Ruuska et al., 2000; Spreitzer and Salvucci, 2002). Based on this understanding, the decline in the activation state of Rubisco at elevated temperature may also be a regulated response to a limitation in electron transport capacity rather than a consequence of a direct effect of heat on the integrity of Rubisco activase. Because reductions in the activation state of Rubisco are associated with the two leading mechanisms proposed for photosynthetic control at elevated temperature, understanding the response of the Rubisco activation state to temperature variation should improve our understanding of the temperature response of photosynthesis. If Rubisco activation becomes the predominant limitation at supraoptimal temperature, then traits that stabilize the activity of Rubisco activase would be valuable to introduce into crop plants. Furthermore, in natural populations, stabilization of Rubisco activase could be an important adaptation to warmer environments (Salvucci and Crafts-Brandner, 2004a) and thus may be a point of natural selection as the global climate warms. Alternatively, if RuBP regeneration is the primary limitation at elevated temperature, then efforts to improve Rubisco activase activity would have minimal impact on photosynthetic performance.
One method to evaluate the limiting role of Rubisco activation versus RuBP regeneration capacity is to vary ambient CO2 levels. Variation in atmospheric CO2 affects the ratio of the RuBP regeneration capacity to the capacity of Rubisco to consume RuBP (RRcap/RCcap), which is a useful index of the energy supply in the leaf cell relative to energy demand. Where RRcap/RCcap is greater than one, there is an abundance of available ATP and reducing power to support high activase activity and maximum activation of Rubisco (Sage et al., 1990b). In most C3 species, the maximum activation state of Rubisco is approximately 90% (Sage, 1990). When the capacity for RuBP regeneration falls below the capacity of Rubisco to consume RuBP (RRcap/RCcap < 1), Rubisco becomes nonlimiting and deactivates. For example, in Chenopodium album and Phaseolus vulgaris, the activation state of Rubisco declined in shaded conditions where RRcap/RCcap was modeled to be less than 1 (Sage et al., 1990b). Subsequent reductions in ambient CO2 around the shaded leaves increased RRcap/RCcap and reactivated Rubisco in both species (Sage et al., 1990b). If elevated temperature also reduces the RuBP regeneration capacity relative to the RuBP consumption capacity of Rubisco, then the activation state of Rubisco should be sensitive to CO2 variation; specifically, it should be possible to reactivate Rubisco at elevated temperatures by lowering CO2 availability. Some evidence from tobacco (Nicotiana tabacum) and cotton (Gossypium hirsutum) supports this possibility (Crafts-Brandner and Salvucci, 2000). By contrast, if the activation state of Rubisco at elevated temperature is reduced because Rubisco activase is heat impaired, then CO2 reduction should have little effect or may promote further declines in the activation state of Rubisco.
In this study, we have examined the temperature response of photosynthesis, Rubisco activity and activation state, electron transport capacity, and the key metabolites RuBP and 3-phosphoglyceric acid (PGA) in sweet potato (Ipomoea batatas). Sweet potato was selected because it performs well at temperatures above 30°C (Lorenz and Maynard, 1988), and therefore was assumed to be less sensitive to heat at 35°C to 40°C than many C3 crops, such as wheat (Triticum aestivum), soybean (Glycine max), or spinach (Spinacia oleracea).
RESULTS
Light Responses of Net CO2 Assimilation
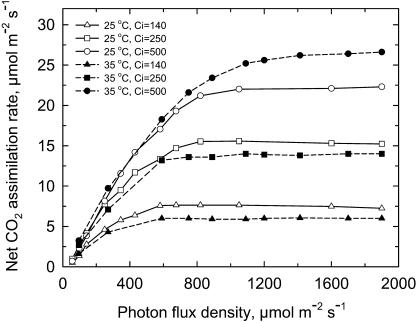
The light saturation point of the net CO2 assimilation rate (A) increased with measurement Ci (Fig. 1). At a Ci of 140 μbar, A was saturated at 600 μmol photons m−2 s−1, while at 500 μbar Ci, 1,000 μmol photons m−2 s−1 were required to saturate A. Increasing temperature from 25°C to 35°C increased the light requirement for saturation by 10% at a Ci of 250 and 500 μbar. Based on these measurements, we conducted our temperature and CO2 response measurements at a light intensity of 1,000 to 1,100 μmol m−2 s−1.
Figure 1.
The light responses of A of sweet potato at leaf temperature of 25°C (solid lines) with Ci of 140 (white triangle), 250 (white square), and 500 (white circle) μbar; or at leaf temperature of 35°C (dashed lines) with Ci of 140 (black triangle), 250 (black square), and 500 (black circle) μbar.
Rubisco Activity, Electron Transport Rate, and Metabolite Pools
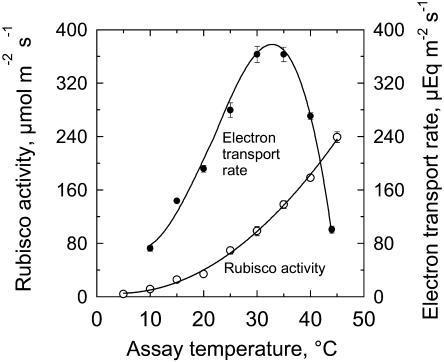
The CO2-saturated rate of Rubisco activity in vitro rose exponentially from 5°C to 45°C (Fig. 2). The lack of an optimum in the response of Rubisco activity to temperature showed Rubisco was not impaired by high temperature up to 45°C. Rubisco is known to be stable above 50°C (Salvucci and Crafts-Brandner, 2004a, 2004b, 2004c). Arrhenius plots indicated there was a thermal break in the response of Rubisco activity to temperature at about 17°C (data not shown). Between 15°C and 40°C, the Q10 was 2.2 and the activation energy was 62.5 kJ mol−1. Between 5°C and 15°C, the Q10 was 7.0 and the activation energy was 130 kJ mol−1.
Figure 2.
The temperature responses of fully activated Rubisco activity (white circles) and electron transport rate (black circles) in sweet potato leaves. Mean ± se, n = 4 per point.
In contrast to the Rubisco response, the rate of whole-chain electron transport increased 5-fold between 10°C and an optimum at about 33°C (Fig. 2). The rate fell off sharply above the optimum, dropping 50% from 35°C to 40°C. Between 10°C and 30°C, the Q10 for electron transport was 2.4.
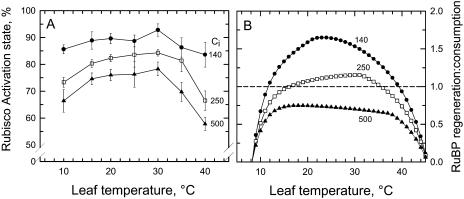
The activation state of Rubisco at an intercellular partial pressure of 250 μbar CO2 (the intercellular CO2 value corresponding to the current ambient of 370 μbar) increased from near 73% at 10°C to a peak value of 83% at 30°C (Fig. 3A). Above 30°C, the activation state of Rubisco declined, most notably above 35°C, where it fell from approximately 80% at 35°C to near 65% at 40°C. Increasing the intercellular CO2 value to 500 μbar reduced the activation state of Rubisco by about six to eight percentage points at all measurement temperatures. The pattern of response at high Ci was the same as at a Ci of 250 μbar. Reducing Ci to 140 μbar increased the activation state of Rubisco at all temperatures, with the degree of enhancement being greatest at the lowest and highest temperatures examined. From 10°C to 35°C, temperature variation had a negligible effect on the activation state of Rubisco at a Ci of 140 μbar. Over this range, the activation state was 85% to 90%, some six to 12 percentage points greater than at a Ci of 250 μbar. Above 35°C, the activation state of Rubisco declined slightly, approaching 84% at 40°C. This value at 40°C was almost 20 percentage points greater than observed at a Ci of 250 μbar.
Figure 3.
A, The temperature response of the Rubisco activation state in sweet potato leaves measured at the indicated Ci of 140 (black circle), 250 (white square), and 500 (black triangle) μbar. Mean ± se, n = 4. In B, the modeled temperature response of the capacity for RuBP regeneration to the capacity of RuBP consumption by Rubisco is shown for the three CO2 levels at which the activation state measurements were conducted. The ratio of the RuBP regeneration capacity to the RuBP consumption capacity was modeled according to Sage (1990) with input parameters as described in the Supplemental Appendix. In determining the RuBP consumption capacity, the maximum capacity of Rubisco was multiplied by 0.9 to account for the observation that the maximum activation state of Rubisco in sweet potato is 90%.
The theoretical model of Farquhar et al. (1980) as modified by Farquhar and Wong (1984) and Sage (1990) was used to predict the ratio of RuBP regeneration capacity to the capacity of Rubisco to consume RuBP (RRcap/RCcap), using inputs derived from the in vitro measurements of Rubisco Vcmax and electron transport (Fig. 3B). At a Ci of 140 μbar, RRcap/RCcap exceeded 1.0 between 12°C and 38°C, but declined sharply below 1.0 at temperatures less than 12°C and greater than 38°C. At a Ci of 250 μbar, RRcap/RCcap was near 1.0 between 16°C and 35°C, and declined sharply below 1.0 outside this temperature range. At a Ci of 500 μbar, RRcap/RCcap never reached a value of 1.0, but instead exhibited a plateau near 0.7 between 14°C and 36°C. RRcap/RCcap declined sharply with cooling below 14°C and heating above 38°C at 500 μbar. At Ci values of 250 and 500 μbar, the reduction in RRcap/RCcap at the two temperature extremes corresponded to marked declines in the activation state of Rubisco (Fig. 3A).
Mean RuBP pools sizes declined with increasing temperatures from 44 to 49 μmol RuBP m−2 at 10°C, to 33 to 36 μmol m−2 at 40°C (Fig. 4A). RuBP pools generally decreased with increasing measurement CO2. On average, the RuBP pool size was 2 μmol m−2 greater at a Ci of 140 than 250 μbar, and 4 μmol m−2 greater at 140 than 500 μbar. PGA pool sizes increased with increasing Ci but were insensitive to temperature variation except above 35°C in the higher CO2 treatments (Fig. 4B). Between 25°C to 40°C at 250 and 500 μbar, the PGA pool size increased about 6% to 8%. RUBP:PGA pool sizes declined slightly with increasing Ci and temperature (Fig. 4C).
Figure 4.
The temperature response of the pool sizes of RuBP (A) and PGA (B), and the RuBP to PGA ratio (C) in sweet potato leaves measured at Ci of 140 (black circle), 250 (white circle) and 500 (black triangle) μbar. Mean ± se, n = 4. The mean Rubisco content in sweet potato was determined to be 22.6 μmol catalytic sites m−2, which is equivalent to 1.6 g Rubisco m−2.
The CO2 Response of the Net CO2 Assimilation Rate
The response of A to Ci showed that temperature increased the CO2-saturated rate of A more than 5-fold between 5°C and 35°C (Fig. 5A), while the initial slope of the A/Ci response (IS) increased two to three times between 10°C and 35°C (Fig. 6). At a Ci of 140 μbar, A corresponded to the IS at all measurement temperatures (Fig. 5A). At a Ci of 250 μbar, A corresponded to the CO2-saturated plateau below 15°C and the IS at 25°C and above. At elevated Ci above 500 μbar, measured A was close to (at 34°C and 38°C) or above (at 11°C to 25°C) the CO2 saturation point (Fig. 5A).
Figure 5.
The rate of net CO2 assimilation in sweet potato leaves as a function of Ci. Data in A were obtained in O2 partial pressure of 200 mbar and at the indicated leaf temperatures. The data in B were obtained in O2 partial pressures of 200 mbar (solid lines) at the indicated leaf temperatures, or in an O2 partial pressure of 30 mbar (dashed lines) at the indicated leaf temperatures. A and B are derived from independent measurements of different sweet potato leaves.
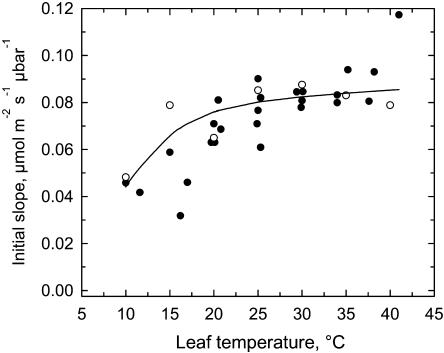
Figure 6.
The temperature response of the IS in sweet potato. Black circles are measured data from A versus CO2 responses. Each point represents the IS of a single A versus Ci response, calculated by fitting a least-squares linear regression through at least two (and usually three to five) gas exchange data points. The open circles are estimated IS calculated according to the equation IS = Vcmax/(Γ* + Kc(1 + O/Ko) from Farquhar and von Caemmerer (1982), using mean Rubisco activities in Figure 2, the kinetic equations in the Supplemental Appendix, and the measured activation state at 140 μbar Ci in Figure 3A. The solid line is the modeled response for the IS using the regression of measured Rubisco Vcmax versus temperature (Eq. A2 in Supplemental Appendix) and the kinetic responses given in the Supplemental Appendix.
When measurements of the A/Ci response at 200 and 30 mbar O2 were compared, there was little effect of O2 variation on A at 10°C, even at 140 μbar (Fig. 5B). At 25°C, A became insensitive to O2 reduction above a Ci of 500 μbar. At 31°C, A became insensitive to O2 reduction at 700 μbar. Below 500 μbar, the rate of A at 31°C and an O2 level of 30 mbar diverged from that measured at an O2 level of 200 mbar, revealing the effect of increasing photorespiration as Ci decreased. We used the O2- and CO2-insensitive values of A to estimate the triose phosphate use rate for our modeled simulations (data at temperatures other than in Fig. 5B are not shown). The appearance of O2 insensitivity of A is a symptom of a Pi regeneration limitation (Sharkey 1985).
The IS increased with increasing temperature up to 25°C, and responded little to temperatures between 25°C and 40°C (Fig. 6, black symbols). The temperature response of IS was also modeled using the individual means of the in vitro Rubisco Vcmax measurements (Fig. 6, white symbols) and the modeled Vcmax values from the regression of the measured Vcmax versus temperature (Fig. 6, solid line). IS values derived from the in vitro Vcmax estimates were similar to the measured IS values, with close correspondence between predicted values and measured values occurring above 25°C.
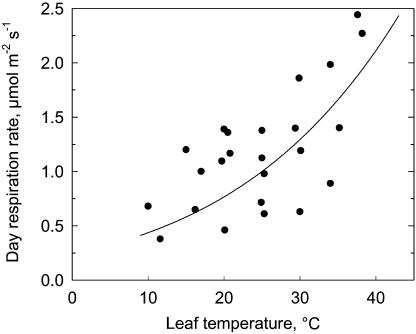
Day respiration increased with temperature such that the rate at 40°C was approximately 5-fold greater than at 10°C (Fig. 7). The Q10 for respiration was 1.7, with no evidence of a break in the Ahrrenius response.
Figure 7.
The temperature response of day respiration rate in sweet potato leaves. Data were calculated using the IS and the CO2 compensation point in the absence of nonphotorespiratory CO2 evolution (Brooks and Farquhar, 1985). The solid line is the modeled response of Rd according to Harley et al. (1985; see the Supplemental Appendix for equation).
Measured and Modeled Responses of Net CO2 Assimilation Rate to Temperature
Rising temperature stimulated A in sweet potato up to a thermal optima of 20°C to 25°C at a Ci of 140 μbar, 25°C to 30°C at 250 μbar, and 30°C to 35°C at 500 μbar (Fig. 8). Increasing CO2 enhanced the sensitivity of A to increasing temperature. At a Ci of 140 μbar, the temperature response of A was relatively flat, while it was pronounced at 500 μbar, with a well defined optimum. At saturating CO2, the Q10 for the response of A to temperature between 10°C and 30°C was 1.7. Rising CO2 had little effect on A at 10°C, a slight effect between 140 and 250 μbar at 17°C, and a pronounced effect at the thermal optimum and above.
Figure 8.
The temperature response of A in sweet potato leaves measured at the Ci of 140 (black circle), 250 (white circle), and 500 (black triangle) μbar; or modeled from fully activated Rubisco activities from Figure 2 modified to account for the Rubisco activation state from Figure 3A at Ci of 140, 250, and 500 μbar (diamonds). In A, the solid lines are theoretical temperature responses of A modeled for conditions assuming the Rubisco capacity is limiting. In B, the dashed lines are modeled temperature responses of A under conditions of an electron transport limitation; the dotted line is the modeled temperature response of A assuming the capacity for Pi regeneration is limiting A. Modeled data were calculated as described in the Supplemental Appendix.
Modeled estimates of A, assuming the capacity of fully activated Rubisco was limiting, were greater than measured values of A at all temperatures at 500 μbar, and above the thermal optimum at 250 and 140 μbar (Fig. 8A). When the Vcmax value in the model was adjusted to account for the measured activation states of Rubisco at each temperature and CO2 level, there was close agreement between measured and modeled values of A, except below 36°C at 250 μbar, where the modeled estimates were slightly less than measured estimates. When we modeled A assuming the capacity for RuBP regeneration was limiting, we had to set up two limitation scenarios, one for a limitation in the maximum rate of electron transport (Jmax), which we measured, and one for a limitation in the capacity of starch and Suc synthesis to regenerate Pi (using Pi regeneration estimates derived from CO2- and O2-saturated A according to Sharkey [1985]). When the Pi regeneration capacity was assumed to be limiting, there was good agreement between measured and modeled values of A at and below the thermal optimum at a Ci of 500 μbar, below 15°C at 250 μbar Ci, and at 10°C at 140 μbar (Fig. 8B). When we modeled A, assuming the measured Jmax values in Figure 2 were limiting, the modeled A was greater than measured A at all temperatures at a Ci of 140 μbar and at all temperatures below 36°C at Ci of 250 μbar. At a Ci of 500 μbar, the measured and modeled values of A corresponded closely above the thermal optimum (Fig. 8B).
DISCUSSION
In numerous plant species, the activity of Rubisco activase declines as temperatures increase above the thermal optimum of photosynthesis (Salvucci and Crafts-Brandner, 2004a, 2000c; Kim and Portis, 2005). This loss of activity causes Rubisco deactivation, which in turn is proposed to reduce photosynthetic capacity at elevated temperature (Feller et al., 1998; Salvucci and Crafts-Brandner, 2004a). Evidence supporting a role for activase lability as a cause of photosynthetic decline are (1) a parallel decline in activase activity and the activation state of Rubisco as temperatures increase above 35°C (Kobza and Edwards, 1987; Feller et al., 1998); (2) a close correlation between activation state reduction and photosynthetic decline (Law and Crafts-Brandner, 1999; Crafts-Brandner and Salvucci, 2000; Haldimann and Feller, 2004); (3) a rise in RuBP:PGA ratios as temperatures rise above the thermal optimum of A (Kobza and Edwards, 1987; Law and Crafts-Brandner, 1999; Crafts-Brandner and Law, 2000); (4) stimulation of A by increasing CO2 under nonphotorespiratory conditions (2% O2; Crafts-Brandner and Salvucci, 2000); and (5) evidence of a regulatory feedback on PSII at elevated temperature (Feller et al., 1998; Law and Crafts-Brandner, 1999; Salvucci and Crafts-Brandner, 2004c).
In contrast to these results, there was little evidence that the Rubisco deactivation limited net CO2 assimilation at elevated temperature in sweet potato. This conclusion is supported by numerous lines of evidence. First, the activation state of Rubisco was manipulated by altering CO2 levels in a manner that is consistent with changes in the ratio of the capacity for RuBP regeneration relative to the capacity of Rubisco to consume RuBP. Reducing CO2 to 140 μbar Ci increased the modeled estimate of RRcap/RCcap, with the most pronounced increase upon CO2 reduction occurring between 10°C and 15°C, and 35°C and 40°C. At these respective temperature ranges, CO2 reduction increased RRcap/RCcap from well below 1.0 to above 1.0, indicating an increase in the energy reserves required for high activase activity and, in turn, a high activation state of Rubisco. Consistently, the observed activation state of Rubisco showed its greatest response to CO2 reduction at these thermal extremes where the CO2 effect on RRcap/RCcap was greatest. Second, the thermal optimum of photosynthesis at elevated CO2 corresponded to the thermal optimum of electron transport (compare Figs. 2 and 8). We did not determine the thermal optimum of Rubisco activase, but it has been reported to be near 42°C in tobacco, a species that is also adapted to warm conditions (Crafts-Brandner and Salvucci, 2000). Third, the pool size of RuBP declined, while PGA levels rose above the thermal optimum. This pattern is consistent with the limitation on A caused by limitations in the RuBP regeneration capacity. By contrast, in wheat and cotton, increasing temperature above the thermal optimum was associated with an increased RuBP pool, which is consistent with a constriction at Rubisco that is proposed to result from heat-induced lability of Rubisco activase (Kobza and Edwards, 1987; Law and Crafts-Brandner, 1999). Fourth, the IS at light saturation is widely regarded to reflect a Rubisco limitation on photosynthesis (von Caemmerer and Farquhar, 1981; Wullschleger, 1993). Anything that reduces the activation state of Rubisco, such as heat-induced impairment of activase function, should reduce Vcmax in vivo and, in turn, the measured IS value. This was not observed in sweet potato. If the activation state of Rubisco was reduced at low CO2, it would not have been possible to predict the IS with the Farquhar and von Caemmerer (1982) model unless deactivation algorithms were used. When we used a maximum activation state of 90% to correct in vitro Vcmax to in vivo Vcmax, there was agreement between the measured IS versus temperature response and the modeled IS versus temperature response derived from Vcmax determinations (Fig. 3B). No deactivation of Rubisco with temperature variation was assumed in our model. The closest agreement was observed at the warmer temperatures where activase is proposed to be heat labile. This is good evidence that Rubisco capacity in vivo has not been impaired by elevated temperature, as should be the case if activase was losing stability. By contrast, in spinach, where there is substantial evidence that activase lability impairs A at elevated temperature (Salvucci and Crafts-Brandner, 2004a), IS exhibits reductions at elevated temperature that are greater than indicated by theoretical assessments assuming constant Rubisco activation state (Yamori et al., 2005).
Modeled assessments are often used to interpret the biochemical limitations on A, with the Farquhar et al. (1980) family of models being the most commonly used. Certain cautions have to be employed when using the models, however. For example, the kinetic constants used for Rubisco have a strong influence upon the output. Recently, it has become popular to estimate Jmax using fluorescence approaches (e.g. June et al., 2004). This approach requires the critical assumption that Jmax is limiting at high CO2 and low O2, which is not the case if the Pi regeneration capacity is limiting at suboptimal temperature or if Rubisco activase function is limiting at superoptimal temperatures. If Jmax is not limiting, then the limiting process will feedback onto PSII efficiency and give a low estimate for Jmax. Because of these limitations, we used direct assessments of Jmax (as in von Caemmerer and Farquhar, 1981) and gas exchange assessments of Pi regeneration capacity (as in Harley and Sharkey, 1991) to estimate RuBP regeneration capacity. To get around a problem associated with a lack of kinetic constants for sweet potato, we pooled published Rubisco constants from spinach, which provided a close match for our low CO2 response of A, and then used these parameters for all other modeling simulations. While this approach has limitations, the output of our model simulations are consistent with the hypothesis that deactivation of Rubisco at high and low temperatures is a response to limitations in RuBP regeneration capacity. When the measured values of electron transport were used to model A in sweet potato, there was good agreement between measured and modeled values of A at elevated temperature in the two higher CO2 treatments. When we modeled A using the measured values of Rubisco activity in vitro that were adjusted to account for the measured activation state, we also had good agreement between measured and predicted values of A at elevated temperature. Since the Rubisco activation state should not affect the in vitro electron transport rate, this indicates the activation state is regulated to match a limitation in electron transport capacity. In turn, at low temperature, the Pi regeneration capacity is predicted to impose a limitation on A at 500 μbar Ci, and, consistently, there is a decline in the activation state of Rubisco at low temperature when the Pi regeneration limitation is most pronounced.
The mechanism by which Rubisco deactivation is linked to a reduction in the ratio of the RuBP regeneration to consumption capacity is well understood at moderate temperatures. At the temperature optimum, reductions in ATP:ADP and redox potential in the chloroplast brought about by low light or high CO2 reduce the activity of Rubisco activase (von Caemmerer and Quick, 2000; Portis, 2003). This decline in ATP:ADP and redox potential corresponds to a reduction in the ratio of the RuBP regeneration to consumption capacities (Sage, 1990; Ruuska et al., 2000). Above the thermal optimum of photosynthesis, similar patterns should occur if the RuBP regeneration capacity is reduced by a reduction in electron transport. At 35°C to 40°C, a reduction in the activity of activase is attributed to the disassociation of functional oligomers into nonfunctional monomeric or dimeric complexes, or by disruption of the physical interactions between activase and Rubisco (Crafts-Brandner et al., 1997; Salvucci and Crafts-Brandner, 2004a). Above 40°C, activase denatures to form insoluble aggregates (Salvucci et al., 2001). Maintenance of activase function in warm conditions is associated with elevated ATP:ADP levels, possibly through stabilization of oligomer structure (Portis, 2003). Higher ATP levels also increase the temperature at which activase denatures by up to 8°C (Salvucci et al., 2001). However, chloroplast ATP:ADP ratios are not known to decline at elevated temperature (Weis, 1981a; Portis, 2003; Schrader et al., 2004). This may occur because cyclic photophosphorylation increases at elevated temperature and is therefore able to maintain high ATP to ADP ratios (Schrader et al., 2004). In this case, linear electron transport is inhibited and the redox state may decline, potentially causing declines in activase function. Sensitivity of activase to redox status is conferred by the larger, 45- to 47-kD isoform of the activase protein (Zhang and Portis, 1999; Law et al., 2001; Zhang et al., 2002). The larger isoform is also more stable at elevated temperature, and its expression increases in plants exposed to heat (Law and Crafts-Brandner, 2001; Salvucci and Crafts-Brandner, 2004a). Because sweet potato is a warm-adapted crop, it is logical to hypothesize that it is enriched in the larger isoform, which would then increase heat stability yet enhance the sensitivity of activase to redox status.
In addition to reduction in activase activity, Rubisco deactivation at elevated temperature is proposed to occur because of an increased rate of misprotonation events (Portis, 2003; Salvucci and Crafts-Brandner, 2004b). Misprotonation occurs prior to CO2 addition (Roy and Andrews, 2000) and therefore should be greater at low CO2 since the rate of CO2 entry into the active site of Rubisco would be slower in CO2-deficient conditions. A reduced rate of CO2 entry should provide more time for misprotonation events to occur. The ability of the sweet potato leaves to fully reactivate Rubisco in low CO2 conditions indicates accelerated misprotonation at elevated temperature is not a serious limitation on the rate of CO2 assimilation above the thermal optimum.
As in prior studies (Law and Crafts-Brandner, 2001; Haldimann and Feller, 2004), we observed a close association between measured A and predicted A when Vcmax was modified to account for deactivation of Rubisco. At a Ci of 140 μbar, our model predicted that Rubisco is the predominant limitation on A over much of the thermal range. In this situation, the activation state of Rubisco contributes to the control of A by reducing the effective Vcmax in vivo. Many studies show that peak activation states for Rubisco are around 80% to 95% (for example, Sage et al., 1989, 1990b), demonstrating some loss of efficiency in Rubisco use is inevitable in C3 plants when Rubisco is limiting. At elevated CO2 and at temperatures away from the thermal optimum, our analysis indicates Rubisco capacity becomes nonlimiting. Under these conditions, deactivation of Rubisco appears sufficient to balance the capacity for RuBP regeneration, as indicated by similarities between modeled A assuming RuBP regeneration is limiting, modeled A assuming deactivated Rubisco is limiting, and measured A (Fig. 8). These results show that parallel reductions in the activation state of Rubisco and A can be explained by regulated reduction of Rubisco capacity in response to a limitation in RuBP regeneration capacity, in addition to the previously proposed idea that declines in the activation state of Rubisco at elevated temperature directly limit A (Law and Crafts-Brandner, 1999; Crafts-Brandner and Salvucci, 2000; Haldimann and Feller, 2004).
Photosynthetic Limitation at Low Temperature
The recovery of the activation state of Rubisco following CO2 reduction at low temperature is consistent with a regulatory feedback reflecting limitations in the RuBP regeneration capacity. Rather than electron transport being limiting, the gas exchange data indicate A becomes limited by the Pi regeneration capacity below 15°C at the higher CO2 treatments. The observed lack of an effect of O2 reduction or CO2 enrichment on A (Fig. 5B) is the main evidence for a Pi regeneration limitation at low temperature (Sage and Sharkey, 1987).
Prior examinations of Rubisco regulation at low temperature generally found no change or an increase in the activation state of Rubisco (Schnyder et al., 1984; Holaday et al., 1992; Hurry et al., 1994; Savitch et al., 1997). We cannot explain this discrepancy, in part because of differences in experimental design between this and other studies. Earlier work often sampled species in growth cabinets, where light levels may have been subsaturating for photosynthesis. Furthermore, in a number of cases, plants examined were acclimated to the growth temperature. During low temperature acclimation, the Pi regeneration capacity often increases relative to other processes in the leaf, such that Pi regeneration limitations are removed (Paul et al., 1990; Holaday et al., 1992; Huner et al., 1993; Strand et al., 1999). This study did not examine acclimation effects, as leaves were exposed to low temperature for less than a few hours.
CONCLUSION
The abundance of recent reports that Rubisco activase controls the response of C3 photosynthesis to elevated temperature has led to the impression that processes contributing to the capacity of RuBP regeneration do not contribute to photosynthetic limitation above the thermal optimum. Our findings in sweet potato, in addition to earlier reports for Pima cotton (Gossypium barbadense) and sunflower (Helianthus annuus; Oja et al., 1988; Schrader et al., 2004; Wise et al., 2004), indicate that activase is not the leading limitation above the thermal optimum. Instead, activase may be mediating imbalances between energy production and utilization in leaves exposed to warm conditions, as occurs in leaves exposed to shade or elevated CO2 at moderate temperatures. We recognize the situation may differ in other species such as tobacco and spinach where gas exchange, fluorescence, and metabolite data indicate activase may be impaired by heat to such a degree that it becomes the primary limitation on photosynthesis. However, we challenge the view that has predominated in the recent literature that activase lability is always the principal limitation on A at elevated temperature. With future increases in atmospheric CO2 and global temperature, plants will increasingly operate in conditions where the activation state of Rubisco is depressed. If we hope to efficiently focus our efforts on improving photosynthesis under these conditions, then it is important to understand when, and in what species, the activation state of Rubisco controls A, as opposed to responding to controls that reside elsewhere within the photosynthetic apparatus.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Sweet potato (Ipomoea batatas) roots were purchased at a local market and grown in a greenhouse in 20-L pots of soil (50% loam, 25% sand, and 25% perlite) at a set-point temperature of 27°C/20°C (day/night temperature). Air temperature in the greenhouse reached 32°C to 35°C on warm days during the growing season. Plants were watered daily and fertilized twice weekly with an all-purpose plant food (Scotts-Miracle Grow). Experiments were conducted between April and October. Maximum light intensities during growth exceeded 1,600 μmol photons m−2 s−1 on sunny days. In all experiments, the most recent, fully expanded leaves were used.
Gas Exchange Analyses
The response of A and stomatal conductance to variation in temperature and atmospheric CO2 was measured using a null-balance gas exchange system modified from that of Sharkey (1985) as described by Pittermann and Sage (2000). Measurements were conducted at 200 or 30 mbar O2 and a vapor pressure difference between leaf and air of 6 to 12 mbar. To determine the light response of A, leaves were equilibrated in the leaf cuvette at an ambient CO2 level of 360 μbar, 1,900 μmol photon m−2 s−1, and either 25°C or 35°C. After this, the atmospheric CO2 partial pressure was adjusted so that the Ci was 140, 250, or 500 μbar, with a variation of ±5 μbar. The photosynthetic photon flux density was then reduced in steps of 80 ± 20 μmol m−2 s−1, with measurements made at each step after 15 min for equilibration of the gas exchange parameters.
The CO2 response of A was measured at 1,000 to 1,100 μmol photons m−2 s−1, which was determined to be saturating in all treatment conditions (Fig. 1). Leaves were first equilibrated at this photosynthetic photon flux density, a temperature between 10°C and 40°C, 200 mbar O2, and an ambient CO2 partial pressure of 360 μbar. The ambient CO2 partial pressure was then increased to more than 760 μbar to give a Ci of at least 500 μbar, and allowed to stabilize for 30 min before measurements were initiated. After the initial measurement, CO2 levels were reduced 10% to 20%, A was allowed to equilibrate again, and the measurements repeated. This procedure was also used to study the CO2 response of A at 30 mbar O2.
The temperature response of day respiration, Rd, was measured according to Brooks and Farquhar (1985) by comparing the difference in CO2 exchange between the CO2 compensation point at 200 mbar O2 and the CO2 compensation point in the absence of photorespiration (Γ*). The value of Γ* at 25°C was measured for sweet potato according to Brooks and Farquhar (1985) and is given in the Supplemental Appendix. Because the value of Γ* at 25°C in sweet potato equaled that in spinach (Spinacia oleracea) determined by Brooks and Farquhar (1985), we used their temperature dependence for Γ* in our estimations of Rd. The triose phosphate utilization rate was estimated from the O2- and CO2-insensitive values of A and the measured Rd at a given temperature (Sharkey, 1985; Harley and Sharkey, 1991). All gas exchange parameters were calculated according to von Caemmerer and Farquhar (1981). Q10 values and activation energies were calculated according to Berry and Raison (1981).
Freeze-Clamp Procedures
Leaf samples for enzyme and metabolite assays were collected using a freeze-clamp system with a hand-operated clamp. Instead of the steady-state leaf chamber used for analysis of gas exchange responses to light, CO2, and temperature, a freeze-clamp leaf cuvette was connected to the null-balance machine described above. The freeze-clamp cuvette consisted of a water-jacketed aluminum block with equal-sized top and bottom sections. A 9-cm2 hole was cut in the center of the each section of the block, and a groove at the edge of holes was milled to fit a brass O-ring. Leaves were sandwiched between the blocks such that they filled the open holes, and the leaf was sealed off from the atmosphere by placing a film of plastic wrap (Saran-Wrap) over the hole and anchoring it in place by inserting the brass ring into a groove surrounding the hole. Silica grease was used to ensure a tight seal between chamber and plastic wrap. Six air channels in both the top and bottom halves of the block connected the cuvette atmosphere with inlet and outlet ports. This created sufficient airflow to prevent still-air pockets and poor heat exchange. Prior to freeze-clamping, leaves were equilibrated in the cuvette at 1,000 μmol photons m−2 s−1, 360 μbar CO2, and 25°C. Once it was determined that leaves were not damaged, the temperature and atmospheric conditions were changed to the desired measurement levels. To provide enough time for the activation state of Rubisco and metabolite levels to stabilize, leaves were sampled 30 min after gas exchange parameters reached the steady state. Gas exchange values were then recorded, and the leaf was freeze-clamped. Freeze-clamping consisted of placing freeze-clamp tongs into a mounting assembly that guided prechilled copper heads on the end of the tongs to precisely close through the leaf chamber opening and clamp the leaf. The tongs were hand operated, and with practice could reliably and repeatedly clamp and freeze the leaf within 0.25 s of interrupting the measurement conditions. Rapid freezing was ensured by prechilling the copper heads in liquid nitrogen. Once frozen, leaves were quickly removed from the clamp and transferred to liquid nitrogen for storage. The copper tongs were milled to produce two equal leaf halves of 3.5 cm2 following clamping. One half was used for Rubisco and chlorophyll assays; the second half was used for the metabolite assay.
Electron Transport Assay
The rate of whole-chain electron transport was assayed after rapidly grinding 2.8-cm2 leaf discs in 5.0 mL of extraction buffer (30 mm NaCl, 5 mm MgCl2, 0.5% bovine serum albumin, 10 mm EDTA, 50 mm Tricine) at pH 7.6 (Sage et al., 1995). Electron transport activity in 0.5-mL aliquots in an assay buffer (30 mm pyrophosphate, pH 8.0, 10 mm MgCl2, 2.5 mm NH4Cl, 0.1 mm methyl viologen) was determined by measuring electron flow to methyl viologen in a Rank Brothers O2 electrode (von Caemmerer and Farquhar, 1981). Assay temperature was controlled by a water bath that circulated water through the electrode water jacket. The procedure was modified to minimize time between extract and assay so that the time between grinding and assay was <120 s. Rapid extraction yielded the high rates required to explain the gas exchange results as estimated using the functions of Farquhar and Wong (1984). However, we observed that Jmax values determined in vitro were 17% higher than values from gas exchange estimated using the Farquhar and Wong (1984) model. We believe this difference occurs because leaves used in the electron transport measurements were produced later in the season than those used for gas exchange measurements.
Enzyme and Metabolite Assays
Rubisco extraction and assay followed procedures modified from Sage et al. (1993). Frozen leaf disks were rapidly (within 90 s) extracted using a Ten Broeck homogenizer in a buffer of 100 mm Bicine, pH 8.2, 10 mm dithiothreitol, 0.4% bovine serum albumin, 2% polyvinylpolypyrolidone, 1% polyethylene glycol, 3% polyoxyethylene sorbitol monoleate, 4 mm amino-n-caproic acids, 0.8 mm benzamide, 20 mm MgCl2, and 150 μm NaHCO3. Immediately after centrifuging the extract at 5,000 to 10,000g for 15 s, the initial activity of Rubisco was assayed at 25°C for 30 s by determining the amount of radiolabeled CO2 (14C-NaHCO3) incorporated into acid-stable products. Rubisco was then activated by bringing the concentration of NaHCO3 in an aliquot of the extract to 10 mm and allowing the extract to incubate at room temperature for 10 to 15 min, at which point the total, fully activated activity was measured by determining the incorporation of 14C-CO2 into acid stable products. The ratio of initial to total activity is termed the activation state of Rubisco, which is a close estimate of the degree at which the enzyme is carbamylated (Butz and Sharkey, 1989). To determine the response of fully activated Rubisco activity to temperature, the enzyme was extracted as above but in the presence of 10 mm rather than 150 μm NaHCO3, and allowed to activate for 5 to 10 min. Assay temperature was controlled between 5°C and 45°C with a series of temperature-regulated water baths.
Rubisco content was determined by incubating activated Rubisco enzyme in the presence of 14C-carboxyarabinitol bisphosphate (CABP) and rabbit anti-Rubisco antibodies for 2 to 3 h at 37°C (Sage et al., 1993). The coagulated complex of Rubisco, 14C-CABP, and antibodies was filtered using a Gelman Supor 450-μm filter, and the collected radiolabeled material was quantified by liquid scintillation counting assuming 6.5 CABP molecules bound to each Rubisco molecule (Butz and Sharkey, 1989). Chlorophyll content was determined by bringing a 200-μL aliquot of a Rubisco extract to 1 mL with N,N-dimethylformamide, and spectrophotometrically assaying the supernatant at 645 and 667 nm (Porra et al., 1989).
For the metabolite assays, leaf samples were extracted by grinding frozen leaf disks in 3.5% perchloric acid at liquid nitrogen temperature. After thawing, the extract was centrifuged at 8,000g for 2 min, and the supernatant was neutralized with 1.6 n KOH and then frozen in liquid nitrogen and stored at −80°C until assay. RuBP and PGA were assayed according to Seemann and Sharkey (1986) by first preincubating the extract with hexokinase and 1 mm Glc to remove any ATP and pentose phosphates other than RuBP in the sample. RuBP was determined by measuring the 14CO2 converted into acid-stable products using purified spinach Rubisco (Seemann and Sharkey, 1986). PGA was determined spectrophotometrically by NADPH oxidation with 0.9 unit mL−1 glycerate-3-phosphate kinase and 0.4 unit mL−1 glyceraldehyde-phosphate dehydrogenase using a diode array spectrophotometer (Hewlett-Packard 8452A; Seemann and Sharkey, 1986).
Modeling
To evaluate potential limitations caused by Rubisco capacity or the RuBP regeneration capacity, we modeled the temperature response of A using established photosynthesis models (Farquhar and Wong, 1984; Brooks and Farquhar, 1985; von Caemmerer, 2000) with modifications to account for limitations in the rate of triose phosphate use or Rubisco activation state (Sage, 1990). Temperature responses of the modeled parameters were derived from Farquhar et al. (1980), Jordan and Ogren (1984), and Brooks and Farquhar (1985), and are given in the Supplemental Appendix. Because the kinetic constants for sweet potato Rubisco are unknown, we had to use published values from other species. We observed that a composite of constants derived by pooling spinach kc values presented by Farquhar et al. (1980) and Jordan and Ogren (1984) yielded the most consistent match between modeled and measured data.
Because of a minor discrepancy between electron transport measurements in vitro and those estimated from gas exchange, we corrected the in vitro data by a factor of 0.86, and then fitted this corrected data to a polynomial function for use in modeling the response of Jmax to temperature (see Eq. A3 in the Supplemental Appendix).
Supplementary Material
Acknowledgments
We thank Debbie Tam for her help in growing the sweet potato plants, and David Kubien, Amane Makino, and Honor McCann for comments on the manuscript.
This work was supported by the National Sciences and Engineering Research Council of Canada (grant no. OGP0154273).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.066233.
References
- Berry JA, Raison JK (1981) Responses of macrophytes to temperature. In OL Lange, PS Nobel, CB Osmond, H Ziegler, eds, Physiological Plant Ecology I: Responses to the Physical Environment, Vol 12A. Springer-Verlag, Berlin, pp 277–338
- Brooks A, Farquhar GD (1985) Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase oxygenase and the rate of respiration in the light—estimates from gas-exchange measurements on spinach. Planta 165: 397–406 [DOI] [PubMed] [Google Scholar]
- Bukhov NG, Wiese C, Neimanis S, Heber U (1999) Heat sensitivity of chloroplasts and leaves: leakage of protons from thylakoids and reversible activation of cyclic electron transport. Photosynth Res 59: 81–93 [Google Scholar]
- Butz ND, Sharkey TD (1989) Activity ratios of ribulose-1,5-bisphosphate carboxylase accurately reflect carbamylation ratios. Plant Physiol 89: 735–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Law RD (2000) Effect of heat stress on the inhibition and recovery of the ribulose-1,5-bisphosphate carboxylase/oxygenase activation state. Planta 212: 67–74 [DOI] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME (2000) Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proc Natl Acad Sci USA 97: 13430–13435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, van deLoo FJ, Salvucci ME (1997) The two forms of ribulose-1,5-bisphosphate carboxylase/oxygenase activase differ in sensitivity to elevated temperature. Plant Physiol 114: 439–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer S (1982) Modelling of photosynthetic response to environmental conditions. In OL Lange, PS Nobel, CB Osmond, H Ziegler, eds, Physiological Plant Ecology II: Water Relations and Carbon Assimilation, Vol 12B. Springer-Verlag, Berlin, pp 549–587
- Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 plants. Planta 149: 78–90 [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Wong SC (1984) An empirical-model of stomatal conductance. Aust J Plant Physiol 11: 191–209 [Google Scholar]
- Feller U, Crafts-Brandner SJ, Salvucci ME (1998) Moderately high temperatures inhibit ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase-mediated activation of Rubisco. Plant Physiol 116: 539–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldimann P, Feller U (2004) Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat-dependent reduction of the activation state of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Cell Environ 27: 1169–1183 [Google Scholar]
- Harley PC, Sharkey TD (1991) An improved model of C3 photosynthesis at high CO2—reversed O2 sensitivity explained by lack of glycerate reentry into the chloroplast. Photosynth Res 27: 169–178 [DOI] [PubMed] [Google Scholar]
- Harley PC, Weber JA, Gates DM (1985) Interactive effects of light, leaf temperature, CO2, and O2 on photosynthesis in soybean. Planta 165: 249–263 [DOI] [PubMed] [Google Scholar]
- Hendrickson L, Chow WS, Furbank RT (2004) Low temperature effects on grapevine photosynthesis: the role of inorganic phosphate. Funct Plant Biol 31: 789–801 [DOI] [PubMed] [Google Scholar]
- Holaday AS, Martindale W, Alred R, Brooks AL, Leegood RC (1992) Changes in activities of enzymes of carbon metabolism in leaves during exposure of plants to low-temperature. Plant Physiol 98: 1105–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huner NPA, Oquist G, Hurry VM, Krol M, Falk S, Griffith M (1993) Photosynthesis, photoinhibition and low-temperature acclimation in cold tolerant plants. Photosynth Res 37: 19–39 [DOI] [PubMed] [Google Scholar]
- Hurry VM, Malmberg G, Gardeström P, Öquist G (1994) Effects of a short-term shift to low-temperature and of long-term cold hardening on photosynthesis and ribulose-1,5-bisphosphate carboxylase oxygenase and sucrose-phosphate synthase activity in leaves of winter rye (Secale cereale L.). Plant Physiol 106: 983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan DB, Ogren WL (1984) The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase oxygenase—dependence on ribulosebisphosphate concentration, pH and temperature. Planta 161: 308–313 [DOI] [PubMed] [Google Scholar]
- June T, Evans JR, Farquhar GD (2004) A simple new equation for the reversible temperature dependence of photosynthetic electron transport: a study on soybean leaf. Funct Plant Biol 31: 275–283 [DOI] [PubMed] [Google Scholar]
- Kim K, Portis AR (2005) Temperature dependence of photosynthesis in Arabidopsis plants with modifications in Rubisco activase and membrane fluidity. Plant Cell Physiol 46: 522–530 [DOI] [PubMed] [Google Scholar]
- Kobza J, Edwards GE (1987) Influences of leaf temperature on photosynthetic carbon metabolism in wheat. Plant Physiol 83: 69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law RD, Crafts-Brandner SJ (1999) Inhibition and acclimation of photosynthesis to heat stress is closely correlated with activation of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiol 120: 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law RD, Crafts-Brandner SJ (2001) High temperature stress increases the expression of wheat leaf ribulose-1,5-bisphosphate carboxylase/oxygenase activase protein. Arch Biochem Biophys 386: 261–267 [DOI] [PubMed] [Google Scholar]
- Law RD, Crafts-Brandner SJ, Salvucci ME (2001) Heat stress induces the synthesis of a new form of ribulose-1,5-bisphosphate carboxylase/oxygenase activase in cotton leaves. Planta 214: 117–125 [DOI] [PubMed] [Google Scholar]
- Lorenz OA, Maynard DN (1988) Knott's Handbook for Vegetable Growers, Ed 3. Wiley-Interscience, New York
- Mott KA, Jensen RG, Oleary JW, Berry JA (1984) Photosynthesis and ribulose 1,5-bisphosphate concentrations in intact leaves of Xanthium strumarium L. Plant Physiol 76: 968–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oja VM, Rasulov BH, Laisk AH (1988) An analysis of the temperature dependence of photosynthesis considering the kinetics of RuP2 carboxylase and the pool of RuP2 in intact leaves. Aust J Plant Physiol 15: 737–748 [Google Scholar]
- Pastenes C, Horton P (1996) Effect of high temperature on photosynthesis in beans. 2. CO2 assimilation and metabolite contents. Plant Physiol 112: 1253–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Lawlor DW, Driscoll SP (1990) The effect of temperature on photosynthesis and carbon fluxes in sunflower and rape. J Exp Bot 41: 547–555 [Google Scholar]
- Pittermann J, Sage RF (2000) Photosynthetic performance at low temperature of Bouteloua gracilis Lag., a high-altitude C4 grass from the Rocky Mountains, USA. Plant Cell Environ 23: 811–823 [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous-equations for assaying chlorophyll-a and chlorophyll-b extracted with 4 different solvents—verification of the concentration of chlorophyll standards by atomic-absorption spectroscopy. Biochim Biophys Acta 975: 384–394 [Google Scholar]
- Portis AR (2003) Rubisco activase—Rubisco's catalytic chaperone. Photosynth Res 75: 11–27 [DOI] [PubMed] [Google Scholar]
- Price GD, von Caemmerer S, Evans JR, Siebke K, Anderson JM, Badger MR (1998) Photosynthesis is strongly reduced by antisense suppression of chloroplastic cytochrome bf complex in transgenic tobacco. Aust J Plant Physiol 25: 445–452 [Google Scholar]
- Roy HA, Andrews TJ (2000) Rubisco: assembly and mechanism. In RC Leegood, TD Sharkey, S von Caemmerer, eds, Photosynthesis: Physiology and Metabolism. Kluwer Academic, Dordrecht, The Netherlands, pp 53–83
- Ruuska SA, Andrews TJ, Badger MR, Price GD, von Caemmerer S (2000) The role of chloroplast electron transport and metabolites in modulating Rubisco activity in tobacco. Insights from transgenic plants with reduced amounts of cytochrome b/f complex or glyceraldehyde 3-phosphate dehydrogenase. Plant Physiol 122: 491–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF (1990) A model describing the regulation of ribulose-1,5-bisphosphate carboxylase, electron-transport, and triose phosphate use in response to light-intensity and CO2 in C3 plants. Plant Physiol 94: 1728–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Reid CD, Moore BD, Seemann JR (1993) Long-term kinetics of the light-dependent regulation of ribulose-1,5-bisphosphate carboxylase oxygenase activity in plants with and without 2-carboxyarabinitol 1-phosphate. Planta 191: 222–230 [Google Scholar]
- Sage RF, Santrucek J, Grise DJ (1995) Temperature effects on the photosynthetic response of C3 plants to long-term CO2 enrichment. Vegetatio 121: 67–77 [Google Scholar]
- Sage RF, Sharkey TD (1987) The effect of temperature on the occurrence of O2 and CO2 insensitive photosynthesis in field-grown plants. Plant Physiol 84: 658–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Sharkey TD, Seemann JR (1988) The in vivo response of the ribulose-1,5-bisphosphate carboxylase activation state and the pool sizes of photosynthetic metabolites to elevated CO2 in Phaseolus vulgaris L. Planta 174: 407–416 [DOI] [PubMed] [Google Scholar]
- Sage RF, Sharkey TD, Seemann JR (1989) Acclimation of photosynthesis to elevated CO2 in 5 C3 species. Plant Physiol 89: 590–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Sharkey TD, Pearcy RW (1990. a) The effect of leaf nitrogen and temperature on the CO2 response of photosynthesis in the C3 dicot Chenopodium album L. Aust J Plant Physiol 17: 135–148 [Google Scholar]
- Sage RF, Sharkey TD, Seemann JR (1990. b) Regulation of ribulose-1,5-bisphosphate carboxylase activity in response to light-intensity and CO2 in the C3 annuals Chenopodium album L. and Phaseolus vulgaris L. Plant Physiol 94: 1735–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci ME, Crafts-Brandner SJ (2004. a) Inhibition of photosynthesis by heat stress: the activation state of Rubisco as a limiting factor in photosynthesis. Physiol Plant 120: 179–186 [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Crafts-Brandner SJ (2004. b) Mechanism for deactivation of Rubisco under moderate heat stress. Physiol Plant 122: 513–519 [Google Scholar]
- Salvucci ME, Crafts-Brandner SJ (2004. c) Relationship between the heat tolerance of photosynthesis and the thermal stability of Rubisco activase in plants from contrasting thermal environments. Plant Physiol 134: 1460–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci ME, Osteryoung KW, Crafts-Brandner SJ, Vierling E (2001) Exceptional sensitivity of Rubisco activase to thermal denaturation in vitro and in vivo. Plant Physiol 127: 1053–1064 [PMC free article] [PubMed] [Google Scholar]
- Savitch LV, Gray GR, Huner NPA (1997) Feedback-limited photosynthesis and regulation of sucrose-starch accumulation during cold acclimation and low-temperature stress in a spring and winter wheat. Planta 201: 18–26 [Google Scholar]
- Seemann JR, Sharkey TD (1986) Salinity and nitrogen effects on photosynthesis, ribulose-1,5-bisphosphate carboxylase and metabolite pool sizes in Phaseolus vulgaris L. Plant Physiol 82: 555–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder H, Mächler F, Nosberger J (1984) Influence of temperature and O2 concentration on photosynthesis and light activation of ribulosebisphosphate carboxylase oxygenase in intact leaves of white clover (Trifolium repens L). J Exp Bot 35: 147–156 [Google Scholar]
- Schrader SM, Wise RR, Wacholtz WF, Ort DR, Sharkey TD (2004) Thylakoid membrane responses to moderately high leaf temperature in Pima cotton. Plant Cell Environ 27: 725–735 [Google Scholar]
- Sharkey TD (1985) O2 insensitive photosynthesis in C3 plants—its occurrence and a possible explanation. Plant Physiol 78: 71–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD (2005) Effects of moderate heat stress on photosynthesis: importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ 28: 269–277 [Google Scholar]
- Spreitzer RJ, Salvucci ME (2002) Rubisco: structure, regulatory interactions, and possibilities for a better enzyme. Annu Rev Plant Biol 53: 449–475 [DOI] [PubMed] [Google Scholar]
- Strand A, Hurry V, Henkes S, Huner N, Gustafsson P, Gardeström P, Stitt M (1999) Acclimation of Arabidopsis leaves developing at low temperatures. Increasing cytoplasmic volume accompanies increased activities of enzymes in the Calvin cycle and in the sucrose-biosynthesis pathway. Plant Physiol 119: 1387–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S (2000) Biochemical Models of Leaf Photosynthesis. CSIRO, Collingwood, Australia
- von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas-exchange of leaves. Planta 153: 376–387 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Quick WP (2000) Rubisco: physiology in vivo. In RC Leegood, TD Sharkey, S von Caemmerer, eds, Photosynthesis: Physiology and Metabolism. Kluwer, Dordrecht, The Netherlands, pp 85–113
- Vu JCV, Allen LH, Boote KJ, Bowes G (1997) Effects of elevated CO2 and temperature on photosynthesis and Rubisco in rice and soybean. Plant Cell Environ 20: 68–76 [Google Scholar]
- Weis E (1981. a) Reversible heat-inactivation of the Calvin cycle—a possible mechanism of the temperature regulation of photosynthesis. Planta 151: 33–39 [DOI] [PubMed] [Google Scholar]
- Weis E (1981. b) The temperature-sensitivity of dark-inactivation and light-activation of the ribulose-1,5-bisphosphate carboxylase in spinach-chloroplasts. FEBS Lett 129: 197–200 [Google Scholar]
- Wise RR, Olson AJ, Schrader SM, Sharkey TD (2004) Electron transport is the functional limitation of photosynthesis in field-grown Pima cotton plants at high temperature. Plant Cell Environ 27: 717–724 [Google Scholar]
- Wullschleger SD (1993) Biochemical limitations to carbon assimilation in C3 plants—a retrospective analysis of the A/Ci curves from 109 species. J Exp Bot 44: 907–920 [Google Scholar]
- Yamori W, Noguchi K, Terashima I (2005) Temperature acclimation of photosynthesis in spinach leaves: analyses of photosynthetic components and temperature dependencies of photosynthetic partial reactions. Plant Cell Environ 28: 536–547 [Google Scholar]
- Zhang N, Kallis RP, Ewy RG, Portis AR (2002) Light modulation of Rubisco in Arabidopsis requires a capacity for redox regulation of the larger Rubisco activase isoform. Proc Natl Acad Sci USA 99: 3330–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Portis AR (1999) Mechanism of light regulation of Rubisco: a specific role for the larger Rubisco activase isoform involving reductive activation by thioredoxin-f. Proc Natl Acad Sci USA 96: 9438–9443 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.